Nitrogen Doped Ordered Mesoporous Carbon as Support of PtRu Nanoparticles for Methanol Electro-Oxidation
Abstract
:1. Introduction
2. Results
2.1. Physico-Chemical Properties of N-Doped Carbon Materials
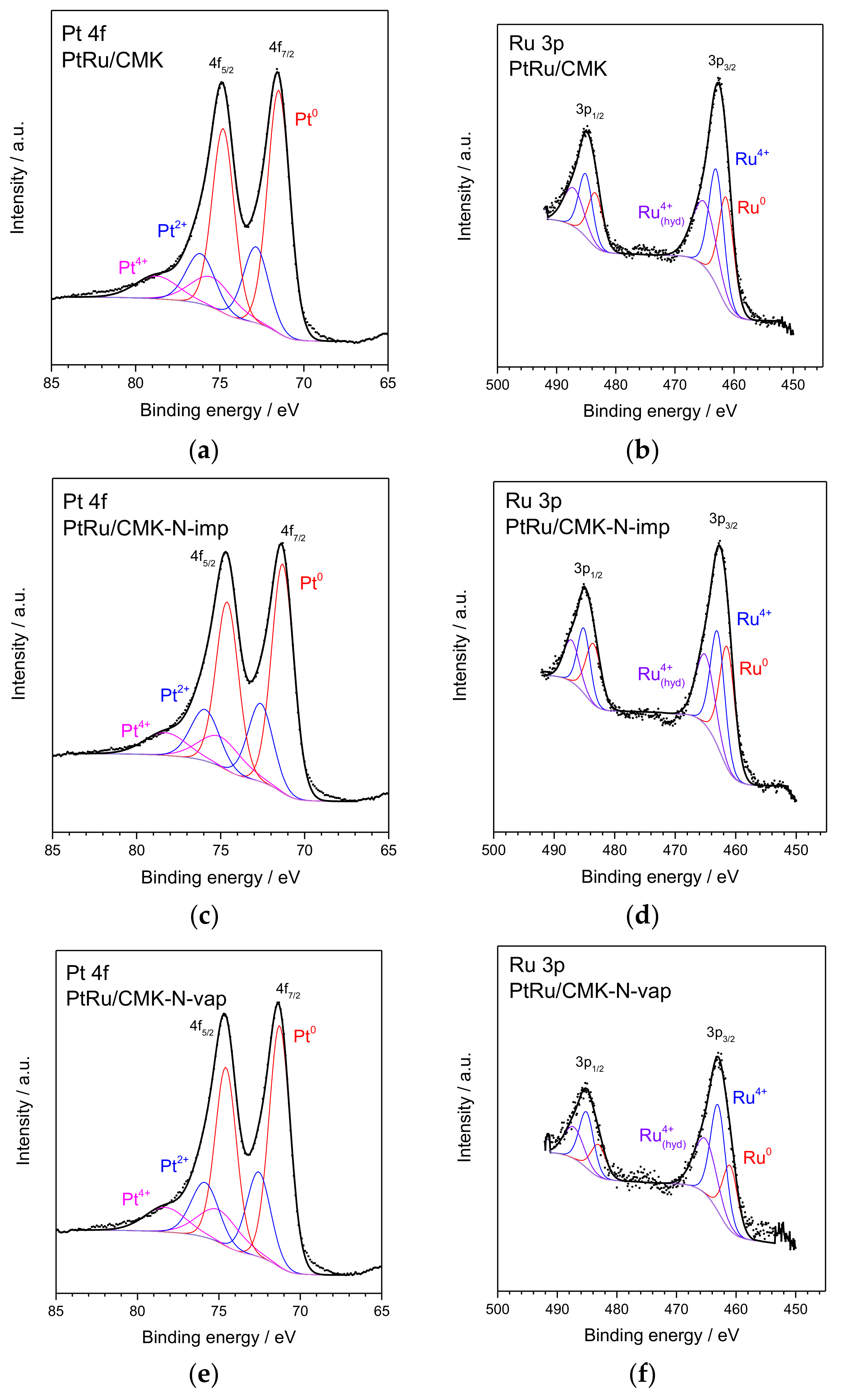
2.2. Physico-Chemical Properties of N-Doped Carbon-Supported PtRu Catalysts
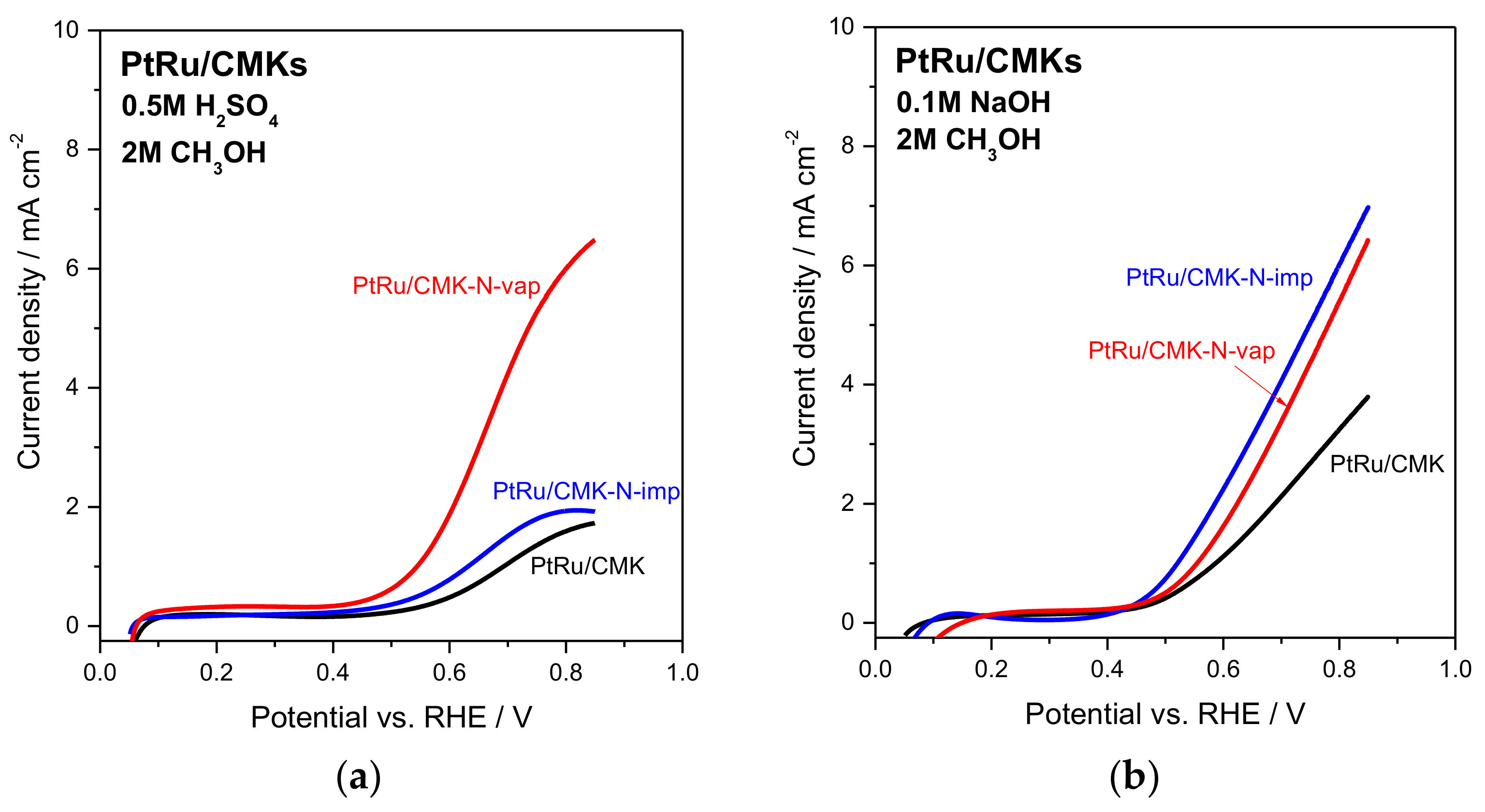
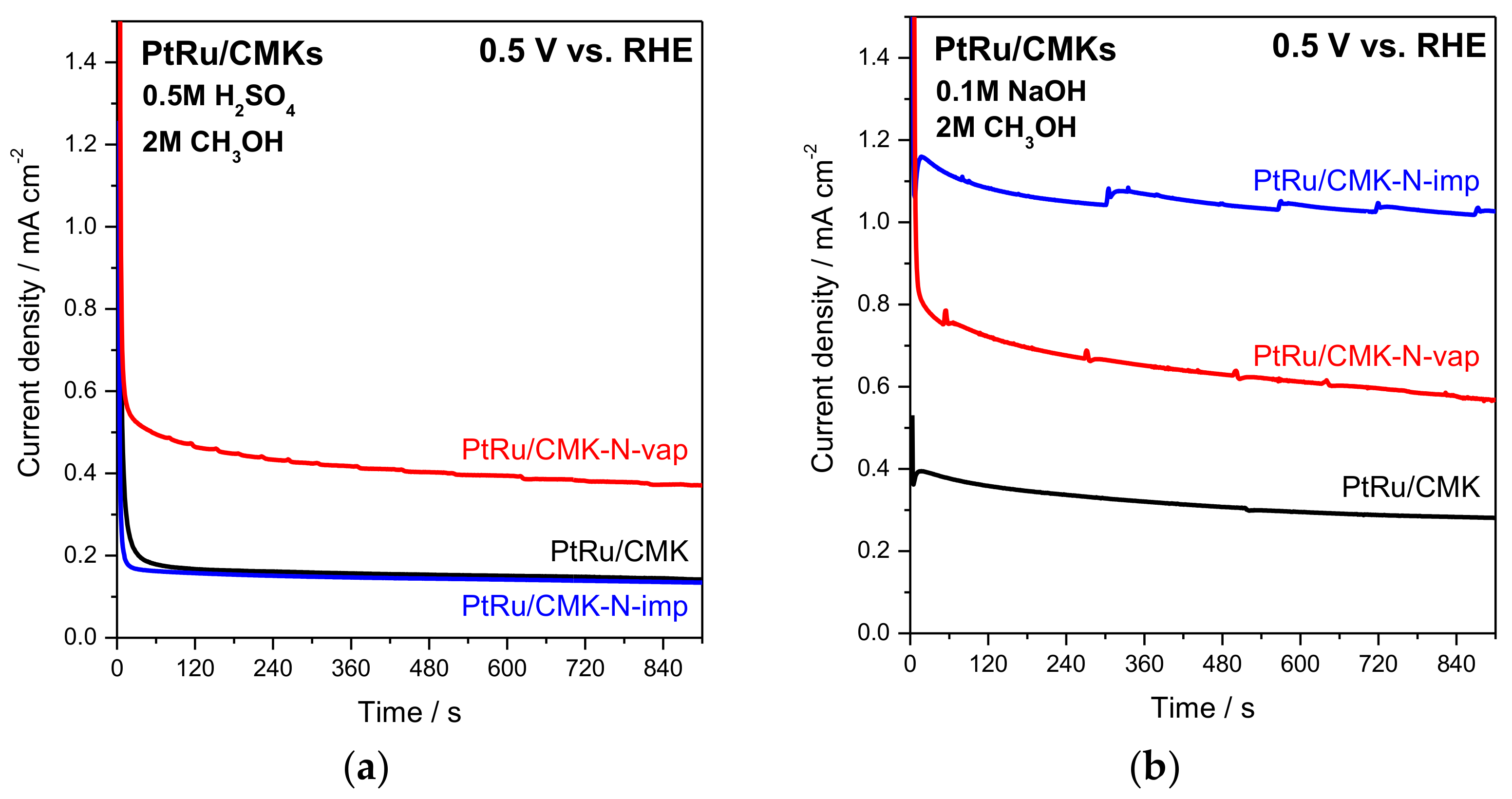
2.3. Electrochemical Assessment of Activity for Methanol Electro-Oxidation on CMK-Supported PtRu Catalysts
3. Materials and Methods
3.1. Preparation of Ordered Mesoporous Carbons
3.2. Synthesis of Carbon-Supported PtRu Electrocatalysts
3.3. Physicochemical Characterization
3.4. Electrochemical Characterization
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Antolini, E.; Gonzalez, E.R. Alkaline direct alcohol fuel cells. J. Power Sources 2010, 195, 3431–3450. [Google Scholar] [CrossRef]
- Aricò, A.S.; Baglio, V.; Antonucci, V. Direct Methanol Fuel Cells; Nova Science Publishers, Inc.: New York, NY, USA, 2010; ISBN 9781608768653. [Google Scholar]
- Sgroi, M.F.; Zedde, F.; Barbera, O.; Stassi, A.; Sebastián, D.; Lufrano, F.; Baglio, V.; Aricò, A.; Bonde, J.L.; Schuster, M. Cost Analysis of Direct Methanol Fuel Cell Stacks for Mass Production. Energies 2016, 9, 1008. [Google Scholar] [CrossRef]
- Sebastián, D.; Serov, A.; Matanovic, I.; Artyushkova, K.; Atanassov, P.; Aricò, A.S.; Baglio, V. Insights on the extraordinary tolerance to alcohols of Fe-N-C cathode catalysts in highly performing direct alcohol fuel cells. Nano Energy 2017, 34, 195–204. [Google Scholar] [CrossRef]
- Zhao, X.; Yin, M.; Ma, L.; Liang, L.; Liu, C.; Liao, J.; Lu, T.; Xing, W. Recent advances in catalysts for direct methanol fuel cells. Energy Environ. Sci. 2011, 4, 2736–2753. [Google Scholar] [CrossRef]
- Lo Vecchio, C.; Aricò, A.S.; Monforte, G.; Baglio, V. EDTA-derived CoNC and FeNC electro-catalysts for the oxygen reduction reaction in acid environment. Renew. Energy 2018, 120, 342–349. [Google Scholar] [CrossRef]
- Hsin, Y.L.; Hwang, K.C.; Yeh, C.-T. Poly(vinylpyrrolidone)-Modified Graphite Carbon Nanofibers as Promising Supports for PtRu Catalysts in Direct Methanol Fuel Cells. J. Am. Chem. Soc. 2007, 129, 9999–10010. [Google Scholar] [CrossRef] [PubMed]
