Reduction of Electric Power Consumption in CO2-PSA with Zeolite 13X Adsorbent
Abstract
:1. Introduction
2. Experimental
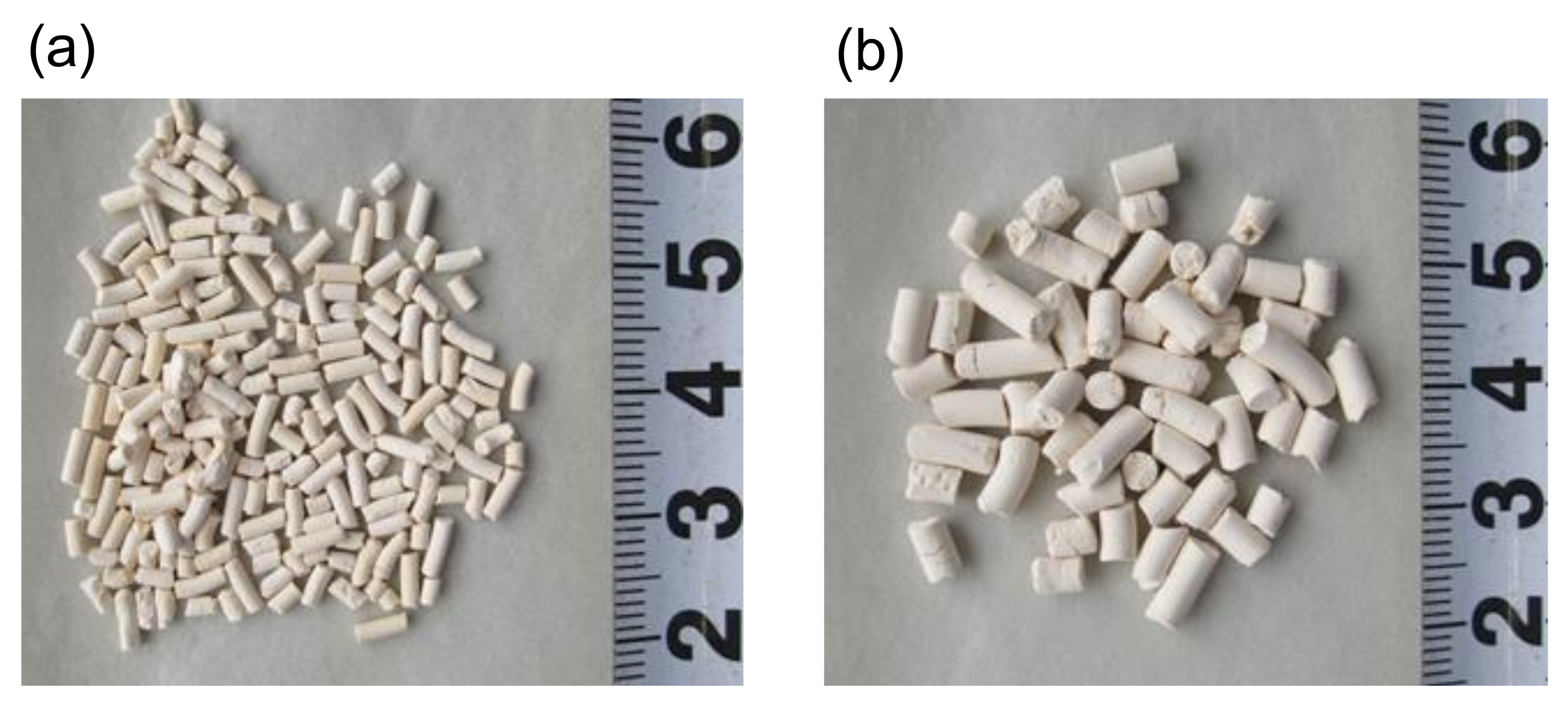
2.1. Preparation of Various Shapes of Adsorbents
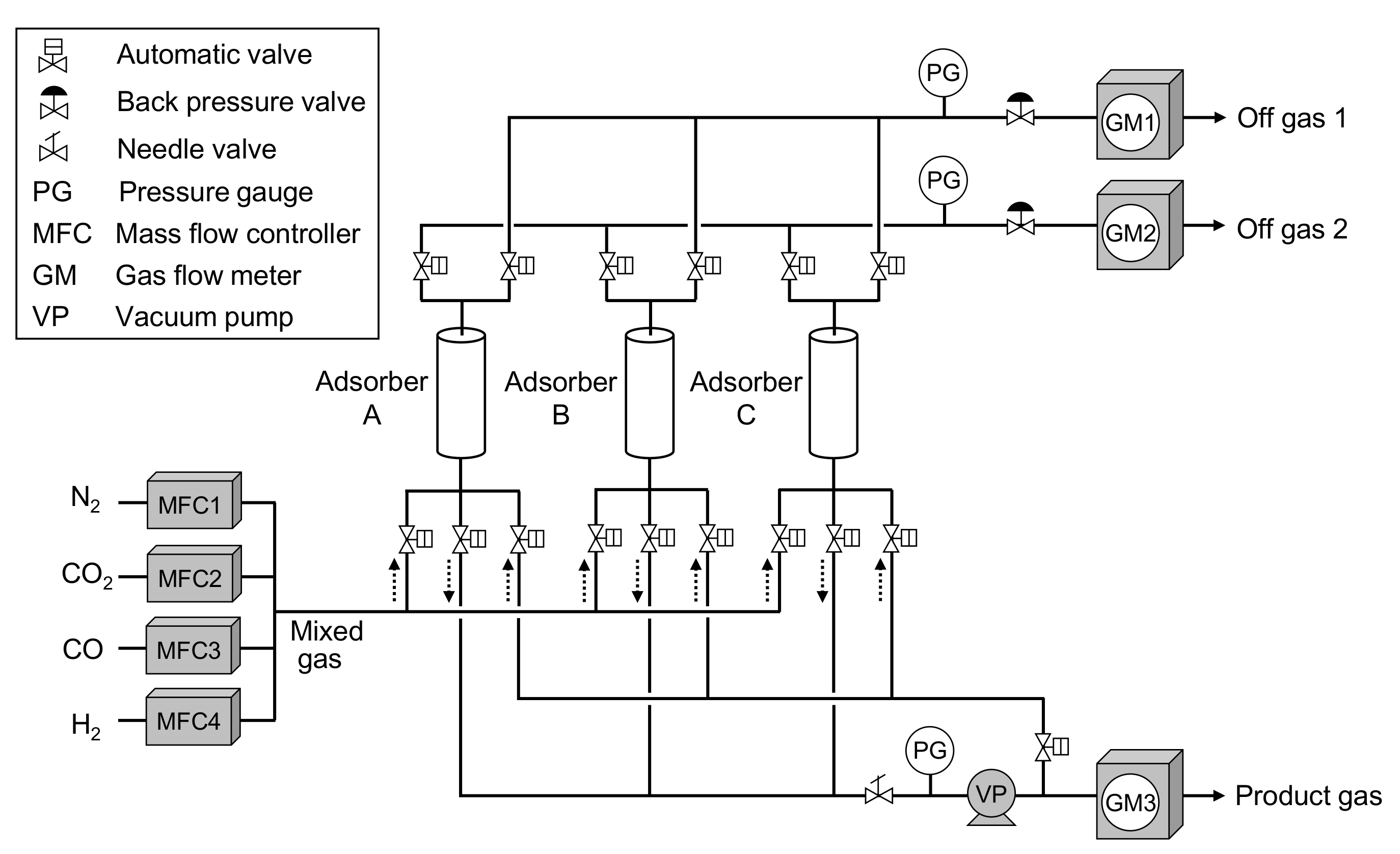
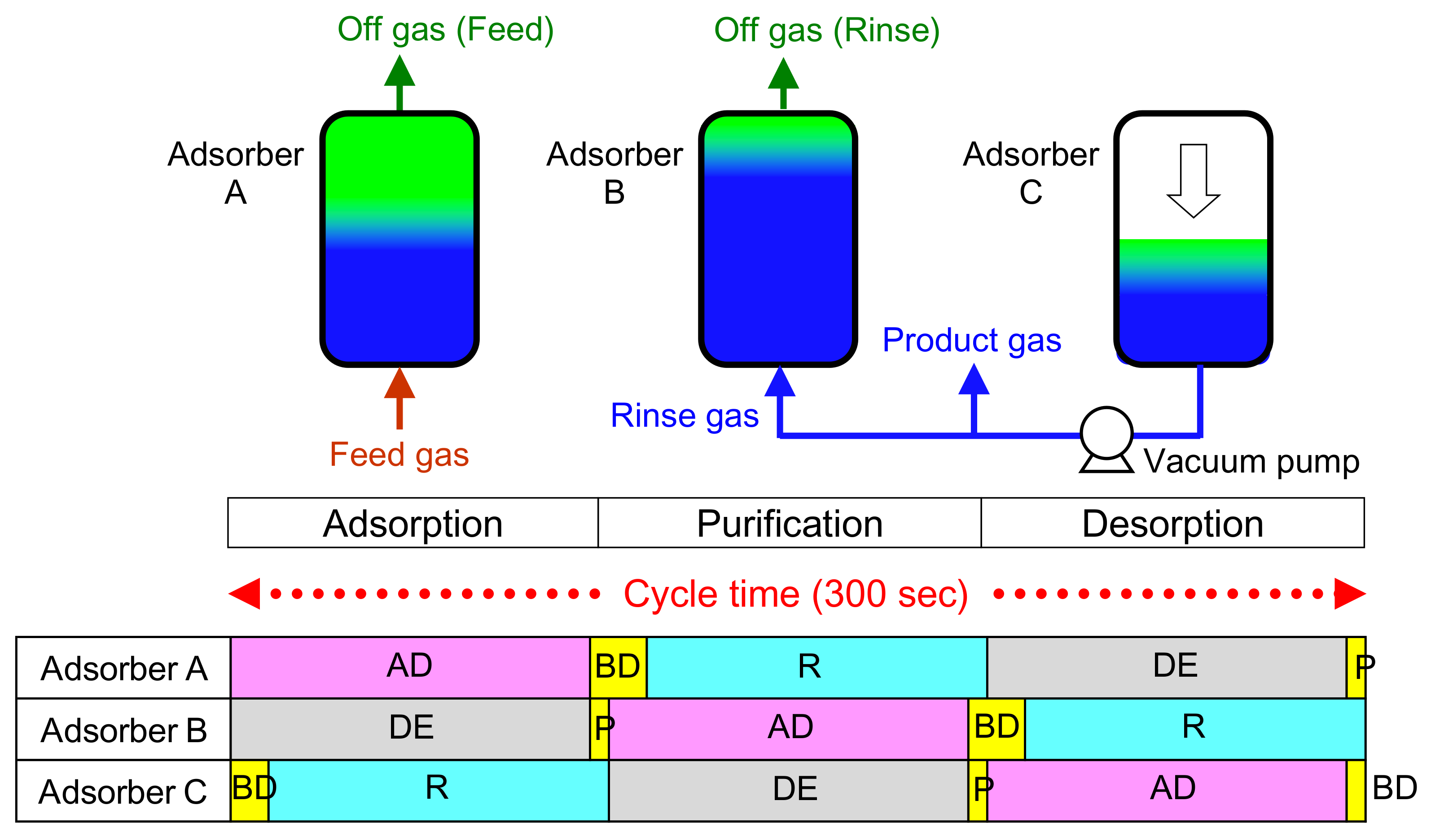
2.2. Laboratory-Scale CO2-PSA Experiment
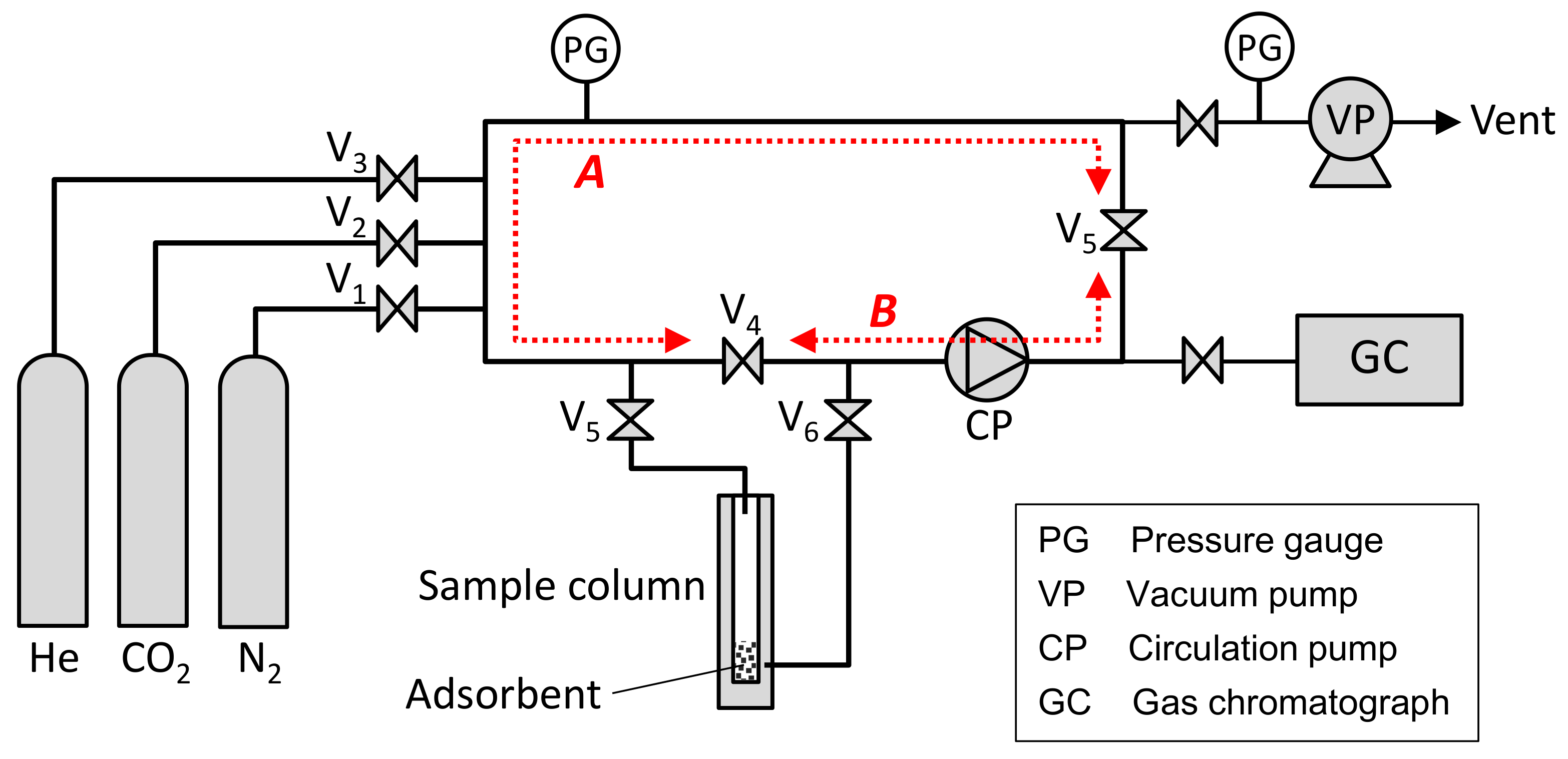
2.3. Measurement of CO2 Adsorption Rate in Early Stage of Adsorption
3. Results
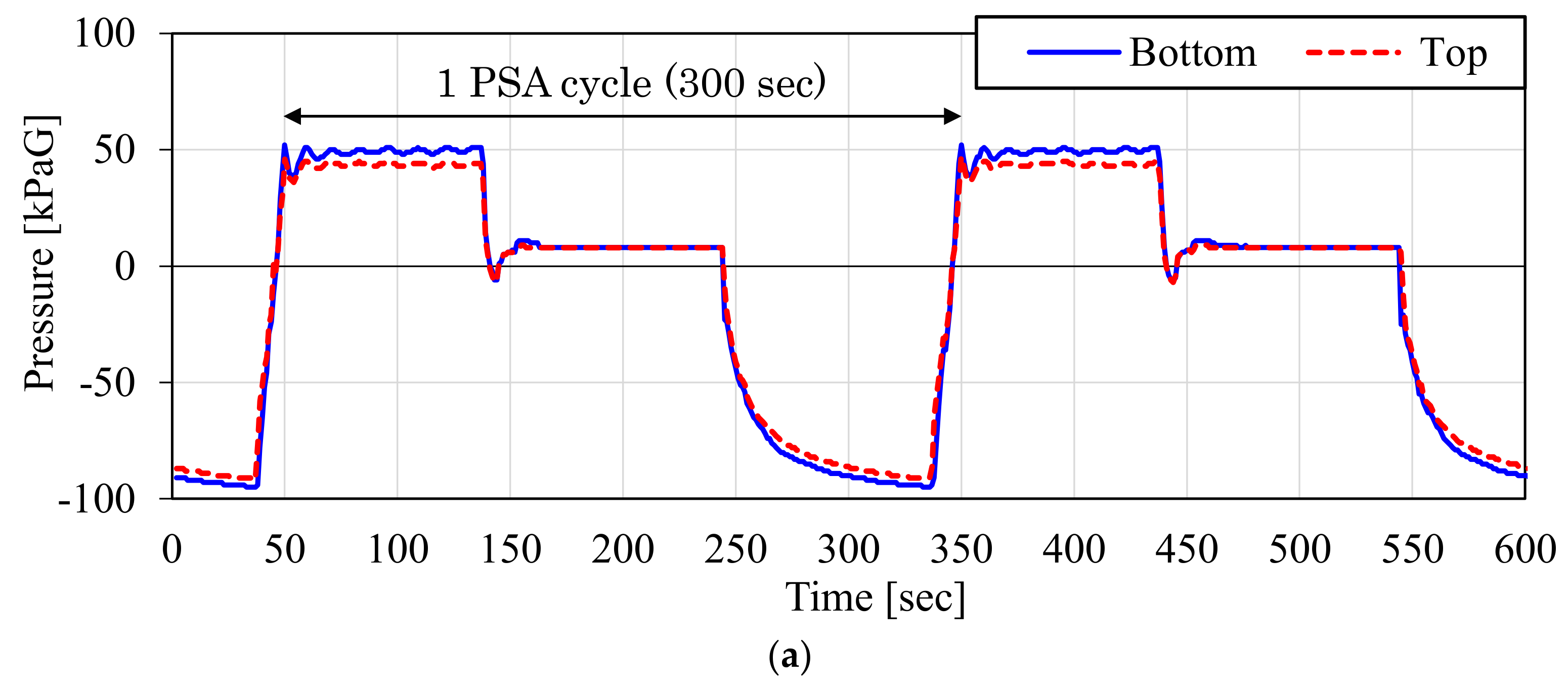
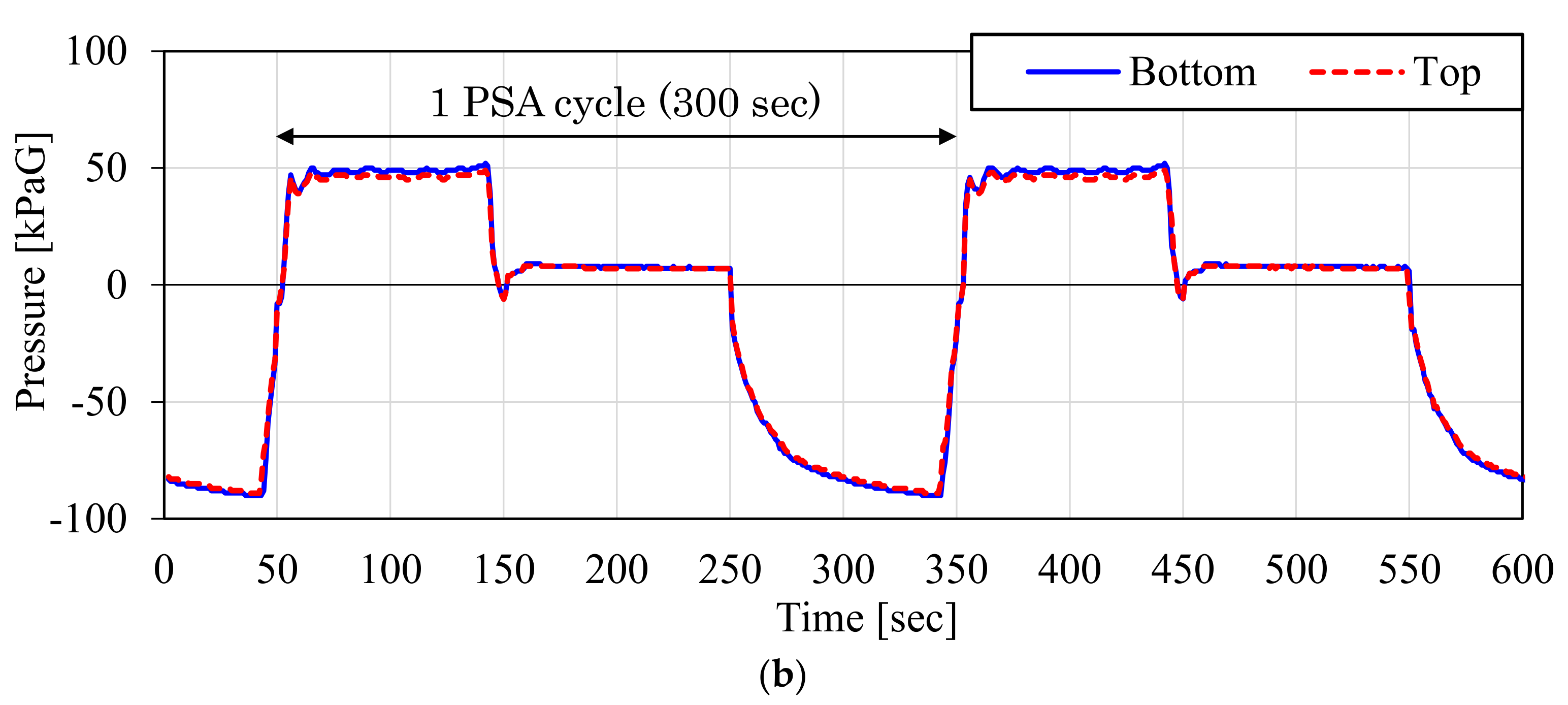
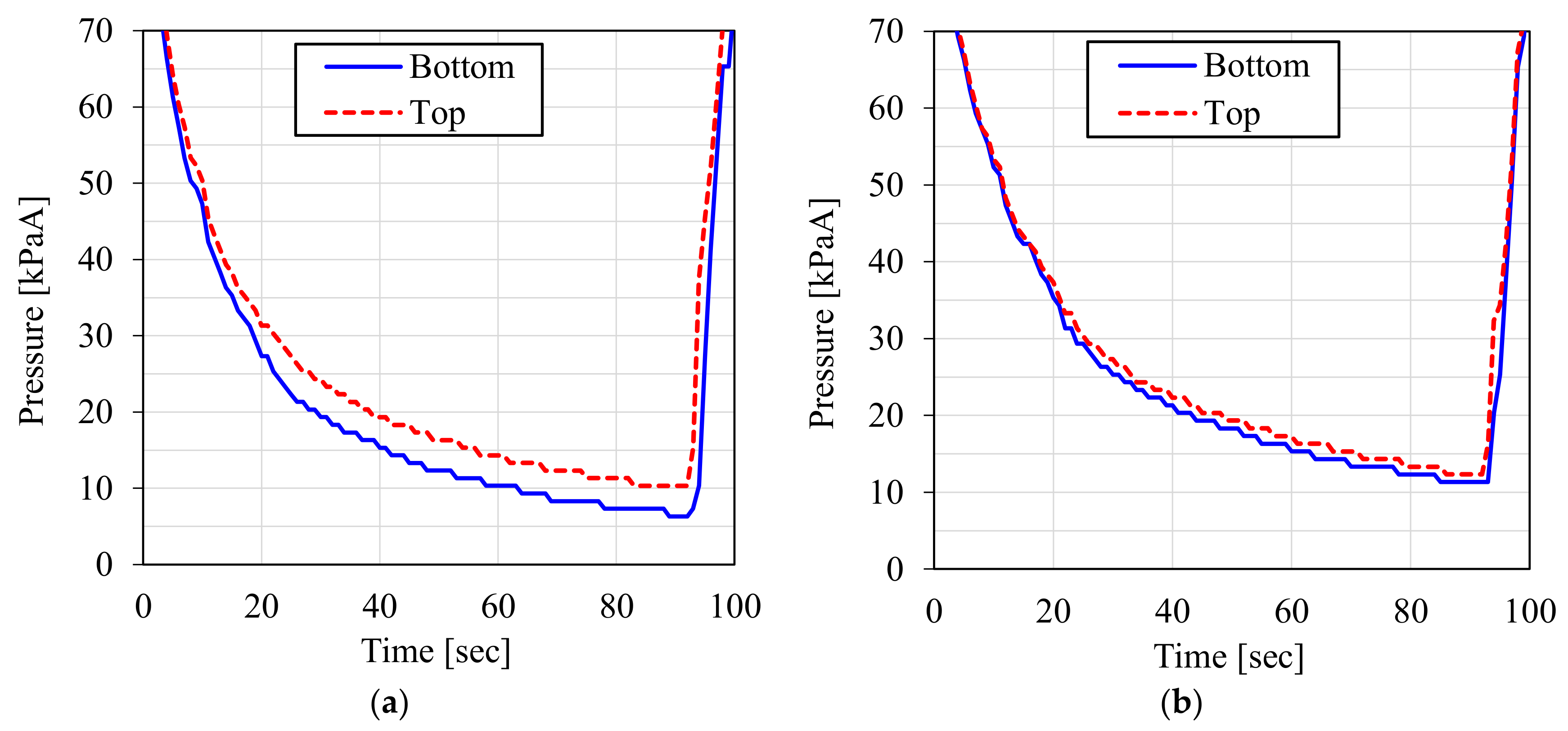
3.1. Result of the Laboratory-Scale CO2-PSA Experiment
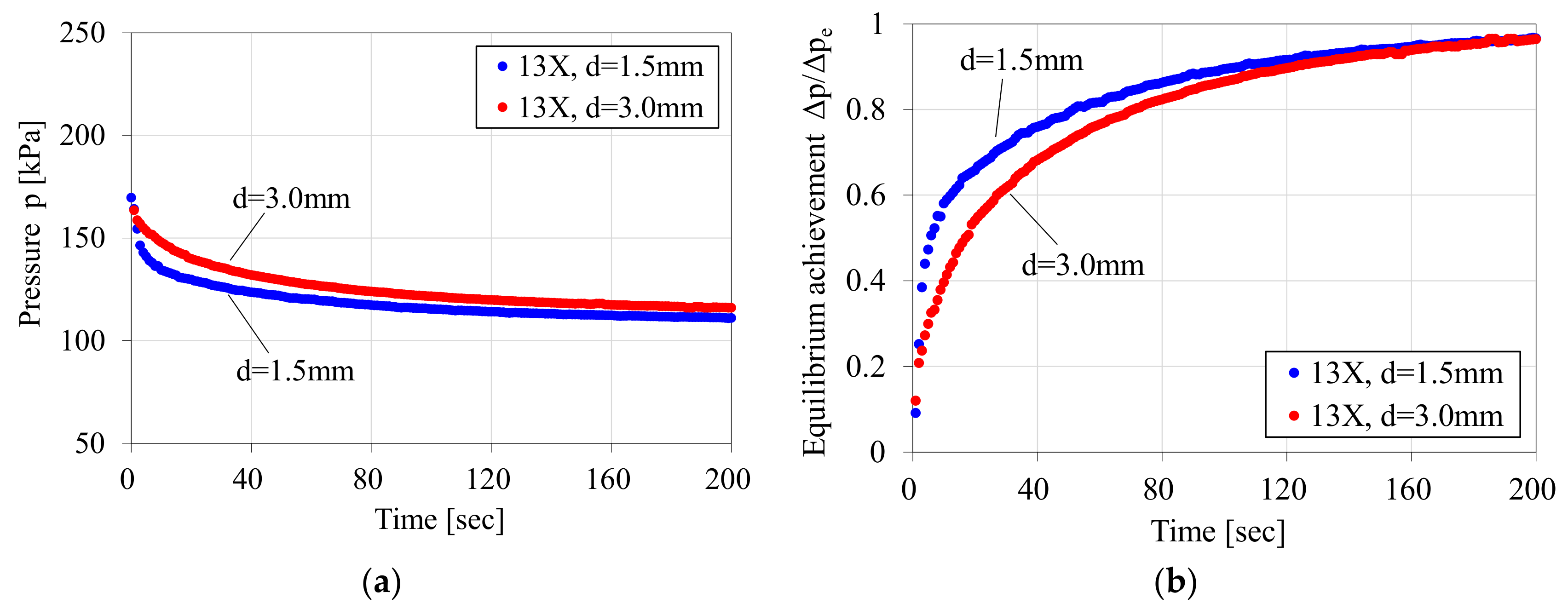
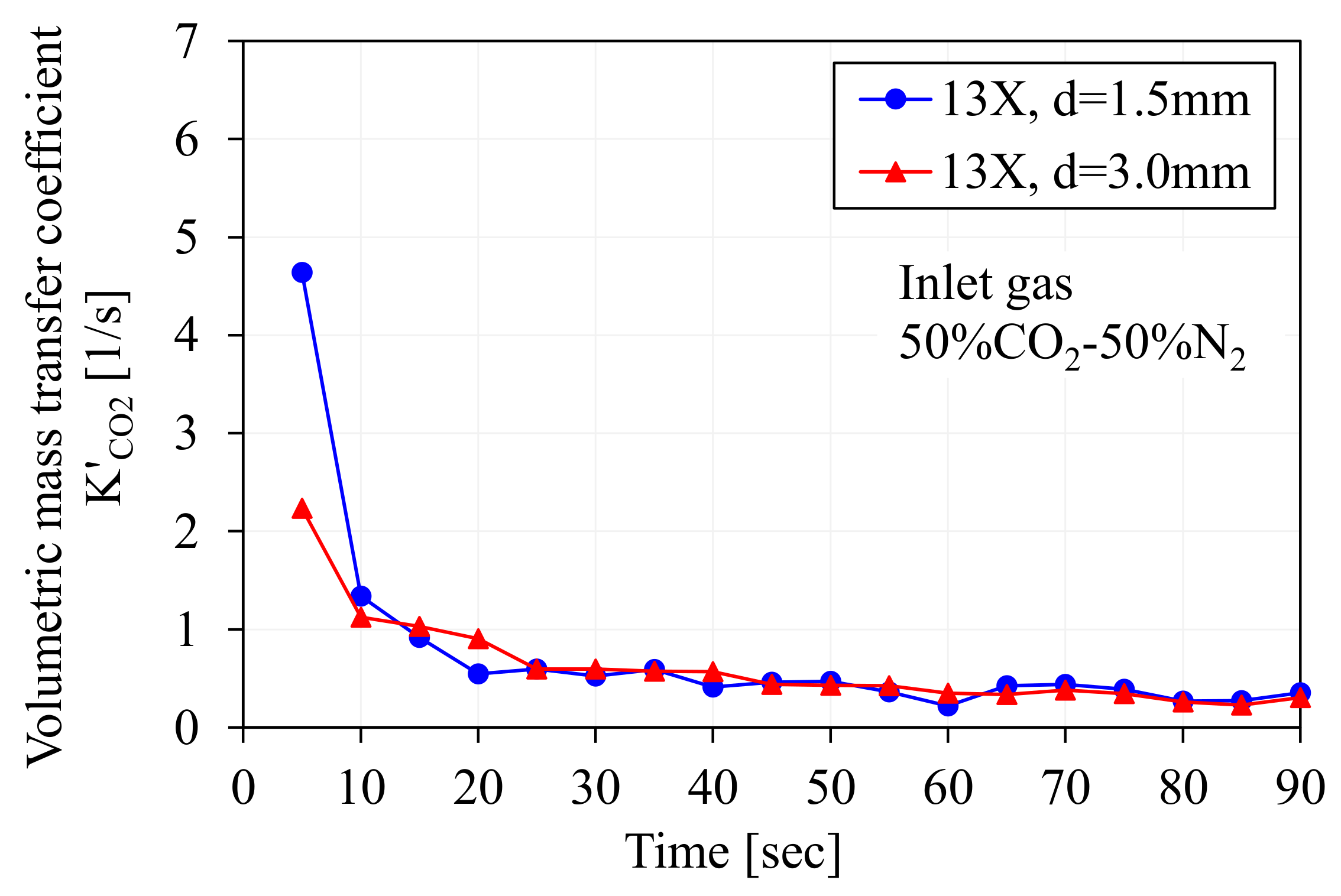
3.2. Result of the Measured CO2 Adsorption Rate in Early Stage of Adsorption
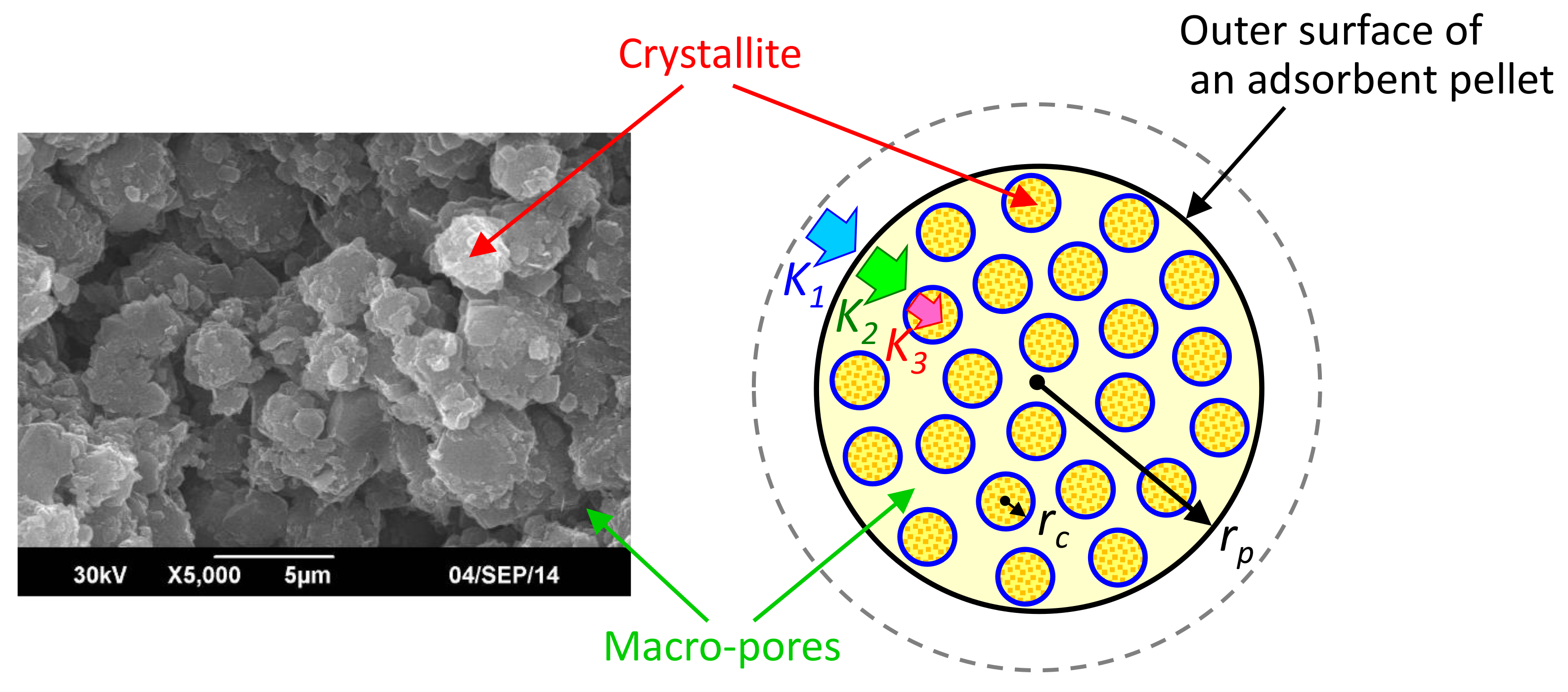
3.3. Discussion
4. Pilot Scale CO2-PSA Experiment
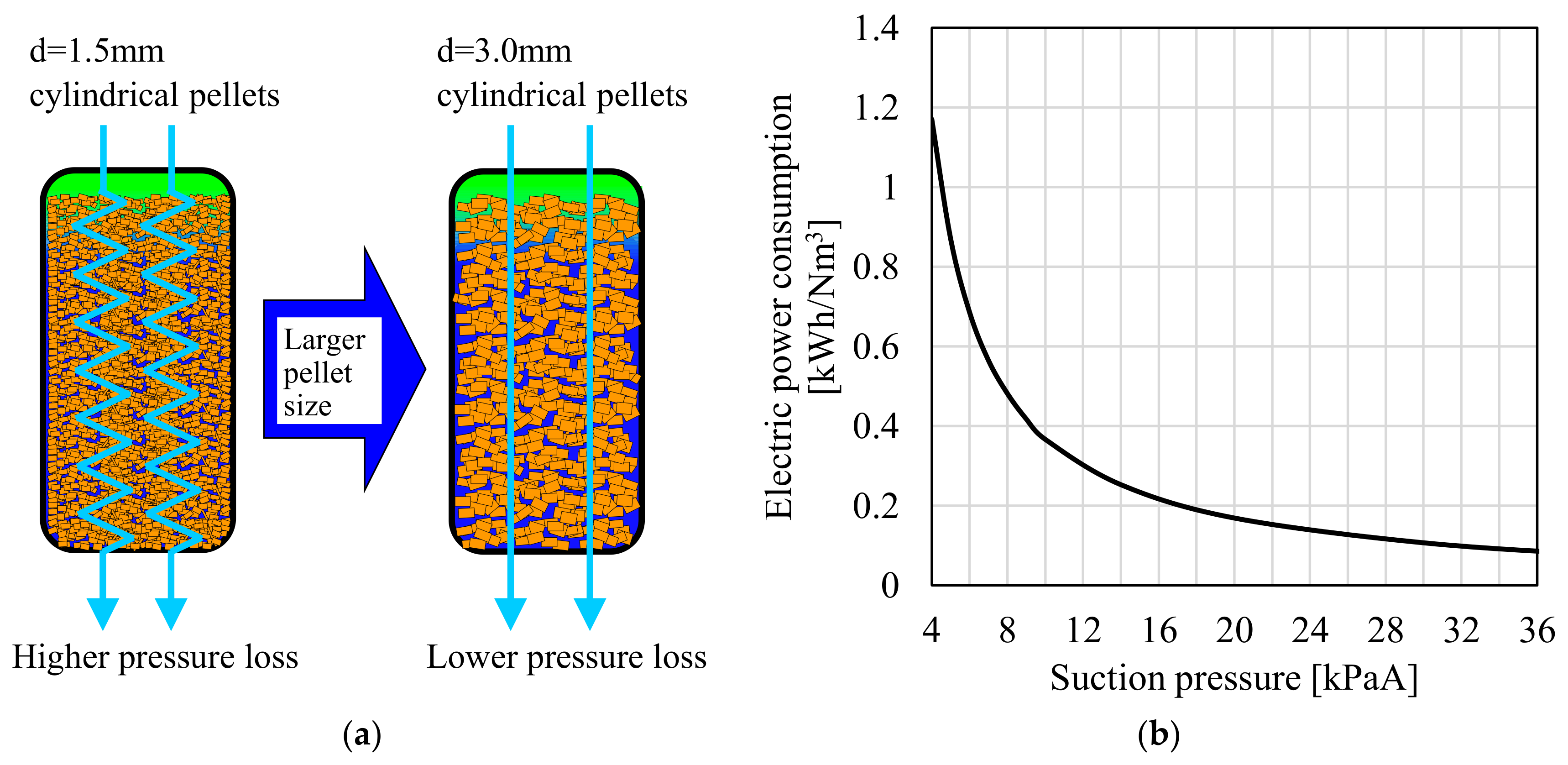
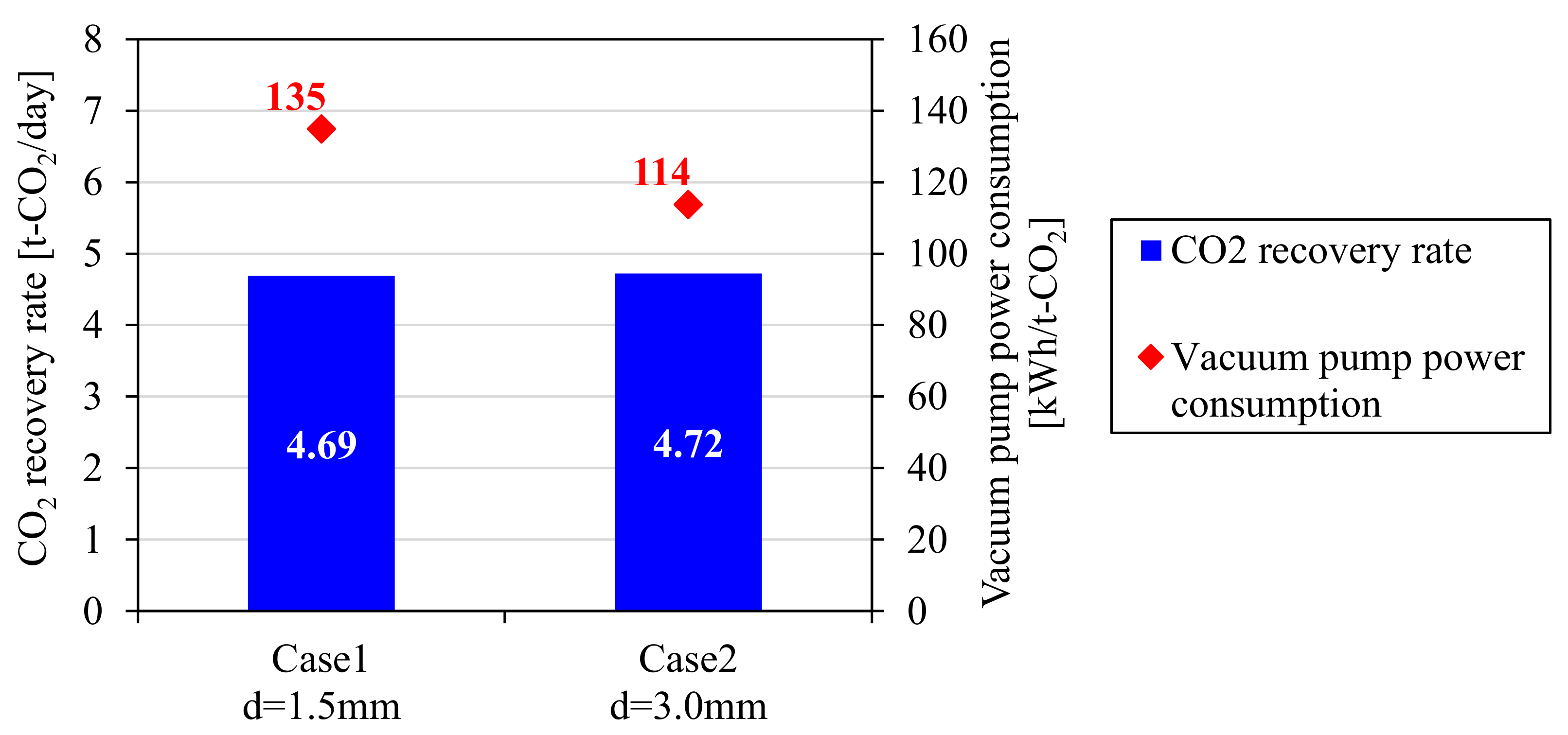
4.1. Reduction of Power Consumption by Effect of Adsorbent Shape
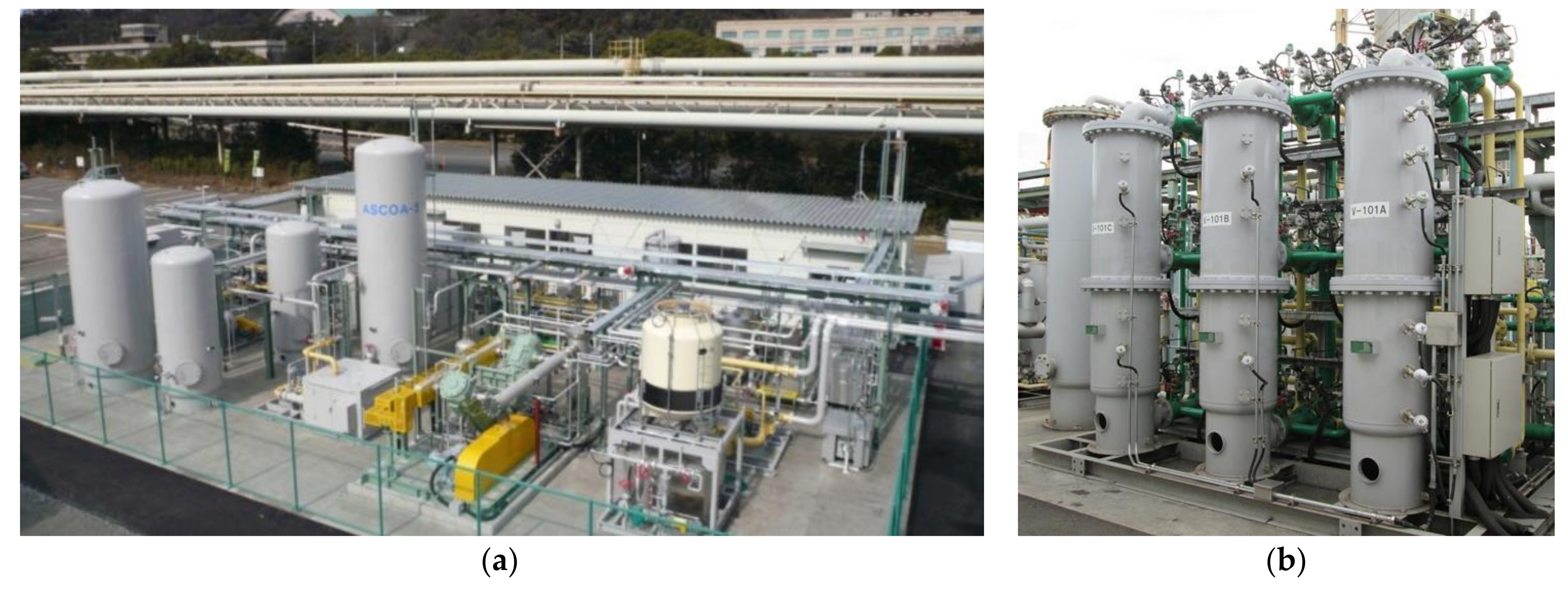
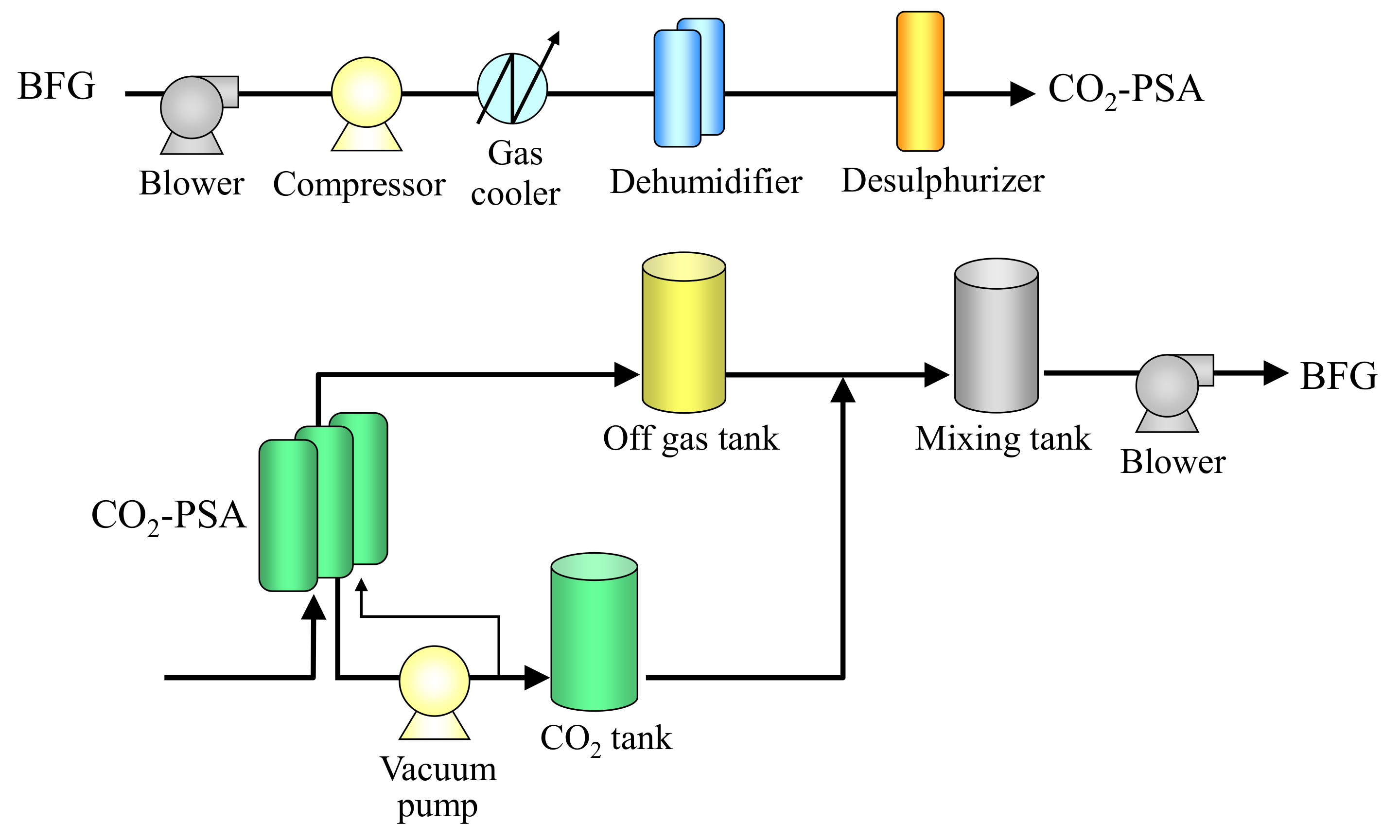
4.2. CO2-PSA Pilot Tests
5. Conclusions
Acknowledgment
Author Contributions
Conflicts of Interest
Nomenclature
| q | Adsorbed gas amount per unit adsorbent weight (g/g) |
| t | Time (s) |
| γ | Packing density of adsorbents (g/m3) |
| KF | Overall mass transfer coefficient (m/s) |
| aV | Surface area per unit volume (m2/m3) |
| c | Gas concentration (g/m3) |
| c* | Equivalent gas concentration (g/m3) |
| K′ | Overall volumetric mass transfer coefficient (1/s) |
| K1 | Volumetric mass transfer coefficient in outer layer of pellet (1/s) |
| K2 | Volumetric mass transfer coefficient in macro-pore of pellet (1/s) |
| K3 | Volumetric mass transfer coefficient in micro-pore of crystallite (1/s) |
| b | Correction factor |
