Effect of Mild-Temperature Thermo-Alkaline Pretreatment on the Solubilization and Anaerobic Digestion of Spent Coffee Grounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Seed Sludge and Spent Coffee Grounds
2.2. Experimental Design for Response Surface Analysis
2.3. Thermo-Alkaline Pretreatment
2.4. Biochemical Methane Potential Assay
2.5. Molecular Analyses
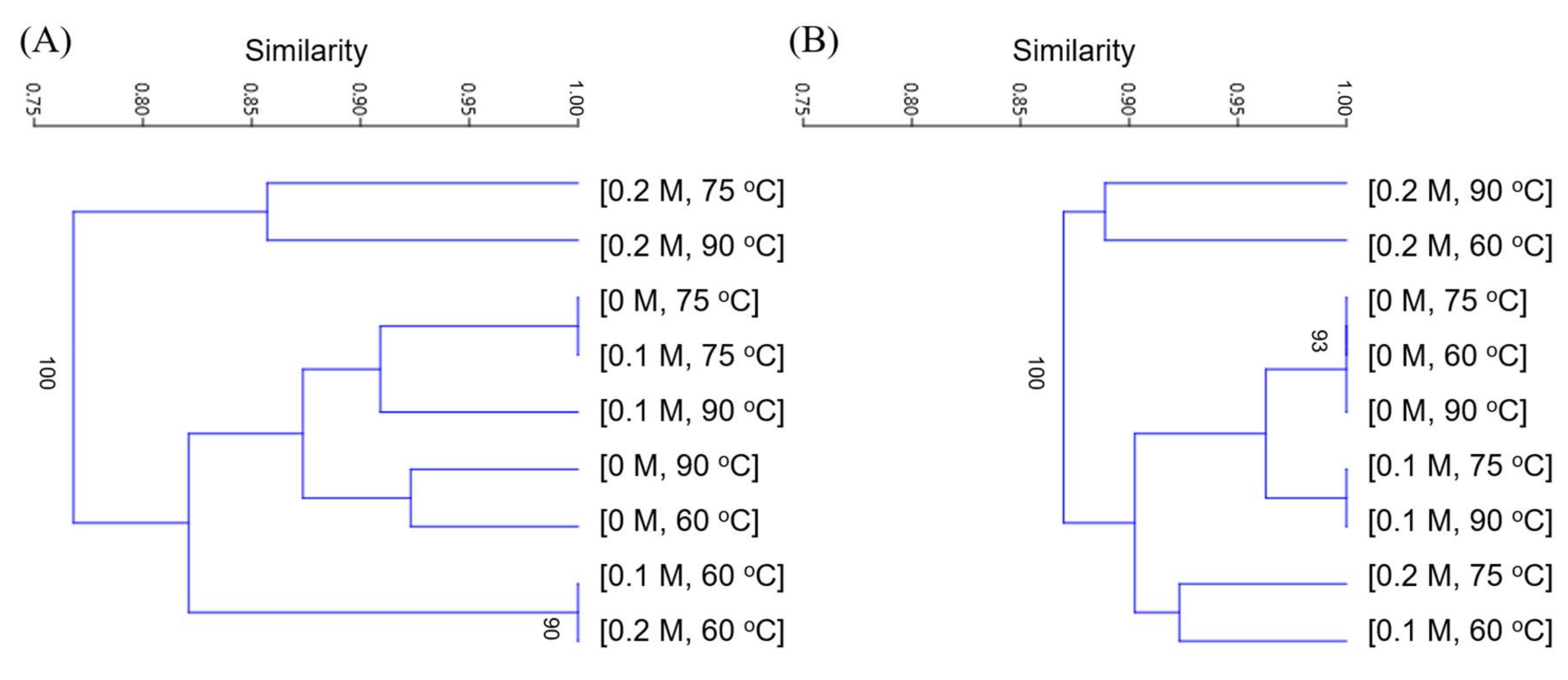
2.6. Cluster Analysis of DGGE Profiles
2.7. Physicochemical Analyses
3. Results and Discussion
3.1. Effects of Pretreatment
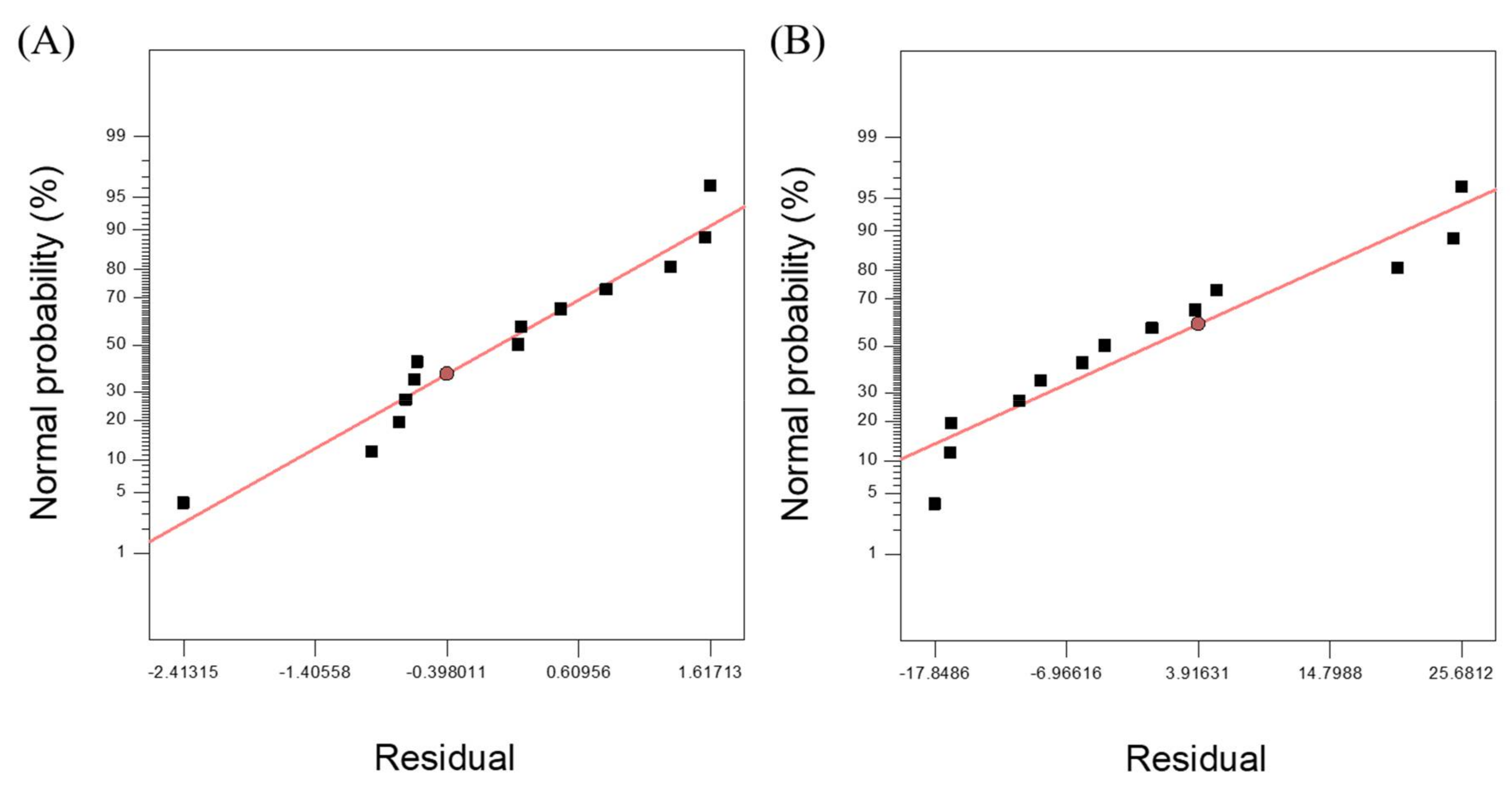
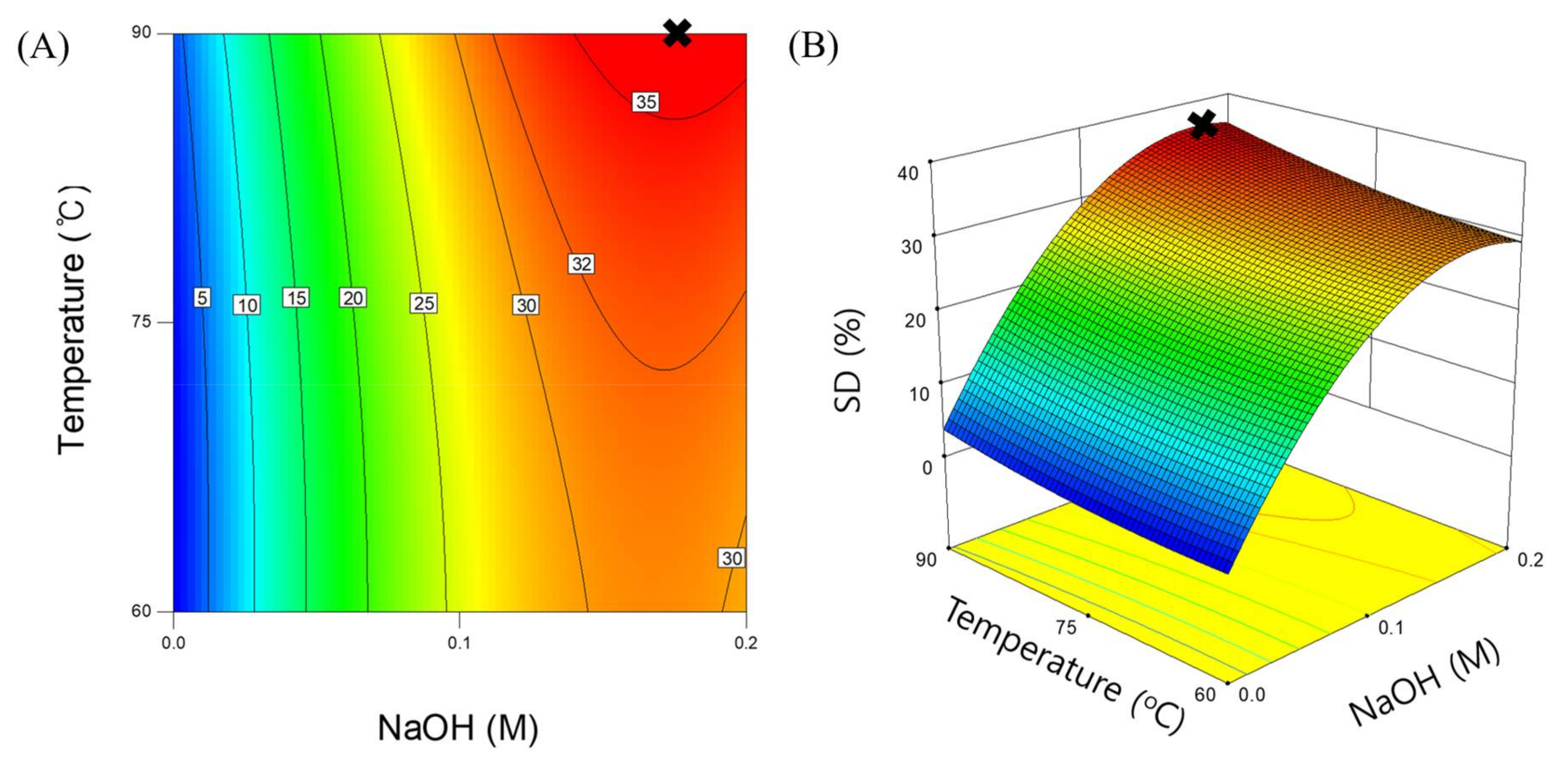
3.2. Response Surface Model for SCG Solubilization
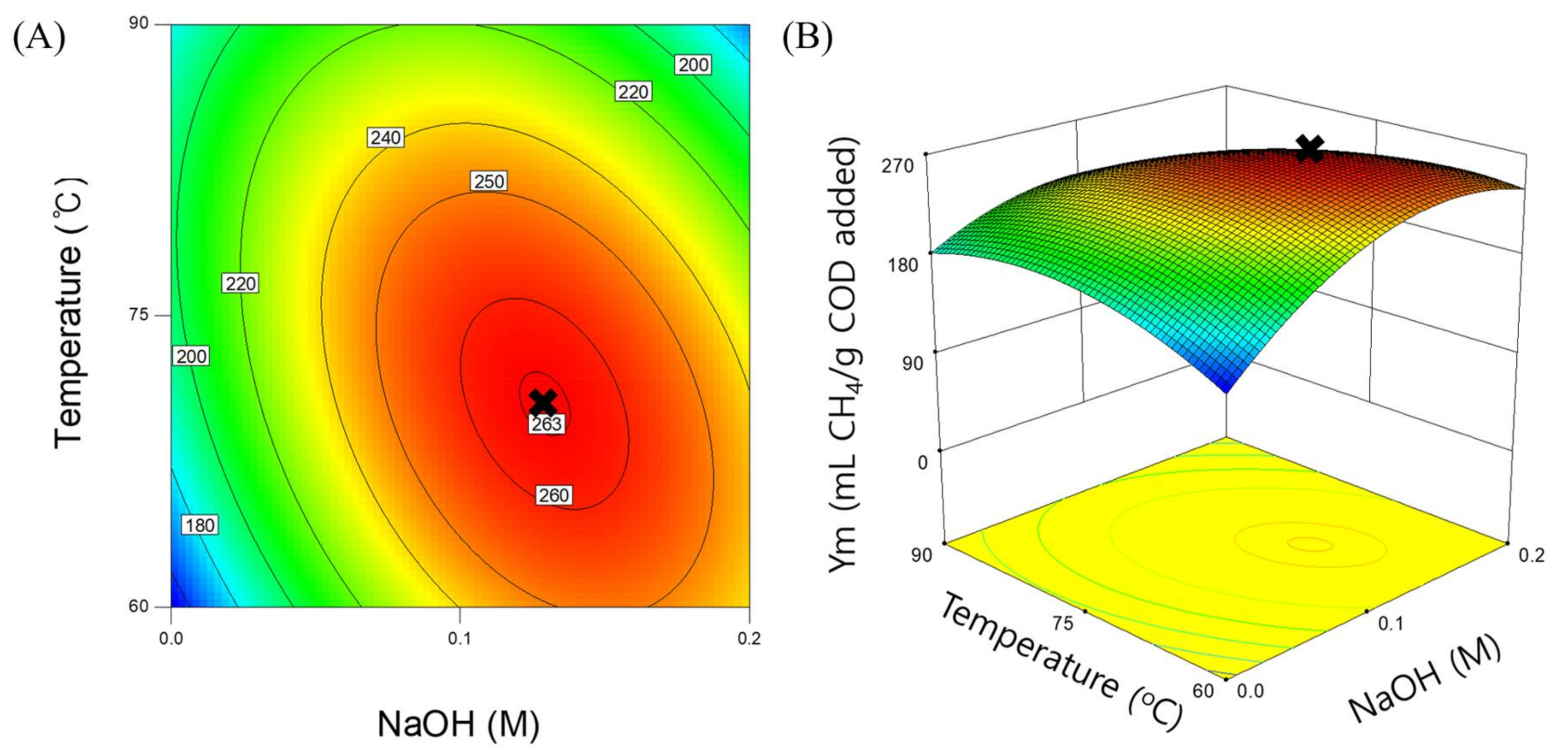
3.3. Response Surface Model for Methane Yield
3.4. Correlation between SD and Ym
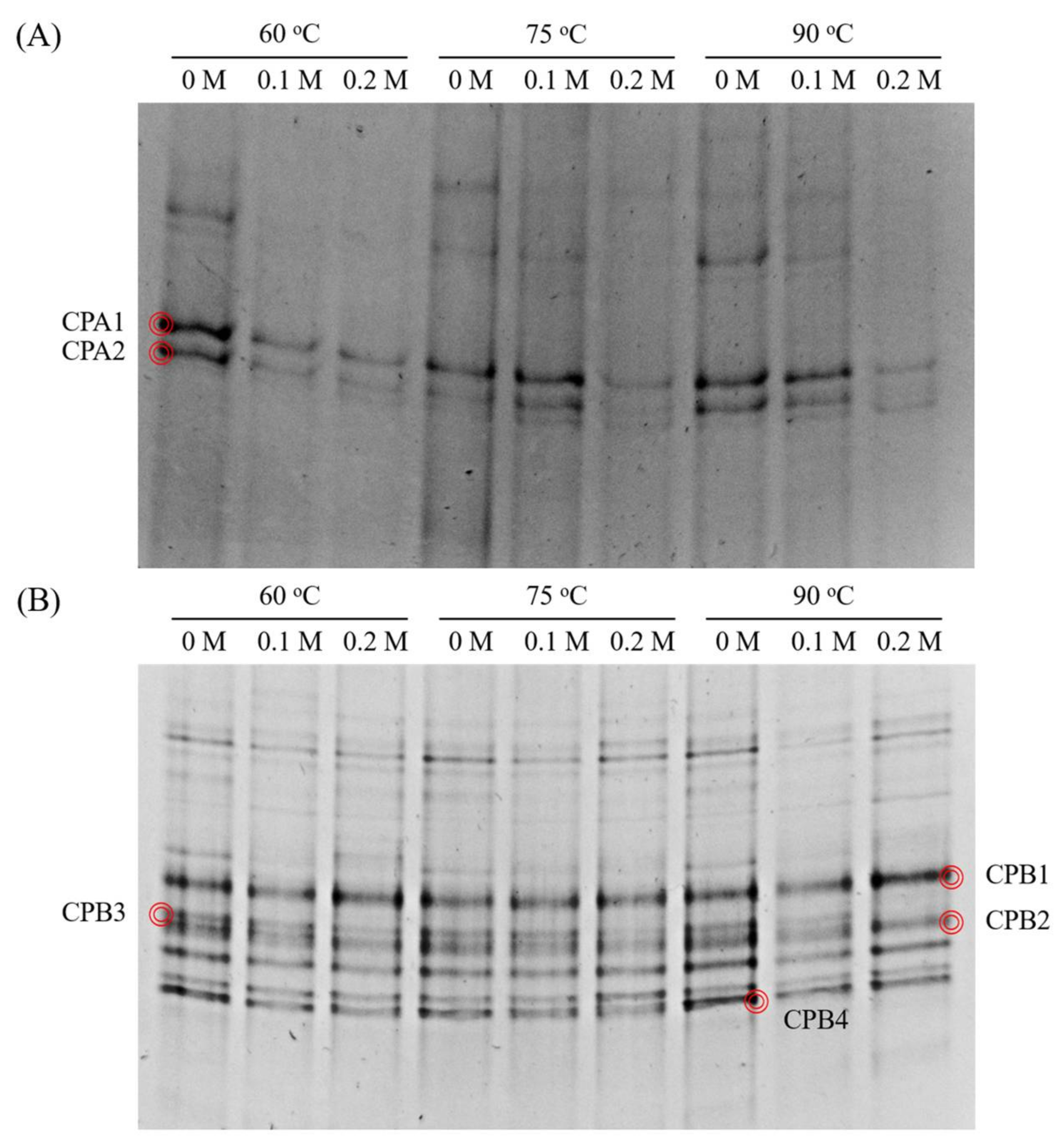
3.5. Microbial Community Structure
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Vardon, D.R.; Moser, B.R.; Zheng, W.; Witkin, K.; Evangelista, R.L.; Strathmann, T.J.; Rajagopalan, K.; Sharma, B.K. Complete utilization of spent coffee grounds to produce biodiesel, bio-oil, and biochar. ACS Sustain. Chem. Eng. 2013, 1, 1286–1294. [Google Scholar] [CrossRef]
- Silva, M.A.; Nebra, S.A.; Machado Silva, M.J.; Sanchez, C.G. The use of biomass residues in the Brazilian soluble coffee industry. Biomass Bioenergy 1998, 14, 457–467. [Google Scholar] [CrossRef]
