Investigation on the Regeneration and Corrosion Characteristics of an Anodized Aluminum Plate Regenerator
Abstract
:1. Introduction
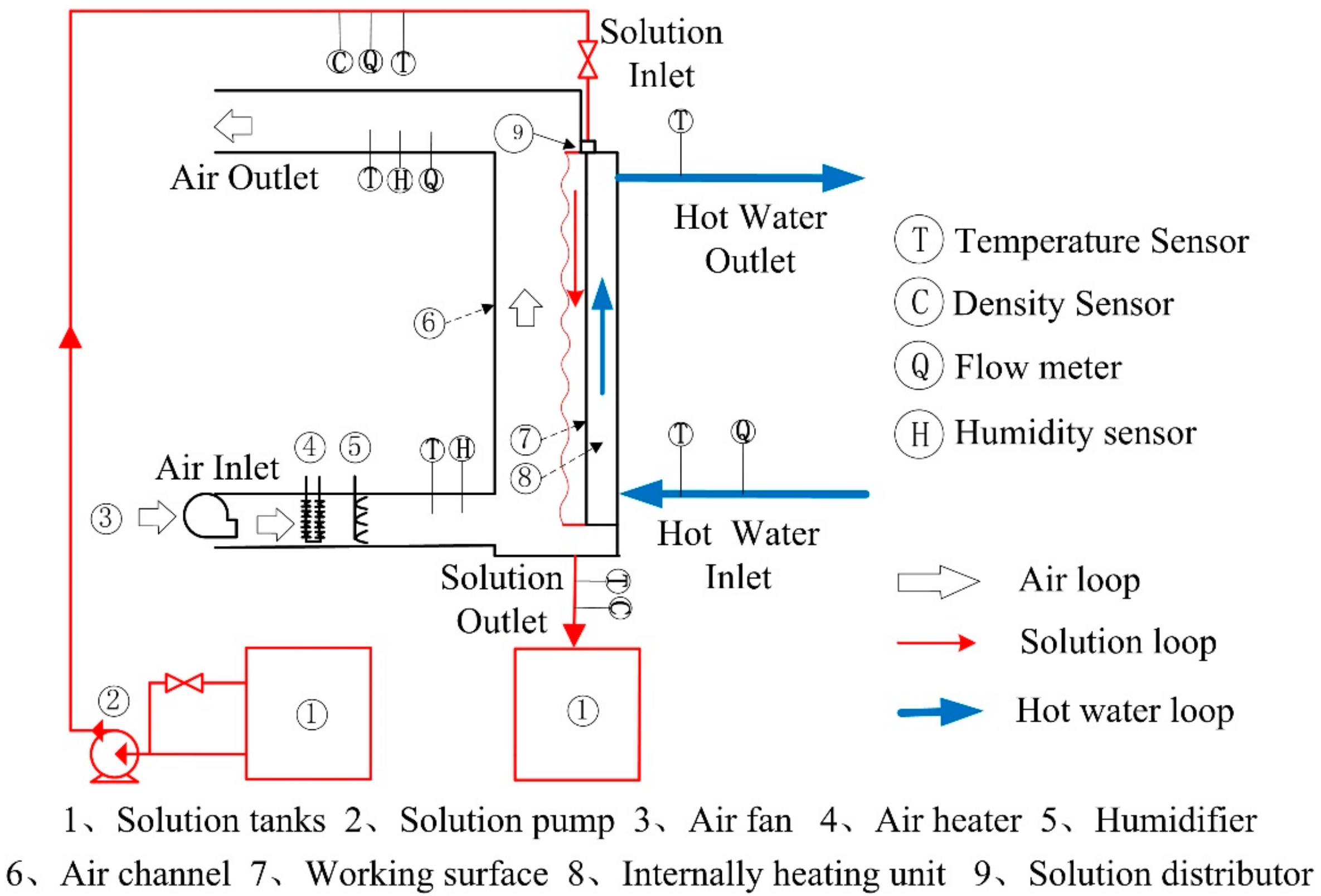
2. Experimental Apparatus
2.1. Experimental Method
2.2. Regeneration Performance Indices
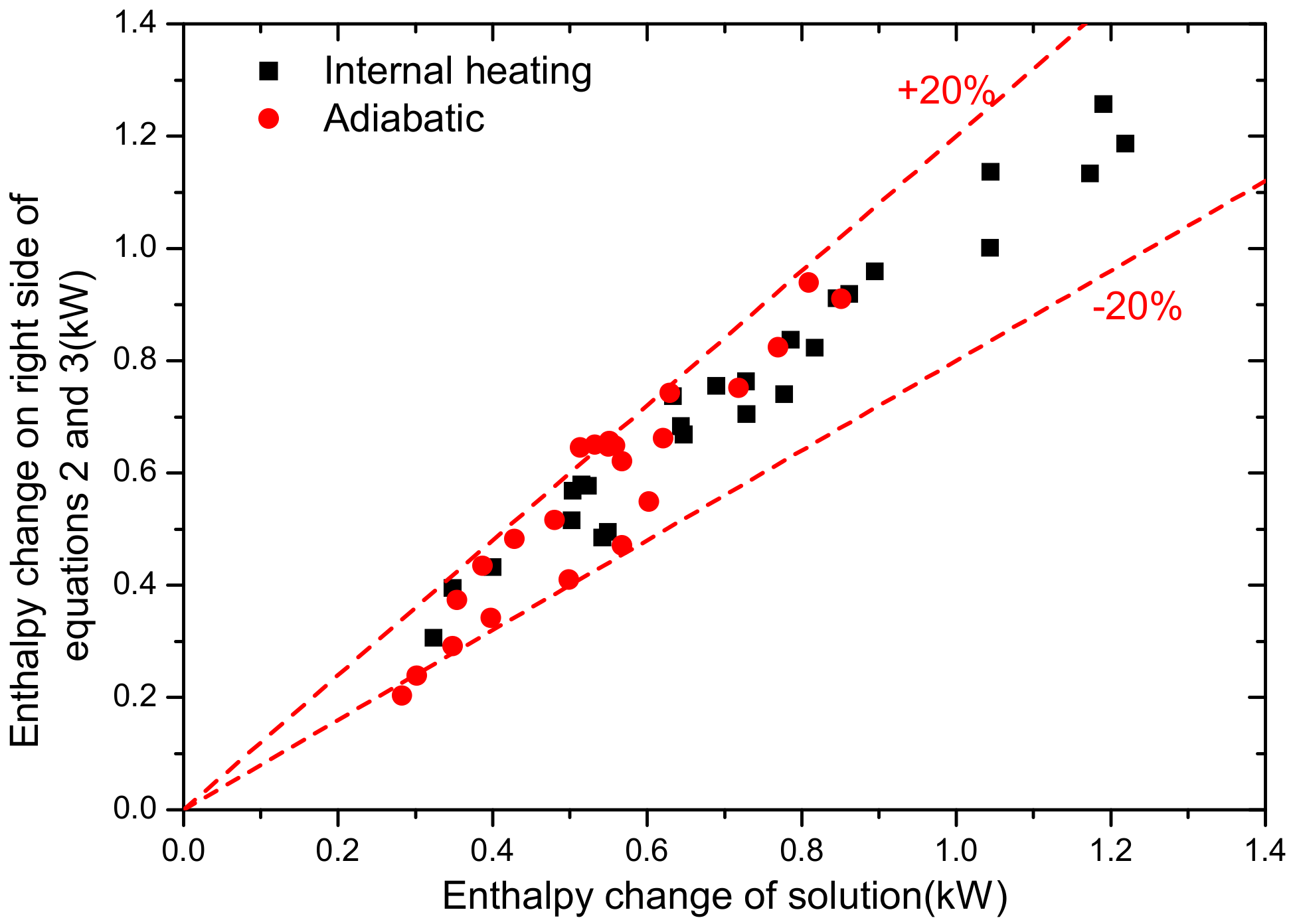
