Evolution of Thermoelectric Properties of Zn4Sb3 Prepared by Mechanical Alloying and Different Consolidation Routes
Abstract
:1. Introduction
2. Experimental
3. Results and Discussion
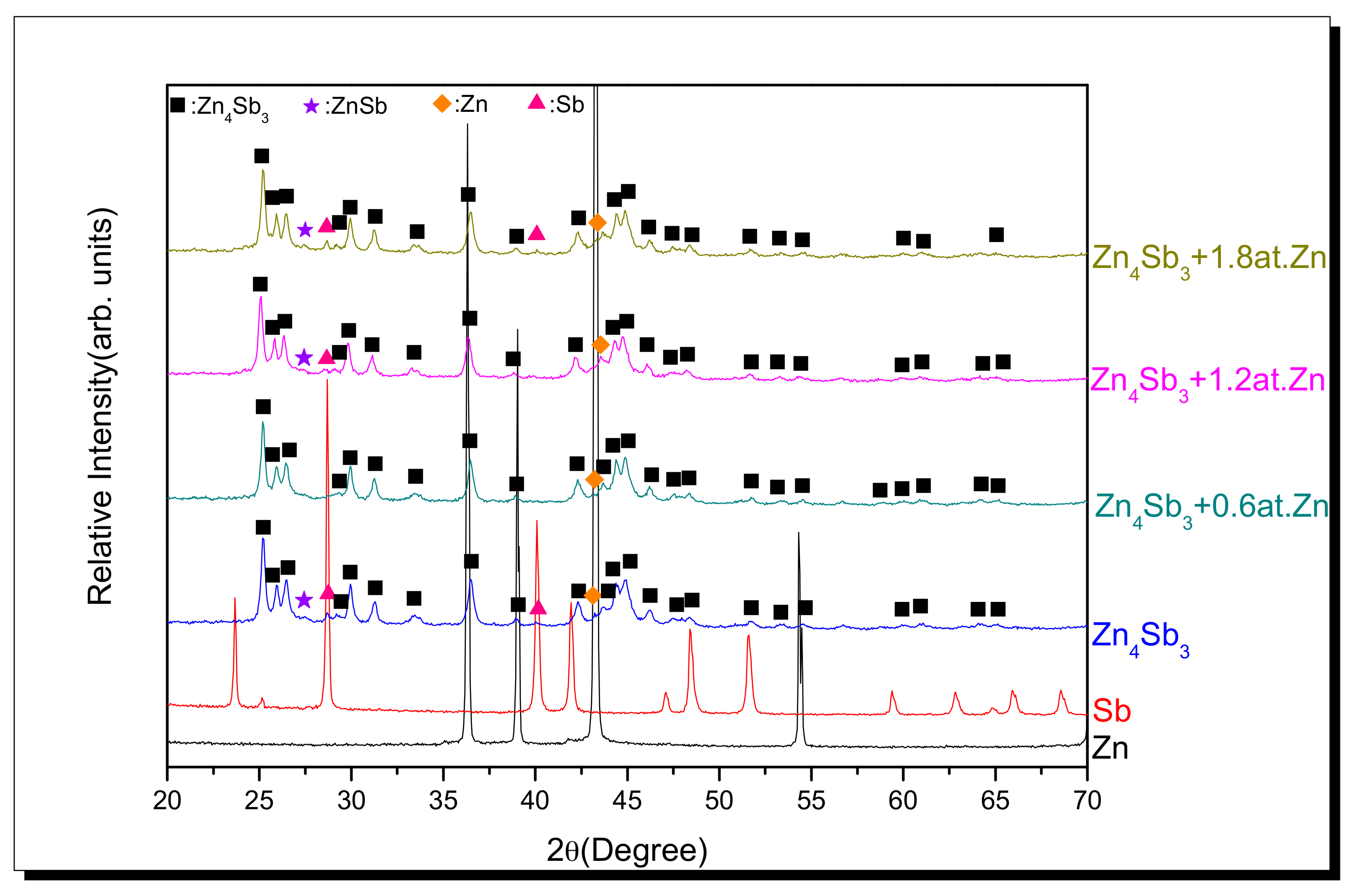