- Chang, S.-T. Overview of mushroom cultivation and utilization as functional foods. In Mushrooms as Functional Foods; Cheung, P.C.K., Ed.; John Wiley & Sons, Inc: Hoboken, NJ, USA, 2008; pp. 1–33. [Google Scholar]
- Qiao, W.; Takayanagi, K.; Niu, Q.; Shofie, M.; Li, Y.Y. Long-term stability of thermophilic co-digestion submerged anaerobic membrane reactor encountering high organic loading rate, persistent propionate and detectable hydrogen in biogas. Bioresour. Technol. 2013, 149, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, H.; Lee, C. Ulva biomass as a co-substrate for stable anaerobic digestion of spent coffee grounds in continuous mode. Bioresour. Technol. 2017, 241, 1182–1190. [Google Scholar] [CrossRef] [PubMed]
- Amin, F.R.; Khalid, H.; Zhang, H.; u Rahman, S.; Zhang, R.; Liu, G.; Chen, C. Pretreatment methods of lignocellulosic biomass for anaerobic digestion. AMB Express 2017, 7, 72. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.; Ding, W.; Bi, J.; Mehryar, E.; Talha, Z.A.A.; Huang, H. Methane enhancement through oxidative cleavage and alkali solubilization pre-treatments for corn stover with anaerobic activated sludge. Bioresour. Technol. 2016, 200, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhao, J.; Xu, F.; Li, Y. Pretreatment of lignocellulosic biomass for enhanced biogas production. Prog. Energy Combust. Sci. 2014, 42, 35–53. [Google Scholar] [CrossRef]