2.3. Uncertainty Analysis and Experimental Validation
3. Comparison of Corrosion and Regeneration Characteristics between Normal and Anodized Aluminum Regenerator
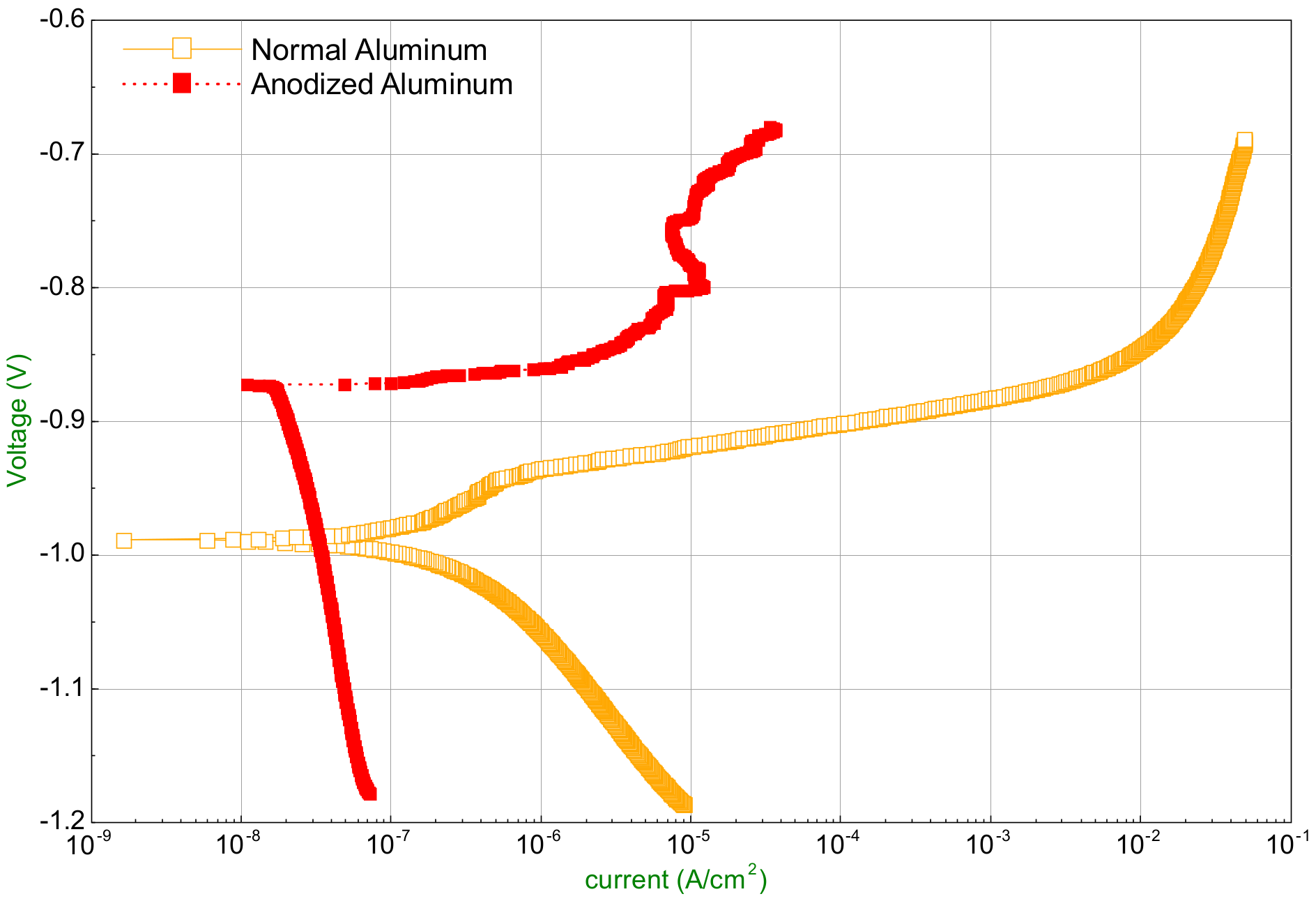
3.1. Corrosion Characteristics Identification by Electrochemical Method
3.2. Comparison of Regeneration Performance