- Iwasita, T.; Hoster, H.; John-Anacker, A.; Lin, W.F.; Vielstich, W. Methanol Oxidation on PtRu Electrodes. Influence of Surface Structure and Pt−Ru Atom Distribution. Langmuir 2000, 16, 522–529. [Google Scholar] [CrossRef]
- Sebastian, D.; Stassi, A.; Siracusano, S.; Vecchio, C.L.; Arico, A.S.; Baglio, V. Influence of Metal Oxide Additives on the Activity and Stability of PtRu/C for Methanol Electro-Oxidation. J. Electrochem. Soc. 2015, 162, F713–F717. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, X.; Wang, Q.; Han, Y.; Fang, Y.; Dong, S. Shape-Control of Pt–Ru Nanocrystals: Tuning Surface Structure for Enhanced Electrocatalytic Methanol Oxidation. J. Am. Chem. Soc. 2018, 140, 1142–1147. [Google Scholar] [CrossRef] [PubMed]
- Sebastián, D.; Suelves, I.; Pastor, E.; Moliner, R.; Lázaro, M.J. The effect of carbon nanofiber properties as support for PtRu nanoparticles on the electrooxidation of alcohols. Appl. Catal. B Environ. 2013, 132, 13–21. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, Z.; Jia, Y.; Wang, M.; Qi, W.; Pang, Y.; Yi, J.; Zhang, Y.; Li, Z.; Zhang, Z. Unique Cu@CuPt Core–Shell Concave Octahedron with Enhanced Methanol Oxidation Activity. ACS Appl. Mater. Interfaces 2017, 9, 36817–36827. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Hu, X.; Zhang, J.; Su, N.; Cheng, J. Facile Fabrication of Platinum-Cobalt Alloy Nanoparticles with Enhanced Electrocatalytic Activity for a Methanol Oxidation Reaction. Sci. Rep. 2017, 7, 45555. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Pollet, B.G. Support materials for PEMFC and DMFC electrocatalysts—A review. J. Power Sources 2012, 208, 96–119. [Google Scholar] [CrossRef]
- Chetty, R.; Kundu, S.; Xia, W.; Bron, M.; Schuhmann, W.; Chirila, V.; Brandl, W.; Reinecke, T.; Muhler, M. PtRu nanoparticles supported on nitrogen-doped multiwalled carbon nanotubes as catalyst for methanol electrooxidation. Electrochim. Acta 2009, 54, 4208–4215. [Google Scholar] [CrossRef]
- Cheng, Y.; Xu, C.; Shen, P.K.; Jiang, S.P. Effect of nitrogen-containing functionalization on the electrocatalytic activity of PtRu nanoparticles supported on carbon nanotubes for direct methanol fuel cells. Appl. Catal. B Environ. 2014, 158–159, 140–149. [Google Scholar] [CrossRef]