- Fernández-Cegrí, V.; Raposo, F.; de la Rubia, M.; Borja, R. Effects of chemical and thermochemical pretreatments on sunflower oil cake in biochemical methane potential assays. J. Chem. Technol. Biotechnol. 2013, 88, 924–929. [Google Scholar] [CrossRef]
- Battista, F.; Fino, D.; Mancini, G. Optimization of biogas production from coffee production waste. Bioresour. Technol. 2016, 200, 884–890. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Yu, Y.; Lee, C. Thermo-alkaline pretreatment of waste activated sludge at low-temperatures: Effects on sludge disintegration, methane production, and methanogen community structure. Bioresour. Technol. 2013, 144, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.A.; Novak, J.T. Hydrolysis of macromolecular components of primary and secondary wastewater sludge by thermal hydrolytic pretreatment. Water Res. 2009, 43, 4489–4498. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.; Baek, G.; Kim, J.; Shin, S.G.; Lee, C. Mild-temperature thermochemical pretreatment of green macroalgal biomass: Effects on solubilization, methanation, and microbial community structure. Bioresour. Technol. 2016, 199, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Lee, C.; Kim, J.; Hwang, S. Group-specific primer and probe sets to detect methanogenic communities using quantitative real-time polymerase chain reaction. Biotechnol. Bioeng. 2005, 89, 670–679. [Google Scholar] [CrossRef] [PubMed]
