Two-Phase Flow Modeling of Solid Dissolution in Liquid for Nutrient Mixing Improvement in Algal Raceway Ponds
Abstract
:1. Introduction
2. Materials and Methods
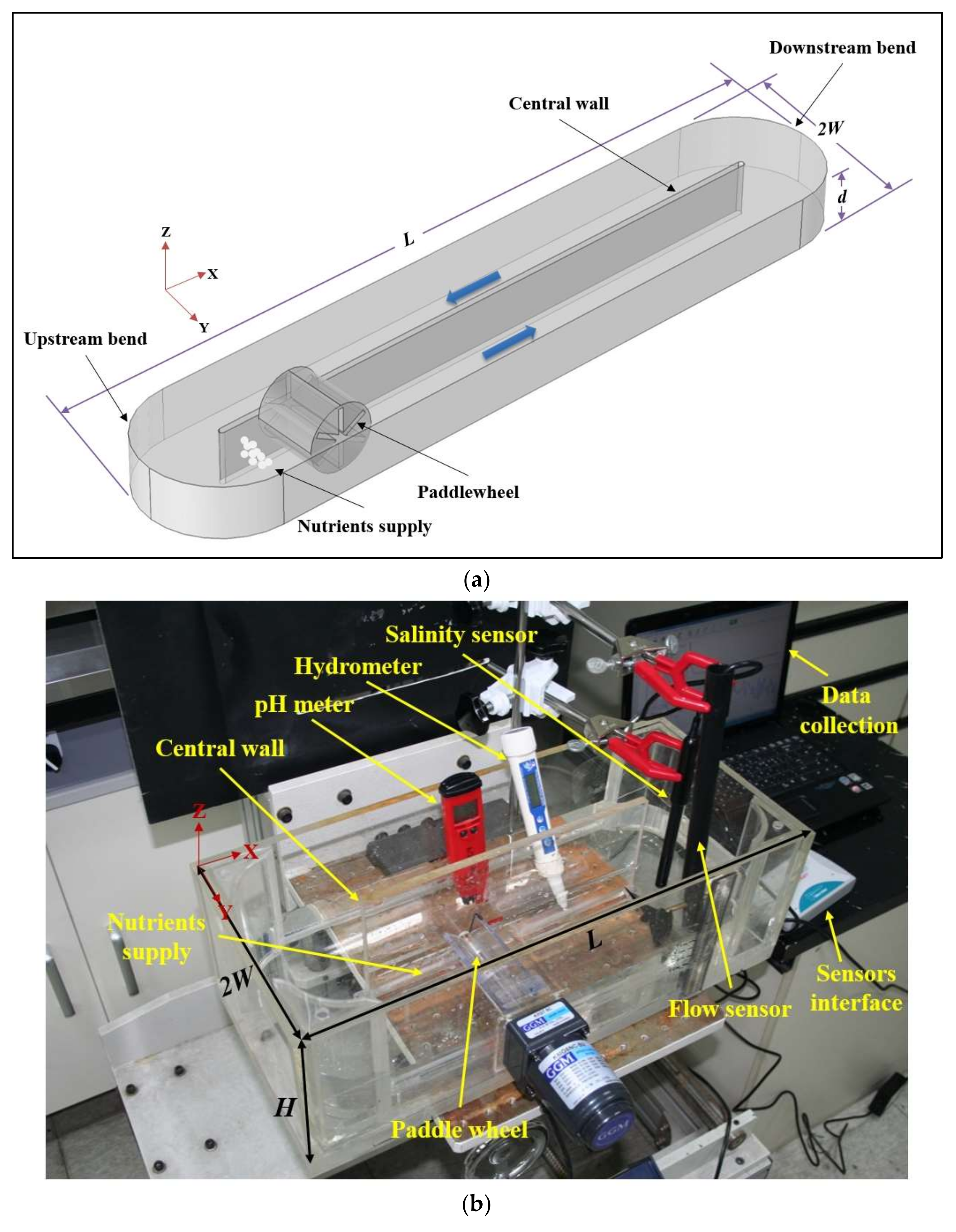
2.1. Raceway Pond
2.2. Numerical Modeling
2.2.1. Solid–Liquid Modeling
2.2.2. Turbulence Modeling
2.2.3. Mass Transfer Modeling
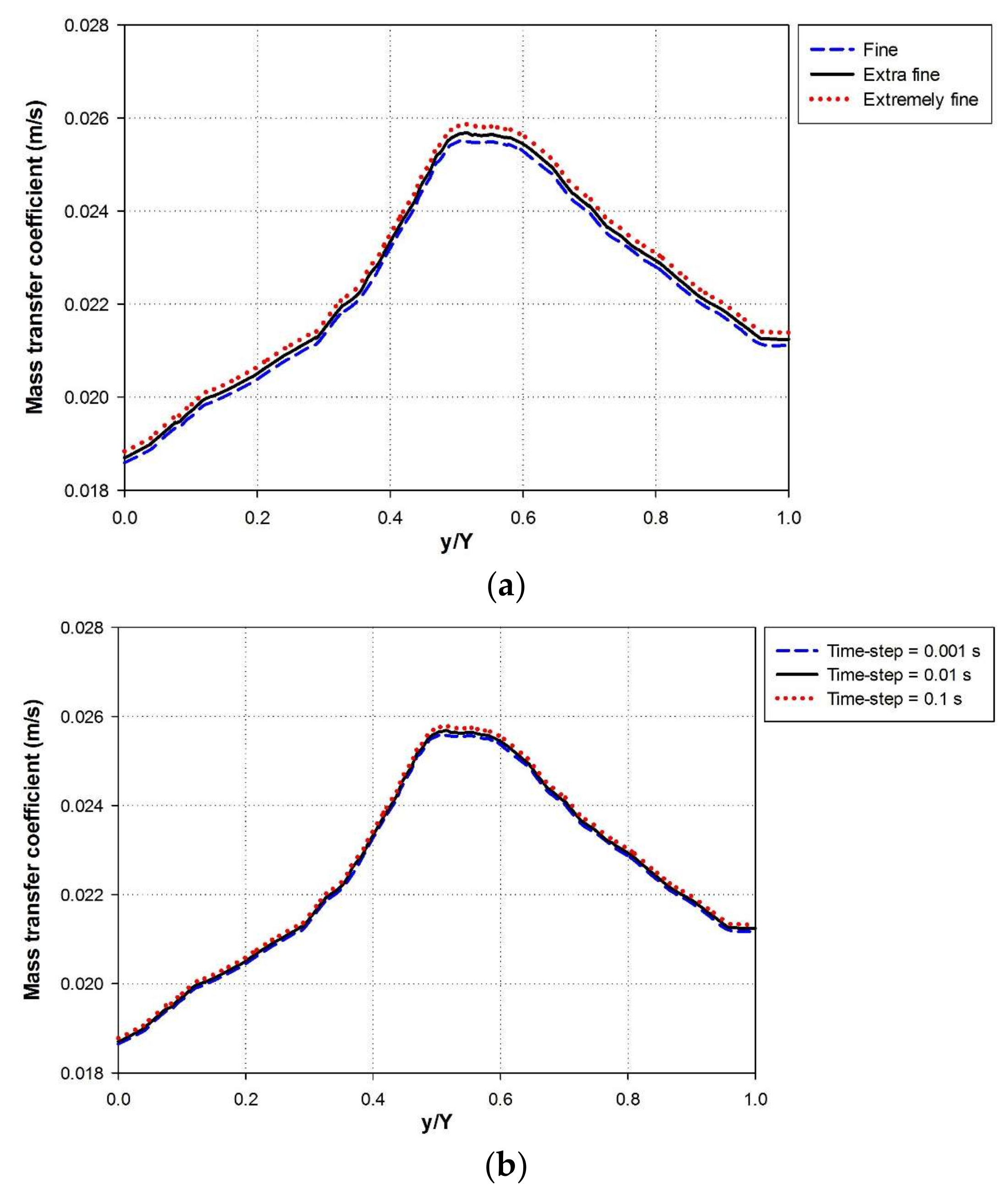
3. Numerical Simulation and Mesh Generation
4. Results and Discussion
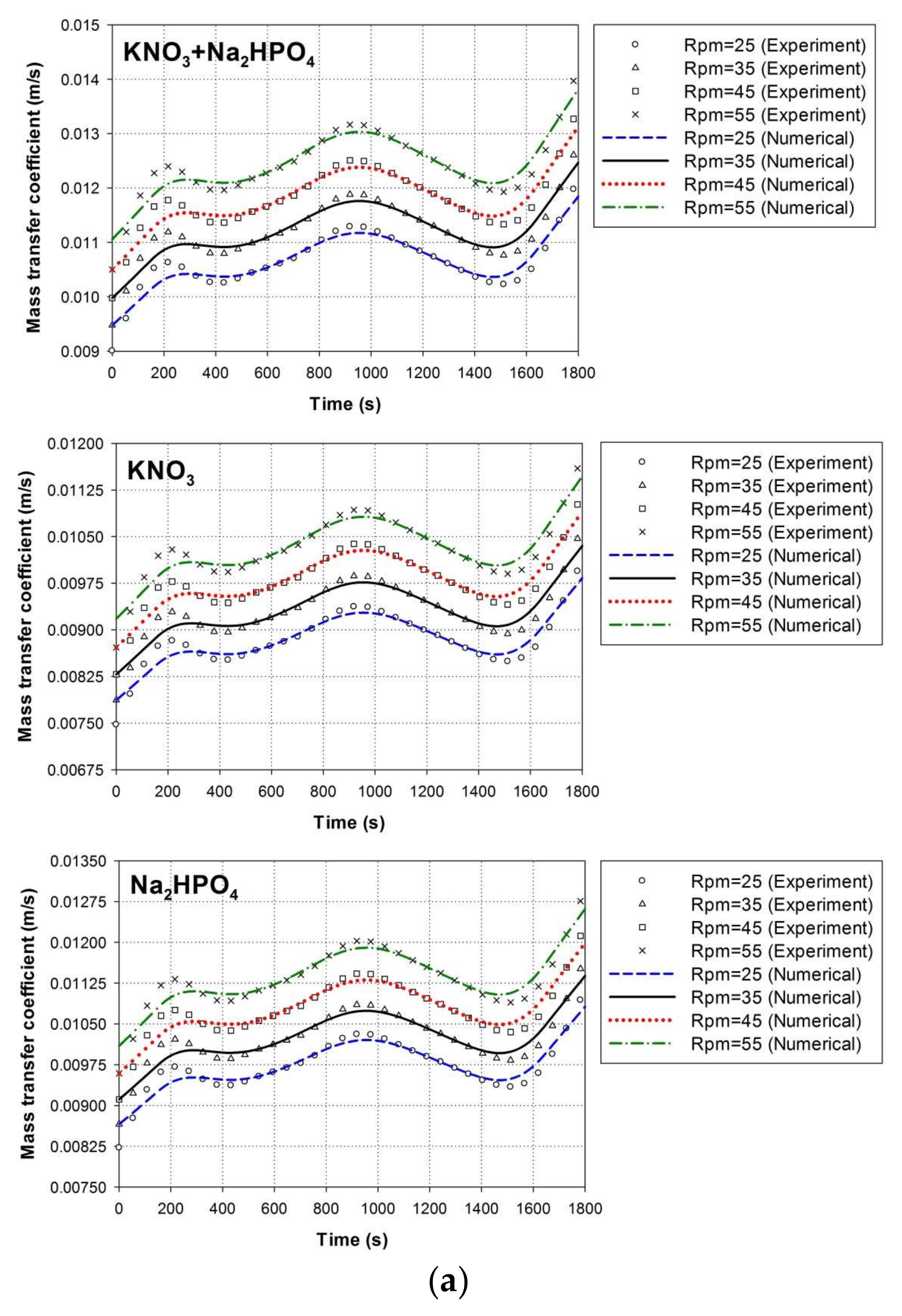
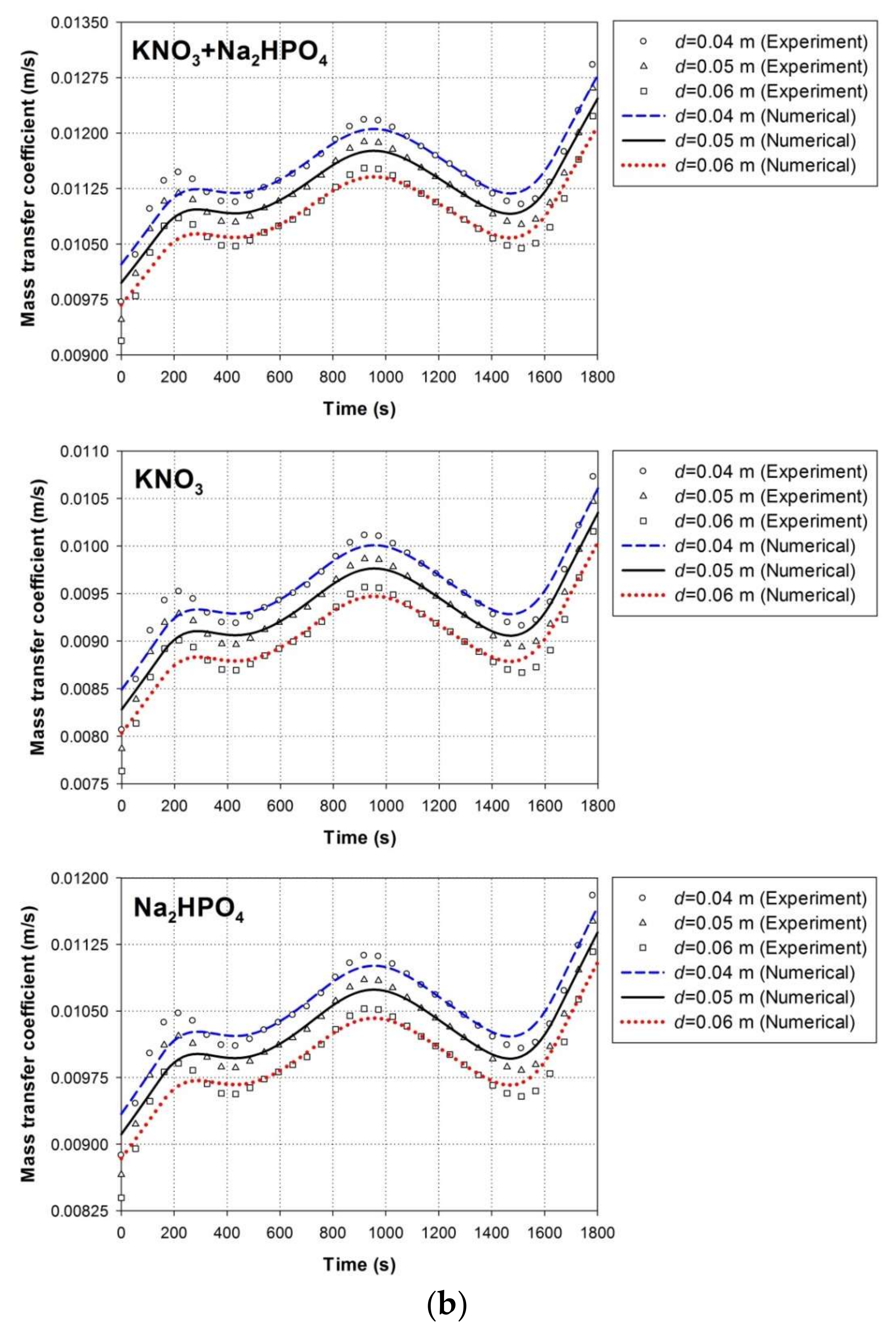
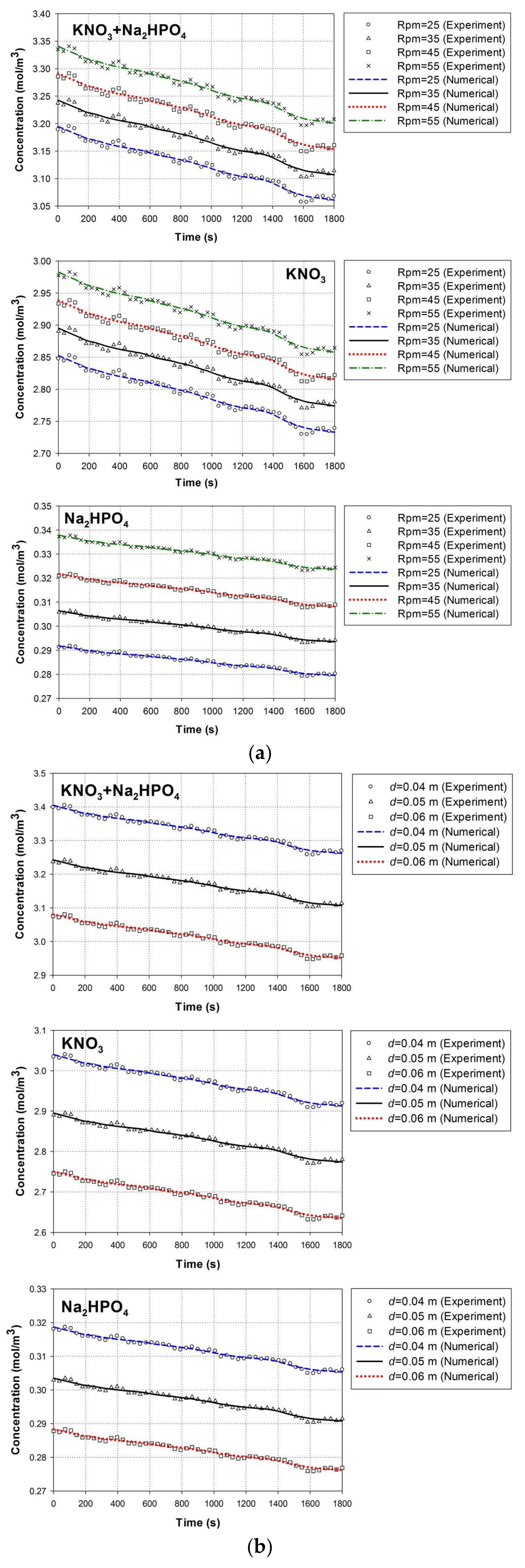
4.1. Experimental Validation
4.1.1. Solid–Liquid Mass Transfer Coefficient
4.1.2. Nutrient Concentrations
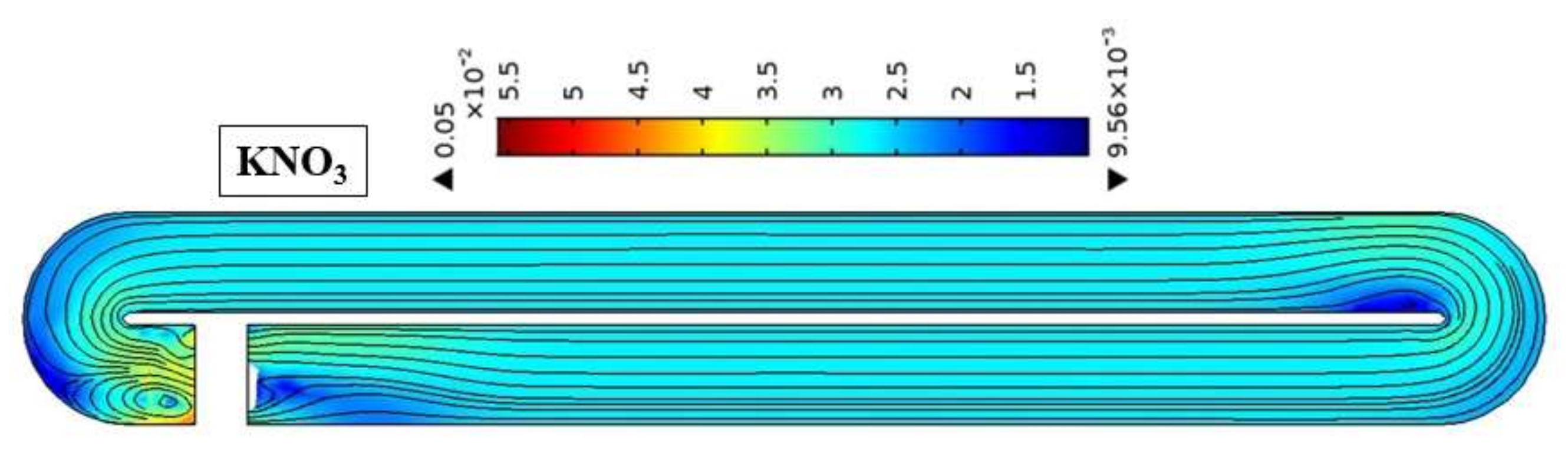
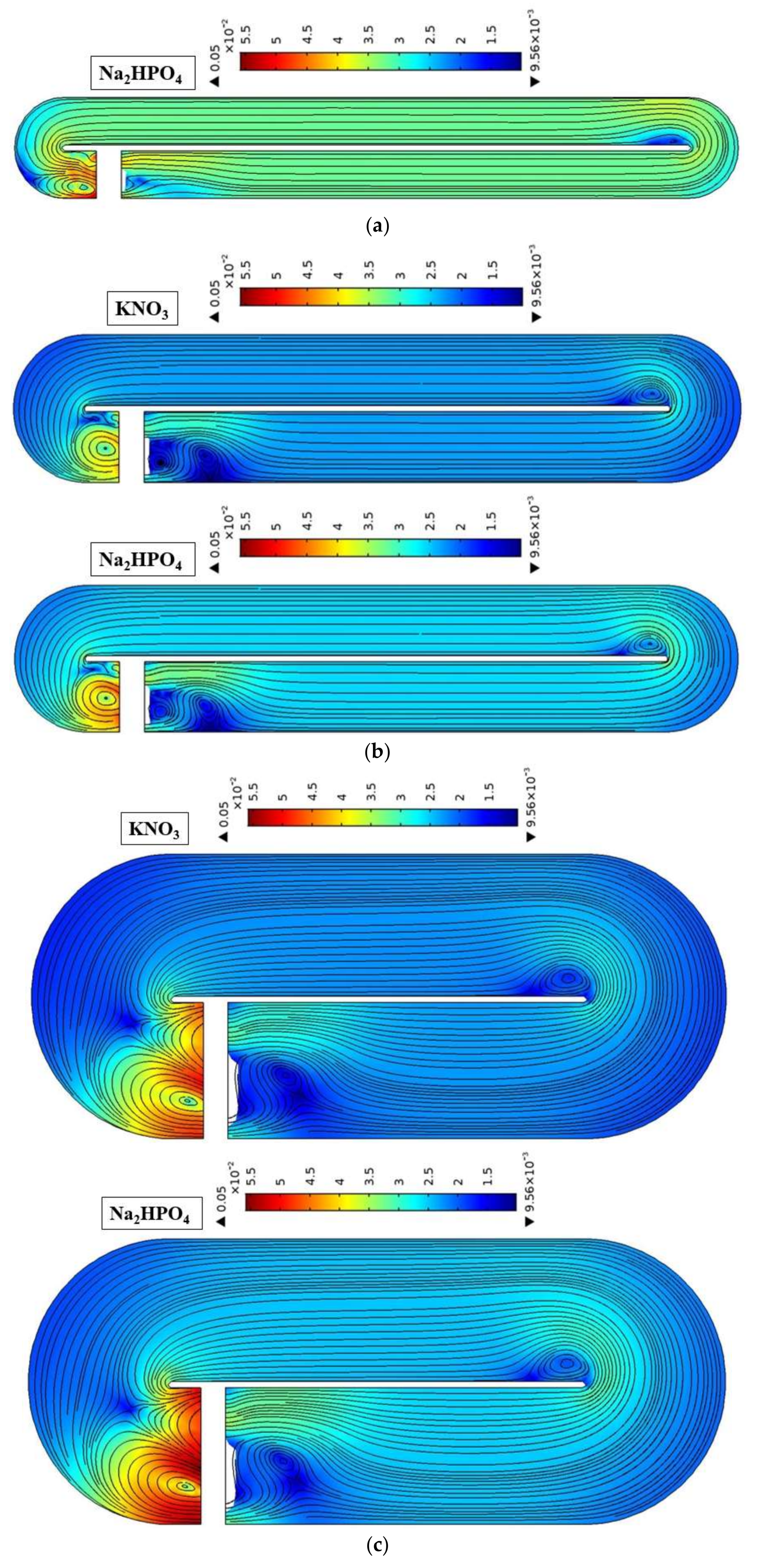
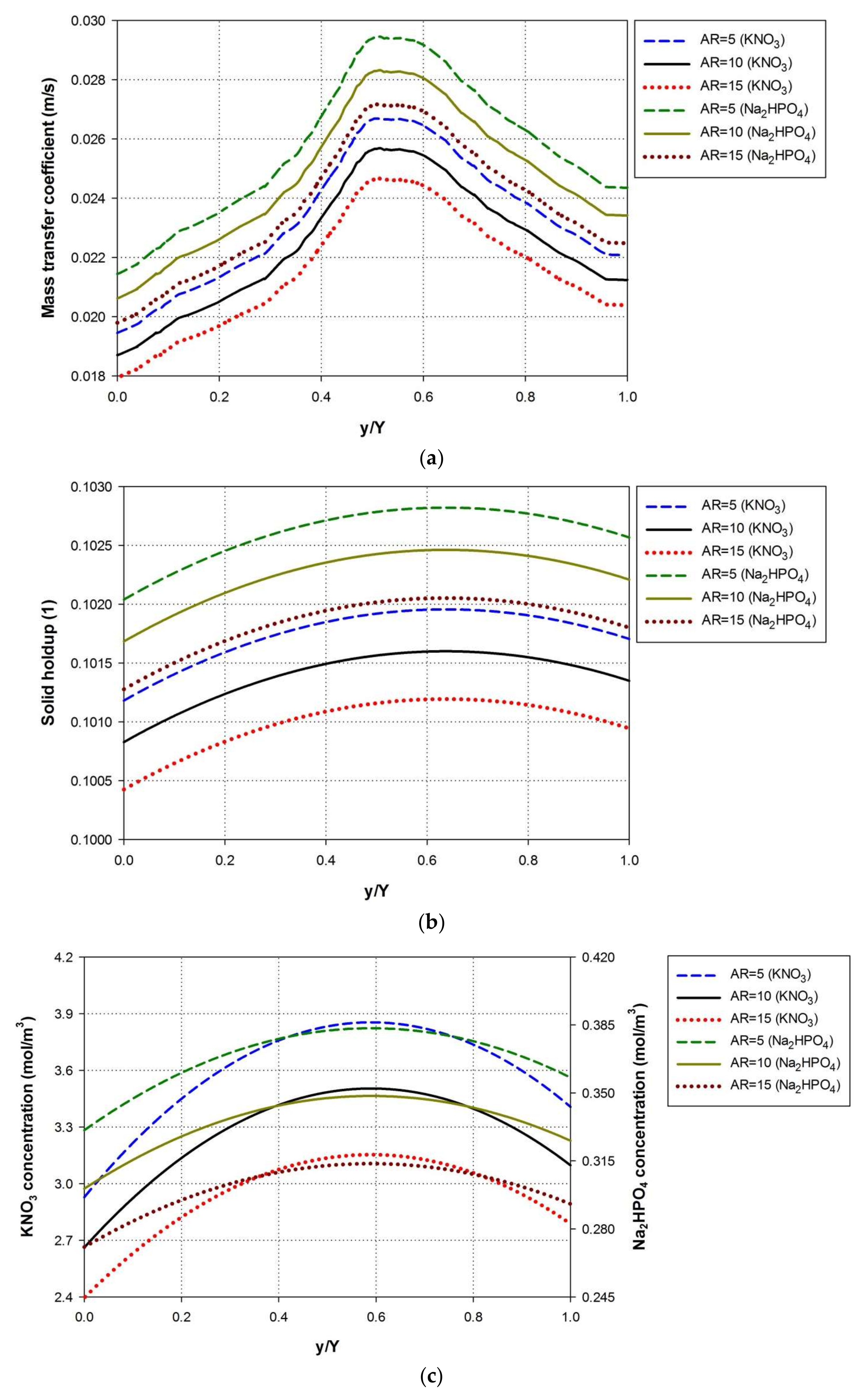
4.2. Effect of Pond Aspect Ratio
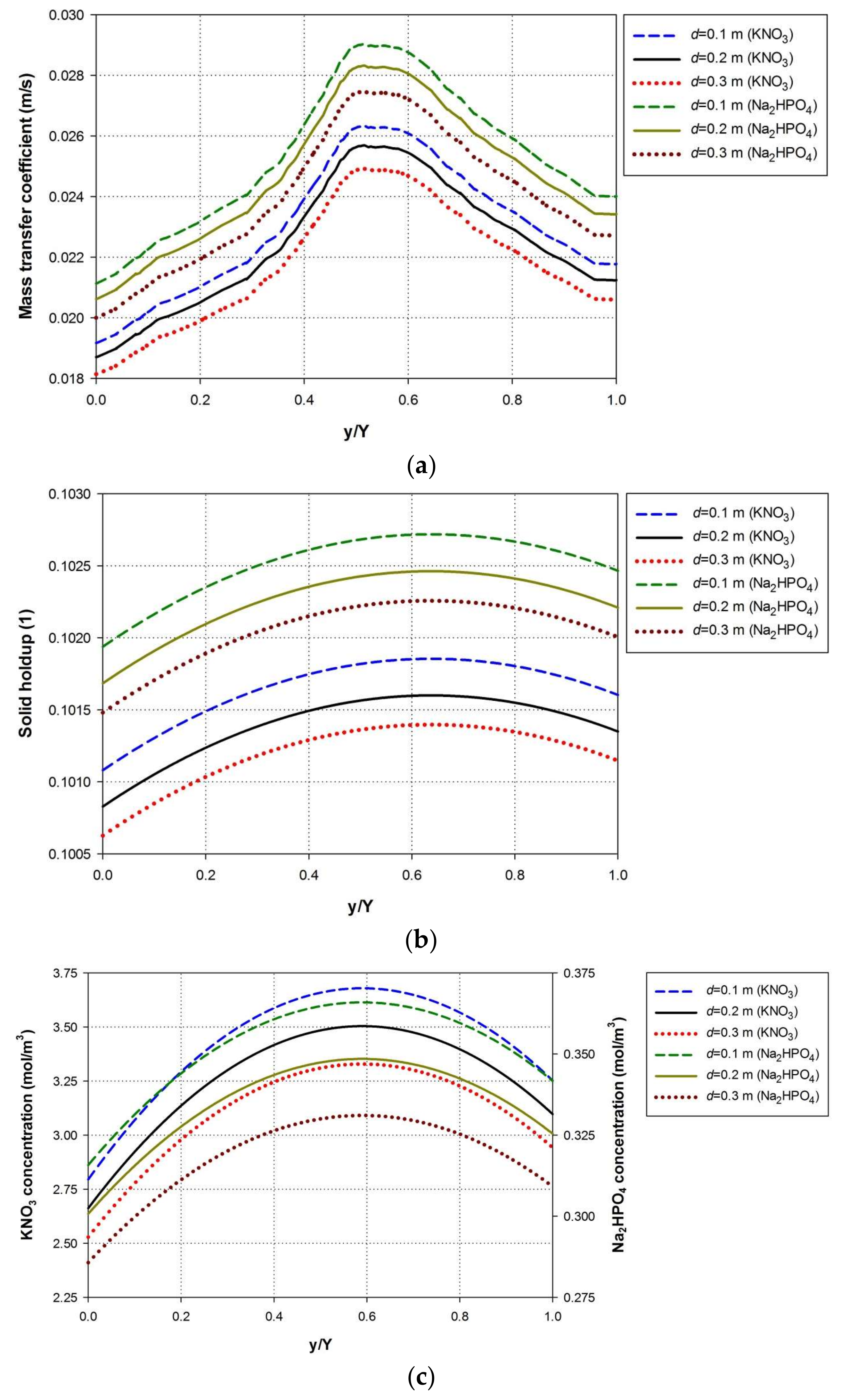
4.3. Effect of Water Depth
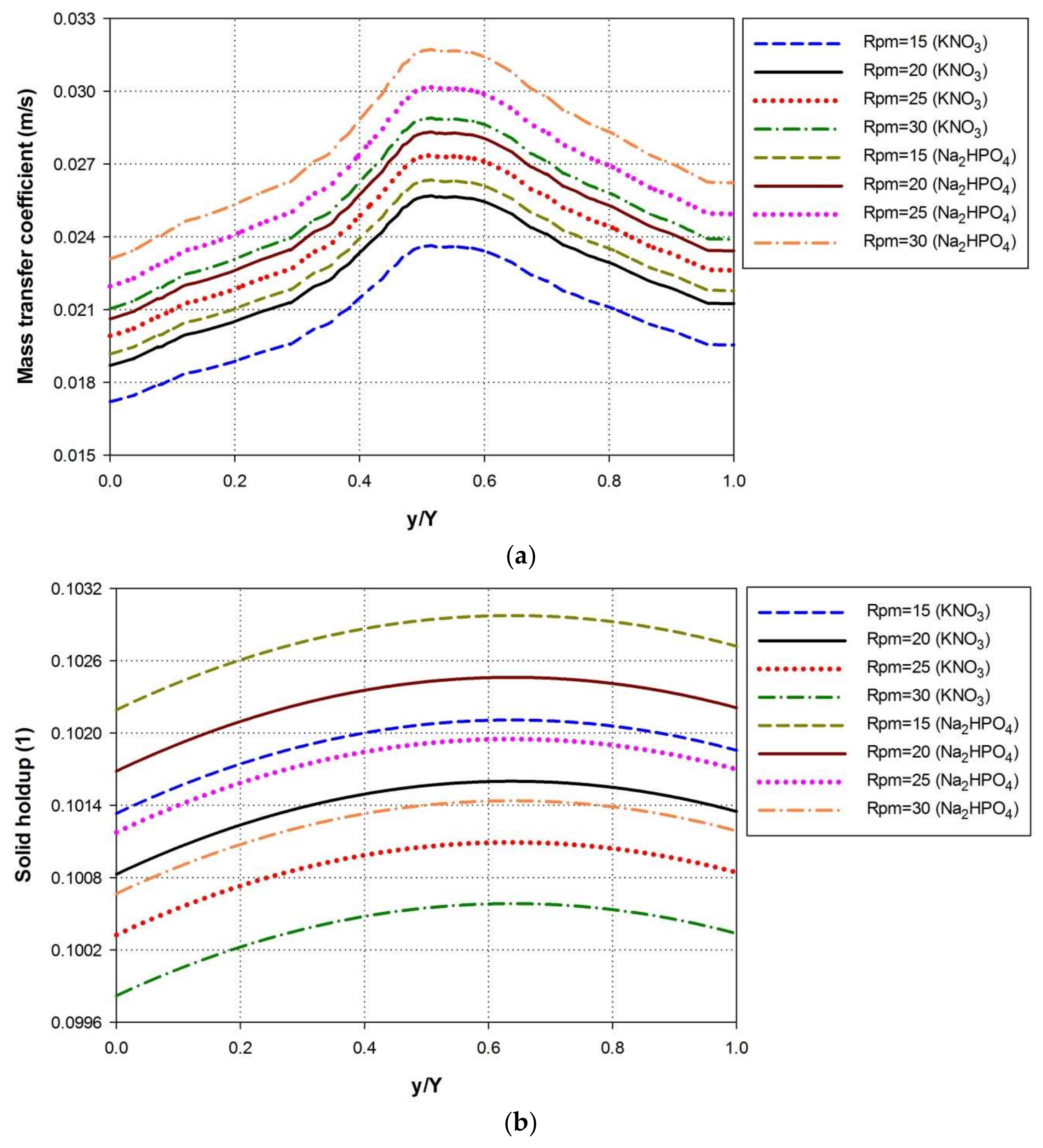
4.4. Effect of Paddle Wheel Rotational Speeds
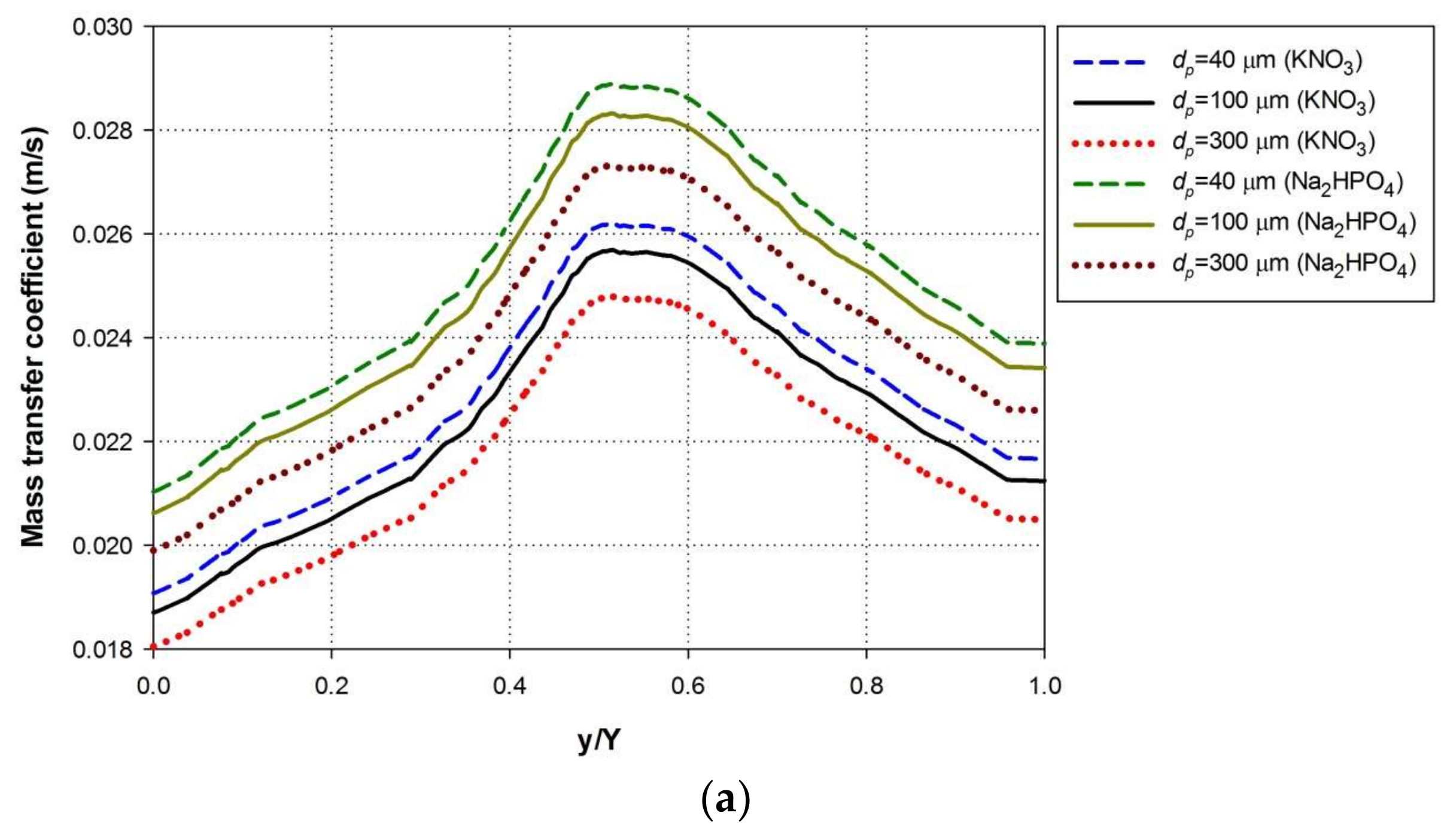
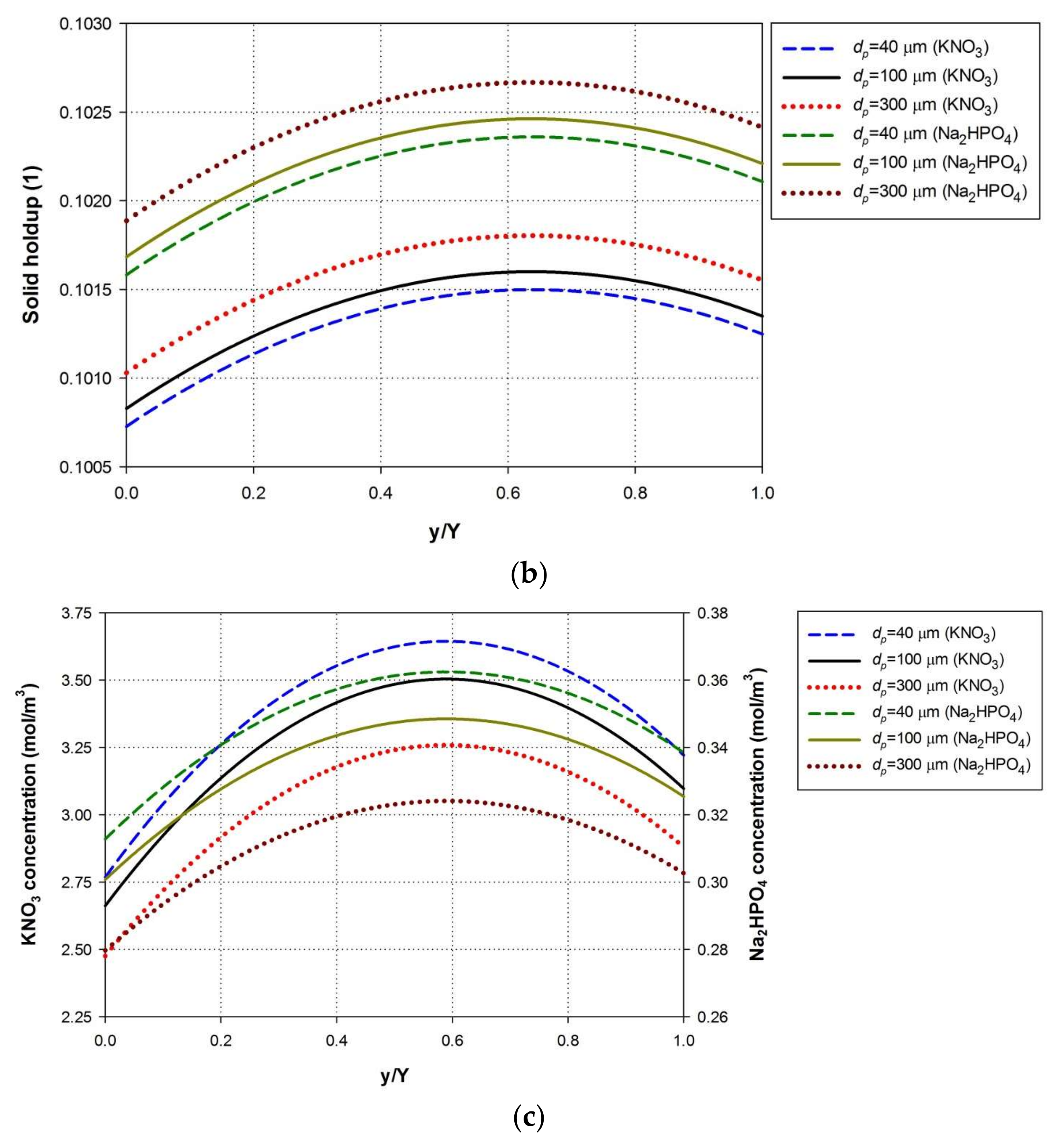
4.5. Effect of Nutrient Particle Size
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Nomenclature
| interfacial area (1/m) | |
| aspect ratio (1) | |
| initial particle concentration in liquid (mol/m3) | |
| dissolved particle concentration in liquid (mol/m3) | |
| mass fraction (kg/kg) | |
| drag coefficient (1) | |
| d | water or pond depth (m) |
| solid particle size (m) | |