3.1. XRD Diffraction Analysis of MA Powder
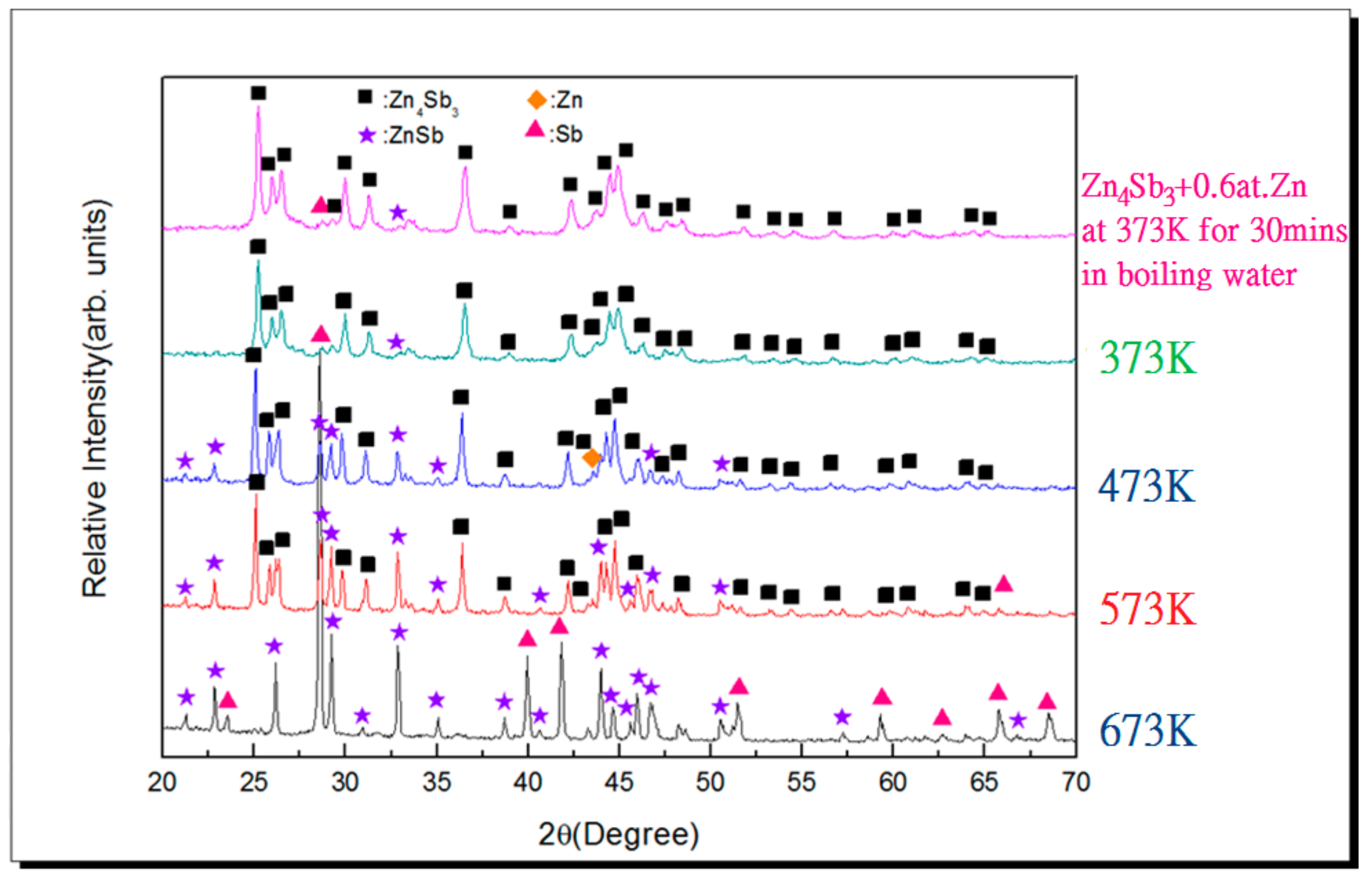
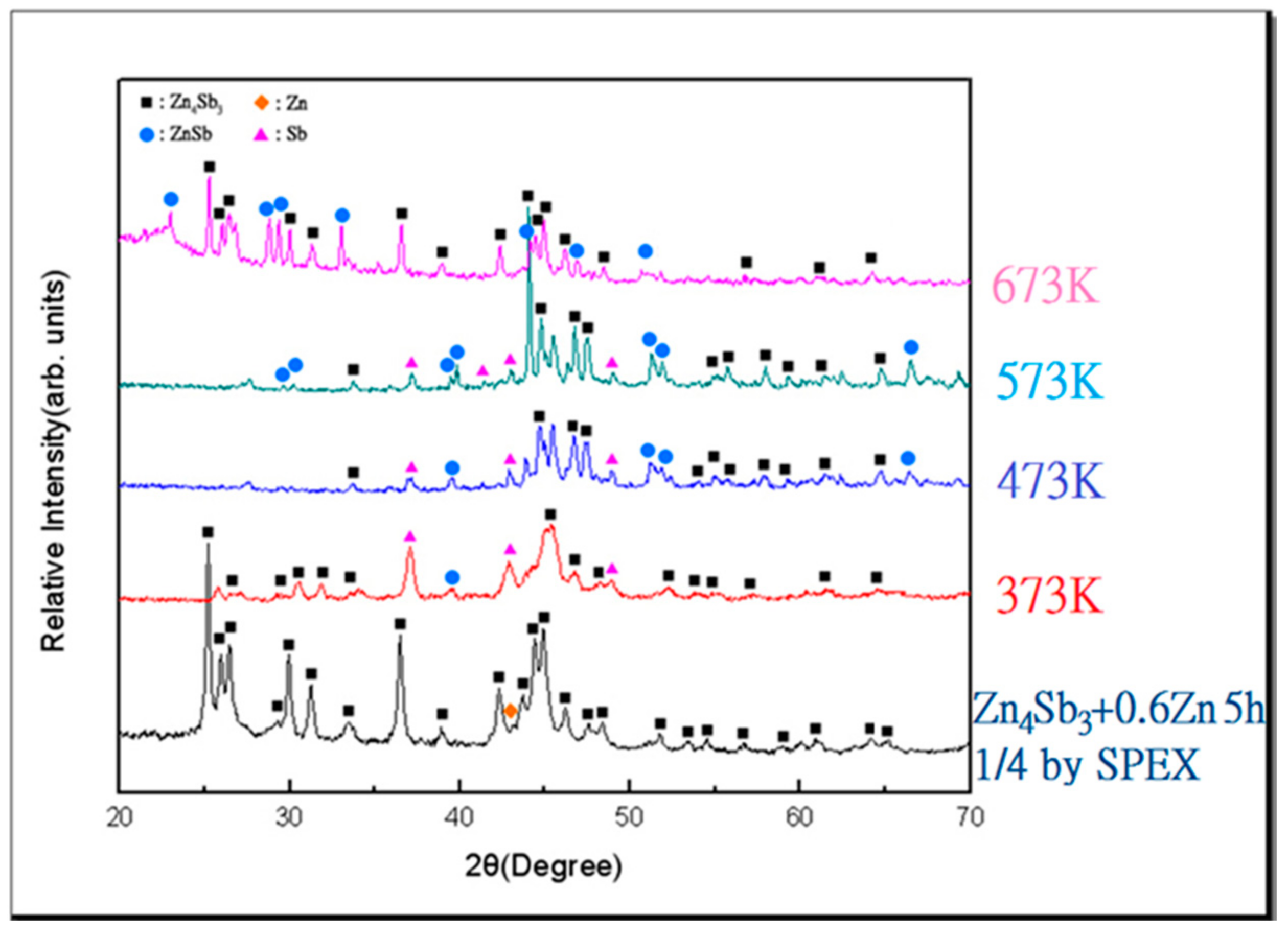
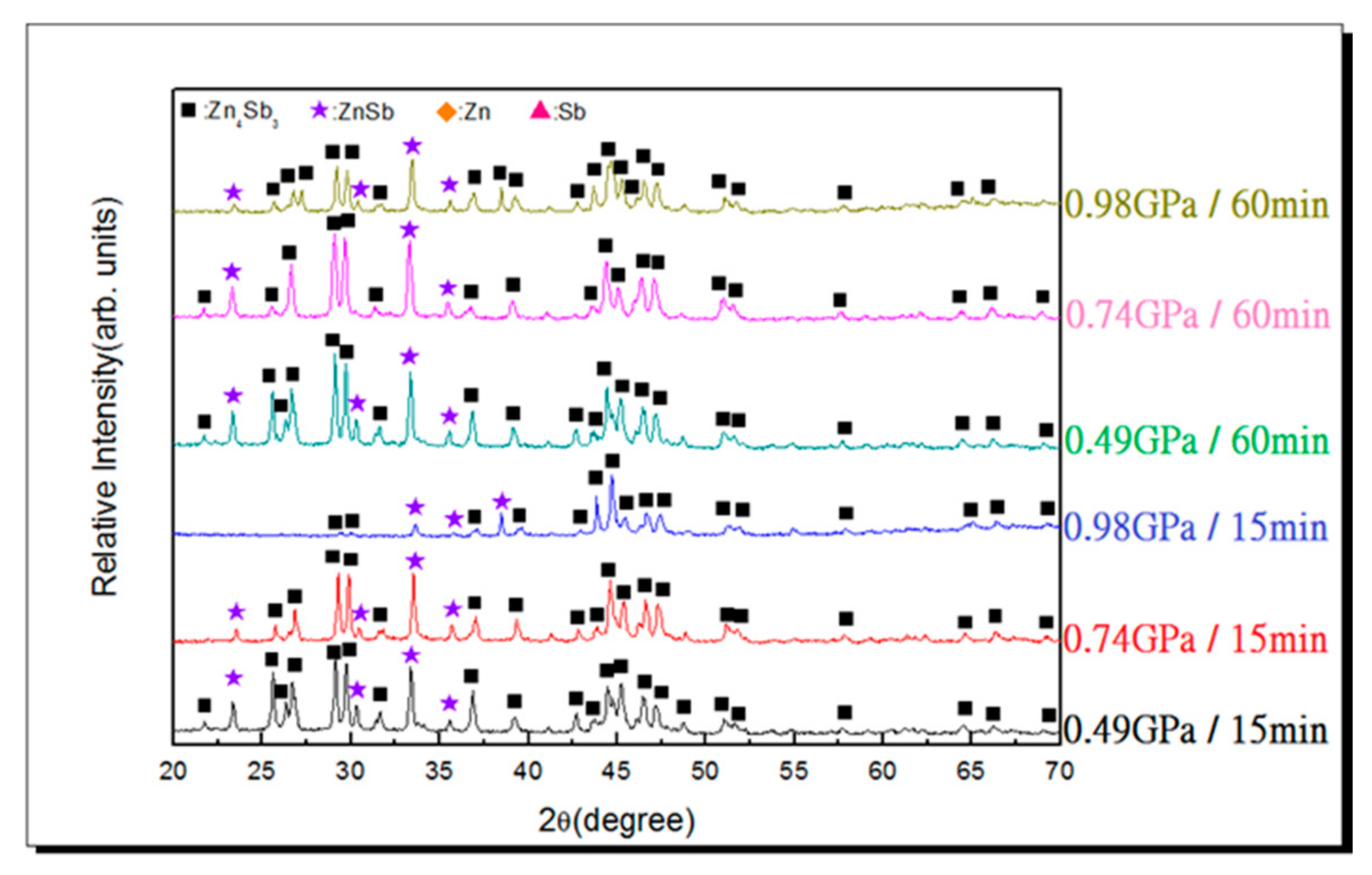
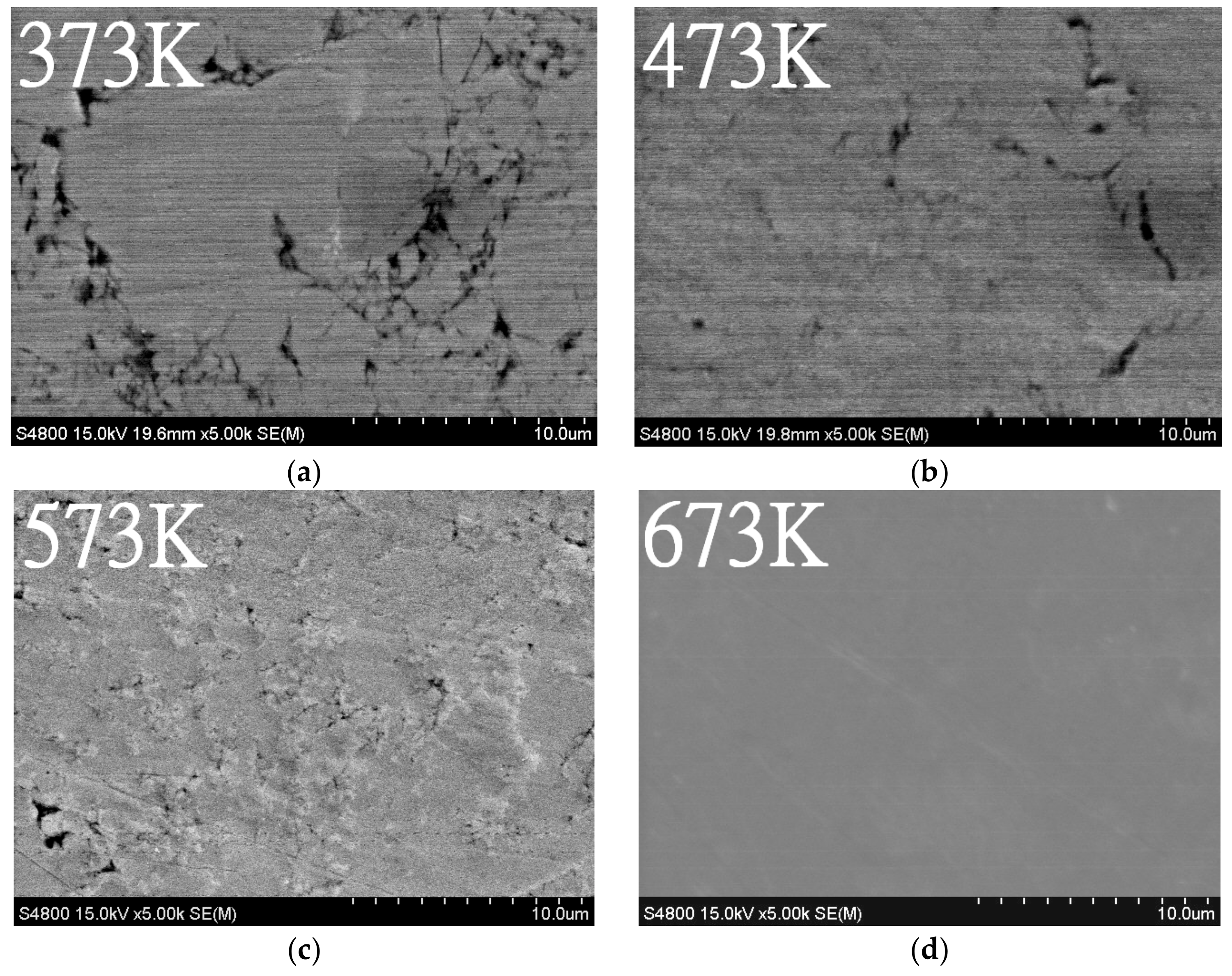
3.2. Vacuum Sintering and Vacuum Hot Pressing of Bulk Zn4Sb3 Alloy
4. Conclusions
- The formation of complete Zn4Sb3 compound phase by mechanical alloying of pure zinc and antimony elemental powders with Zn4Sb3 composition is unsuccessful due to the partial volatility of the zinc during milling process.