- APHA-AWWA-WEF. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Tyagi, V.K.; Lo, S.-L. Enhancement in mesophilic aerobic digestion of waste activated sludge by chemically assisted thermal pretreatment method. Bioresour. Technol. 2012, 119, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, C.; Liu, W.; Zou, S. Optimized alkaline pretreatment of sludge before anaerobic digestion. Bioresour. Technol. 2012, 123, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Feijoo, G.; Soto, M.; Méndez, R.; Lema, J.M. Sodium inhibition in the anaerobic digestion process: Antagonism and adaptation phenomena. Enzyme Microb. Technol. 1995, 17, 180–188. [Google Scholar] [CrossRef]
- Manu, B.; Chaudhari, S. Anaerobic decolorisation of simulated textile wastewater containing azo dyes. Bioresour. Technol. 2002, 82, 225–231. [Google Scholar] [CrossRef]
- Larsson, S.; Palmqvist, E.; Hahn-Hägerdal, B.; Tengborg, C.; Stenberg, K.; Zacchi, G.; Nilvebrant, N.-O. The generation of fermentation inhibitors during dilute acid hydrolysis of softwood. Enzyme Microb. Technol. 1999, 24, 151–159. [Google Scholar] [CrossRef]
- Harmsen, P.; Huijgen, W.; Bermudez, L.; Bakker, R. Literature Review of Physical and Chemical Pretreatment Processes for Lignocellulosic Biomass; 9085857570; Wageningen UR-Food & Biobased Research: Wageningen, The Netherland, 2010. [Google Scholar]
- Girotto, F.; Lavagnolo, M.C.; Pivato, A. Spent coffee grounds alkaline pre-treatment as biorefinery option to enhance their anaerobic digestion yield. Waste Biomass Valoriz. 2017, 1–6. [Google Scholar] [CrossRef]