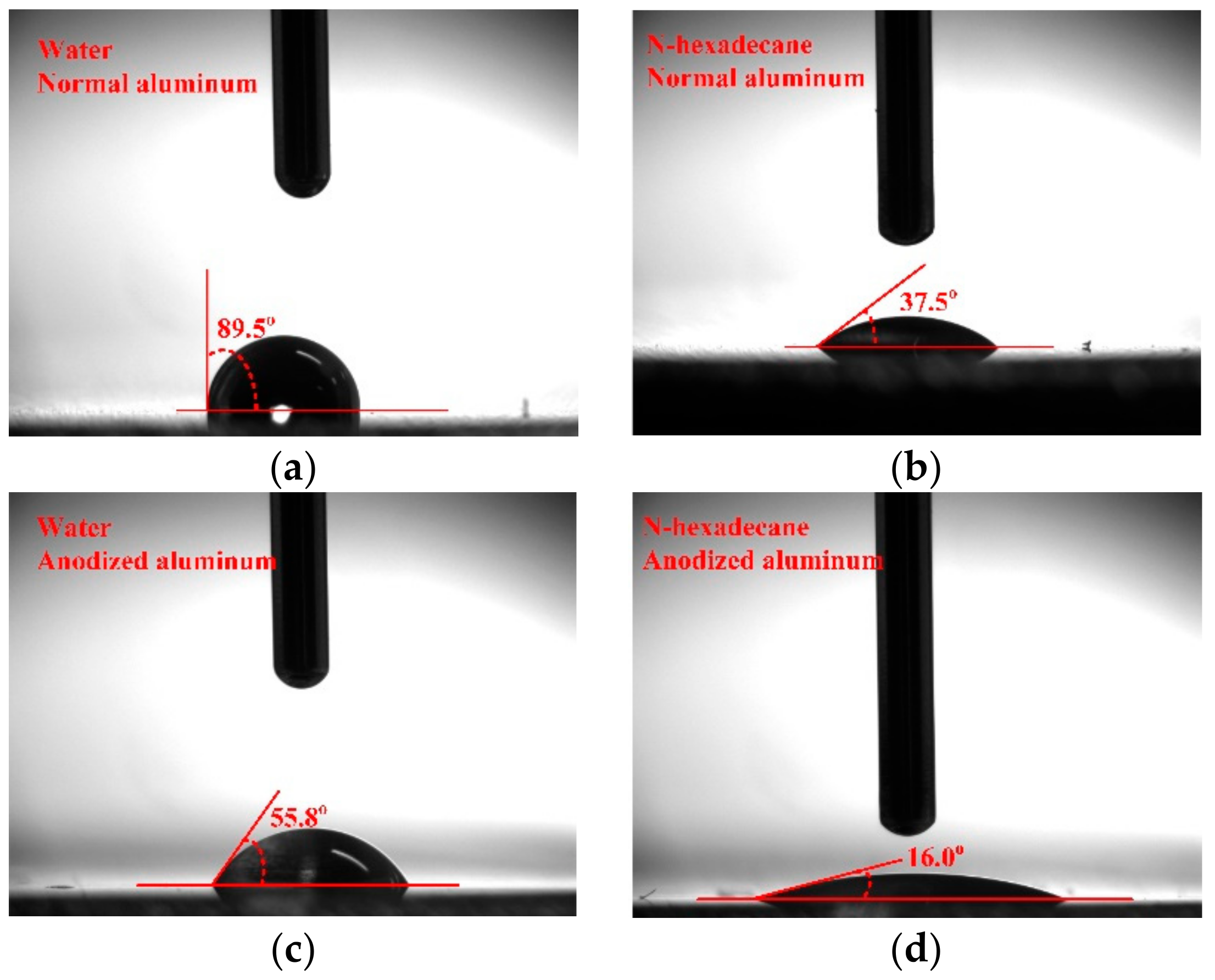
3.3. Discussion on Regeneration Performance Enhancement by Anodized Aluminum
4. Comparison of Regeneration Performance with and without Internal Heating
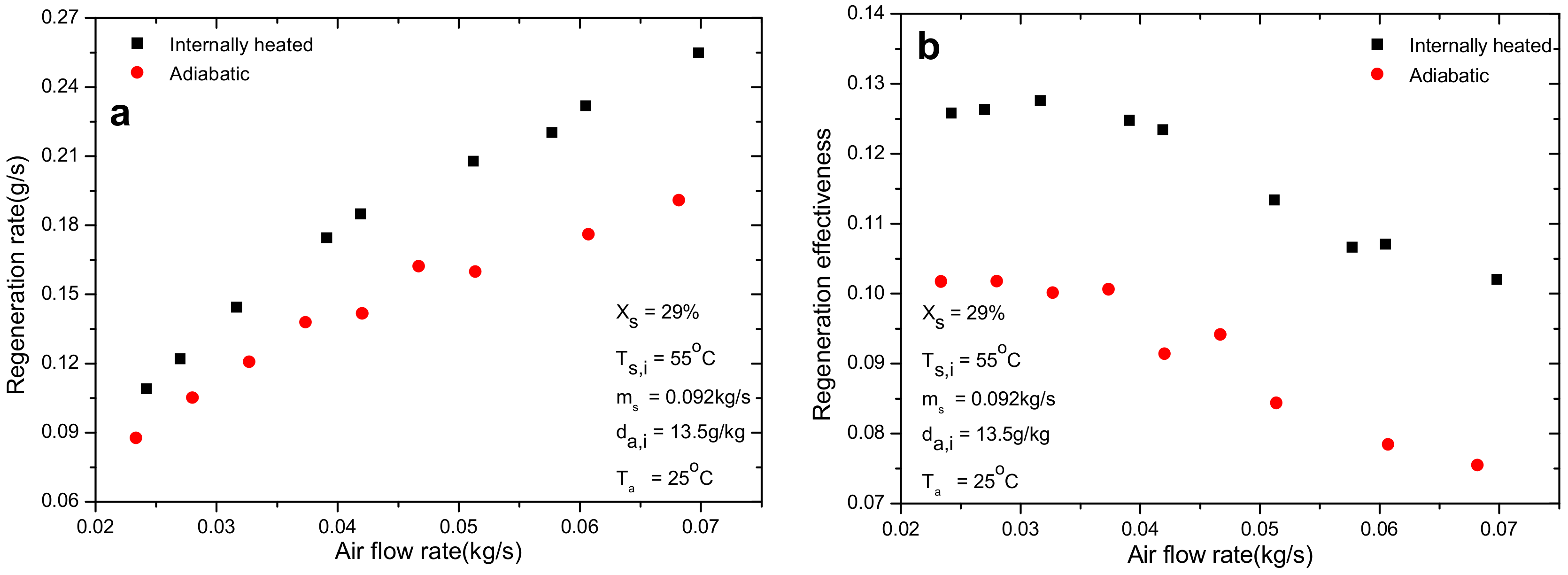
4.1. Effect of Air Flow Rate
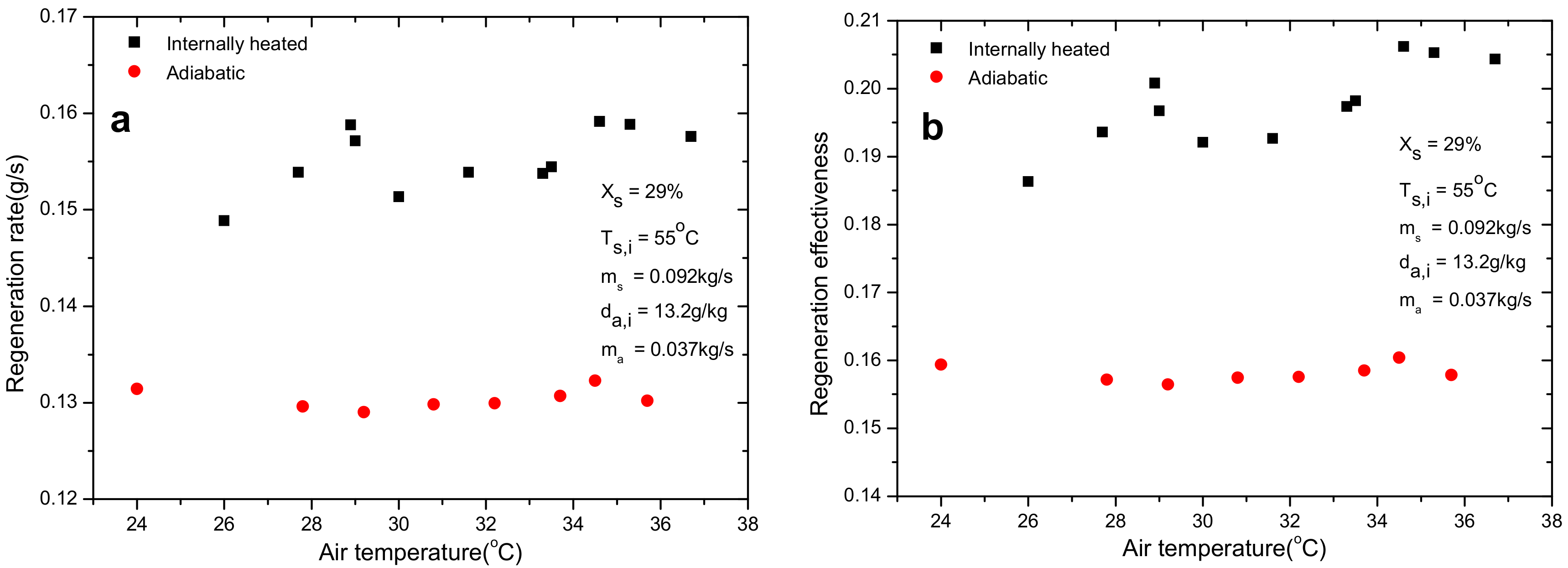