- Sebastián, D.; Lázaro, M.J.; Moliner, R.; Suelves, I.; Aricò, A.S.; Baglio, V. Oxidized carbon nanofibers supporting PtRu nanoparticles for direct methanol fuel cells. Int. J. Hydrog. Energy 2014, 39, 5414–5423. [Google Scholar] [CrossRef]
- Gálvez, M.E.; Calvillo, L.; Alegre, C.; Sebastián, D.; Suelves, I.; Pérez-Rodríguez, S.; Celorrio, V.; Pastor, E.; Pardo, J.I.; Moliner, R.; et al. Nanostructured carbon materials as supports in the preparation of direct methanol fuel cell electrocatalysts. Catalysts 2013, 3, 671–682. [Google Scholar] [CrossRef]
- Zhao, S.; Yin, H.; Du, L.; Yin, G.; Tang, Z.; Liu, S. Three dimensional N-doped graphene/PtRu nanoparticle hybrids as high performance anode for direct methanol fuel cells. J. Mater. Chem. A 2014, 2, 3719–3724. [Google Scholar] [CrossRef]
- Alegre, C.; Baquedano, E.; Galvez, M.E.; Moliner, R.; Lazaro, M.J. Tailoring carbon xerogels’ properties to enhance catalytic activity of Pt catalysts towards methanol oxidation. Int. J. Hydrog. Energy 2015, 40, 14736–14745. [Google Scholar] [CrossRef]
- Alegre, C.; Gálvez, M.; Moliner, R.; Lázaro, M. Influence of the Synthesis Method for Pt Catalysts Supported on Highly Mesoporous Carbon Xerogel and Vulcan Carbon Black on the Electro-Oxidation of Methanol. Catalysts 2015, 5, 392–405. [Google Scholar] [CrossRef]
- Maiyalagan, T.; Alaje, T.O.; Scott, K. Highly Stable Pt–Ru Nanoparticles Supported on Three-Dimensional Cubic Ordered Mesoporous Carbon (Pt–Ru/CMK-8) as Promising Electrocatalysts for Methanol Oxidation. J. Phys. Chem. C 2012, 116, 2630–2638. [Google Scholar] [CrossRef]
- Dong, C.-D.; Chen, C.-W.; Chen, C.-F.; Hung, C.-M. Platinum particles supported on mesoporous carbons: Fabrication and electrocatalytic performance in methanol-tolerant oxygen-reduction reactions. Sci. Rep. 2015, 4, 5790. [Google Scholar] [CrossRef] [PubMed]
- Walcarius, A. Mesoporous materials and electrochemistry. Chem. Soc. Rev. 2013, 42, 4098–4140. [Google Scholar] [CrossRef] [PubMed]
- Joo, S.H.; Pak, C.; You, D.J.; Lee, S.-A.; Lee, H.I.; Kim, J.M.; Chang, H.; Seung, D. Ordered mesoporous carbons (OMC) as supports of electrocatalysts for direct methanol fuel cells (DMFC): Effect of carbon precursors of OMC on DMFC performances. Electrochim. Acta 2006, 52, 1618–1626. [Google Scholar] [CrossRef]