- Wang, P.; Wang, H.; Qiu, Y.; Ren, L.; Jiang, B. Microbial characteristics in anaerobic digestion process of food waste for methane production—A review. Bioresour. Technol. 2017, 248, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, C. Response of a continuous anaerobic digester to temperature transitions: A critical range for restructuring the microbial community structure and function. Water Res. 2016, 89, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, A.; Tindall, B.J.; Bardin, V.; Blanchet, D.; Jeanthon, C. Petrimonas sulfuriphila gen. Nov., sp. Nov., a mesophilic fermentative bacterium isolated from a biodegraded oil reservoir. Int. J. Syst. Evol. Microbiol. 2005, 55, 1113–1121. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Cabrol, L.; Ruiz-Filippi, G.; Pullammanappallil, P. Microbial ecology in anaerobic digestion at agitated and non-agitated conditions. PLoS ONE 2014, 9, e109769. [Google Scholar] [CrossRef] [PubMed]
- Morrison, M.; Miron, J. Adhesion to cellulose by ruminococcus albus: A combination of cellulosomes and pil-proteins? FEMS Microbiol. Lett. 2000, 185, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Ziganshin, A.M.; Liebetrau, J.; Pröter, J.; Kleinsteuber, S. Microbial community structure and dynamics during anaerobic digestion of various agricultural waste materials. Appl. Microbiol. Biotechnol. 2013, 97, 5161–5174. [Google Scholar] [CrossRef] [PubMed]
