Changes in Lignin Chemistry of Switchgrass due to Delignification by Sodium Hydroxide Pretreatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Sodium Hydroxide Pretreatment
2.3. Enzymatic Hydrolysis
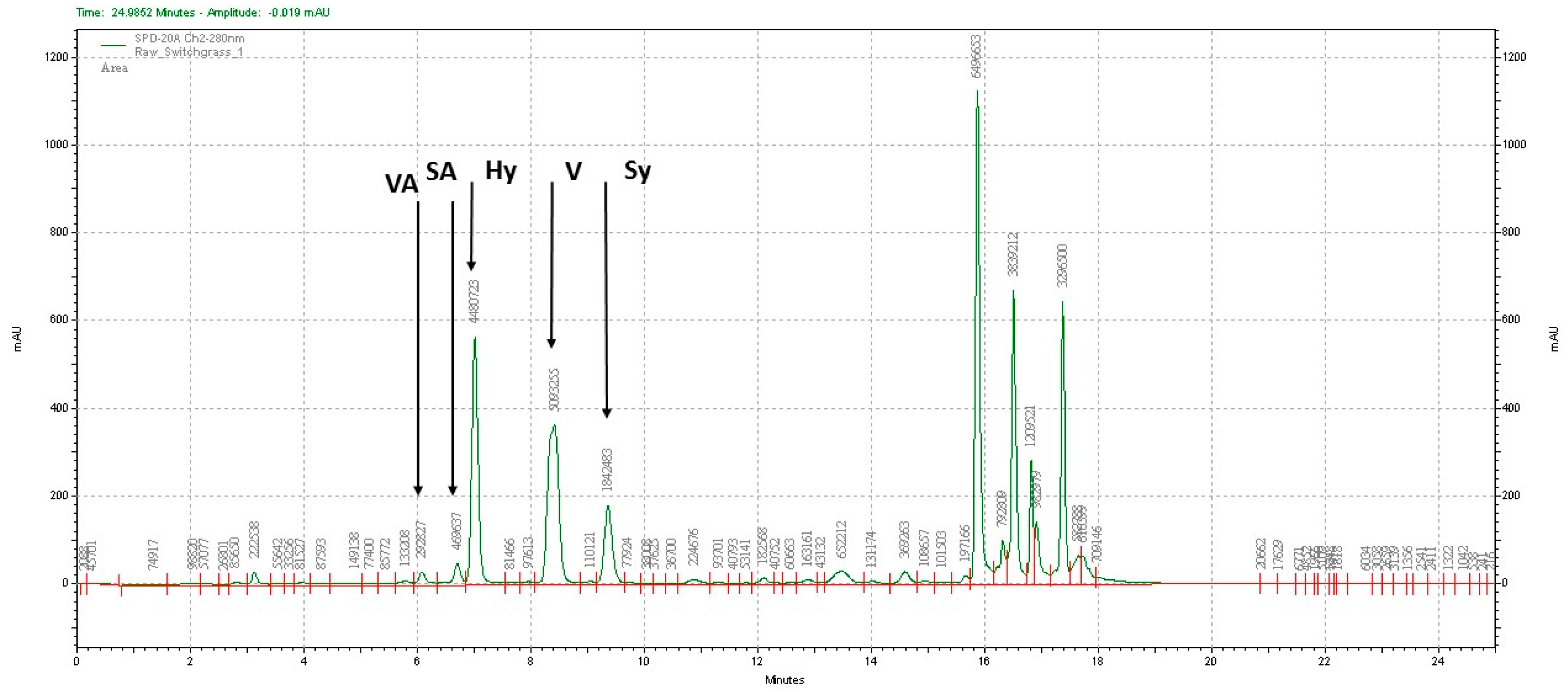
2.4. Analytical Methods
2.5. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Switchgrass
3.2. Chemical Composition of Sodium Hydroxide-Pretreated Switchgrass
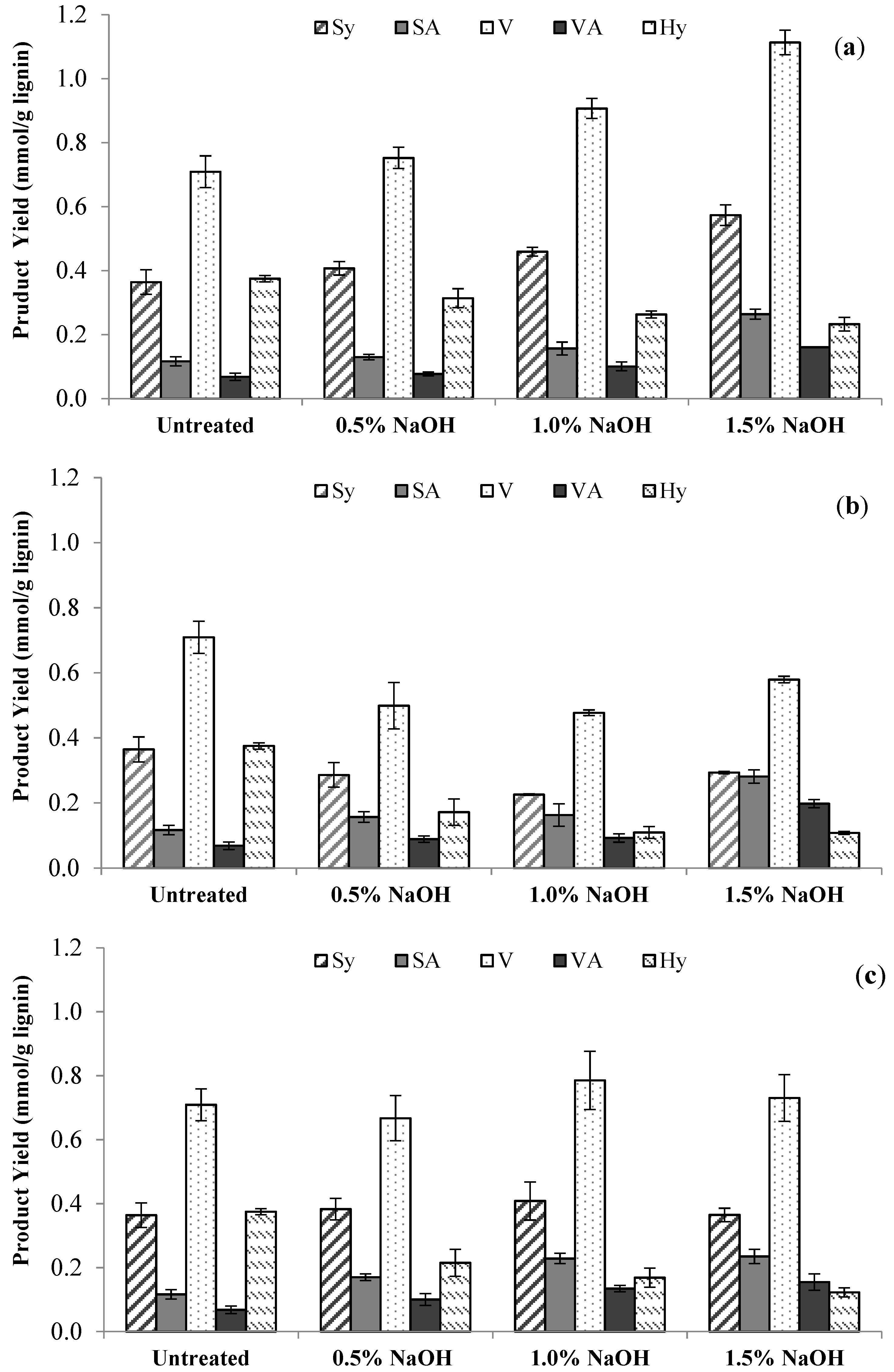
3.3. Lignin Chemistry Changes in Switchgrass due to Sodium Hydroxide Pretreatment
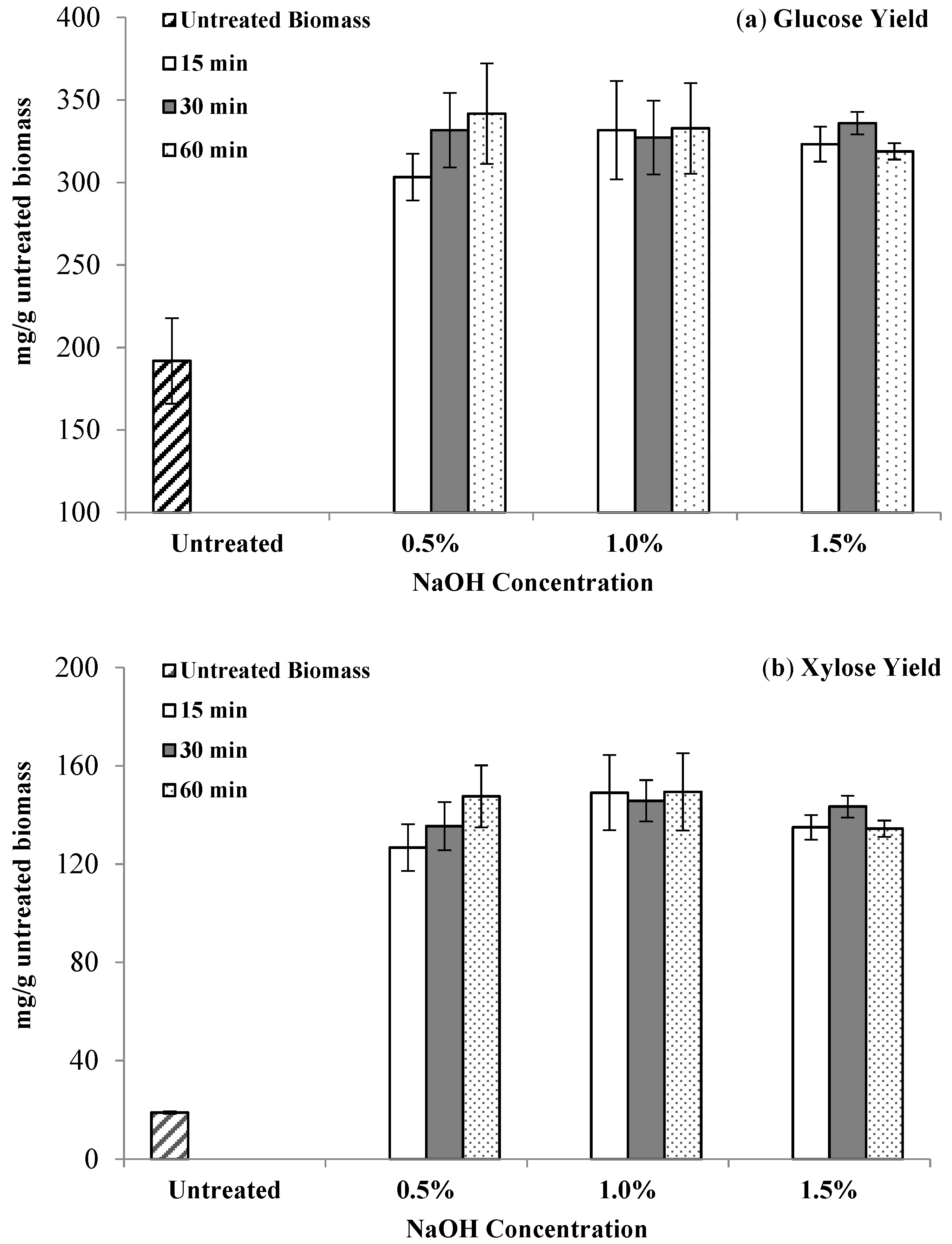
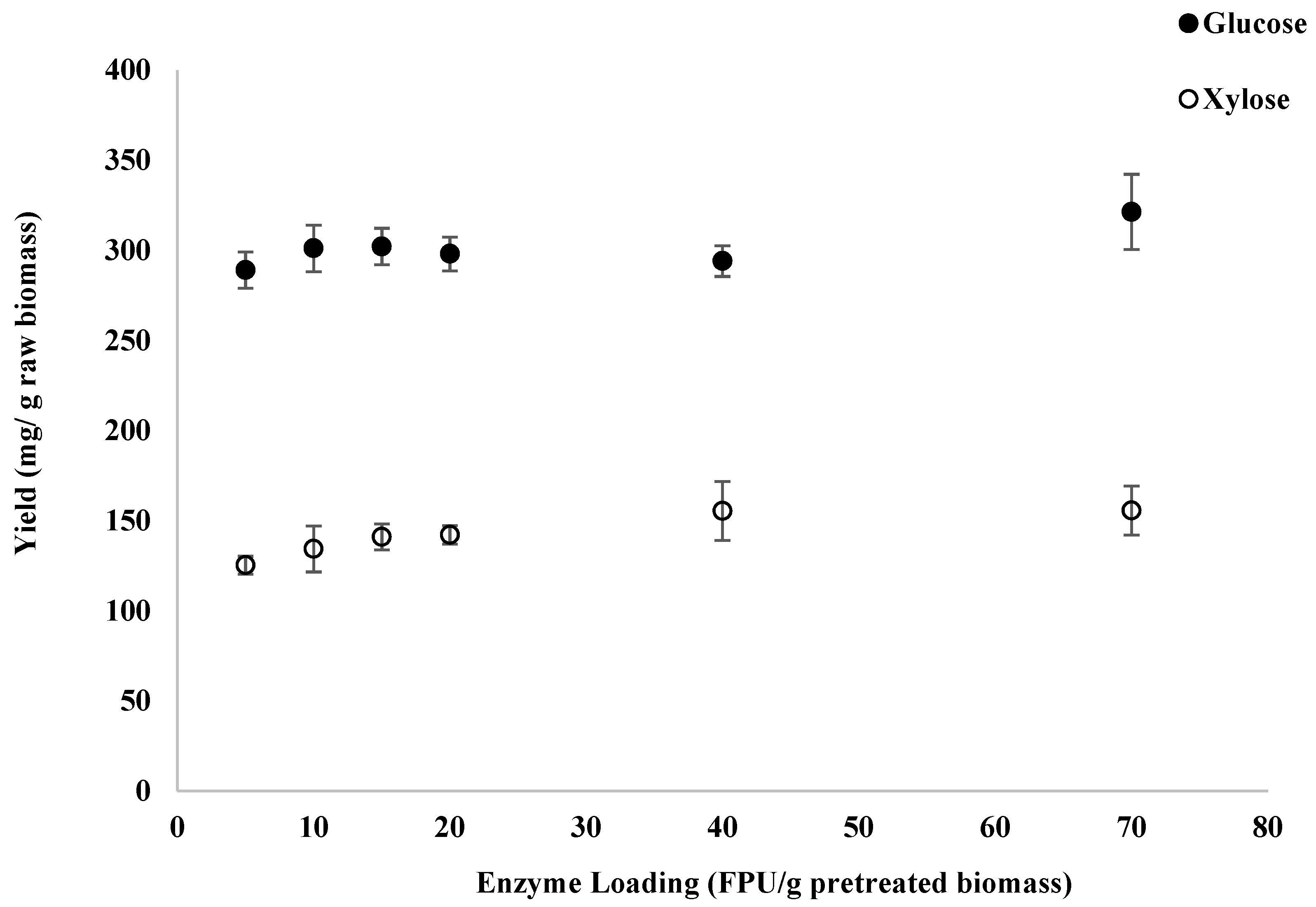
3.4. Enzymatic Hydrolysis with NaOH-Pretreated Switchgrass
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kishimoto, T.; Chiba, W.; Saito, K.; Fukushima, K.; Uraki, Y.; Ubukata, M. Influence of syringyl to guaiacyl ratio on the structure of natural and synthetic lignins. J. Agric. Food Chem. 2010, 58, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Q.; Li, C.L.; Sang, T.; Xu, J. Pretreatment on Miscanthus lutarioriparious by liquid hot water for efficient ethanol production. Biotechnol. Biofuels 2013, 6, 76. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Padmanabhan, S.; Cheng, K.; Xie, H.; Gokhale, A.; Afzal, W.; Na, H.; Pauly, M.; Bell, A.T.; Prausnitz, J.M. Two-step delignification of miscanthus to enhance enzymatic hydrolysis: Aqueous ammonia followed by sodium hydroxide and oxidants. Energy Fuels 2014, 28, 542–548. [Google Scholar] [CrossRef]