- Bruno, M.M.; Petruccelli, M.A.; Viva, F.A.; Corti, H.R. Mesoporous carbon supported PtRu as anode catalyst for direct methanol fuel cell: Polarization measurements and electrochemical impedance analysis of mass transport. Int. J. Hydrog. Energy 2013, 38, 4116–4123. [Google Scholar] [CrossRef]
- Calderón, J.; Calvillo, L.; Lázaro, M.; Rodríguez, J.; Pastor, E. Effect of the Dendrimer Generation Used in the Synthesis of Pt-Ru Nanoparticles Supported on Carbon Nanofibers on the Catalytic Activity towards Methanol Oxidation. Energies 2017, 10, 159. [Google Scholar] [CrossRef]
- Alegre, C.; Sebastián, D.; Gálvez, M.E.; Baquedano, E.; Moliner, R.; Aricò, A.S.; Baglio, V.; Lázaro, M.J. N-Doped Carbon Xerogels as Pt Support for the Electro-Reduction of Oxygen. Materials 2017, 10, 1092. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Neyerlin, K.; Olson, T.S.; Pylypenko, S.; Bult, J.; Dinh, H.N.; Gennett, T.; Shao, Z.; O’Hayre, R. Enhancement of Pt and Pt-alloy fuel cell catalyst activity and durability via nitrogen-modified carbon supports. Energy Environ. Sci. 2010, 3, 1437–1446. [Google Scholar] [CrossRef]
- Su, F.; Tian, Z.; Poh, C.K.; Wang, Z.; Lim, S.H.; Liu, Z.; Lin, J. Pt Nanoparticles Supported on Nitrogen-Doped Porous Carbon Nanospheres as an Electrocatalyst for Fuel Cells. Chem. Mater. 2010, 22, 832–839. [Google Scholar] [CrossRef]
- Lei, M.; Wang, J.; Li, J.R.; Wang, Y.G.; Tang, H.L.; Wang, W.J. Emerging methanol-tolerant AlN nanowire oxygen reduction electrocatalyst for alkaline direct methanol fuel cell. Sci. Rep. 2014, 4, 6013. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Nie, H.; Yang, Z.; Zhang, J.; Jin, Z.; Lu, Y.; Xiao, Z.; Huang, S. Sulfur–nitrogen co-doped three-dimensional carbon foams with hierarchical pore structures as efficient metal-free electrocatalysts for oxygen reduction reactions. Nanoscale 2013, 5, 3283–3288. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Cochell, T.; Manthiram, A. Boron-doped carbon nanotube-supported Pt nanoparticles with improved CO tolerance for methanol electro-oxidation. Phys. Chem. Chem. Phys. 2012, 14, 13910–13913. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Shi, Q.; Peng, F.; Wang, H.; Yu, H.; Li, J.; Wei, X. Enhanced methanol oxidation activity of Pt catalyst supported on the phosphorus-doped multiwalled carbon nanotubes in alkaline medium. Catal. Commun. 2012, 22, 34–38. [Google Scholar] [CrossRef]