| diffusion coefficient (m2/s) | |
| hydraulic diameter (m) | |
| dispersion coefficient (m2/s) | |
| Froude number (1) | |
| gravity vector (m/s2) | |
| pond height (m) | |
| turbulent kinetic energy (m2/s2) | |
| solid–liquid mass transfer coefficient (m/s) | |
| L | pond length (m) |
| mass transfer rate (kg/(m3∙s)) | |
| molecular weight (kg/mol) | |
| density number (1) | |
| number of particles per volume (1/m3) | |
| pressure (Pa) | |
| Reynolds number (1) | |
| Schmidt number (1) | |
| velocity vector (m/s) | |
| slip velocity vector (m/s) | |
| liquid velocity (m/s) | |
| W | water width (m) |
| gradient operator | |
| Greek Symbols | |
| density (kg/m3) | |
| turbulent energy dissipation rate (m2/s3) | |
| dynamic viscosity (Pa·s) | |
| turbulent or eddy viscosity (Pa·s) | |
| angular velocity (rad/s) | |
| Prandtl number for kinetic energy (1) | |
| turbulent particle Schmidt number (1) | |
| Prandtl number for dissipation rate (1) | |
| volume fraction or holdup (m3/m3) | |
| Subscripts | |
| continuous phase | |
| dispersed phase | |
| liquid phase | |
| particle | |
| solid phase | |
References
- Mostert, E.S.; Grobbelaar, J.U. The influence of nitrogen and phosphorus on algal growth and quality in outdoor mass algal cultures. Biomass 1987, 13, 219–233. [Google Scholar] [CrossRef]
- Grobbelaar, J.U.; Soeder, C.J.; Stengel, E. Modeling algal productivity in large outdoor cultures and waste treatment systems. Biomass 1990, 21, 297–314. [Google Scholar] [CrossRef]