- Ohra-Aho, T.; Gomes, F.J.B.; Colodette, J.L.; Tamminen, T. S/G ratio and lignin structure among Eucalyptus hybrids determined by Py-GC/MS and nitrobenzene oxidation. J. Anal. Appl. Pyrolysis 2013, 101, 166–171. [Google Scholar] [CrossRef]
- Santos, R.B.; Hart, P.W.; Jameel, H.; Chang, H.M. Wood based lignin reactions important to the biorefinery and pulp and paper industries. BioResources 2013, 8, 1456–1477. [Google Scholar] [CrossRef]
- Boerjan, W.; Ralph, J.; Baucher, M. Lignin biosynthesis. Annu. Rev. Plant Biol. 2003, 54, 519–546. [Google Scholar] [CrossRef] [PubMed]
- Umezawa, T. The cinnamate/monolignol pathway. Phytochem. Rev. 2010, 9, 1–17. [Google Scholar] [CrossRef]
- Haque, M.A.; Barman, D.N.; Kang, T.H.; Kim, M.K.; Kim, J.; Kim, H.; Yun, H.D. Effect of dilute alkali pretreatment on structural features and enhanced enzymatic hydrolysis of Miscanthus sinensis at boiling temperature with low residence time. Biosyst. Eng. 2013, 114, 294–305. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, J.; Pandey, P.; Cheng, J.J.; Li, R.; Qu, R. Improvement of sugar production from transgenic switchgrass with low-temperature alkali pretreatment. Energy Fuels 2012, 26, 3054–3061. [Google Scholar] [CrossRef]
- Xu, J.; Chen, Y.; Cheng, J.J.; Sharma-Shivappa, R.R.; Burns, J.C. Delignification of switchgrass cultivars for bioethanol production. BioResources 2011, 6, 707–720. [Google Scholar]
- Fu, C.; Mielenz, J.R.; Xiao, X.; Ge, Y.; Hamilton, C.Y.; Rodriguez, M.; Chen, F.; Foston, M.; Ragauskas, A.; Bouton, J.; et al. Genetic manipulation of lignin reduces recalcitrance and improves ethanol production from switchgrass. Proc. Natl. Acad. Sci. USA 2011, 108, 3803–3808. [Google Scholar] [CrossRef] [PubMed]
