A Study of Sewage Sludge Co-Combustion with Australian Black Coal and Shiitake Substrate
Abstract
1. Introduction
2. Materials and Methodology
2.1. Sample Pretreatment
2.2. Fuel Properties
2.3. Thermogravimetric Analysis
2.4. Synergistic Effect Analysis
2.5. Combustion Characteristics Parameters
2.6. Kinetic Parameters Analysis
2.7. Fourier Transform Infrared Spectroscopy
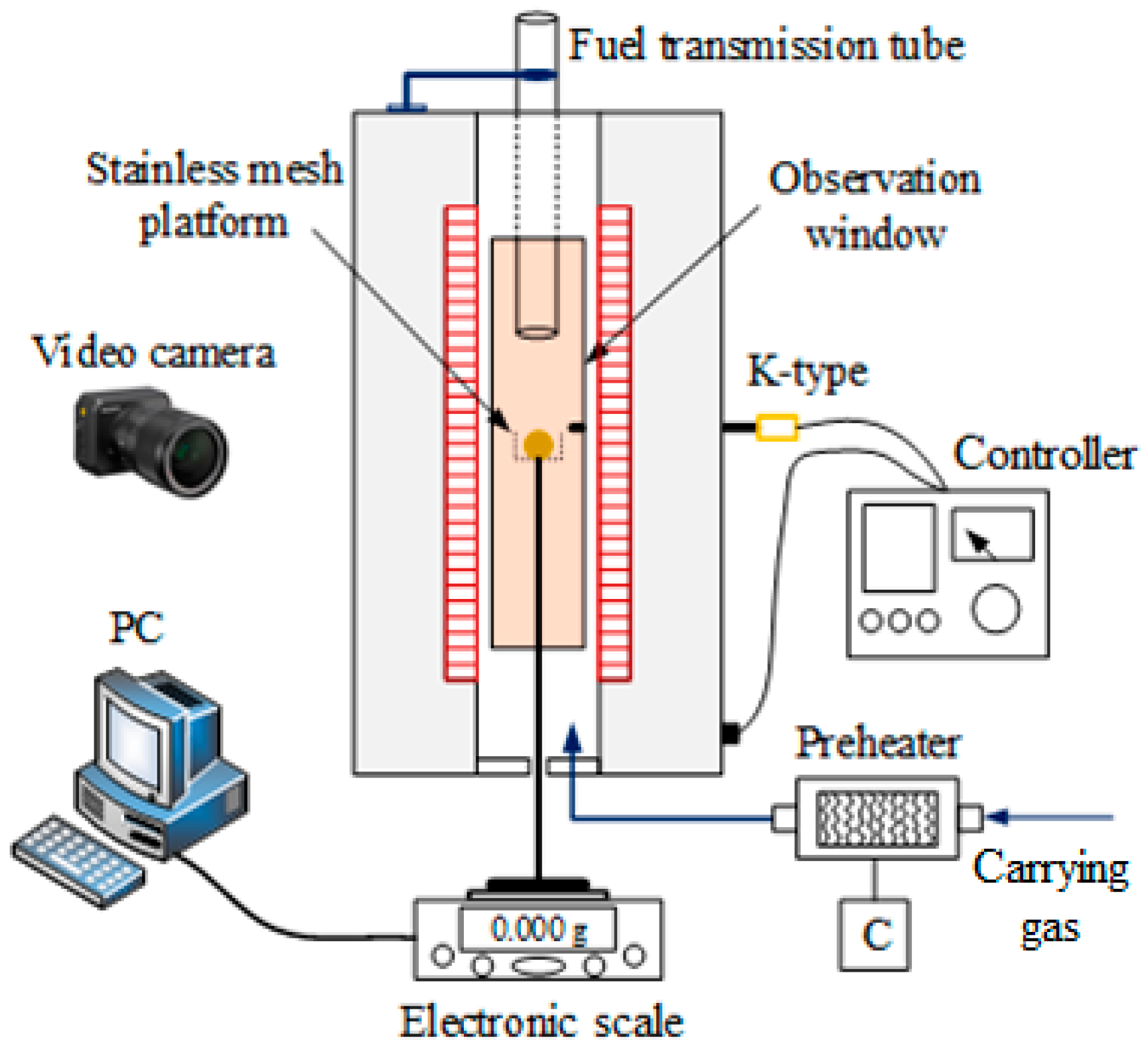
2.8. Single-Pellet Combustion
3. Results and Discussion
3.1. Fuel Properties