| kF | Mass transfer coefficient in outer layer of pellet (m/s) |
| Sc | Schmidt number |
| Re | Reynolds number |
| μ | Gas viscosity (Pa·S) |
| ρ | Gas density (kg/m3) |
| u | Gas superficial velocity (m/s) |
| Dm | Gas molecular diffusion coefficient (m2/s) |
| T | Temperature (K) |
| Mi | Molecular weight (K) |
| P | Pressure (atm) |
| σ12 | Collision diameter (Å) |
| ΩD | Collision integral |
| R | Gas constant (J/mol K) |
| Dep | Effective diffusion coefficient in macro-pore of pellet (m2/s) |
| Dkp | Knudsen diffusion coefficient in macro-pore of pellet (m2/s) |
| Dec | Effective diffusion coefficient in micro-pore of crystallite (m2/s) |
| Dkc | Knudsen diffusion coefficient in micro-pore of crystallite (m2/s) |
| dp | Adsorbent pellet diameter (m) |
| x | Crystallite diameter (m) |
| εp | Marco-pore porosity inside pellet |
| εc | Micro-pore porosity inside crystallite |
| τp | Tortuosity factor inside pellet |
| τc | Tortuosity factor inside crystallite |
| dmacro | Macro-pore diameter in pellet (m) |
| dmicro | Micro-pore diameter in crystallite (m) |
| Subscripts | |
| p | Pellet |
| c | Crystallite |
| macro | Macro-pore |
| micro | Micro-pore |
| i | Gas species |
References
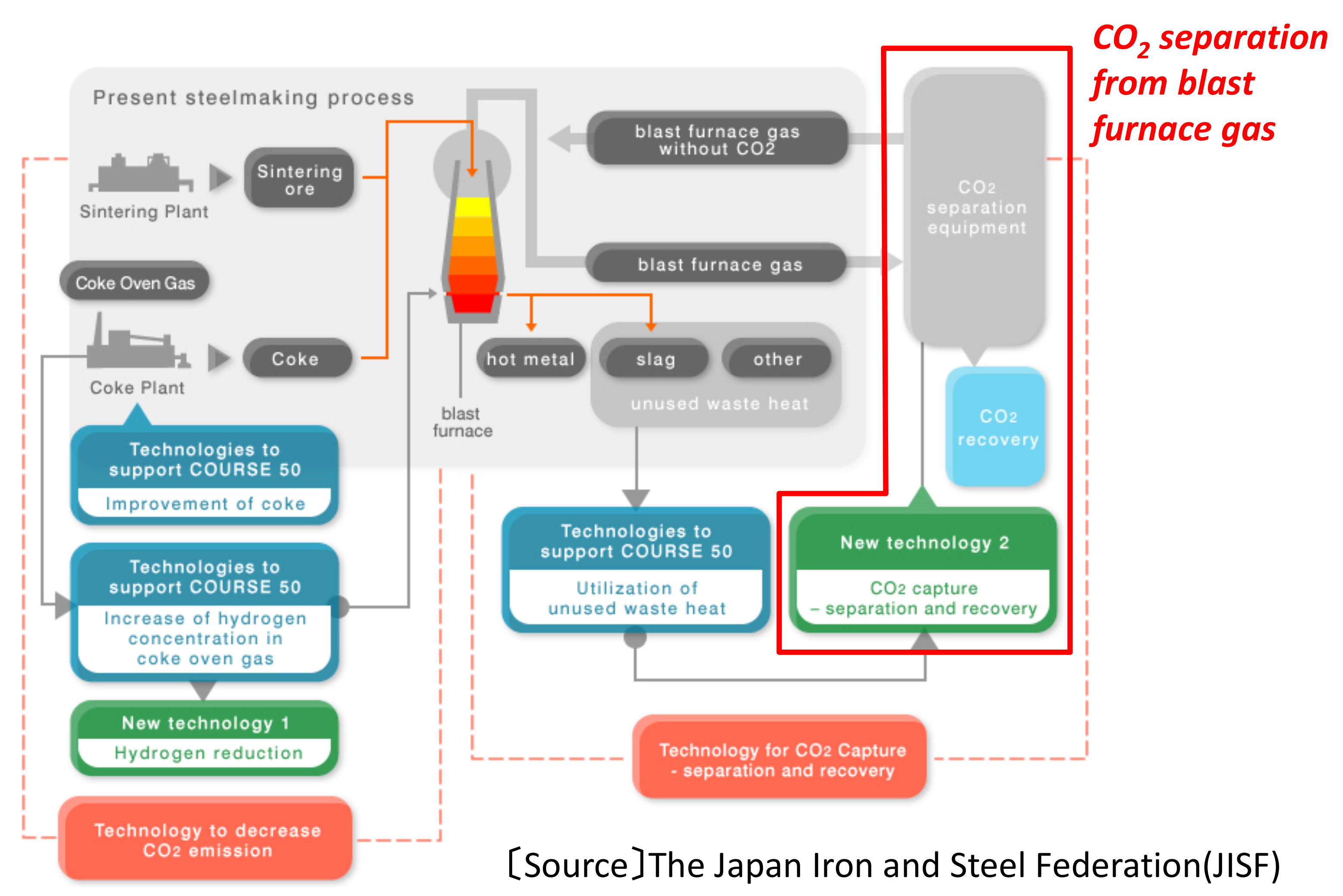
- Miwa, T.; Okuda, H. CO2 Ultimate Reduction in Steelmaking Process by Innovative Technology for Cool Earth 50 (COURSE50). J. Jpn. Inst. Energy 2010, 89, 28–35. [Google Scholar]
- Tonomura, S. Outline of Course 50. Energy Procedia 2013, 37, 7160–7167. [Google Scholar] [CrossRef]
- Ujisawa, Y.; Tonomura, S.; Ishiwata, N.; Nabeshima, Y.; Saito, K. CO2 breakthrough program by COURSE50 in Japanese steel industry sector. In Energy Technology Roadmaps of Japan: Future Energy Systems Based on Feasible Technologies Beyond 2030; Springer: Berlin, Germany, 2016; pp. 431–439. [Google Scholar]