- Han, G.-W.; Kim, Y.; Koo, B.-C.; Choi, G.-W. Bioethanol production by Miscanthus as a lignocellulosic biomass: Focus on high efficiency conversion to glucose and ethanol. Bioresources 2011, 6, 1939–1953. [Google Scholar]
- Jensen, K.; Clark, C.D.; Ellis, P.; English, B.; Menard, J.; Walsh, M.; de la Torre Ugarte, D. Farmer willingness to grow switchgrass for energy production. Biomass Bioenergy 2007, 31, 773–781. [Google Scholar] [CrossRef]
- Keshwani, D.R.; Cheng, J.J. Switchgrass for bioethanol and other value-added applications: A review. Bioresour. Technol. 2009, 100, 1515–1523. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Palled, V.; Sharma-Shivappa, R.R.; Osborne, J. Potential of potassium hydroxide pretreatment of switchgrass for fermentable sugar production. Appl. Biochem. Biotechnol. 2013, 169, 761–772. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, R.A.; Chen, Y.; Sharma-Shivappa, R.R.; Boyette, M.D.; Osborne, J.O. A comparison of chemical pretreatment methods for improving saccharification of cotton stalks. Bioresour. Technol. 2007, 98, 3000–3011. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Cheng, J.J.; Sharma-Shivappa, R.R.; Burns, J.C. Sodium Hydroxide Pretreatment of Switchgrass for Ethanol Production. Energy Fuels 2010, 24, 2113–2119. [Google Scholar] [CrossRef]
- Wang, Z.; Li, R.; Xu, J.; Marita, J.M.; Hatfield, R.D.; Qu, R.; Cheng, J.J. Sodium hydroxide pretreatment of genetically modified switchgrass for improved enzymatic release of sugars. Bioresour. Technol. 2012, 110, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Lawther, J.M.; Banks, W.B. Influence of alkaline pre-treatments on the cell wall components of wheat straw. Ind. Crops Prod. 1995, 4, 127–145. [Google Scholar] [CrossRef]