- A nearly single Zn4Sb3 compound phase can be obtained in mechanically alloyed β-Zn4Sb3 alloy powder with superfluous 0.6 at. % of Zn.
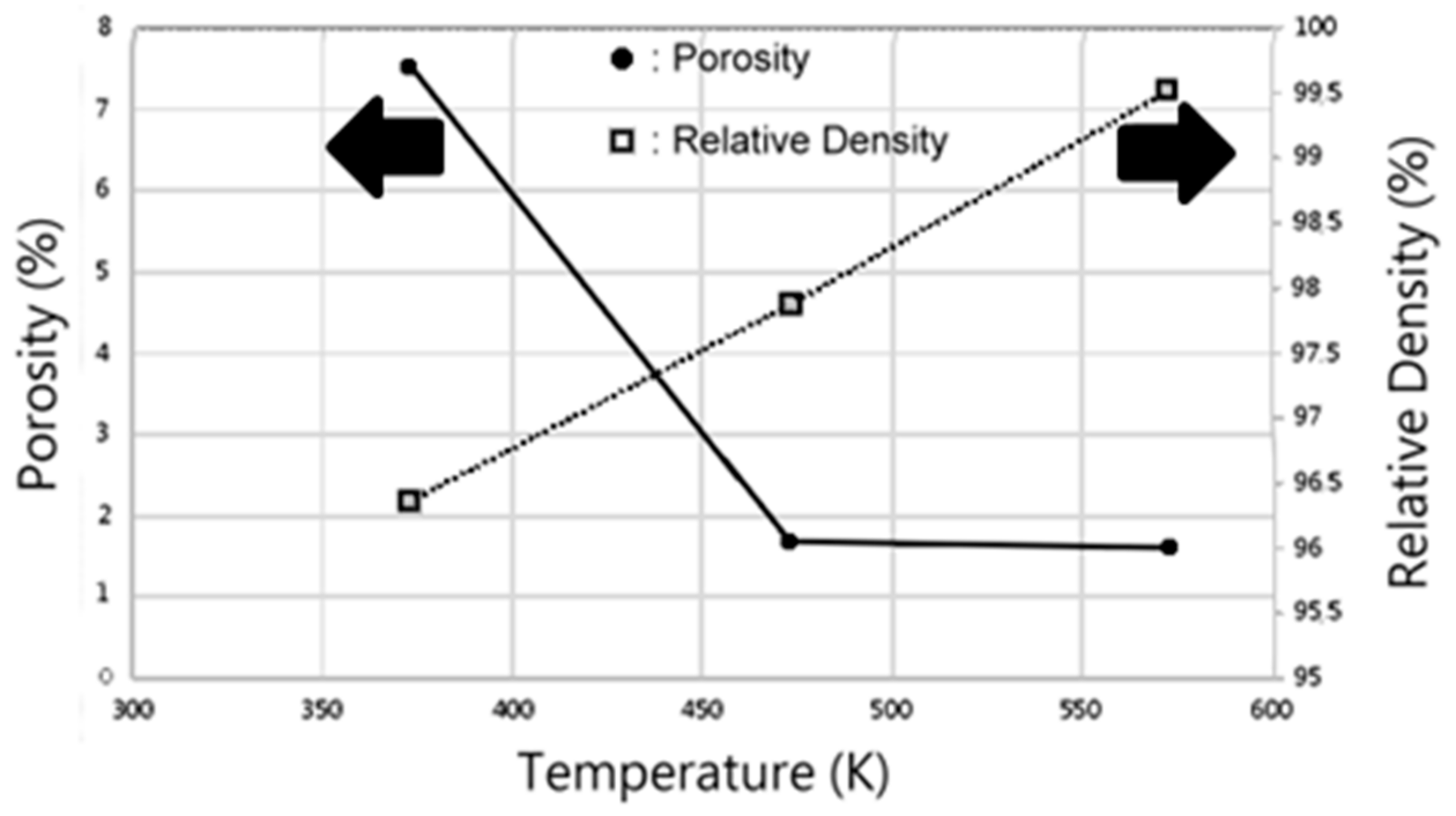
- The β-Zn4Sb3 phase was found to be the main phase in vacuum-sintered specimens after sintering around 373–473 K. As the sintering temperature increased to 573 K, ZnSb phase gradually emerges and the amount of β-Zn4Sb3 phase gradually decreases. The evaporation of Zn during high-temperature sintering at 673 K can cause the complete disappearance of β-Zn4Sb3 phase, leading to the formation of an Sb and ZnSb equilibrium phase.