- Hayashi, M.; Mimura, T. Steel Industries in Japan Achieve Most Efficient Energy Cut-off Chemical Absorption Process for Carbon Dioxide Capture from Blast Furnace Gas. Energy Procedia 2013, 37, 7134–7138. [Google Scholar] [CrossRef]
- Saima, H.; Mogi, Y.; Haraoka, T. Development of PSA Technology for the Separation of Carbon Dioxide from Blast Furnace Gas. JFE Tech. Rep. 2014, 19, 133–138. [Google Scholar]
- Shigaki, N.; Tobo, H.; Ozawa, S.; Ta, Y.; Hagiwara, K. Heat Recovery Process from Packed Bed of Hot Slag Plates. ISIJ Int. 2015, 55, 2258–2265. [Google Scholar] [CrossRef]
- Shigaki, N.; Ozawa, S.; Ikuhiro, S. Effect of gas velocity distribution on heat recovery process in packed bed of plate-shaped slag. Energies 2017, 10, 755. [Google Scholar] [CrossRef]
- Goto, K.; Okabe, H.; Chowdhury, F.A.; Shimizu, S.; Fujioka, Y.; Onoda, M. Development of novel absorbents for CO2 capture from blast furnace gas. Int. J. Greenh. Gas Control 2011, 5, 1214–1219. [Google Scholar] [CrossRef]
- Chowdhury, F.A.; Okabe, H.; Shimizu, S.; Onoda, M.; Fujioka, Y. Development of novel tertiary amine absorbents for CO2 capture. Energy Procedia 2009, 1, 1241–1248. [Google Scholar] [CrossRef]
- Chowdhury, F.A.; Yamada, H.; Matsuzaki, Y.; Goto, K.; Higashii, T.; Onoda, M. Development of Novel Synthetic Amine Absorbents for CO2 capture. Energy Procedia 2014, 63, 572–579. [Google Scholar] [CrossRef]
- Chowdhury, F.A.; Goto, K.; Yamada, H.; Matsuzaki, Y.; Yamamoto, S.; Higashii, T.; Onoda, M. Results of RITE’s Advanced Liquid Absorbents Develop for Low Temperature CO2 Capture. Energy Procedia 2017, 114, 1716–1720. [Google Scholar] [CrossRef]
- Rashidi, N.A.; Yusup, S.; Borhan, A. Isotherm and Thermodynamic Analysis of Carbon Dioxide on Activated Carbon. Procedia Eng. 2016, 148, 630–637. [Google Scholar] [CrossRef]