- Adney, B.; Nrel, J.B. Measurement of Cellulase Activities Laboratory Analytical Procedure (LAP): Issue Date 08/12/1996; National Renewable Energy Laboratory: Golden, CO, USA, 2008; p. 8. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Ash in Biomass: Laboratory Analytical Procedure (LAP); Nrel/Tp-510-42622; National Renewable Energy Laboratory: Golden, CO, USA, 2008; p. 18. [Google Scholar]
- Sluiter, A.; Ruiz, R.O.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Extractives in Biomass; Technical Report; National Renewable Energy Laboratory: Golden, CO, USA, 2004; pp. 1–8. [Google Scholar]
- Sluiter, A.; Hames, B.; Hyman, D.; Payne, C.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Nrel, J.W. Determination of Total Solids in Biomass and Total Dissolved Solids in Liquid Process Samples; NREL/TP-510-42621; National Renewable Energy Laboratory: Golden, CO, USA, 2008; p. 9. [Google Scholar]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass: Laboratory Analytical Procedure (LAP); Nrel/Tp-510-42618; National Renewable Energy Laboratory: Golden, CO, USA, 2012; p. 15. [Google Scholar]
- Kajita, S.; Katayama, Y.; Omori, S. Alterations in the biosynthesis of lignin in transgenic plants with chimeric genes for 4-coumarate:coenzyme a ligase. Plant Cell Physiol. 1996, 37, 957–965. [Google Scholar] [CrossRef] [PubMed]