- Alegre, C.; Sebastián, D.; Gálvez, M.E.; Moliner, R.; Lázaro, M.J. Sulfurized carbon xerogels as Pt support with enhanced activity for fuel cell applications. Appl. Catal. B Environ. 2016, 192, 260–267. [Google Scholar] [CrossRef]
- Liu, Z.; Su, F.; Zhang, X.; Tay, S.W. Preparation and characterization of PtRu nanoparticles supported on nitrogen-doped porous carbon for electrooxidation of methanol. ACS Appl. Mater. Interfaces 2011, 3, 3824–3830. [Google Scholar] [CrossRef] [PubMed]
- Lv, R.; Cui, T.; Jun, M.-S.; Zhang, Q.; Cao, A.; Su, D.S.; Zhang, Z.; Yoon, S.-H.; Miyawaki, J.; Mochida, I.; et al. Open-Ended, N-Doped Carbon Nanotube-Graphene Hybrid Nanostructures as High-Performance Catalyst Support. Adv. Funct. Mater. 2011, 21, 999–1006. [Google Scholar] [CrossRef]
- Luque-Centeno, J.M.; Martínez-Huerta, M.V.; Sebastián, D.; Lemes, G.; Pastor, E.; Lázaro, M.J. Bifunctional N-doped graphene Ti and Co. nanocomposites for the oxygen reduction and evolution reactions. Renew. Energy 2018, 125, 182–192. [Google Scholar] [CrossRef]
- Pels, J.R.; Kapteijn, F.; Moulijn, J.A.; Zhu, Q.; Thomas, K.M. Evolution of nitrogen functionalities in carbonaceous materials during pyrolysis. Carbon 1995, 33, 1641–1653. [Google Scholar] [CrossRef]
- Garcia, G.; Baglio, V.; Stassi, A.; Pastor, E.; Antonucci, V.; Aricò, A.S. Investigation of Pt–Ru nanoparticle catalysts for low temperature methanol electro-oxidation. J. Solid State Electrochem. 2007, 11, 1229–1238. [Google Scholar] [CrossRef]
- Gan, L.; Du, H.; Li, B.; Kang, F. Influence of reaction temperature on the particle-composition distributions and activities of polyol-synthesized Pt-Ru/C catalysts for methanol oxidation. J. Power Sources 2009, 191, 233–239. [Google Scholar] [CrossRef]
- Guo, J.; Sun, G.; Shiguo, S.; Shiyou, Y.; Weiqian, Y.; Jing, Q.; Yushan, Y.; Qin, X. Polyol-synthesized PtRu/C and PtRu black for direct methanol fuel cells. J. Power Sources 2007, 168, 299–306. [Google Scholar] [CrossRef]