- Langer, S.G.; Ahmed, S.; Einfalt, D.; Bengelsdorf, F.R.; Kazda, M. Functionally redundant but dissimilar microbial communities within biogas reactors treating maize silage in co-fermentation with sugar beet silage. Microb. Biotechnol. 2015, 8, 828–836. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Unit | Value a |
|---|---|---|
| Total COD | mg/L | 20951.0 (570.6) |
| Soluble COD | mg/L | 714.9 (0.9) |
| Total solids | mg/L | 13,550.0 (70.7) |
| Volatile solids | mg/L | 13,350.0 (70.7) |
| Carbon | % | 54.3 (0.1) |
| Hydrogen | % | 7.4 (0.1) |
| Nitrogen | % | 2.1 (0.1) |
| Sulfur | % | 0 |
| Oxygen | % | 36.9 (0.4) |
| pH | 5.91 |
| Run | NaOH (M) | Temperature (°C) | ||
|---|---|---|---|---|
| Coded | Actual | Coded | Actual | |
| 1 | –1 | 0 | –1 | 60 |
| 2 | 0 | 0.1 | –1 | 60 |
| 3 | 1 | 0.2 | –1 | 60 |
| 4 | –1 | 0 | 0 | 75 |
| 5 a | 0 | 0.1 | 0 | 75 |
| 6 | 1 | 0.2 | 0 | 75 |
| 7 | –1 | 0 | 1 | 90 |
| 8 | 0 | 0.1 | 1 | 90 |
| 9 | 1 | 0.2 | 1 | 90 |
| Run | Degree of Solubilization (%) | Methane Yield (mL CH4/g COD added) b |
|---|---|---|
| 1 | 1.0 | 155.1 |
| 2 | 25.1 | 240.3 |
| 3 | 30.0 | 235.7 |
| 4 | 2.2 | 201.4 |
| 5 a | 26.8 (1.7) | 254.0 (17.5) |
| 6 | 31.8 | 253.2 |
| 7 | 2.9 | 173.8 |
| 8 | 31.9 | 246.1 |
| 9 | 35.1 | 151.9 |
| Terms | Thermo-Alkaline Pretreatment (NaOH) | ||
|---|---|---|---|
| Coefficient | F-Value | p-Value | |
| Intercept | 26.99 | - | - |