- Goldman, J.C. Outdoor algal mass cultures—II. Photosynthetic yield limitations. Water Res. 1979, 13, 119–136. [Google Scholar] [CrossRef]
- Ruiz-Marin, A.; Mendoza-Espinosa, L.G.; Stephenson, T. Growth and nutrient removal in free and immobilized green algae in batch and semi-continuous cultures treating real wastewater. Bioresour. Technol. 2010, 101, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, A.; Parker, M.S.; Moccia, L.P.; Lin, E.O.; Arrieta, A.L.; Ribalet, F.; Murphy, M.E.P.; Maldonado, M.T.; Armbrust, E.V. Ferritin is used for iron storage in bloom-forming marine pennate diatoms. Nature 2009, 457, 467–470. [Google Scholar] [CrossRef] [PubMed]
- Borowitzka, M.A. Commercial production of microalgae: ponds, tanks, tubes and fermenters. J. Biotechnol. 1999, 70, 313–321. [Google Scholar] [CrossRef]
- Grobbelaar, J.U.; Kroon, B.M.A.; Burger-Wiersma, T.; Mur, L.R. Influence of medium frequency light/dark cycles of equal duration on the photosynthesis and respiration of Chlorella pyrenoidosa. Hydrobiologia 1992, 238, 53–62. [Google Scholar] [CrossRef]
- Terry, K.L.; Raymond, L.P. System design for the autotrophic production of microalgae. Enzyme Microb. Technol. 1985, 7, 474–487. [Google Scholar] [CrossRef]
- Yang, Z.; del Ninno, M.; Wen, Z.; Hu, H. An experimental investigation on the multiphase flows and turbulent mixing in a flat-panel photobioreactor for algae cultivation. J. Appl. Phycol. 2014, 26, 2097–2107. [Google Scholar] [CrossRef]