- Lima, C.F.; Barbosa, L.C.A.; Marcelo, C.R.; Silvério, F.O.; Colodette, J.L. Comparison between analytical pyrolysis and nitrobenzene oxidation for determination of syringyl/guaiacyl ratio in Eucalyptus spp. Lignin. BioResources 2008, 3, 701–712. [Google Scholar] [CrossRef]
- Schultz, T.; Templeton, M. Proposed Mechanism for the Nitrobenzene Oxidation of Lignin. Holzforschung 1986, 40, 93–97. [Google Scholar] [CrossRef]
- Cheng, H.; Zhan, H.; Fu, S.; Lucia, L.A. Alkali extraction of hemicellulose from depithed corn stover and effects on soda-AQ pulping. BioResources 2010, 11, 196–206. [Google Scholar]
- Kim, J.S.; Lee, Y.Y.; Kim, T.H. A review on alkaline pretreatment technology for bioconversion of lignocellulosic biomass. Bioresour. Technol. 2016, 199, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Belmokhtar, N.; Habrant, A.; Lopes Ferreira, N.; Chabbert, B. Changes in Phenolics Distribution After Chemical Pretreatment and Enzymatic Conversion of Miscanthus × giganteus Internode. Bioenergy Res. 2013, 6, 506–518. [Google Scholar] [CrossRef]
- Yamamura, M.; Noda, S.; Hattori, T.; Shino, A.; Kikuchi, J.; Takabe, K.; Tagane, S.; Gau, M.; Uwatoko, N.; Mii, M.; et al. Characterization of lignocellulose of Erianthus arundinaceus in relation to enzymatic saccharification efficiency. Plant Biotechnol. 2013, 30, 25–35. [Google Scholar] [CrossRef]
- Russell, W.R.; Burkitt, M.J.; Scobbie, L.; Chesson, A. EPR investigation into the effects of substrate structure on peroxidase-catalyzed phenylpropanold oxidation. Biomacromolecules 2006, 7, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Binod, P.; Sindhu, S.; Singhania, R.R.; Vikram, S.; Devi, L.; Nagalakshmi, S.; Kurien, N.; Sukumaran, R.K.; Pandey, A. Bioethanol production from rice straw: An overview. Bioresour. Technol. 