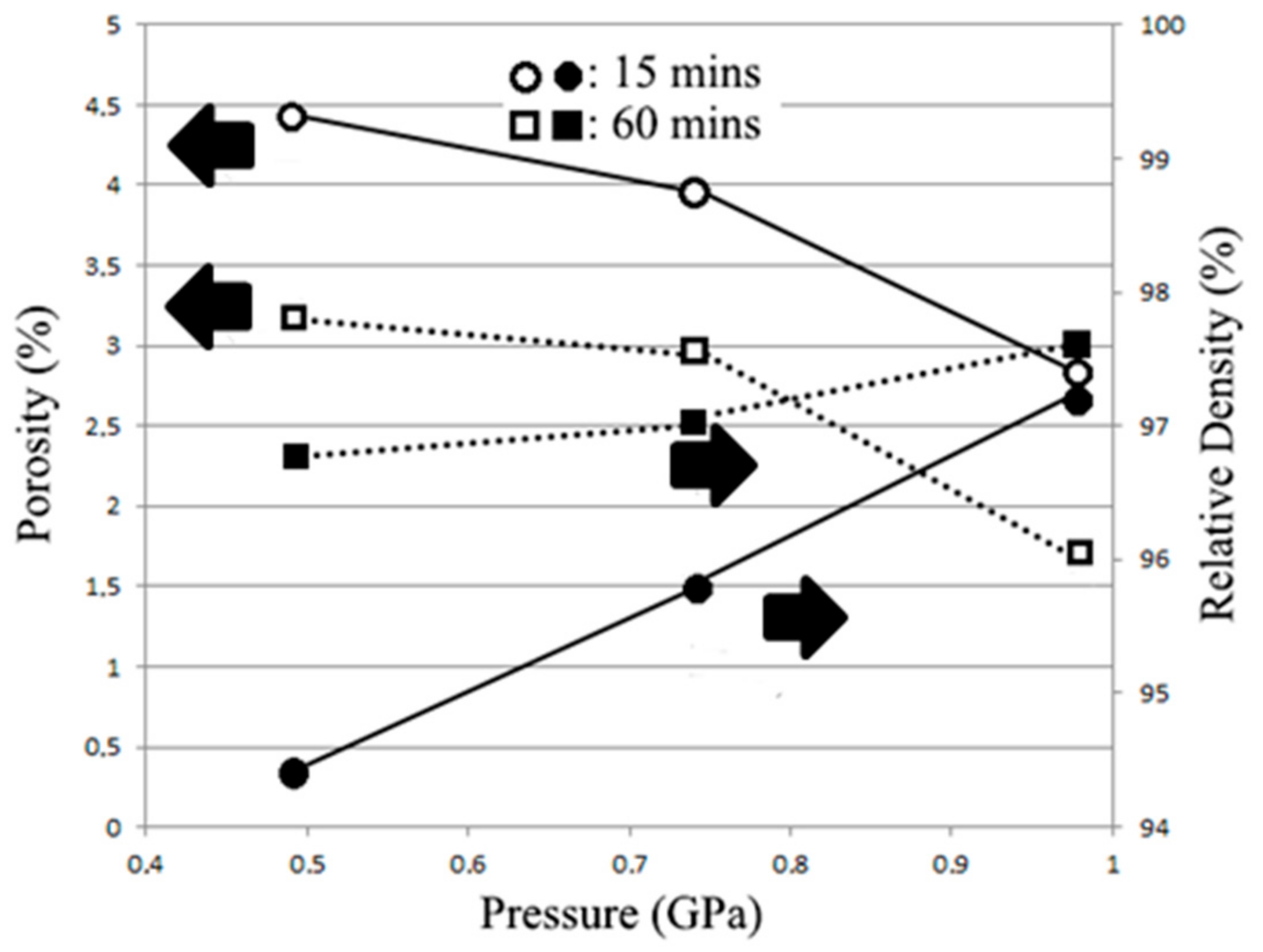
- Under an applied pressure of 0.98 GPa at 473 K for a duration of 30 min, β-Zn4Sb3 powder can be vacuum hot pressed into a single β-Zn4Sb3 bulk with a density of 97.87%. Raising the applied pressure, duration, and temperature can moderately increase the relative density of the hot pressed bulk materials. However, excessive hot pressing temperature will reduce the proportion of the β-Zn4Sb3 phase.