| Xn | 15.14 | 585.79 | <0.0001 |
| Xt | 2.3 | 13.57 | 0.0078 |
| XnXt | 0.83 | 1.17 | 0.3153 |
| Xn2 | –10.5 | 129.6 | <0.0001 |
| Xt2 | 1.02 | 1.21 | 0.307 |
| Model | p-value | R2 | AP |
| Regression | <0.0001 | 0.9907 | 33.515 |
| Lack-of-fit | 0.6121 | - | - |
| Terms | Thermo-Alkaline Pretreatment (NaOH) | ||
|---|---|---|---|
| Coefficient | F-Value | p-Value | |
| Intercept | 259.12 | - | - |
| Xn | 18.4 | 4.98 | 0.0608 |
| Xt | –9.91 | 1.45 | 0.2683 |
| XnXt | –25.62 | 6.44 | 0.0388 |
| Xn2 | –44.73 | 13.56 | 0.0078 |
| Xt2 | –28.82 | 5.63 | 0.0495 |
| Model | p-value | R2 | AP |
| Regression | 0.0067 | 0.8602 | 7.848 |
| Lack-of-fit | 0.2939 | - | - |
| Band | Closest Species | Similarity (%) | Accession No. | Classification a |
|---|---|---|---|---|
| Archaea | ||||
| CPA1 | Methanospirillum hungatei strain JF-1 | 97.8 | CP000254 | Methanospirillum |
| Methanospirillum lacunae strain Ki8-1 | 97.8 | NR_112981 | ||
| Methanospirillum stamsii strain ps | 97.8 | NR_117705 | ||
| CPA2 | Methanospirillum stamsii strain ps 16S | 98.2 | NR_117705 | Methanospirillum |
| Methanospirillum lacunae strain Ki8-1 | 98.2 | NR_112981 | ||
| Methanospirillum hungatei strain JF-1 | 98.2 | CP000254 | ||
| Bacteria | ||||
| CPB1 | Clostridiales bacterium clone 1G044 | 92.2 | JN713505 | Ruminococcaceae |
| Uncultured bacterium clone B36 | 100.0 | HM066994 | ||
| Uncultured bacterium clone 12Bac R8 | 99.8 | JF421649 | ||