2010, 101, 4767–4774. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J. Biomass to Renewable Energy Processes; CRC Press, Taylor and Francis: New York, NY, USA, 2010. [Google Scholar]
- Ziebell, A.; Gracom, K.; Katahira, R.; Chen, F.; Pu, Y.; Ragauskas, A.; Dixon, R.A.; Davis, M. Increase in 4-coumaryl alcohol units during lignification in alfalfa (Medicago sativa) alters the extractability and molecular weight of lignin. J. Biol. Chem. 2010, 285, 38961–38968. [Google Scholar] [CrossRef] [PubMed]
- Mood, S.H.; Golfeshan, H.H.; Tabatabaei, M.; Jouzani, G.S.; Najafi, G.H.; Gholami, M.; Mehdi Ardjmand, M. Lignocellulosic biomass to bioethanol, a comprehensive review with a focus on pretreatment. Renew. Sustain. Energy Rev. 2013, 27, 77–93. [Google Scholar] [CrossRef]
- Nlewem, K.C.; Thrash, M.E., Jr. Comparison of different pretreatment methods based on residual lignin effect on the enzymatic hydrolysis of switchgrass. Bioresour. Technol. 2010, 101, 5426–5430. [Google Scholar] [CrossRef] [PubMed]
- Barman, D.N.; Haque, M.A.; Kang, T.H.; Kim, G.H.; Kim, T.Y.; Kim, M.K.; Yun, H.D. Effect of mild alkali pretreatment on structural changes of reed (Phragmites communis Trinius) straw. Environ. Technol. (UK) 2014, 35, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Öhgren, K.; Bura, R.; Saddler, J.; Zacchi, G. Effect of hemicellulose and lignin removal on enzymatic hydrolysis of steam pretreated corn stover. Bioresour. Technol. 