- Weissman, J.C.; Goebel, R.P.; Benemann, J.R. Photobioreactor design: Mixing, carbon utilization, and oxygen accumulation. Biotechnol. Bioeng. 1988, 31, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Conley, D.J.; Paerl, H.W.; Howarth, R.W.; Boesch, D.F.; Seitzinger, S.P.; Havens, K.E.; Lancelot, C.; Likens, G.E. Ecology: Controlling Eutrophication: Nitrogen and Phosphorus. Science 2009, 323, 1014–1015. [Google Scholar] [CrossRef] [PubMed]
- Talling, J.F.; Fogg, G.E. Algal Cultures and Phytoplankton Ecology. J. Appl. Ecol. 1966, 3, 215. [Google Scholar] [CrossRef]
- Eustance, E.; Wray, J.T.; Badvipour, S.; Sommerfeld, M.R. The effects of cultivation depth, areal density, and nutrient level on lipid accumulation of Scenedesmus acutus in outdoor raceway ponds. J. Appl. Phycol. 2016, 28, 1459–1469. [Google Scholar] [CrossRef]
- Marra, J. Algae Biomass: Production and Use. Gedaliah Shelef, Carl J. Soeder. Q. Rev. Biol. 1981, 56, 496–497. [Google Scholar] [CrossRef]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Kalaga, D.V.; Dhar, A.; Dalvi, S.V.; Joshi, J.B. Particle-liquid mass transfer in solid-liquid fluidized beds. Chem. Eng. J. 2014, 245, 323–341. [Google Scholar] [CrossRef]
- Tang, C.; Liu, M.; Li, Y. Experimental investigation of hydrodynamics of liquid–solid mini-fluidized beds. Particuology 2016, 27, 102–109. [Google Scholar] [CrossRef]