- Hosseini, S.; Bayesti, I.; Marahel, E.; Babadi, F.E.; Abdullah, L.C.; Choong, T.S.Y. Adsorption of carbon dioxide using activated carbon impregnated with Cu promoted by zinc. J. Taiwan Inst. Chem. Eng. 2015, 52, 109–117. [Google Scholar] [CrossRef]
- Plaza, M.G.; Pevida, C.; Pis, J.J.; Rubiera, F. Evaluation of the cyclic capacity of low-cost carbon adsorbents for post-combustion CO2 capture. Energy Procedia 2011, 4, 1228–1234. [Google Scholar] [CrossRef]
- Sarker, A.I.; Aroonwilas, A.; Veawab, A. Equilibrium and Kinetic Behaviour of CO2 Adsorption onto Zeolites, Carbon Molecular Sieve and Activated Carbons. Energy Procedia 2017, 114, 2450–2459. [Google Scholar] [CrossRef]
- Mendes, P.A.P.; Ribeiro, A.M.; Gleichmann, K.; Ferreira, A.F.P.; Rodrigues, A.E. Separation of CO2/N2 on binderless 5A zeolite. J. CO2 Util. 2017, 20, 224–233. [Google Scholar] [CrossRef]
- McEwen, J.; Hayman, J.D.; Yazaydin, A.O. A comparative study of CO2, CH4 and N2 adsorption in ZIF-8, Zeolite-13X and BPL activated carbon. Chem. Phys. 2013, 412, 72–76. [Google Scholar] [CrossRef]
- Walton, K.S.; Abney, M.B.; LeVan, M.D. CO2 adsorption in Y and X zeolites modified by alkali metal cation exchange. Microporous Mesoporous Mater. 2006, 91, 78–84. [Google Scholar] [CrossRef]
- Campo, M.C.; Ribeiro, A.M.; Ferreira, A.F.P.; Santos, J.C.; Lutz, C.; Loureiro, J.M.; Rodrigues, A.E. Carbon dioxide removal for methane upgrade by a VSA process using an improved 13X zeolite. Fuel Process. Technol. 2016, 143, 185–194. [Google Scholar] [CrossRef]
- Khunpolgrang, J.; Yosantea, S.; Kongnoo, A.; Phalakornkule, C. Alternative PSA process cycle with combined vacuum regeneration and nitrogen purging for CH4/CO2 separation. Fuel 2015, 140, 171–177. [Google Scholar] [CrossRef]
- Ga, S.; Jang, H.; Lee, J.H. New Performance Indicators for Evaluation of Adsorbents for CO2 Capture with PSA processes. IFAC-PapersOnLine 2016, 49, 651–656. [Google Scholar] [CrossRef]