- Celorrio, V.; Calvillo, L.; Pérez-Rodríguez, S.; Lázaro, M.J.; Moliner, R. Modification of the properties of carbon nanocoils by different treatments in liquid phase. Microporous Mesoporous Mater. 2011, 142, 55–61. [Google Scholar] [CrossRef]
- Kim, J.; Jang, J.-S.; Peck, D.-H.; Lee, B.; Yoon, S.-H.; Jung, D.-H. Methanol-Tolerant Platinum-Palladium Catalyst Supported on Nitrogen-Doped Carbon Nanofiber for High Concentration Direct Methanol Fuel Cells. Nanomaterials 2016, 6, 148. [Google Scholar] [CrossRef] [PubMed]
- Wood, K.N.; O’Hayre, R.; Pylypenko, S. Recent progress on nitrogen/carbon structures designed for use in energy and sustainability applications. Energy Environ. Sci. 2014, 7, 1212–1249. [Google Scholar] [CrossRef]
- Calderón, J.C.; García, G.; Calvillo, L.; Rodríguez, J.L.; Lázaro, M.J.; Pastor, E. Electrochemical oxidation of CO and methanol on Pt–Ru catalysts supported on carbon nanofibers: The influence of synthesis method. Appl. Catal. B Environ. 2015, 165, 676–686. [Google Scholar] [CrossRef]
- Dos Santos, L.; Colmati, F.; Gonzalez, E.R. Preparation and characterization of supported Pt-Ru catalysts with a high Ru content. J. Power Sources 2006, 159, 869–877. [Google Scholar] [CrossRef]
- Nieto-Márquez, A.; Toledano, D.; Sánchez, P.; Romero, A.; Valverde, J.L. Impact of nitrogen doping of carbon nanospheres on the nickel-catalyzed hydrogenation of butyronitrile. J. Catal. 2010, 269, 242–251. [Google Scholar] [CrossRef]
- Roy, S.C.; Harding, A.W.; Russell, A.E.; Thomas, K.M. Spectroelectrochemical Study of the Role Played by Carbon Functionality in Fuel Cell Electrodes. J. Electrochem. Soc. 1997, 144, 2323–2328. [Google Scholar] [CrossRef]
- Tripkovic, A.V.; Popovic, K.D.; Grgur, B.N.; Blizanac, B.; Ross, P.N.; Markovic, N.M. Methanol electrooxidation on supported Pt and PtRu catalysts in acid and alkaline solutions. Electrochim. Acta 2002, 47, 3707–3714. [Google Scholar] [CrossRef]
- Zhang, L.; Gao, A.; Liu, Y.; Wang, Y.; Ma, J. PtRu nanoparticles dispersed on nitrogen-doped carbon nanohorns as an efficient electrocatalyst for methanol oxidation reaction. Electrochim. Acta 2014, 132, 416–422. [Google Scholar] [CrossRef]