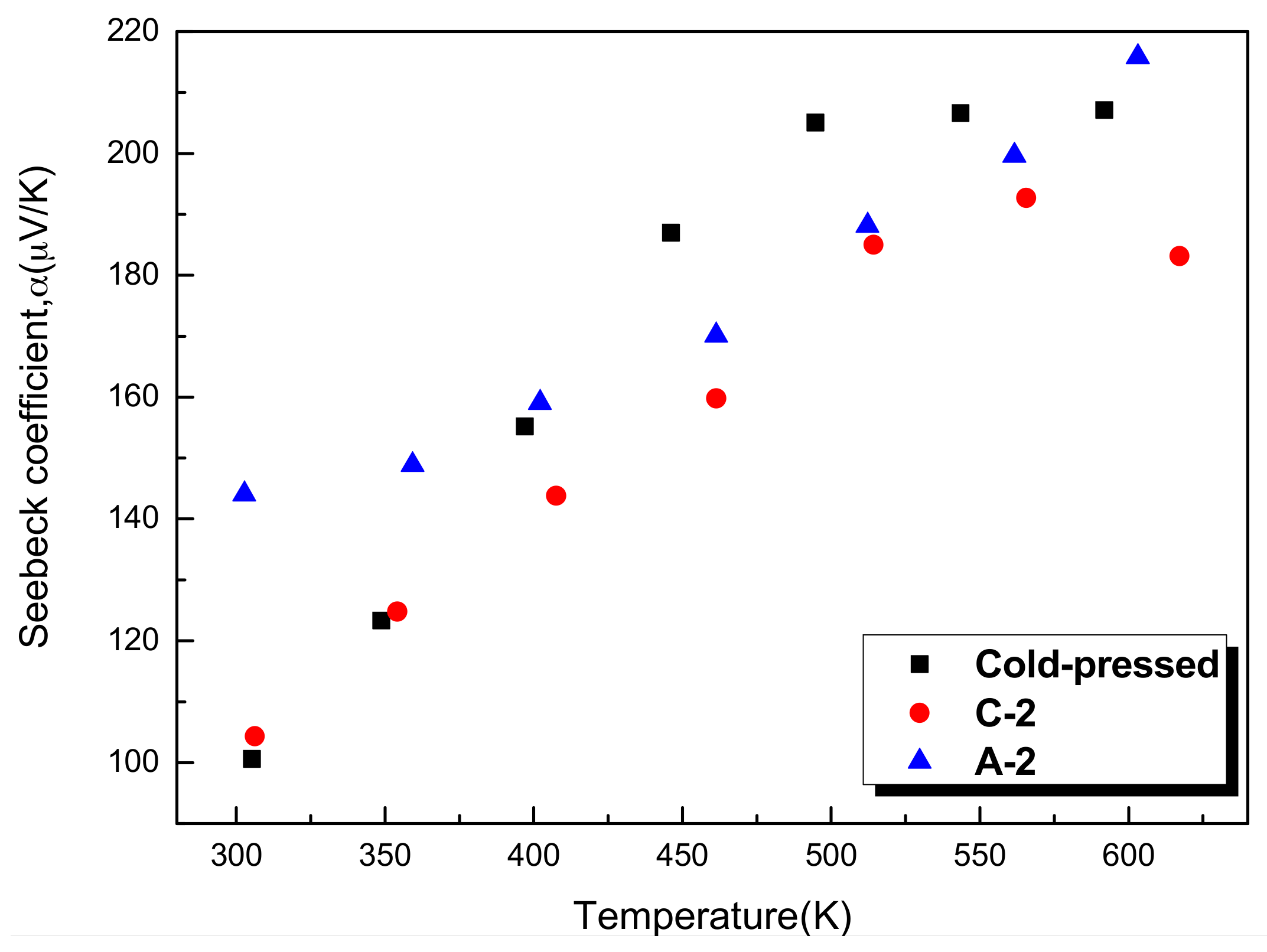
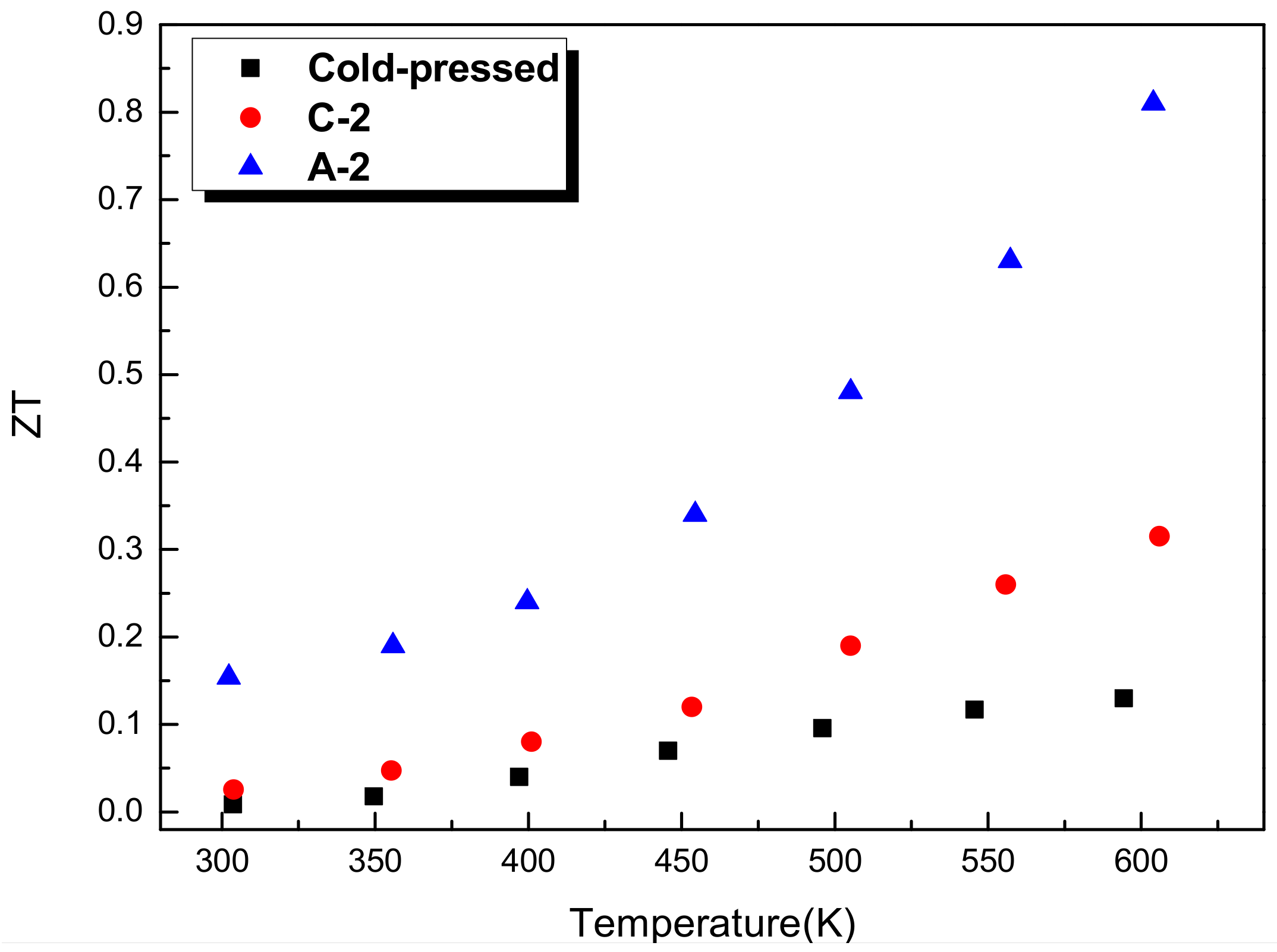
- The β-Zn4Sb3 bulk exhibits p-type semiconducting properties. As the proportion of Zn4Sb3 increases, the Seebeck coefficient increases but the electrical conductivity decreases. The bulk specimen with single β-Zn4Sb3 phase exhibits the best thermoelectric properties with the power factor and ZT value is 1.31 × 10−3 W/mK2 and 0.81, respectively.