- Ga, S.; Jang, H.; Lee, J.H. New performance indicators for adsorbent evaluation derived from a reduced order model of an idealized PSA process for CO2 capture. Comput. Chem. Eng. 2017, 102, 188–212. [Google Scholar] [CrossRef]
- Zhang, J.; Xiao, P.; Li, G.; Webley, P.A. Effect of Flue Gas Impurities on CO2 Capture Performance from Flue Gas at Coal-fired Power Stations by Vacuum Swing Adsorption. Energy Procedia 2009, 1, 1115–1122. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Rübner, K.; Hoffmann, D. Characterization of Mineral Building Materials by Mercury Intrusion Porosimetry. Part. Part. Syst. Charact. 2006, 23, 20–28. [Google Scholar] [CrossRef]
- Yoshida, F.; Ramaswami, D.; Hougen, O.A. Temperatures and partial pressures at the surfaces of catalyst particles. AIChE J. 1962, 8, 5–11. [Google Scholar] [CrossRef]
- Ye, G.; Duan, X.; Zhu, K.; Zhou, X.; Coppens, M.O.; Yuan, W. Optimizing spatial pore-size and porosity distributions of adsorbents for enhanced adsorption and desorption performance. Chem. Eng. Sci. 2015, 132, 108–117. [Google Scholar] [CrossRef]
- Silva, J.A.C.; Schumann, K.; Rodrigues, A.E. Sorption and kinetics of CO2 and CH4 in binderless beads of 13X zeolite. Microporous Mesoporous Mater. 2012, 158, 219–228. [Google Scholar] [CrossRef]
- Sun, Z.; Tang, X.; Cheng, G. Numerical simulation for tortuosity of porous media. Microporous Mesoporous Mater. 2013, 173, 37–42. [Google Scholar] [CrossRef]
- Itoh, T.; Wanibe, Y.; Sakao, H. Analysis of Packing Density by Randomly Packed Models of Binary Powders. J. Jpn. Inst. Met. 1986, 50, 475–479. [Google Scholar] [CrossRef]
- Zhao, J.; Li, S.; Lu, P.; Meng, L.; Li, T.; Zhu, H. Shape influences on the packing density of frustums. Powder Technol. 2011, 214, 500–505. [Google Scholar] [CrossRef]