2007, 98, 2503–2510. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Song, X.; Sun, R.; Jiang, J. Effect of lignin content on enzymatic hydrolysis of furfural residues. BioResources 2011, 6, 317–328. [Google Scholar] [CrossRef]
| Material | Alamo Switchgrass (% Dry Basis) |
|---|---|
| Glucan | 36.7 ± 0.37 |
| Xylan | 21.5 ± 0.31 |
| Arabinan | 2.7 ± 0.01 |
| Galactan | 0.8 ± 0.02 |
| Lignin | - |
| Acid-Soluble Lignin (ASL) | 2.6 ± 0.02 |
| Acid-Insoluble Lignin (AIL) | 21.7 ± 0.27 |
| Extractives | 3.2 ± 0.48 |
| Ash | 2.8 ± 0.05 |
| Parameter | Time (min) | NaOH Concentration (%) | ||
|---|---|---|---|---|
| 0.5 | 1.0 | 1.5 | ||
| Solid Recovery (wt %) 1 | 15 | 82.3 ± 1.3 | 69.8 ± 2.2 | 65.9 ± 1.1 |
| 30 | 80.9 ± 1.3 | 68.3 ± 1.0 | 62.8 ± 0.6 | |
| 60 | 79.0 ± 3.1 | 66.0 ± 2.1 | 61.6 ± 0.5 | |
| Glucan (wt %) 2 | 15 | 39.6 ± 3.6 | 36.7 ± 1.5 | 36.7 ± 0.6 |
| 30 | 39.3 ± 1.5 | 37.7 ± 1.5 | 35.4 ± 2.8 | |
| 60 | 38.1 ± 3.0 | 36.5 ± 3.6 | 34.5 ± 2.2 | |
| Xylan (wt %) 2 | 15 | 22.6 ± 2.0 | 18.7 ± 0.7 | 16.0 ± 0.3 |
| 30 | 22.0 ± 1.0 | 18.7 ± 0.9 | 16.3 ± 1.3 | |
| 60 | 20.6 ± 1.3 | 17.7 ± 1.9 | 15.3 ± 1.1 | |
| Lignin (wt %) 2 | 15 | 13.7 ± 0.6 | 7.1 ± 0.9 | 4.3 ± 0.3 |
| 30 | 11.4 ± 0.7 | 6.6 ± 0.8 | 3.7 ± 0.5 | |
| 60 | 10.8 ± 0.3 | 4.8 ± 0.2 | 3.8 ± 0.2 | |
| Delignification (wt %) 3 | 15 | 44.0 ± 2.3 | 71.0 ± 3.7 | 82.5 ± 1.4 |
| 30 | 53.5 ± 2.7 | 72.9 ± 3.2 | 84.6 ± 1.9 | |
| 60 | 55.9 ± 1.3 | 80.2 ± 0.8 | 84.3 ± 0.9 | |
| Time, min | Conc., % | S/G Ratio | H/G Ratio | S:G:H | Total Yield 1, % |
|---|---|---|---|---|---|