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Zhu, T.; Liu, Y.; Fu, C.; Heremans, J.P.; Snyder, J.G.; Zhao, X. Compromise and Synergy in High-Efficiency Thermoelectric Materials. Adv. Mater. 2017, 29, 1605884. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.; Zhao, L.D.; Kanatzidis, M.G. Rationally Designing High-Performance Bulk Thermoelectric Materials. Chem. Rev. 2016, 116, 12123–12149. [Google Scholar] [CrossRef] [PubMed]
- DiSalvo, F.J. Thermoelectric cooling and power generation. Science 1999, 285, 703–706. [Google Scholar] [CrossRef] [PubMed]
- Mahan, G.; Sales, B.; Sharp, J. Thermoelectric materials: New approaches to an old problem. Phys. Today 1997, 50, 42–47. [Google Scholar] [CrossRef]
- Dmitriev, A.V.; Zvyagin, I.P. Current trends in the physics of thermoelectric materials. Phys. Usp. 2010, 53, 789–803. [Google Scholar] [CrossRef]
- Poudel, B.; Hao, Q.; Ma, Y.; Lan, Y.; Minnich, A.; Yu, B.; Yan, X.; Wang, D.; Muto, A.; Vashaee, D.; et al. High-Thermoelectric Performance of Nanostructured Bismuth Antimony Telluride Bulk Alloys. Science 2008, 320, 634–638. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.; Chasapis, T.C.; Chen, Z.G.; Yang, L.; Kanatzidis, M.G.; Snyder, G.J.; Zou, J. n-Type Bi2Te3−xSex Nanoplates with Enhanced Thermoelectric Efficiency Driven by Wide-Frequency Phonon Scatterings and Synergistic Carrier Scatterings. ACS Nano 2016, 10, 4719–4727. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.; Chen, Z.G.; Yang, L.; Zou, J. BixSb2−xTe3 nanoplates with enhanced thermoelectric performance due to sufficiently decoupled electronic transport properties and strong wide-frequency phonon scatterings. Nano Energy 2016, 20, 144–155. [Google Scholar] [CrossRef]
- Hong, M.; Chen, Z.G.; Yang, L.; Chasapis, T.C.; Kang, S.D.; Zou, Y.; Auchterlonie, G.J.; Kanatzidis, M.G.; Snyder, G.J.; Zou, J. Enhancing thermoelectric performance of SnSe1−xTex nanoplates through band engineering. J. Mater. Chem. A 2017, 5, 10713–10721. [Google Scholar] [CrossRef]
- Zhao, L.D.; Tan, G.; Hao, S.; He, J.; Pei, Y.; Chi, H.; Wang, H.; Gong, S.; Xu, H.; Dravid, V.P.; et al. Ultrahigh power factor and thermoelectric performance in hole-doped single-crystal SnSe. Science 2016, 351, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.R.; Tan, G.; Zhang, X.; Wu, C.F.; Li, J.F.; Dravid, V.P.; Snyder, G.J.; Kanatzidis, M.G. Distinct Impact of Alkali-Ion Doping on Electrical Transport Properties of Thermoelectric p-Type Polycrystalline SnSe. J. Am. Chem. Soc. 2016, 138, 8875–8888. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, Z.; Zhang, X.; Sun, Y.; Yang, J.; Pei, Y. Electronic origin of the high thermoelectric performance of GeTe among the p-type group IV monotellurides. NPG Asia Mater. 2017, 9, e353. [Google Scholar] [CrossRef]
- Li, J.; Zhang, X.; Lin, S.; Chen, Z.; Pei, Y. Realizing the High Thermoelectric Performance of GeTe by Sb-Doping and Se-Alloying. Chem. Mater. 2017, 29, 605–611. [Google Scholar] [CrossRef]
- Hong, M.; Chen, Z.G.; Yang, L.; Zou, Y.C.; Dargusch, M.S.; Wang, H.; Zou, J. Realizing zT of 2.3 in Ge1−x−ySbxInyTe via Reducing the Phase-Transition Temperature and Introducing Resonant Energy Doping. Adv. Mater. 2018, 30, 1705942. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.; Chen, Z.G.; Yang, L.; Liao, Z.M.; Zou, Y.C.; Chen, Y.H.; Matsumura, S.; Zou, J. Achieving zT > 2 in p-Type AgSbTe2−xSex Alloys via Exploring the Extra Light Valence Band and Introducing Dense Stacking Faults. Adv. Energy Mater. 2018, 8, 1702333. [Google Scholar] [CrossRef]
- Caillat, T.; Fleurial, J.P.; Borshchevsky, A. Preparation and thermoelectric properties of semiconducting Zn4Sb3. J. Phys. Chem. Solids 1997, 58, 1119–1125. [Google Scholar] [CrossRef]
- Zhu, T.J.; Zhao, X.B.; Yan, M.; Hu, S.H.; Li, T.; Zhou, B.C. Transport properties of β-Zn4Sb3 prepared by vacuum melting. Mater. Lett. 2000, 46, 44–48. [Google Scholar] [CrossRef]
- Izard, V.; Record, M.C.; Tedenac, J.C. Mechanical alloying of a new promising thermoelectric material, Zn4Sb3. J. Alloys Compd. 2002, 345, 257–264. [Google Scholar] [CrossRef]
- Aizawa, T.; Iwaisako, Y. Solid-state synthesis of single-phase Zn4Sb3 bulk sample on the route of the bulk mechanical alloying. In Proceedings of the IEEE 18th International Conference on Thermoelectrics, Baltimore, MD, USA, 29 August–2 September 1999; pp. 173–176. [Google Scholar]
- Rowe, D.M.; Schuka, V.S. The effect of phonon-grain boundary scattering on the lattice thermal conductivity and thermoelectric conversion efficiency of heavily doped fine-grained, hot-pressed silicon germanium alloy. J. Appl. Phys. 1981, 52, 7421–7426. [Google Scholar] [CrossRef]
- Okamura, C.; Ueda, T.; Hasezaki, K. Preparation of single phase β-Zn4Sb3 thermoelectric materials by mechanical grinding process. Mater. Trans. 2010, 51, 152–155. [Google Scholar] [CrossRef]
- Okamura, C.; Ueda, T.; Hasezaki, K. Preparation of single-phase ZnSb thermoelectric materials using mechanical grinding process. Mater. Trans. 2010, 51, 860–862. [Google Scholar] [CrossRef]