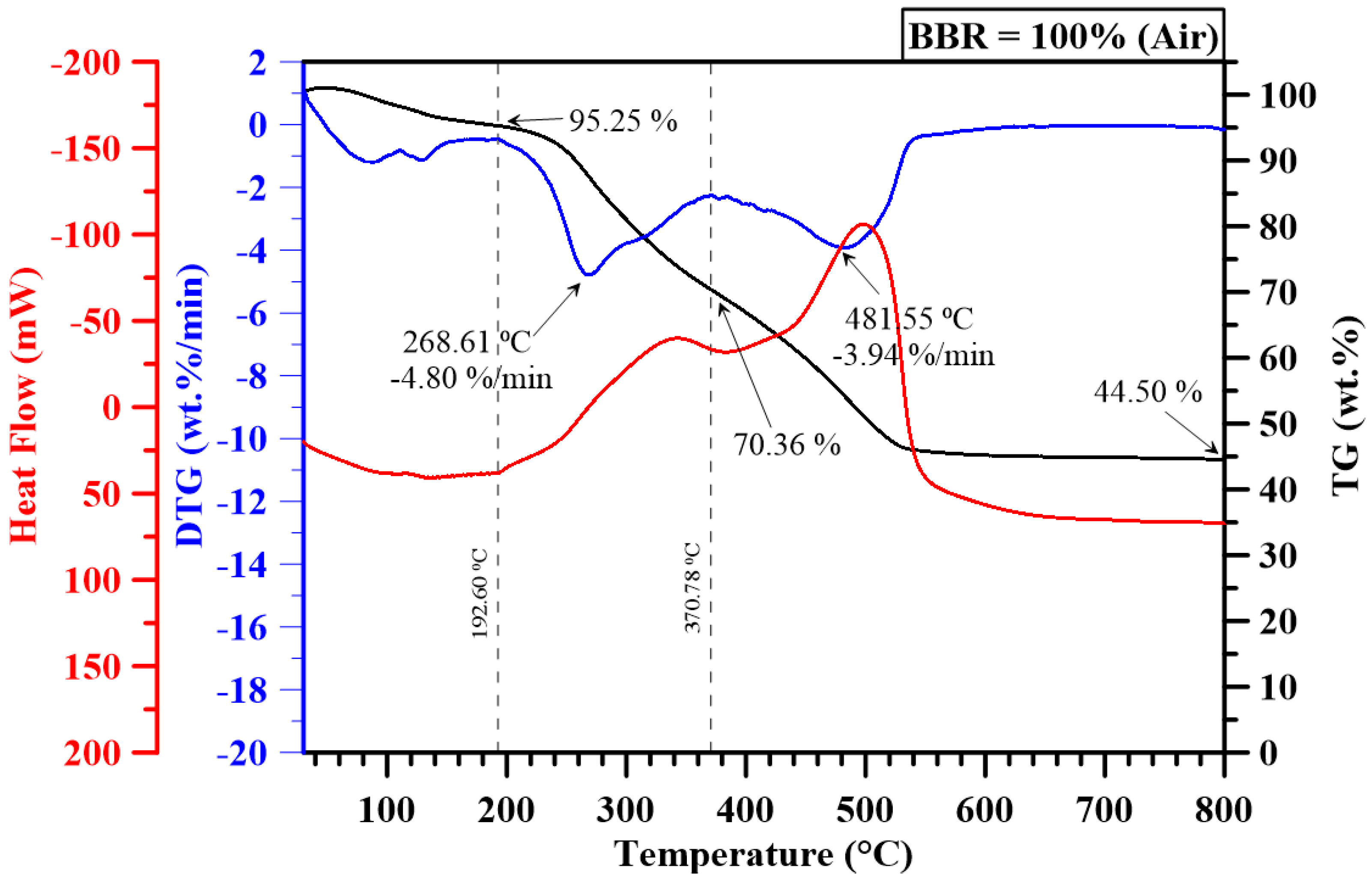
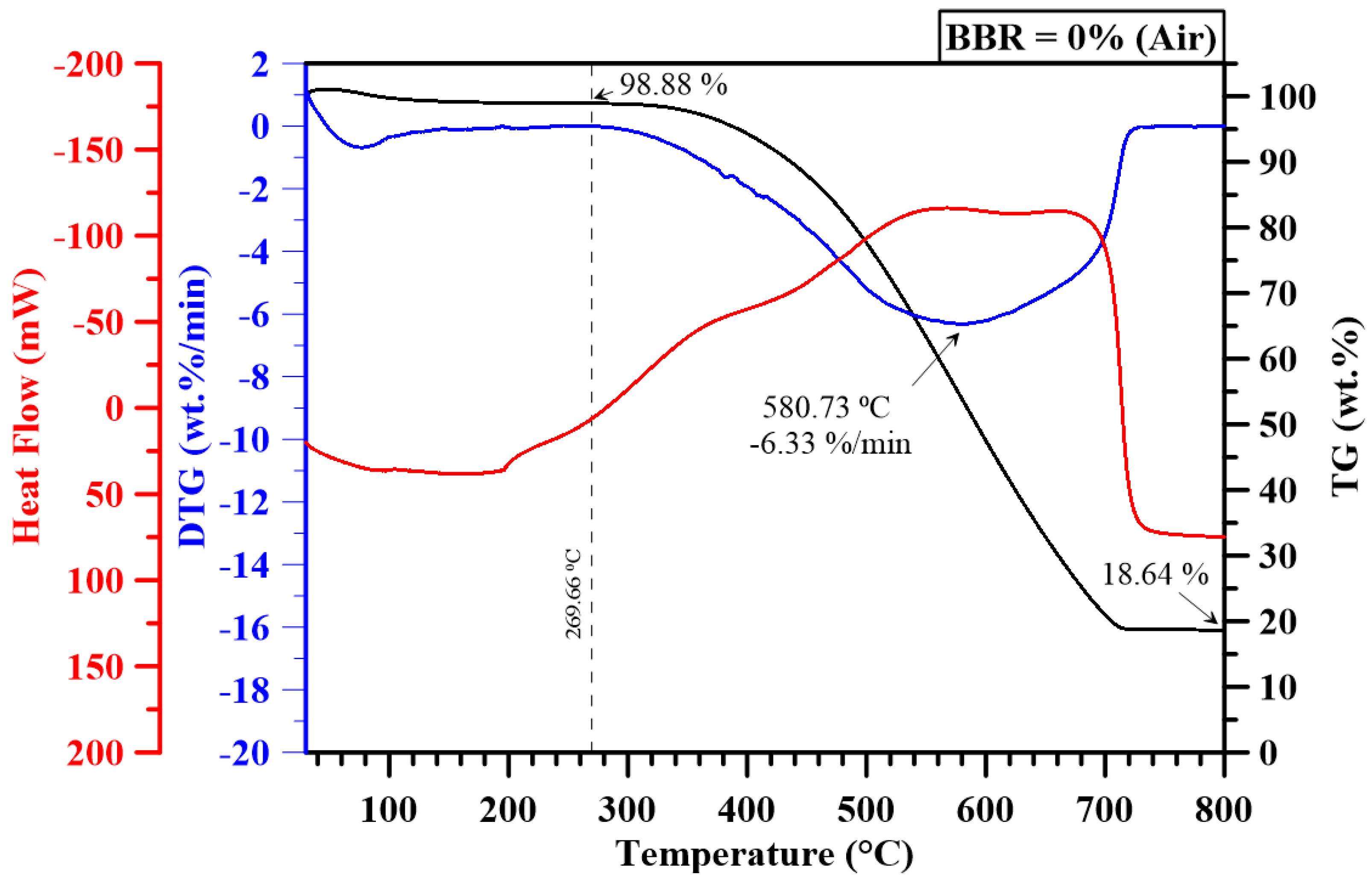
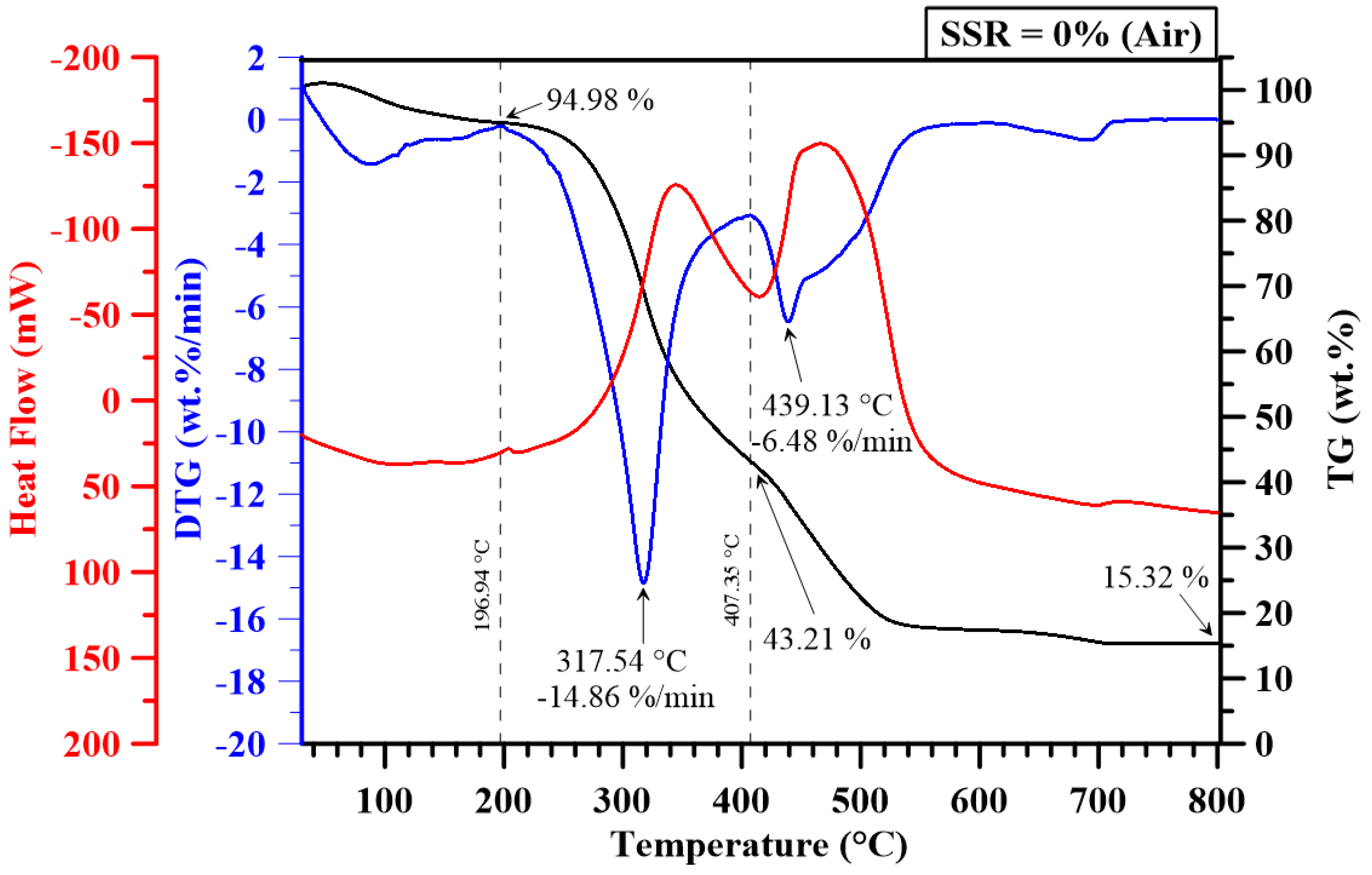