4.2. Effect of Air Inlet Dry Bulb Temperature
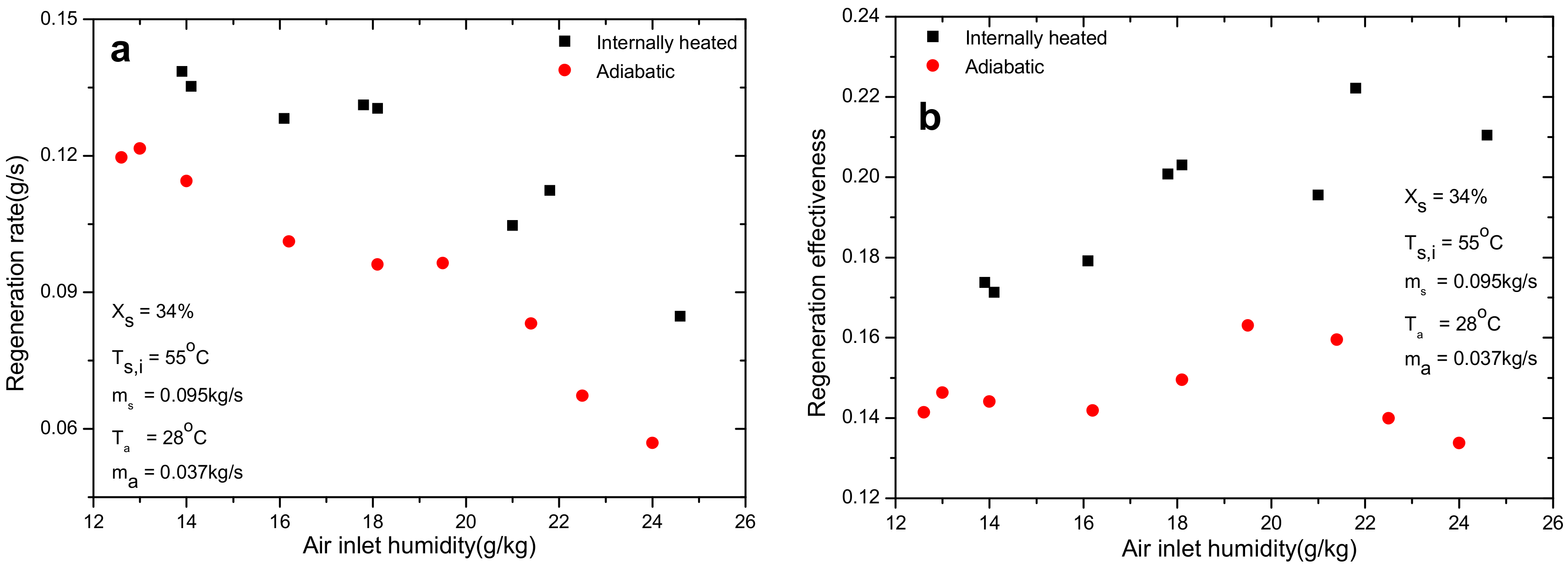
4.3. Effect of Air Inlet Absolute Humidity
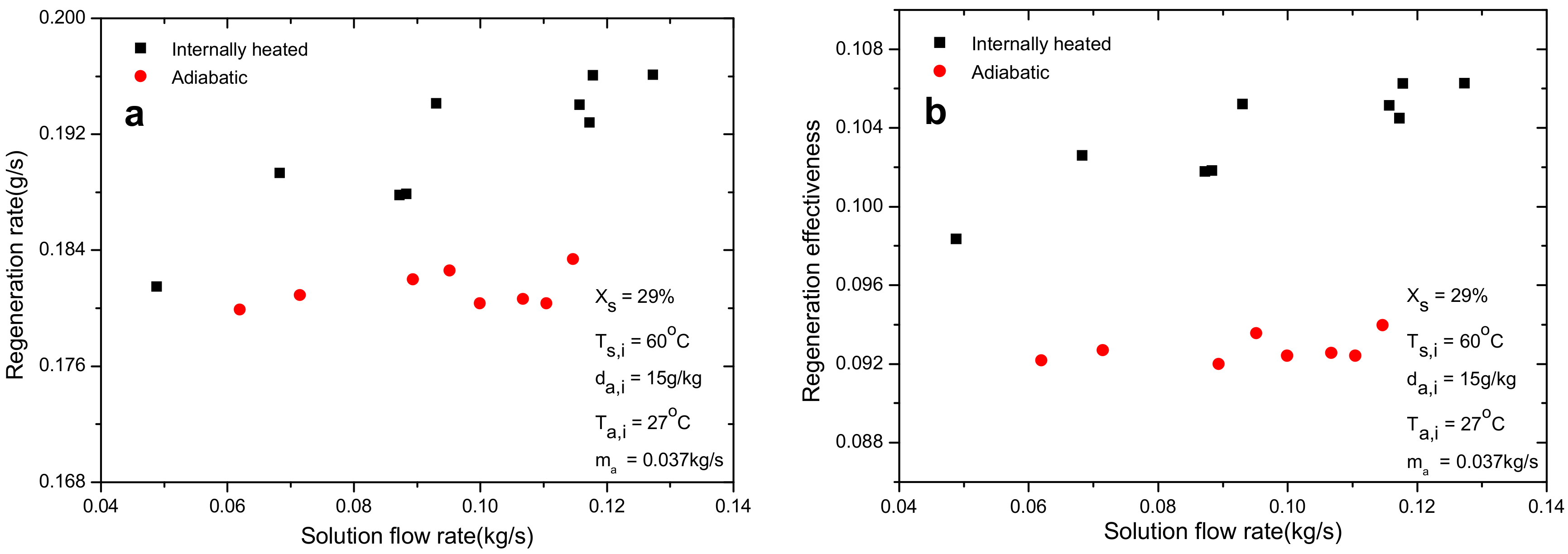
4.4. Effect of Solution Flow Rate
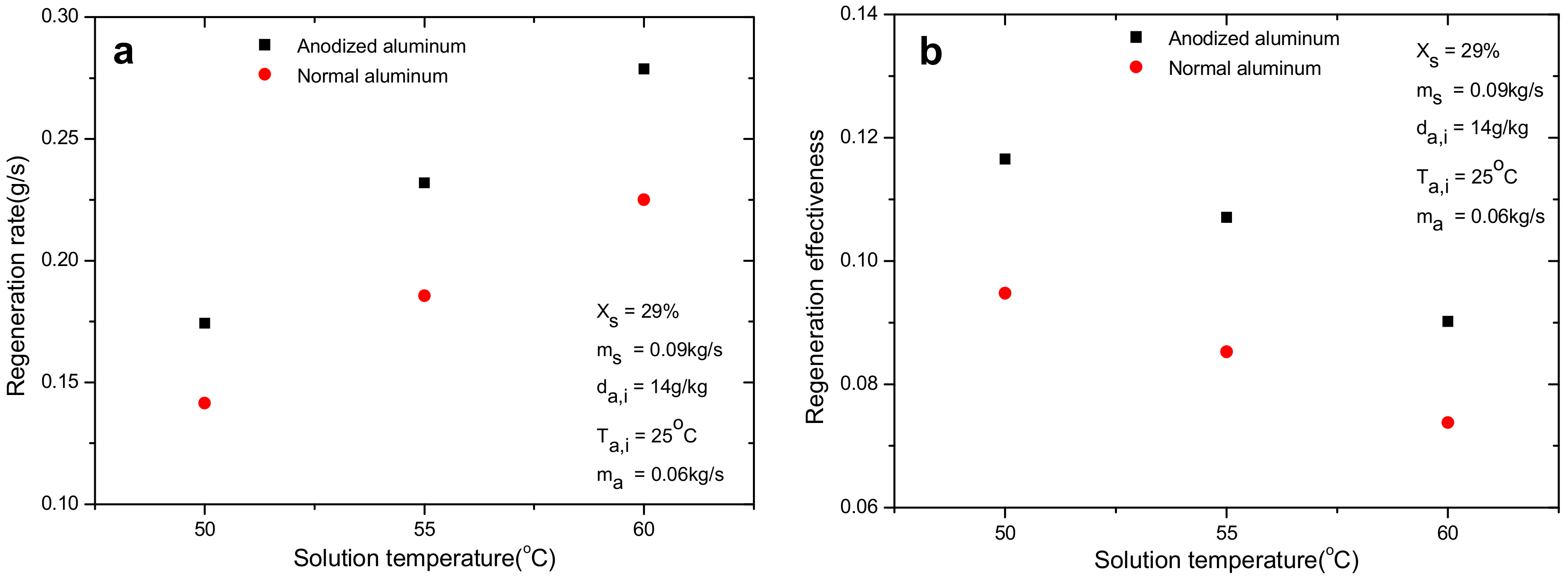
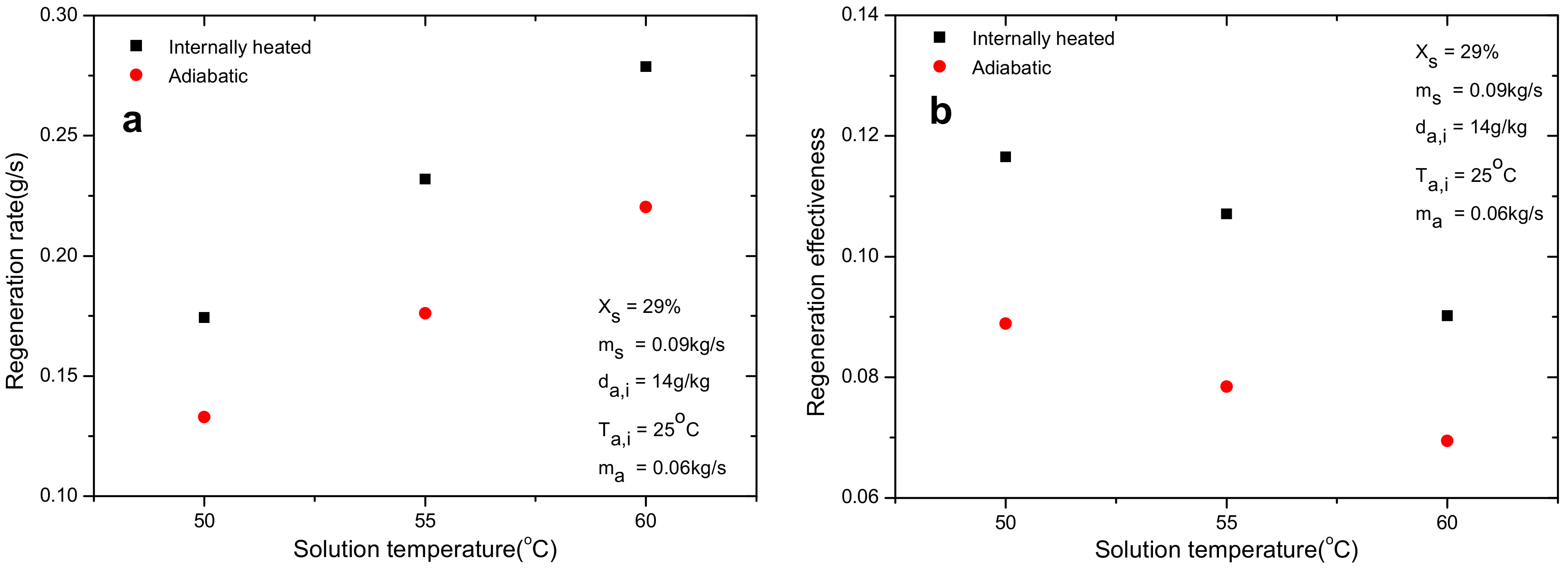