- Palkar, R.R.; Shilapuram, V. Development of a model for the prediction of hydrodynamics of a liquid–solid circulating fluidized beds: A full factorial design approach. Powder Technol. 2015, 280, 103–112. [Google Scholar] [CrossRef]
- Grisafi, F.; Brucato, A.; Rizzuti, L. Solid-liquid mass transfer coefficients in gas-solid-liquid agitated vessels. Can. J. Chem. Eng. 1998, 76, 446–455. [Google Scholar] [CrossRef]
- Chiesa, M.; Mathiesen, V.; Melheim, J.A.; Halvorsen, B. Numerical simulation of particulate flow by the Eulerian–Lagrangian and the Eulerian–Eulerian approach with application to a fluidized bed. Comput. Chem. Eng. 2005, 29, 291–304. [Google Scholar] [CrossRef]
- Enwald, H.; Peirano, E.; Almstedt, A.-E. Eulerian two-phase flow theory applied to fluidization. Int. J. Multiph. Flow 1996, 22, 21–66. [Google Scholar] [CrossRef]
- Cornelissen, J.T.; Taghipour, F.; Escudié, R.; Ellis, N.; Grace, J.R. CFD modelling of a liquid–solid fluidized bed. Chem. Eng. Sci. 2007, 62, 6334–6348. [Google Scholar] [CrossRef]
- Yang, N.; Wang, W.; Ge, W.; Li, J. CFD simulation of concurrent-up gas–solid flow in circulating fluidized beds with structure-dependent drag coefficient. Chem. Eng. J. 2003, 96, 71–80. [Google Scholar] [CrossRef]
- Ali, H.; Park, C.W. Numerical multiphase modeling of CO2 absorption and desorption in microalgal raceway ponds to improve their carbonation efficiency. Energy 2017, 127, 358–371. [Google Scholar] [CrossRef]