| CPB2 | Petrimonas sulfuriphila strain BN3 | 99.8 | NR_042987 | Petrimonas |
| CPB3 | Proteiniphilum acetatigenes strain TB107 | 90.5 | NR_043154 | Porphyromonadaceae |
| Uncultured bacterium clone LC5 | 99.6 | FJ982805 | ||
| Uncultured bacterium clone 5Bac R8 | 99.6 | JF421645 | ||
| CPB4 | Egibacter rhizosphaerae strain EGI 80759 | 88.6 | NR_147752 | Bacteria |
| Uncultured Firmicutes bacterium clone B5 | 100.0 | AB721095 | ||
| Uncultured Firmicutes bacterium clone QEEA1DA02 | 100.0 | CU918783 | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.; Kim, J.; Lee, C. Effect of Mild-Temperature Thermo-Alkaline Pretreatment on the Solubilization and Anaerobic Digestion of Spent Coffee Grounds. Energies 2018, 11, 865. https://doi.org/10.3390/en11040865
Kim D, Kim J, Lee C. Effect of Mild-Temperature Thermo-Alkaline Pretreatment on the Solubilization and Anaerobic Digestion of Spent Coffee Grounds. Energies. 2018; 11(4):865. https://doi.org/10.3390/en11040865
Chicago/Turabian StyleKim, Danbee, Jaai Kim, and Changsoo Lee. 2018. "Effect of Mild-Temperature Thermo-Alkaline Pretreatment on the Solubilization and Anaerobic Digestion of Spent Coffee Grounds" Energies 11, no. 4: 865. https://doi.org/10.3390/en11040865
APA StyleKim, D., Kim, J., & Lee, C. (2018). Effect of Mild-Temperature Thermo-Alkaline Pretreatment on the Solubilization and Anaerobic Digestion of Spent Coffee Grounds. Energies, 11(4), 865. https://doi.org/10.3390/en11040865