4.5. Effect of Solution Temperature
4.6. Effect of Solution Concentration
4.7. Discussion on Results and Phenomenon
5. Conclusions
- (1)
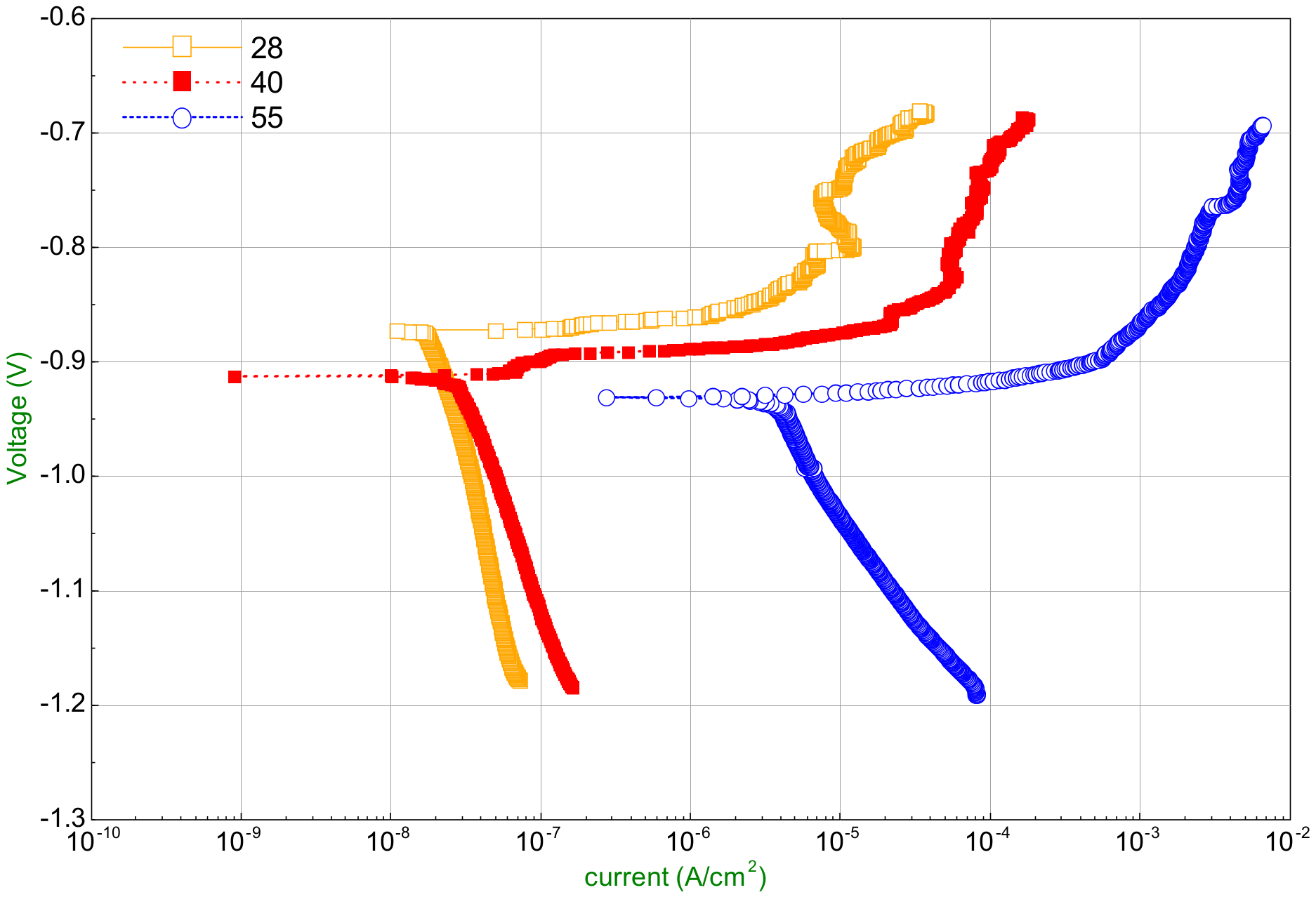
- The anodized aluminum regenerator can resist the corrosion by lithium chloride solution greatly compared with the ordinary aluminum one at relative low solution temperature. The corrosion rate decreases from 0.0005218 mm/year to 0.000011 m/year in 35% LiCl solution at 28 °C. However, when the solution temperature is higher, pitting corrosion occurs and enlarges gradually which is caused by the damage of oxide layer of anodizing.