- Ali, H.; Cheema, T.A.; Park, C.W. Numerical prediction of heat transfer characteristics based on monthly temperature gradient in algal open raceway ponds. Int. J. Heat Mass Transf. 2017, 106, 7–17. [Google Scholar] [CrossRef]
- Ali, H.; Cheema, T.; Park, C. Determination of the Structural Characteristics of Microalgal Cells Walls under the Influence of Turbulent Mixing Energy in Open Raceway Ponds. Energies 2018, 11, 388. [Google Scholar] [CrossRef]
- Ali, H.; Cheema, T.A.; Park, C.W. Effect of Paddle-Wheel Pulsating Velocity on the Hydrodynamic Performance of High-Rate Algal Ponds. J. Energy Eng. 2015, 141, 4014039. [Google Scholar] [CrossRef]
- Ali, H.; Cheema, T.A.; Yoon, H.-S.; Do, Y.; Park, C.W. Numerical prediction of algae cell mixing feature in raceway ponds using particle tracing methods. Biotechnol. Bioeng. 2015, 112, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Cooper, A.R. Effect of Aspect Ratio and Viscosity Gradients on Flow Through Open Channels. J. Am. Ceram. Soc. 1960, 43, 97–103. [Google Scholar] [CrossRef]
- Auel, C.; Albayrak, I.; Boes, R.M. Turbulence Characteristics in Supercritical Open Channel Flows: Effects of Froude Number and Aspect Ratio. J. Hydraul. Eng. 2014, 140, 4014004. [Google Scholar] [CrossRef]
- Pang, M.J.; Wei, J.J. Analysis of drag and lift coefficient expressions of bubbly flow system for low to medium Reynolds number. Nucl. Eng. Des. 2011, 241, 2204–2213. [Google Scholar] [CrossRef]