| Untreated Sample | 0.62 ± 0.02 | 0.48 ± 0.03 | 29:48:23 | 26.1 ± 2.1 | |
| 15 | 0.5 | 0.64 ± 0.01 | 0.37 ± 0.03 | 32:50:18 | 27.5 ± 0.7 |
| 1.0 | 0.61 ± 0.02 | 0.26 ± 0.01 | 33:53:14 | 31.6 ± 0.3 | |
| 1.5 | 0.65 ± 0.01 | 0.18 ± 0.02 | 35:55:10 | 39.9 ± 1.8 | |
| 30 | 0.5 | 0.75 ± 0.02 | 0.29 ± 0.03 | 37:49:14 | 20.4 ± 2.9 |
| 1.0 | 0.66 ± 0.03 | 0.19 ± 0.03 | 36:54:10 | 17.8 ± 0.8 | |
| 1.5 | 0.73 ± 0.03 | 0.14 ± 0.01 | 39:54:7 | 26.2 ± 0.8 | |
| 60 | 0.5 | 0.72 ± 0.03 | 0.28 ± 0.02 | 36:50:14 | 25.7 ± 2.8 |
| 1.0 | 0.69 ± 0.01 | 0.18 ± 0.01 | 37:53:10 | 30.1 ± 3.1 | |
| 1.5 | 0.68 ± 0.05 | 0.14 ± 0.01 | 37:55:8 | 29.1 ± 1.6 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, W.; Savithri, D.; Sharma-Shivappa, R.; Kolar, P. Changes in Lignin Chemistry of Switchgrass due to Delignification by Sodium Hydroxide Pretreatment. Energies 2018, 11, 376. https://doi.org/10.3390/en11020376
Jung W, Savithri D, Sharma-Shivappa R, Kolar P. Changes in Lignin Chemistry of Switchgrass due to Delignification by Sodium Hydroxide Pretreatment. Energies. 2018; 11(2):376. https://doi.org/10.3390/en11020376
Chicago/Turabian StyleJung, Woochul, Dhanalekshmi Savithri, Ratna Sharma-Shivappa, and Praveen Kolar. 2018. "Changes in Lignin Chemistry of Switchgrass due to Delignification by Sodium Hydroxide Pretreatment" Energies 11, no. 2: 376. https://doi.org/10.3390/en11020376
APA StyleJung, W., Savithri, D., Sharma-Shivappa, R., & Kolar, P. (2018). Changes in Lignin Chemistry of Switchgrass due to Delignification by Sodium Hydroxide Pretreatment. Energies, 11(2), 376. https://doi.org/10.3390/en11020376