- (2)
- Compared with the normal aluminum plate regenerator, the anodized one improves the regeneration performance significantly in terms of regeneration rate and effectiveness by 24% and 23.7%, respectively. The enhancement is directly ascribed to the improvement of wettability of falling film on regenerator, which is caused by the enlargement of surface energy after anodizing. The surface energy increases from 26.4 mN/m for normal aluminum to 47.6 mN/m for anodized one.
- (3)
- Relative improvements of 6.0–38% and 6.3–32% for regeneration rate and effectiveness were detected in the internal heating regenerator compared with the adiabatic one. The introduction of internal heating greatly alleviates the decrease of solution temperature and maintains the regeneration performance at higher level.
- (4)
- Air inlet humidity, solution temperature and solution concentration can directly determine the mass transfer driving force and affect the regeneration performance greatly. However, other parameters, such as air inlet temperature and solution temperature, show negligible influence on regeneration performance. The regeneration rate increases with the increase of air flow rate. Nevertheless, opposite trend is observed for regeneration effectiveness.
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviation
| Nomenclature | |||
| Absolute humidity () | Surface energy () | ||
| Potential () | Change value | ||
| Flow rate () | Subscripts | ||
| Enthalpy () | Air | ||
| Current () | Dispersion force | ||
| Liquid desiccant cooling system | corrosion | ||
| Molar mass () | Dry bulb | ||
| Regeneration rate (g/s) | Equilibrium | ||
| Temperature () | Inlet | ||
| Corrosion rate () | Liquid | ||
| Concentration () | Outlet | ||
| Greek symbols | Polar force | ||
| Relative humidity () | Solution/solid | ||
| Density () | Hot water | ||
References
- Buker, M.S.; Riffat, S.B. Recent developments in solar assisted liquid desiccant evaporative cooling technology—A review. Energy Build. 2015, 96, 95–108. [Google Scholar] [CrossRef]
- Rafique, M.M.; Gandhidasan, P.; Bahaidarah, H.M.S. Liquid desiccant materials and dehumidifiers—A review. Renew. Sustain. Energy Rev. 2016, 56, 179–195. [Google Scholar] [CrossRef]
- Abdel-Salam, A.H.; Simonson, C.J. State-of-the-art in liquid desiccant air conditioning equipment and systems. Renew. Sustain. Energy Rev. 2016, 58, 1152–1183. [Google Scholar] [CrossRef]
- Ahmed, M.H.; Kattab, N.M.; Fouad, M. Evaluation and optimization of solar desiccant wheel performance. Renew. Energy 2005, 30, 305–325. [Google Scholar] [CrossRef]
- Fumo, N.; Goswami, D.Y. Study of an aqueous lithium chloride desiccant system: Air dehumidification and desiccant regeneration. Sol. Energy 2002, 72, 351–361. [Google Scholar] [CrossRef]
- Zhang, L.; Hihara, E.; Matsuoka, F.; Dang, C. Experimental analysis of mass transfer in adiabatic structured packing dehumidifier/regenerator with liquid desiccant. Int. J. Heat Mass Transf. 2010, 53, 2856–2863. [Google Scholar] [CrossRef]
- Yin, Y.; Zhang, X.; Chen, Z. Experimental study on dehumidifier and regenerator of liquid desiccant cooling air conditioning system. Build. Environ. 2007, 42, 2505–2511. [Google Scholar] [CrossRef]
- Bassuoni, M.M. An experimental study of structured packing dehumidifier/regenerator operating with liquid desiccant. Energy 2011, 36, 2628–2638. [Google Scholar] [CrossRef]
- Liu, X.H.; Jiang, Y.; Chang, X.M.; Yi, X.Q. Experimental investigation of the heat and mass transfer between air and liquid desiccant in a cross-flow regenerator. Renew. Energy 2007, 32, 1623–1636. [Google Scholar] [CrossRef]
- Cannistraro, M.; Galvagno, A.; Trovato, G. Analysis and measures for energy savings in operating theaters. Int. J. Heat Technol. 2017, 35, S422–S448. [Google Scholar] [CrossRef]
- Liu, X.H.; Jiang, Y.; Yi, X.Q. Effect of regeneration mode on the performance of liquid desiccant packed bed regenerator. Renew. Energy 2009, 34, 209–216. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, Y.; Xia, J.; Chang, X. Analytical solutions of coupled heat and mass transfer processes in liquid desiccant air dehumidifier/regenerator. Energy Convers. Manag. 2007, 48, 2221–2232. [Google Scholar] [CrossRef]
- Yin, Y.; Zhang, X.; Wang, G.; Luo, L. Experimental study on a new internally cooled/heated dehumidifier/regenerator of liquid desiccant systems. Int. J. Refrig. 2008, 31, 857–866. [Google Scholar] [CrossRef]
- Yin, Y.; Zhang, X.; Peng, D.; Li, X. Model validation and case study on internally cooled/heated dehumidifier/regenerator of liquid desiccant systems. Int. J. Therm. Sci. 2009, 48, 1664–1671. [Google Scholar] [CrossRef]