- Meng, L.; Lu, P.; Li, S.; Zhao, J.; Li, T. Shape and size effects on the packing density of binary spherocylinders. Powder Technol. 2012, 228, 284–294. [Google Scholar] [CrossRef]
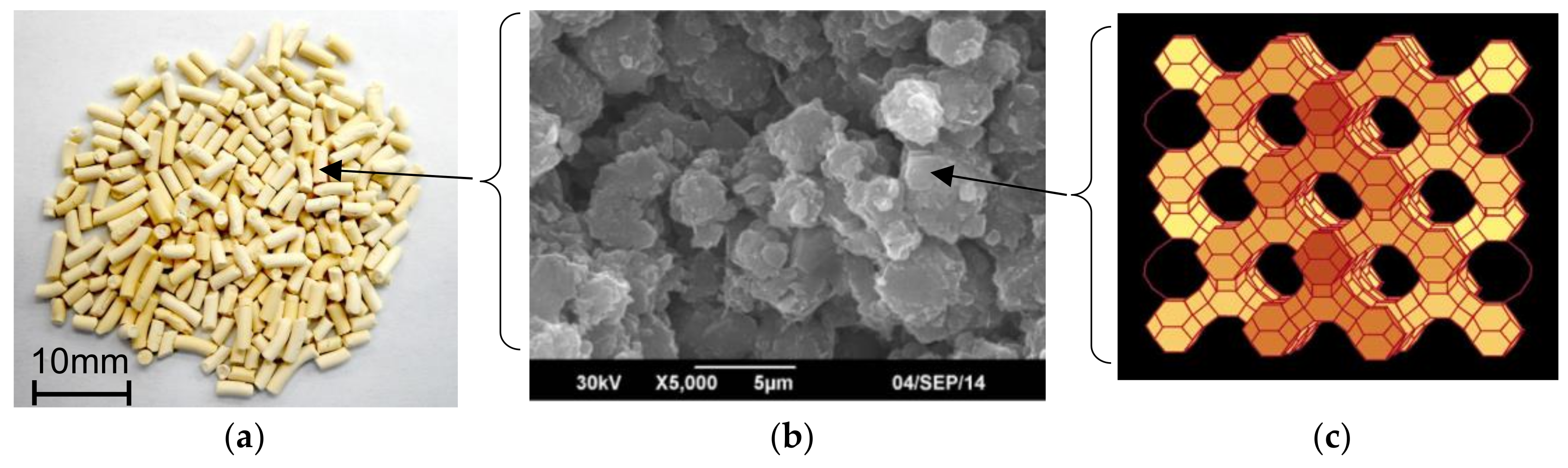

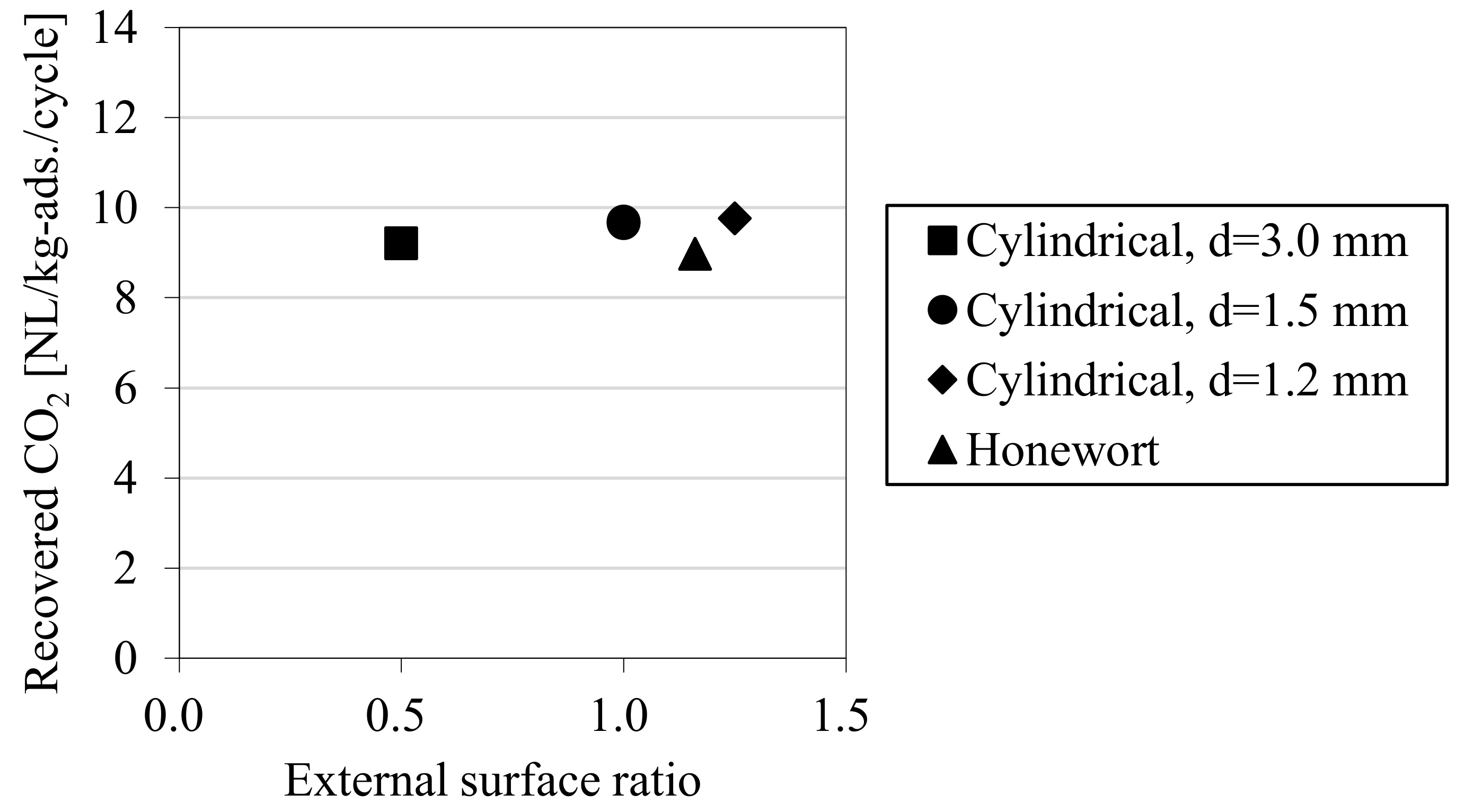
| Shape | Cylindrical d = 3.0 mm | Cylindrical d = 1.5 mm | Cylindrical d = 1.2 mm | Honewort d = 1.63 mm |
|---|---|---|---|---|
| External surface ratio | 0.50 | 1.00 | 1.25 | 1.16 |
| Pellet shape drawing |  |  |  |  |
| Adsorbent Type | Zeolite13X (NaX) |
|---|---|
| Macro-Meso pore volume (cm3/g) | 0.27 |
| Average macro-meso pore diameter (nm) | 126 |
| BET surface area (m2/g) | 802 |
| Packing density (g/cm3) | 0.67 |
| Particle density (g/cm3) | 1.58 |
| Si/Al ratio | 1.4 |
| Parameter | Value | |
|---|---|---|
| Adsorber | Number of adsorbers | 3 |
| Weight of adsorbent in an adsorber (g) | 120 | |
| Inner diameter (mm) | 40 | |
| PSA operation | Feed gas volume in 1 cycle (NL/kg-adsorbent/cycle) | 40 |
| Feed gas composition (vol %) | 32CO2, 33CO, 30N2, 5H2 | |
| Pressure at the bottom of the adsorber (kPaA) | Adsorption 151 | |
| Desorption 9 | ||
| Cycle time (sec/cycle) | 300 | |
| CO2 concentration of recovered gas (vol %) | 90 | |
| Property | Symbol | Value | |
|---|---|---|---|
| Adsorbent pellet diameter (mm) | Dp | 1.5 | 3.0 |
| Equivalent adsorbent pellet diameter (mm) | dp′ | 1.82 | 3.44 |
| Surface area per unit volume (m2/m3) | av | 2052 | 1088 |
| Crystallite diameter (m) | dc | 3.00 × 10−6 | |
| Macro-pore diameter in the pellet (m) | dmacro | 1.26 × 10−7 | |
| Micro-pore diameter in the crystallite (m) | dmicro | 7.35 × 10−10 | |
| Pressure (Pa) | P | 151,000 | |
| Gas constant (J/mol K) | R | 8.314 | |
| Temperature (K) | T | 298.15 | |
| Molecular weight of CO2 (g/mol) | M1 | 44.01 | |
| Molecular weight of N2 (g/mol) | M2 | 28.01 | |