- Pérez-Rodríguez, S.; Rillo, N.; Lázaro, M.J.; Pastor, E. Pd catalysts supported onto nanostructured carbon materials for CO2 valorization by electrochemical reduction. Appl. Catal. B Environ. 2015, 163, 83–95. [Google Scholar] [CrossRef]
- Lázaro, M.J.; Calvillo, L.; Bordejé, E.G.; Moliner, R.; Juan, R.; Ruiz, C.R. Functionalization of ordered mesoporous carbons synthesized with SBA-15 silica as template. Microporous Mesoporous Mater. 2007, 103, 158–165. [Google Scholar] [CrossRef]
- Valle-Vigón, P.; Sevilla, M.; Fuertes, A.B. Functionalization of mesostructured silica–carbon composites. Mater. Chem. Phys. 2013, 139, 281–289. [Google Scholar] [CrossRef]
- Naumkin, A.V.; Kraut-Vass, A.; Gaarenstroom, S.W.; Powell, C.J. NIST X-ray Photoelectron Spectroscopy Database, NIST Standard Reference Database 20, Version 4.1. 2017. Available online: http://srdata.nist.gov/xps/Default.aspx (accessed on 20 December 2017).
- Briggs, D. Handbook of X-Ray Photoelectron Spectroscopy; Wanger, C.D., Riggs, W.M., Davis, L.E., Moulder, J.F., Muilenberg, G.E., Eds.; Perkin-Elmer Corp.: Eden Prairie, MN, USA, 1979. [Google Scholar]
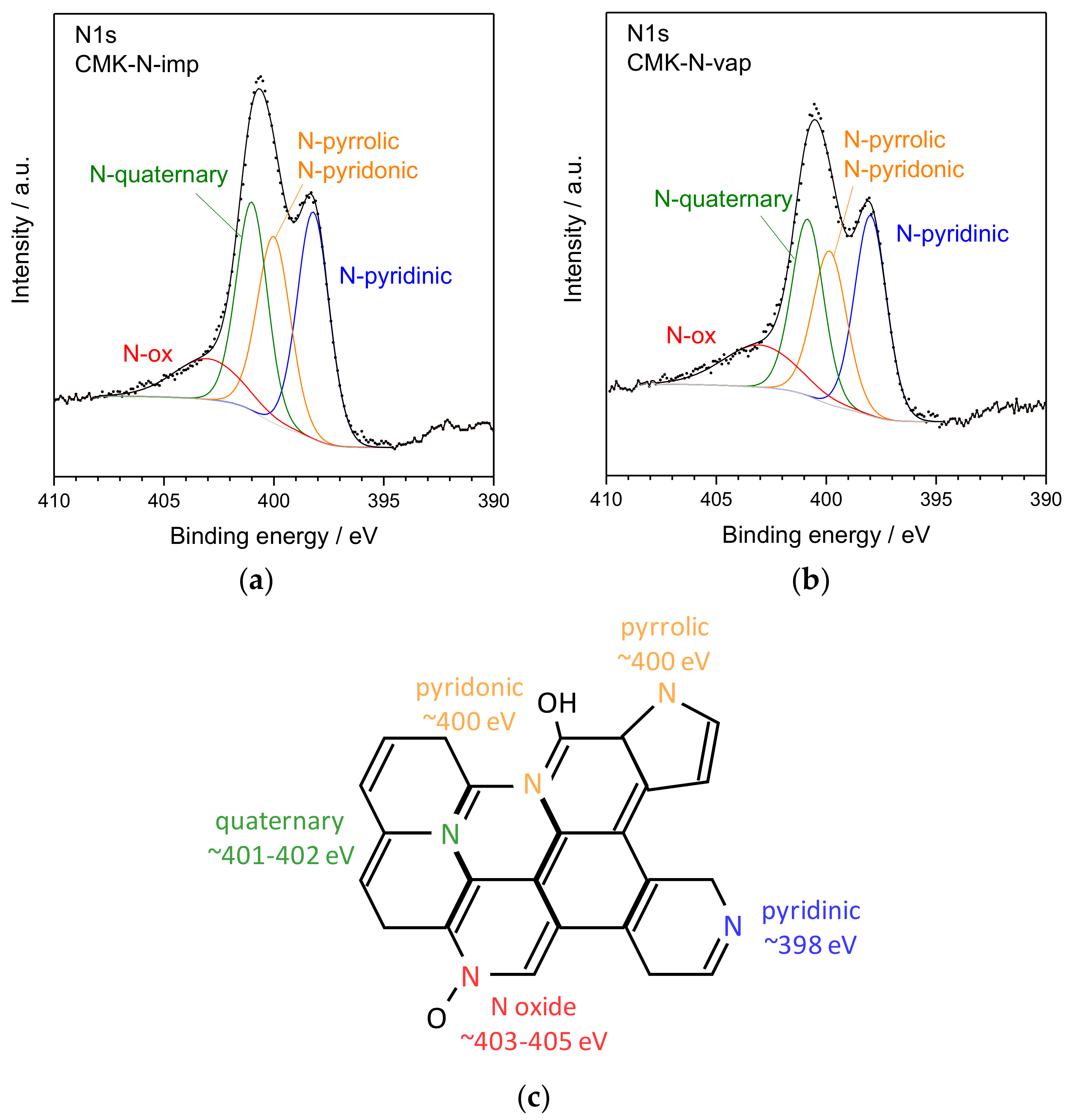
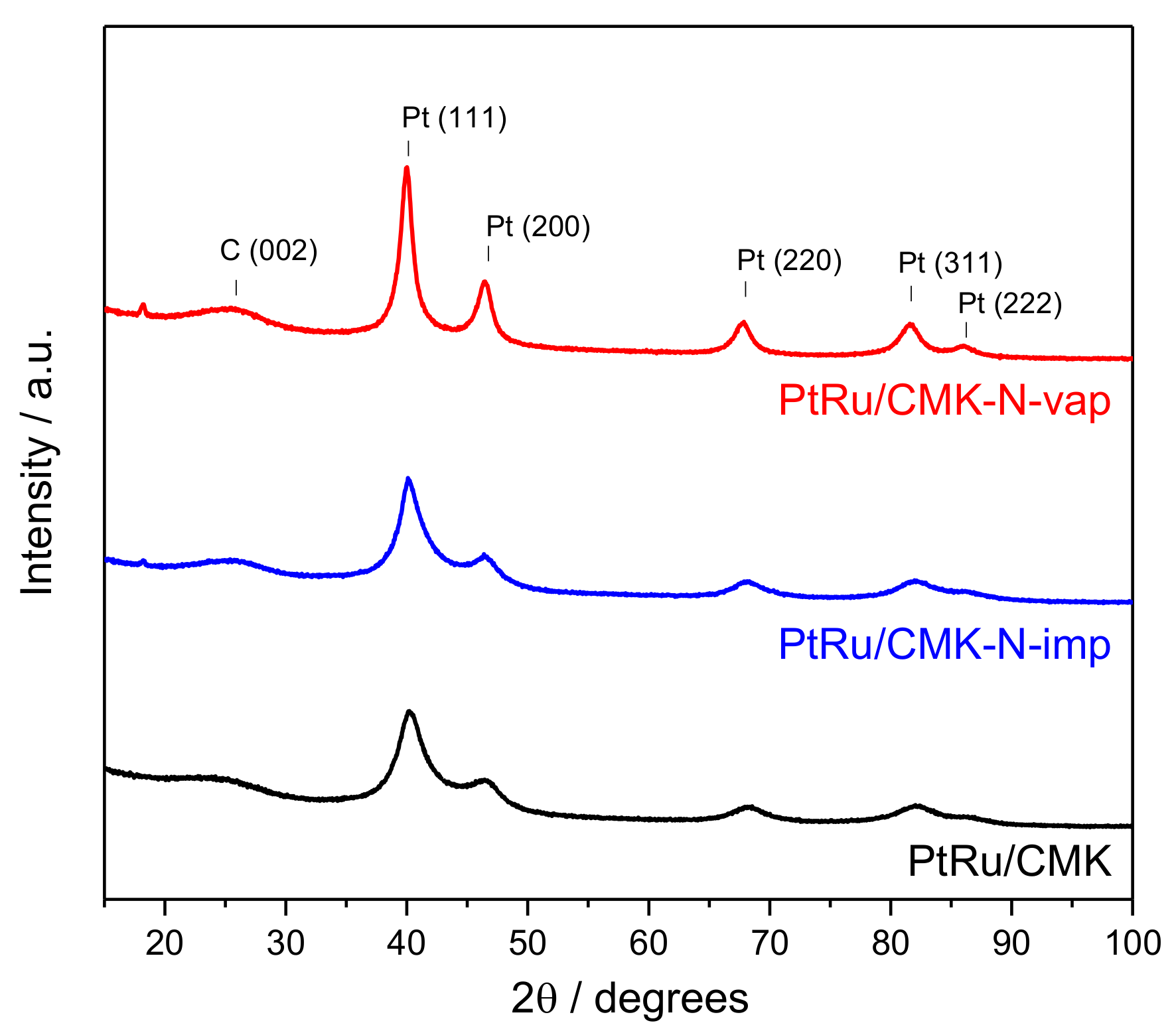
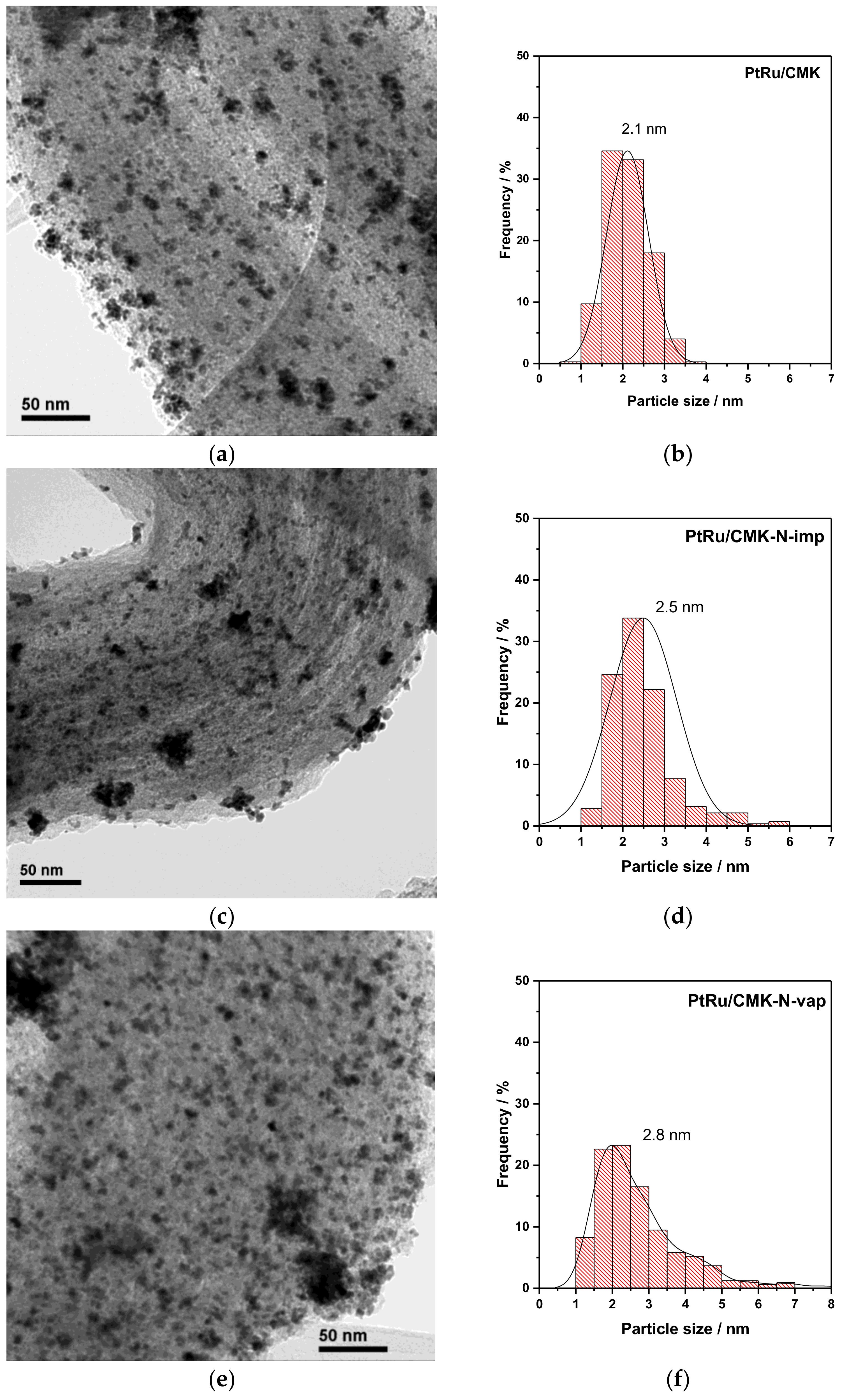
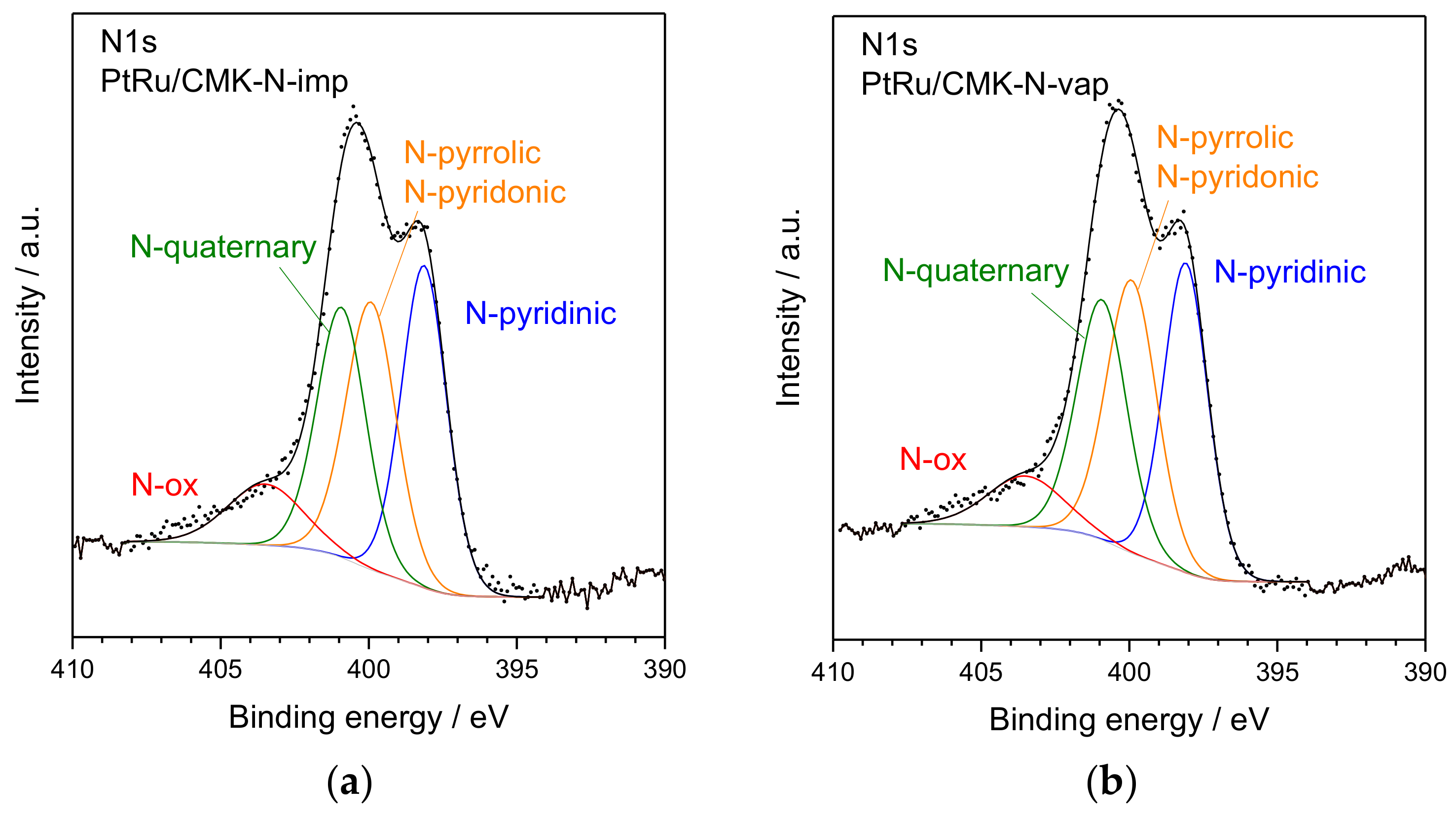
| Material | Nitrogen Content/wt. % (EA) | Nitrogen Content/at % (XPS) | SBET/m2 g−1 | Vpore/cm3 g−1 | Vmicropore/cm3 g−1 | Vmesopore/cm3 g−1 |
|---|---|---|---|---|---|---|
| CMK | <0.1 | 0.1 | 1006 | 0.68 | 0.47 | 0.21 |
| CMK-N-imp | 14.2 | 7.9 | 151 | 0.14 | 0.01 | 0.13 |
| CMK-N-vap | 13.7 | 10.1 | 886 | 0.88 | 0.24 | 0.64 |
| Material | Pyridinic N (398.2 eV)/% | Pyrrolic/Pyridonic N (400 eV)/% | Quaternary N (401 eV)/% | N Oxides (403–405 eV)/% |
|---|---|---|---|---|
| CMK-N-imp | 32.1 | 27.3 | 28.5 | 12.1 |
| CMK-N-vap | 31.4 | 25.6 | 27.6 | 15.4 |
| Catalyst | N Content (XPS) /at % | PtRu Crystallite Size (XRD) 1/nm | Pt (111) 2θ (°) | Lattice Parameter (nm) | XRu in PtRu Alloy | Pt:Ru Atomic Ratio (EDX) | Pt:Ru Atomic Ratio (XPS) |
|---|---|---|---|---|---|---|---|
| PtRu/CMK | - | 2.8 | 40.26 | 0.388 | 0.4 | 44:56 | 52:48 |
| PtRu/CMK-N-imp | 5.5 | 2.9 | 40.13 | 0.389 | 0.3 | 47:53 | 63:37 |
| PtRu/CMK-N-vap | 9.0 | 5.8 | 40.00 | 0.390 | 0.2 | 58:42 | 70:30 |
| Species | PtRu/CMK | PtRu/CMK-N-imp | PtRu/CMK-N-vap | |||