- Yin, Y.; Zhang, X. Comparative study on internally heated and adiabatic regenerators in liquid desiccant air conditioning system. Build. Environ. 2010, 45, 1799–1807. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, T.; Liu, X.; Jiang, J. Experimental analysis of an internally-cooled/heated liquid desiccant dehumidifier/regenerator made of thermally conductive plastic. Energy Build. 2015, 99, 75–86. [Google Scholar] [CrossRef]
- Flamensbeck, M.; Summerer, F.; Riesch, P.; Ziegler, F.; Alefeld, G. A cost effective absorption chiller with plate heat exchangers using water and hydroxides. Appl. Therm. Eng. 1998, 18, 413–425. [Google Scholar] [CrossRef]
- Estiot, E.; Natzer, S.; Behr, C.; Eichel, P.; Kren, C.; Schweigler, C. Warmetauscherentwicklung fur Kompakte Wasser/LIBR-Absorptions-Kalteanlagen; DKV Tagungsbericht: Dresden, Germany, 2006; Volume 33, pp. 153–166. [Google Scholar]
- Kim, D.S.; Ferreira, C.A.I. Flow patterns and heat and mass transfer coefficients of low Reynolds number falling film flows on vertical plates: Effects of a wire screen and an additive. Int. J. Refrig. 2009, 32, 138–149. [Google Scholar] [CrossRef]
- Luo, Y.; Shao, S.; Xu, H.; Tian, C.; Yang, H. Experimental and theoretical research of a fin-tube type internally-cooled liquid desiccant dehumidifier. Appl. Energy 2014, 133, 127–134. [Google Scholar] [CrossRef]
- Wen, T.; Lu, L.; Dong, C.; Luo, Y. Investigation on the regeneration performance of liquid desiccant by adding surfactant PVP-K30. Int. J. Heat Mass Transf. 2018, 123, 445–454. [Google Scholar] [CrossRef]
- Coleman, H.W.; Steele, W.G. Experimentation, Validation, and Uncertainty Analysis for Engineers; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Roberge, P. Handbook of Corrosion Engineering 2/E; McGraw Hill Professional: New York, NY, USA, 2012. [Google Scholar]
- Wen, T.; Lu, L.; Dong, C.; Luo, Y. Development and experimental study of a novel plate dehumidifier made of anodized aluminum. Energy 2018, 144, 169–177. [Google Scholar] [CrossRef]
- Adamson, A.W.; Gast, A.P. Physical Chemistry of Surfaces; Interscience: Geneva, Switzerland, 1967. [Google Scholar]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, L.; Su, Y.; Mou, X.; Xiao, Y.; Liu, J. Investigation on the corrosion behavior of aluminum alloys 3A21 and 7A09 in chloride aqueous solution. Mater. Des. 2013, 50, 15–21. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, F.; Xue, C.; Li, L.; Yin, Y. Structure stability and corrosion resistance of nano-TiO2 coatings on aluminum in seawater by a vacuum dip-coating method. Surf. Coat. Technol. 2010, 205, 2335–2339. [Google Scholar] [CrossRef]
- Piccolo, A.; Siclari, R.; Rando, F.; Cannistraro, M. Comparative performance of thermoacoustic heat exchangers with different pore geometries in oscillatory flow. implementation of experimental techniques. Appl. Sci. 2017, 7, 784. [Google Scholar] [CrossRef]



| Parameter | Uncertainty | Parameter | Uncertainty |
|---|---|---|---|
| Temperature/ | Hot water flow rate/ | ||
| Solution flow rate/ | Solution concentration/ | ||
| Solution density/ | Air absolute humidity/ | ||
| Air flow rate/ | Regenerator rate/ | ||
| Air relative humidity/ | Regenerator effectiveness/ |
| Solution | |||
|---|---|---|---|
| Normal Aluminum | −0.9886 | 0.1597 | 0.0005218 |
| Anodized Aluminum | −0.8713 | 0.0261 | 0.000011 |
| Liquid | |||
|---|---|---|---|
| water | 51 mN/m | 21.8 mN/m | 72.8 mN/m |
| n-hexadecane | 0 mN/m | 27.6 mN/m | 27.6 mN/m |
| Surface | Water Contact Angle | n-Hexadecane Contact Angle | Surface Energy |
|---|---|---|---|
| Normal Al | 89.5° | 37.5° | 26.4 mN/m |
| Anodized Al | 55.8° | 16.0° | 47.6 mN/m |
| Temperature/°C | |||
|---|---|---|---|
| 28 | −0.8713 | 0.0261 | 0.000011 |
| 40 | −0.9127 | 0.0548 | 0.000179 |
| 55 | −0.9314 | 5.19 | 0.00217 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, T.; Lu, L.; Yang, H.; Luo, Y. Investigation on the Regeneration and Corrosion Characteristics of an Anodized Aluminum Plate Regenerator. Energies 2018, 11, 1209. https://doi.org/10.3390/en11051209
Wen T, Lu L, Yang H, Luo Y. Investigation on the Regeneration and Corrosion Characteristics of an Anodized Aluminum Plate Regenerator. Energies. 2018; 11(5):1209. https://doi.org/10.3390/en11051209
Chicago/Turabian StyleWen, Tao, Lin Lu, Hongxing Yang, and Yimo Luo. 2018. "Investigation on the Regeneration and Corrosion Characteristics of an Anodized Aluminum Plate Regenerator" Energies 11, no. 5: 1209. https://doi.org/10.3390/en11051209
APA StyleWen, T., Lu, L., Yang, H., & Luo, Y. (2018). Investigation on the Regeneration and Corrosion Characteristics of an Anodized Aluminum Plate Regenerator. Energies, 11(5), 1209. https://doi.org/10.3390/en11051209