- Wilcox, D.C. Turbulence Modeling for CFD; Turbulence Modeling for CFD; DCW Industries: Flintridge, CA, USA, 2006; ISBN 9781928729082. [Google Scholar]
- Silva, R.; Cotas, C.; Garcia, F.A.P.; Faia, P.M.; Rasteiro, M.G. Particle Distribution Studies in Highly Concentrated Solid-liquid Flows in Pipe Using the Mixture Model. Procedia Eng. 2015, 102, 1016–1025. [Google Scholar] [CrossRef]
- Ali, H.; Cheema, T.A.; Park, C.W. Numerical modeling of two-phase bubbly flow mixing with mass transport in an effective microorganism odor removing system. J. Chem. Technol. Biotechnol. 2016, 91, 1012–1022. [Google Scholar] [CrossRef]
- Randolph, A.D.; Larson, M.A.; Randolph, A.D.; Larson, M.A. Chapter 3—The population balance. In Theory of Particulate Processes; Academic Press Inc.: San Diego, CA, USA, 1988; pp. 50–79. ISBN 9780125796521. [Google Scholar]
- Harned, H.S.; Hudson, R.M. The Differential Diffusion Coefficient of Potassium Nitrate in Dilute Aqueous Solutions at 25°. J. Am. Chem. Soc. 1951, 73, 652–654. [Google Scholar] [CrossRef]
- Camacho, F.G.; Gómez, A.C.; Sobczuk, T.M.; Grima, E.M. Effects of mechanical and hydrodynamic stress in agitated, sparged cultures of Porphyridium cruentum. Process Biochem. 2000, 35, 1045–1050. [Google Scholar] [CrossRef]