| Collision diameter (m) | σ12 | 3.87 × 10−10 | |
| Marco-pore porosity inside the pellet | εp | 0.314 | |
| Micro-pore porosity inside the crystallite | εc | 0.274 | |
| Gas density (kg/m3) | ρ | 1.613735 | |
| Gas superficial velocity (m/s) | u | 0.003 | |
| Gas viscosity (Pa·s) | μ | 1.62 × 10−5 | |
| Property | Symbol | Value | |
|---|---|---|---|
| Gas molecular diffusion coefficient (C-E eq.) (m2/s) | Dm | 8.699 × 10−6 | |
| Knudsen diffusion coefficient (m2/s) | macro-pore of the pellet | Dkp | 1.591 × 10−5 |
| micro-pore of the crystallite | Dkc | 9.281 × 10−8 | |
| Effective diffusion coefficient (m2/s) | macro-pore of the pellet | Dep | 8.828 × 10−7 |
| micro-pore of the crystallite | Dec | 1.258 × 10−8 | |
| Property | Symbol | Value | ||
|---|---|---|---|---|
| d = 1.5 mm | d = 3.0 mm | |||
| Volumetric mass transfer coefficient (1/s) | outer layer of the pellet | K1 | 12.2 | 4.7 |
| macro-pore of the pellet | K2 | 9.9 | 2.8 | |
| micro-pore of the crystallite | K3 | 35,857 | 35,857 | |
| Overall volumetric mass transfer coefficient (1/s) | K′calc | 5.47 | 1.75 | |
| Equipment | Units/System | Specifications | |
|---|---|---|---|
| Blower | 1 | Type | Centrifugal type |
| Flow rate | 500 Nm3/h | ||
| Pressure | Outlet 4.9 kPaG | ||
| Gas compressor | 1 | Type | Reciprocating type |
| Flow rate | 500 Nm3/h | ||
| Pressure | Outlet 300 kPaG | ||
| Gas cooler | 1 | Type | Refrigerator cooling type |
| Temperature | Outlet gas 283 K | ||
| Dehumidifier | 2 | Type | Heating regeneration type |
| Desiccant | Silica gel, Alumina gel | ||
| Dew point | Outlet gas < 213 K | ||
| Adsorber | 3 | Dimensions | 600 ID × 1500 TTH |
| Volume | 0.42 m3 | ||
| Vacuum pump | 1 | Type | Roots type dry pump |
| Pumping speed | 1284 m3/h | ||
| Rated output | 45 kW | ||
| Pellet Shape | Length (mm) | Bulk Density of Packed Bed(kg/L) | Crushing Strength (N) | ||||