|---|---|---|---|---|---|---|
| BE/eV | % | BE/eV | % | BE/eV | % | |
| Ru 3p 3/2 | ||||||
| Metallic Ru | 461.3 | 33.1 | 461.4 | 38.8 | 461.0 | 25.5 |
| Ru4+ | 463.0 | 35.0 | 463.0 | 33.9 | 463.0 | 42.0 |
| Ru4+ hydrate | 465.1 | 31.9 | 465.1 | 27.3 | 465.2 | 32.5 |
| Pt 4f 7/2 | ||||||
| Metallic Pt | 71.5 | 62.7 | 71.3 | 61.9 | 71.3 | 60.2 |
| Pt2+ | 72.8 | 22.6 | 72.7 | 22.4 | 72.6 | 23.8 |
| Pt4+ | 75.4 | 14.7 | 75.3 | 15.7 | 75.0 | 16.0 |
| N 1s | ||||||
| Pyridinic N | - | - | 398.2 | 34.2 | 398.1 | 33.2 |
| Pyrrolic/Pyridonic N | - | - | 400.0 | 30.9 | 400.0 | 32.5 |
| Quaternary N | - | - | 401.0 | 24.9 | 401.0 | 25.9 |
| N oxides | - | - | 403.5 | 10.0 | 403.6 | 8.4 |
| Catalyst | LV 1 MOR Activity at 0.55 V vs. RHE in 0.5 M H2SO4/mA mgPt−1 | LV 1 MOR Activity at 0.55 V vs. RHE in 0.1 M NaOH/mA mgPt−1 | CA 2 (t = 10 min) MOR Activity at 0.5 V vs. RHE in 0.5 M H2SO4/mA mgPt−1 | CA 2 (t = 10 min) MOR Activity at 0.5 V vs. RHE in 0.1 M NaOH/mA mgPt−1 |
|---|---|---|---|---|
| PtRu/CMK | 11.6 | 24.2 | 4.6 | 10.0 |
| PtRu/CMK-N-imp | 16.2 | 45.3 | 4.4 | 32.9 |
| PtRu/CMK-N-vap | 30.0 | 25.6 | 11.0 | 16.8 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sebastián, D.; Nieto-Monge, M.J.; Pérez-Rodríguez, S.; Pastor, E.; Lázaro, M.J. Nitrogen Doped Ordered Mesoporous Carbon as Support of PtRu Nanoparticles for Methanol Electro-Oxidation. Energies 2018, 11, 831. https://doi.org/10.3390/en11040831
Sebastián D, Nieto-Monge MJ, Pérez-Rodríguez S, Pastor E, Lázaro MJ. Nitrogen Doped Ordered Mesoporous Carbon as Support of PtRu Nanoparticles for Methanol Electro-Oxidation. Energies. 2018; 11(4):831. https://doi.org/10.3390/en11040831
Chicago/Turabian StyleSebastián, David, María Jesús Nieto-Monge, Sara Pérez-Rodríguez, Elena Pastor, and María Jesús Lázaro. 2018. "Nitrogen Doped Ordered Mesoporous Carbon as Support of PtRu Nanoparticles for Methanol Electro-Oxidation" Energies 11, no. 4: 831. https://doi.org/10.3390/en11040831
APA StyleSebastián, D., Nieto-Monge, M. J., Pérez-Rodríguez, S., Pastor, E., & Lázaro, M. J. (2018). Nitrogen Doped Ordered Mesoporous Carbon as Support of PtRu Nanoparticles for Methanol Electro-Oxidation. Energies, 11(4), 831. https://doi.org/10.3390/en11040831