- Ueno, K.; Yamamoto, A.; Noguchi, T.; Inoue, T.; Sodeoka, S.; Takazawa, H.; Lee, C.H.; Obara, H. Optimization of hot-press conditions of Zn4Sb3 for high thermoelectric performance: I. Physical properties and thermoelectric performance. J. Alloys Compd. 2004, 384, 254–260. [Google Scholar] [CrossRef]
- Mozharivskyj, Y.; Pecharsky, A.O.; Bud’ko, S.; Miller, G.J. A Promising Thermoelectric Material: Zn4Sb3 or Zn6−δSb5. Its Composition, Structure, Stability, and Polymorphs. Structure and Stability of Zn1−δSb. Chem. Mater. 2004, 16, 1580–1589. [Google Scholar] [CrossRef]
- Ueno, K.; Yamamoto, A.; Noguchi, T.; Li, C.H.; Inoue, T.; Sodeoka, S.; Obara, H. Effect of impurity oxygen concentration on the thermoelectric properties of hot pressed Zn4Sb3. J. Alloys Compd. 2006, 417, 259–263. [Google Scholar] [CrossRef]
- Cui, J.L.; Mao, L.D.; Chen, D.Y.; Qian, X.; Liu, X.L.; Yang, W. Effects of a Cu-contained compound on the microstructures and thermoelectric properties of Zn–Sb based alloys. Curr. Appl. Phys. 2009, 9, 713–716. [Google Scholar] [CrossRef]
- Itoh, T.; Shan, J.; Kitagawa, K. Thermoelectric properties of β-Zn4Sb3 synthesized by mechanical alloying and pulse discharge sintering. J. Propul. Power 2008, 24, 353–358. [Google Scholar] [CrossRef]
- Lee, J.S.; Oh, T.S.; Hyun, D.B. Thermoelectric properties of the hot-press (Bi0.2Sb0.8)2Te3 alloy with addition of BN and WO3 powders. J. Mater. Sci. 2000, 35, 881–887. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, Z.; Ruan, X. First principles calculation of lattice thermal conductivity of metals considering phonon-phonon and phonon–electron scattering. J. Appl. Phys. 2016, 119, 225109. [Google Scholar] [CrossRef]
- Liao, B.; Qiu, B.; Zhou, J.; Huberman, S.; Esfarjani, K.; Chen, G. Significant reduction of lattice thermal conductivity by the electron-phonon interaction in silicon with high carrier concentrations: A first-principles study. Phys. Rev. Lett. 2015, 114, 115901. [Google Scholar] [CrossRef] [PubMed]
- Ur, S.C.; Nash, P.; Kim, I.H. Mechanical alloying and thermoelectric properties of Zn4Sb3. J. Mater. Sci. 2003, 38, 3553–3558. [Google Scholar] [CrossRef]
- Ur, S.C.; Kim, I.H.; Nash, P. Thermoelectric properties of Zn4Sb3 directly synthesized by hot pressing. Mater. Lett. 2004, 58, 2132–2136. [Google Scholar] [CrossRef]
- Cui, J.L.; Fu, H.; Chen, D.Y.; Mao, L.D.; Liu, X.L.; Yang, W. Thermoelectric properties of Cu-added Zn-Sb based alloys with multi-phase equilibrium. Mater. Charact. 2009, 60, 824–828. [Google Scholar] [CrossRef]
- Cui, J.; Liu, X.; Yang, W.; Chen, D.; Mao, L.; Qian, X. Effects of Al addition on the thermoelectric properties of Zn-Sb based alloys. Chin. J. Mech. Eng. 2009, 22, 270–275. [Google Scholar] [CrossRef]
- Ruan, X.; Xiao, W. Preparation and thermoelectric properties of SiO2/β-Zn4Sb3 nanocomposite materials. J. Wuhan Univ. Technol.-Mater. 2009, 24, 694–697. [Google Scholar] [CrossRef]



| Sample Codes | Pressure (GPa) | Temperature (K) | Time (min) |
|---|---|---|---|
| A-1 | 0.98 | 373 | 30 |
| A-2 | 0.98 | 473 | 30 |
| A-3 | 0.98 | 573 | 30 |
| B-1 | 0.49 | 473 | 15 |
| B-2 | 0.74 | 473 | 15 |
| B-3 | 0.98 | 473 | 15 |
| C-1 | 0.49 | 473 | 60 |
| C-2 | 0.74 | 473 | 60 |
| C-3 | 0.98 | 473 | 60 |
| Code | α (μV/K) | σ (Ω−1cm−1) | θ (W/mK2) | κ (W/mK) | ZT | Relative Density (%) | Phases |
|---|---|---|---|---|---|---|---|
| cold-pressed | 207.1 | 59.5 | 0.00026 | 1.14 | 0.13 | 81.05 | Zn4Sb3 + Zn |
| C-2 | 183.2 | 185.8 | 0.00062 | 1.2 | 0.31 | 97.02 | Zn4Sb3 + ZnSb |
| A-2 | 215.8 | 281.1 | 0.00131 | 0.982 | 0.81 | 97.87 | Zn4Sb3 |
| Preparation Methods * | κ (W/mK) | ZT | Relative Density (%) | Phases | References |
|---|---|---|---|---|---|
| MA→HT (673 K) | 0.7 | 0.24 | >95 | ZnSb + Zn | Itoh [27] |
| MA→HT (723 K) | 0.45 | 1.0 | >95 | Zn4Sb3 | |
| 0.7 | 1.0 | 96–98 | Zn4Sb3 | Caillat [16] | |
| Vacuum melting | 0.7 a | 0.32 | - | Zn4Sb3 | Zhu [17] |
| HP | 0.7 a | 1.2 | 98.5 | Zn4Sb3 | Ur [32] |
| HP | 0.83 | 0.9 | 99.6 | Zn4Sb3 | Ueno [23] |
| Melting→MA→HT | 1.0 | 0.61 | - | Zn4Sb3 + ZnSb + Cu2Sb + Zn | Cui [33] |
| 0.9 | 0.47 | - | Zn4Sb3 | ||
| Melting→MA→HT | 0.62 | 0.5 | - | Zn4Sb3 + ZnSb + AlSb | Cui [34] |
| Melting to MA, the ingot were coated by SiO2, then HT | 0.6 | 0.7 | - | Zn4Sb3 + SiO2 | Ruan [35] |
| 0.84 | 0.56 | - | Zn4Sb3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, P.-Y.; Lin, P.-H. Evolution of Thermoelectric Properties of Zn4Sb3 Prepared by Mechanical Alloying and Different Consolidation Routes. Energies 2018, 11, 1200. https://doi.org/10.3390/en11051200
Lee P-Y, Lin P-H. Evolution of Thermoelectric Properties of Zn4Sb3 Prepared by Mechanical Alloying and Different Consolidation Routes. Energies. 2018; 11(5):1200. https://doi.org/10.3390/en11051200
Chicago/Turabian StyleLee, Pee-Yew, and Pei-Ho Lin. 2018. "Evolution of Thermoelectric Properties of Zn4Sb3 Prepared by Mechanical Alloying and Different Consolidation Routes" Energies 11, no. 5: 1200. https://doi.org/10.3390/en11051200
APA StyleLee, P.-Y., & Lin, P.-H. (2018). Evolution of Thermoelectric Properties of Zn4Sb3 Prepared by Mechanical Alloying and Different Consolidation Routes. Energies, 11(5), 1200. https://doi.org/10.3390/en11051200