- Thomas, W.H.; Gibson, C.H. Effects of small-scale turbulence on microalgae. J. Appl. Phycol. 1990, 2, 71–77. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Yeh, K.-L.; Aisyah, R.; Lee, D.-J.; Chang, J.-S. Cultivation, photobioreactor design and harvesting of microalgae for biodiesel production: A critical review. Bioresour. Technol. 2011, 102, 71–81. [Google Scholar] [CrossRef] [PubMed]

| Nutrient | Density (kg/m3) | Particle Size (µm) | Molar Mass (g/mol) |
|---|---|---|---|
| KNO3 | 2110 | 100 | 101.1 |
| Na2HPO4 | 1700 | 100 | 141.96 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, H.; Zhang, D.; Wagner, J.L.; Park, C.W. Two-Phase Flow Modeling of Solid Dissolution in Liquid for Nutrient Mixing Improvement in Algal Raceway Ponds. Energies 2018, 11, 899. https://doi.org/10.3390/en11040899
Ali H, Zhang D, Wagner JL, Park CW. Two-Phase Flow Modeling of Solid Dissolution in Liquid for Nutrient Mixing Improvement in Algal Raceway Ponds. Energies. 2018; 11(4):899. https://doi.org/10.3390/en11040899
Chicago/Turabian StyleAli, Haider, Dongda Zhang, Jonathan L. Wagner, and Cheol Woo Park. 2018. "Two-Phase Flow Modeling of Solid Dissolution in Liquid for Nutrient Mixing Improvement in Algal Raceway Ponds" Energies 11, no. 4: 899. https://doi.org/10.3390/en11040899
APA StyleAli, H., Zhang, D., Wagner, J. L., & Park, C. W. (2018). Two-Phase Flow Modeling of Solid Dissolution in Liquid for Nutrient Mixing Improvement in Algal Raceway Ponds. Energies, 11(4), 899. https://doi.org/10.3390/en11040899