|---|---|---|---|---|---|---|---|
| Max. | Min. | Ave. | Max./Min. | Standard Deviation | |||
| Cylindrical, d = 1.5 mm | 5.66 | 1.62 | 3.19 | 3.49 | 1.02 | 0.64 | 40 |
| Cylindrical, d = 3.0 mm | 6.80 | 2.69 | 4.84 | 2.53 | 0.89 | 0.63 | 89 |
| Parameter | Value | |||
|---|---|---|---|---|
| Case 1 | Case 2 | |||
| Adsorbent | Type | Zeolite13X | ||
| Shape | Cylindrical | |||
| Pellet diameter (mm) | 1.5 | 3.0 | ||
| Adsorber | Number of adsorbers | 3 | ||
| Total weight of adsorbent in an adsorber (kg) | 240 | |||
| Packing height of adsorbent (mm) | 1350 | |||
| Inner diameter (mm) | 600 | |||
| PSA operation | Feed gas flow rate (Nm3/h) | 400 | ||
| Dew point of the feed gas (K) | <213 | |||
| Pressure at the bottom of the adsorber (kPaA) | Adsorption | 151 | ||
| Desorption | From 6 to 10 | |||
| Cycle time (sec/cycle) | 300 | |||
| CO2 recovery rate (t-CO2/day) | 4.7 | |||
| CO2 concentration of recovered gas (%) | 90 | |||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shigaki, N.; Mogi, Y.; Haraoka, T.; Sumi, I. Reduction of Electric Power Consumption in CO2-PSA with Zeolite 13X Adsorbent. Energies 2018, 11, 900. https://doi.org/10.3390/en11040900
Shigaki N, Mogi Y, Haraoka T, Sumi I. Reduction of Electric Power Consumption in CO2-PSA with Zeolite 13X Adsorbent. Energies. 2018; 11(4):900. https://doi.org/10.3390/en11040900
Chicago/Turabian StyleShigaki, Nobuyuki, Yasuhiro Mogi, Takashi Haraoka, and Ikuhiro Sumi. 2018. "Reduction of Electric Power Consumption in CO2-PSA with Zeolite 13X Adsorbent" Energies 11, no. 4: 900. https://doi.org/10.3390/en11040900
APA StyleShigaki, N., Mogi, Y., Haraoka, T., & Sumi, I. (2018). Reduction of Electric Power Consumption in CO2-PSA with Zeolite 13X Adsorbent. Energies, 11(4), 900. https://doi.org/10.3390/en11040900