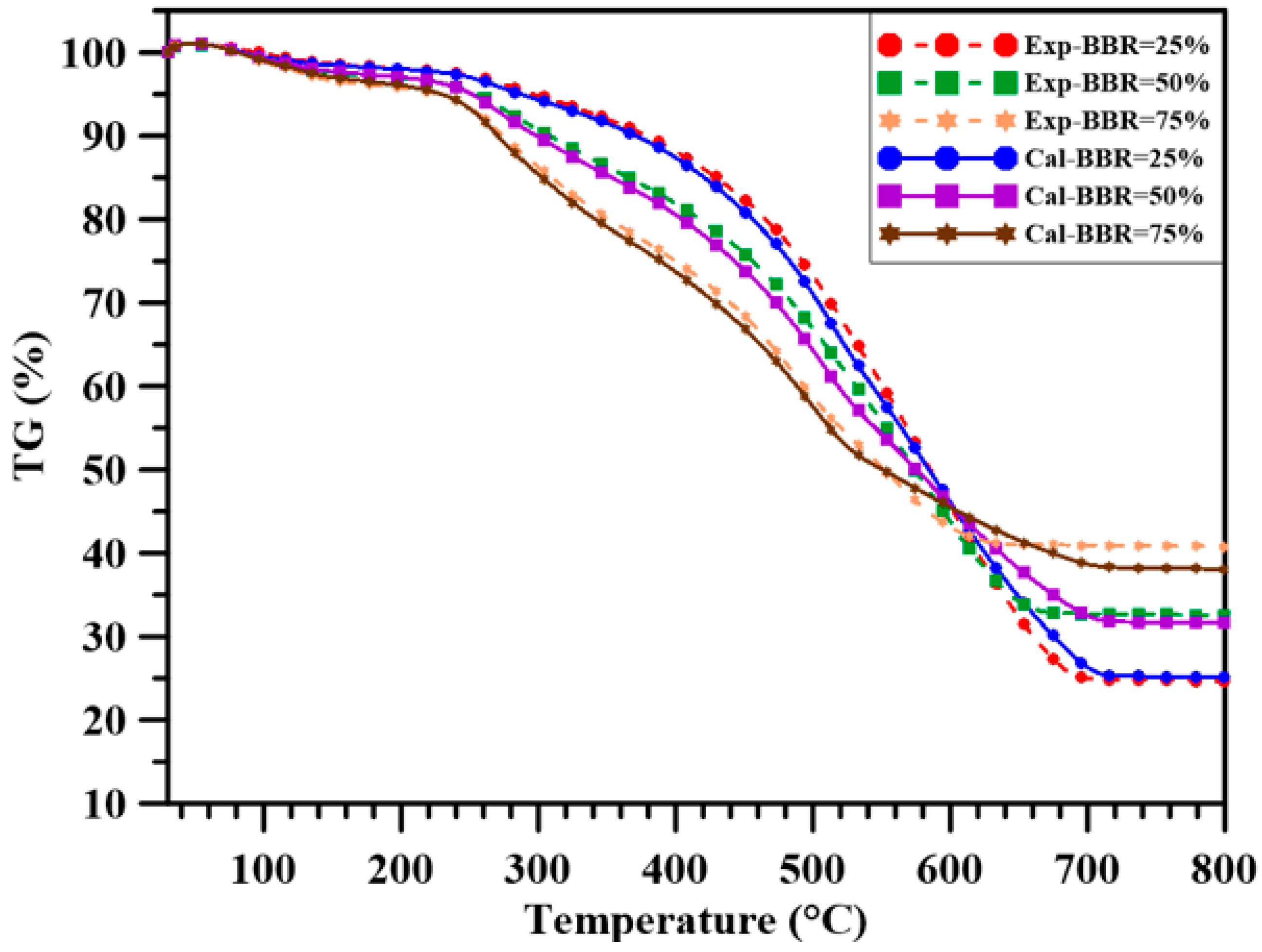
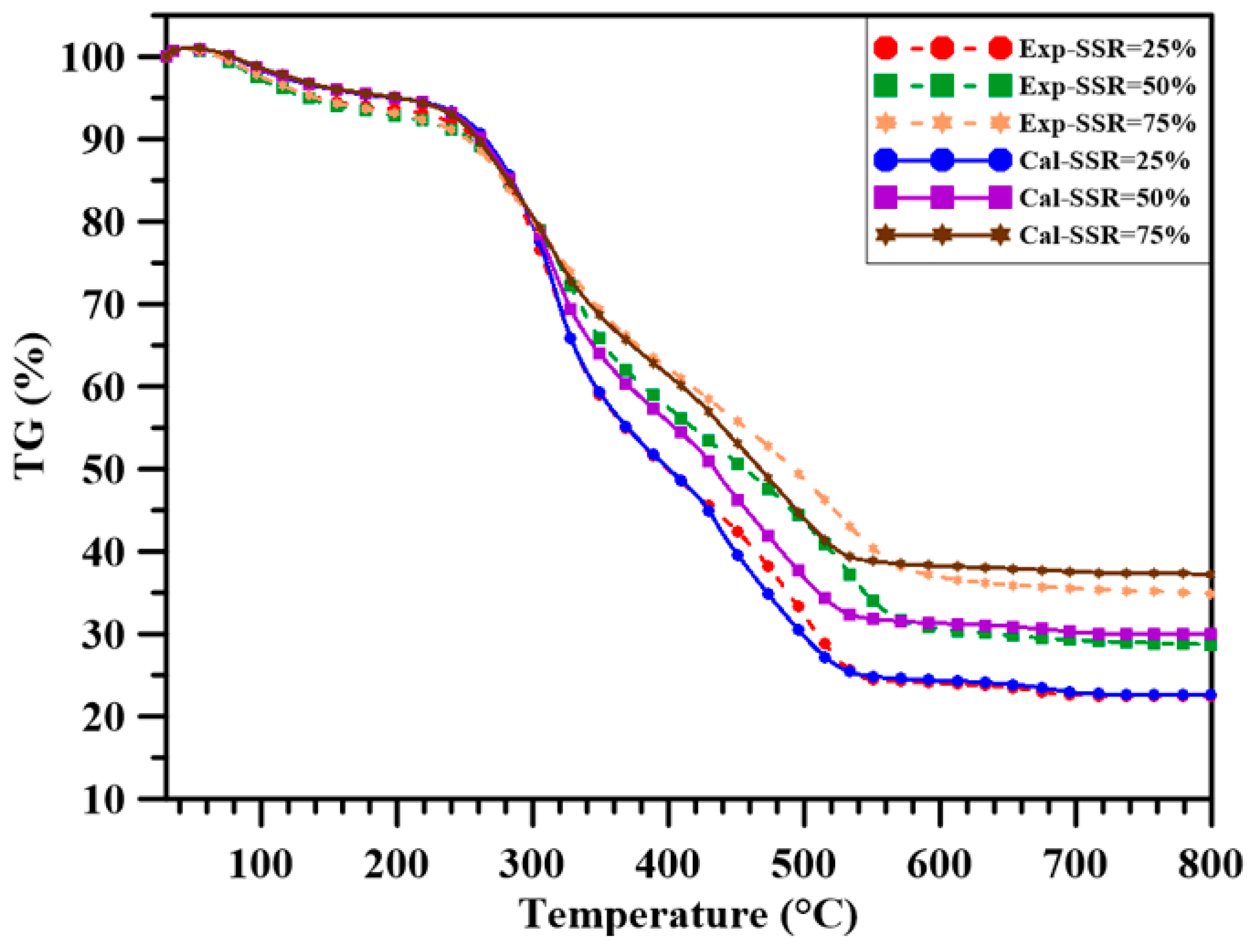
3.2. Thermogravimetric Analysis
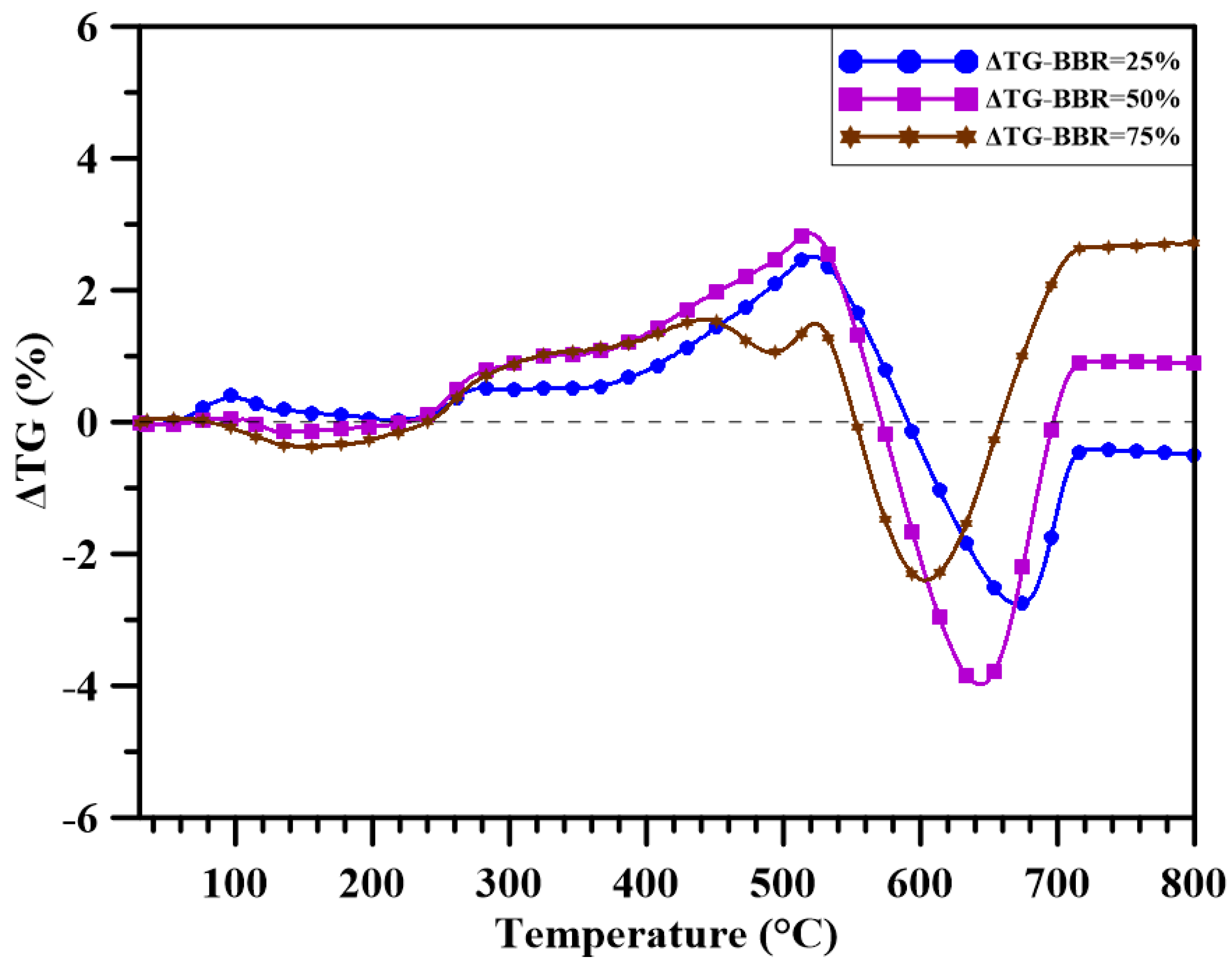
3.3. Synergistic Effect Analysis
3.4. Combustion Characteristics Parameters
3.5. Kinetic Parameters Analysis
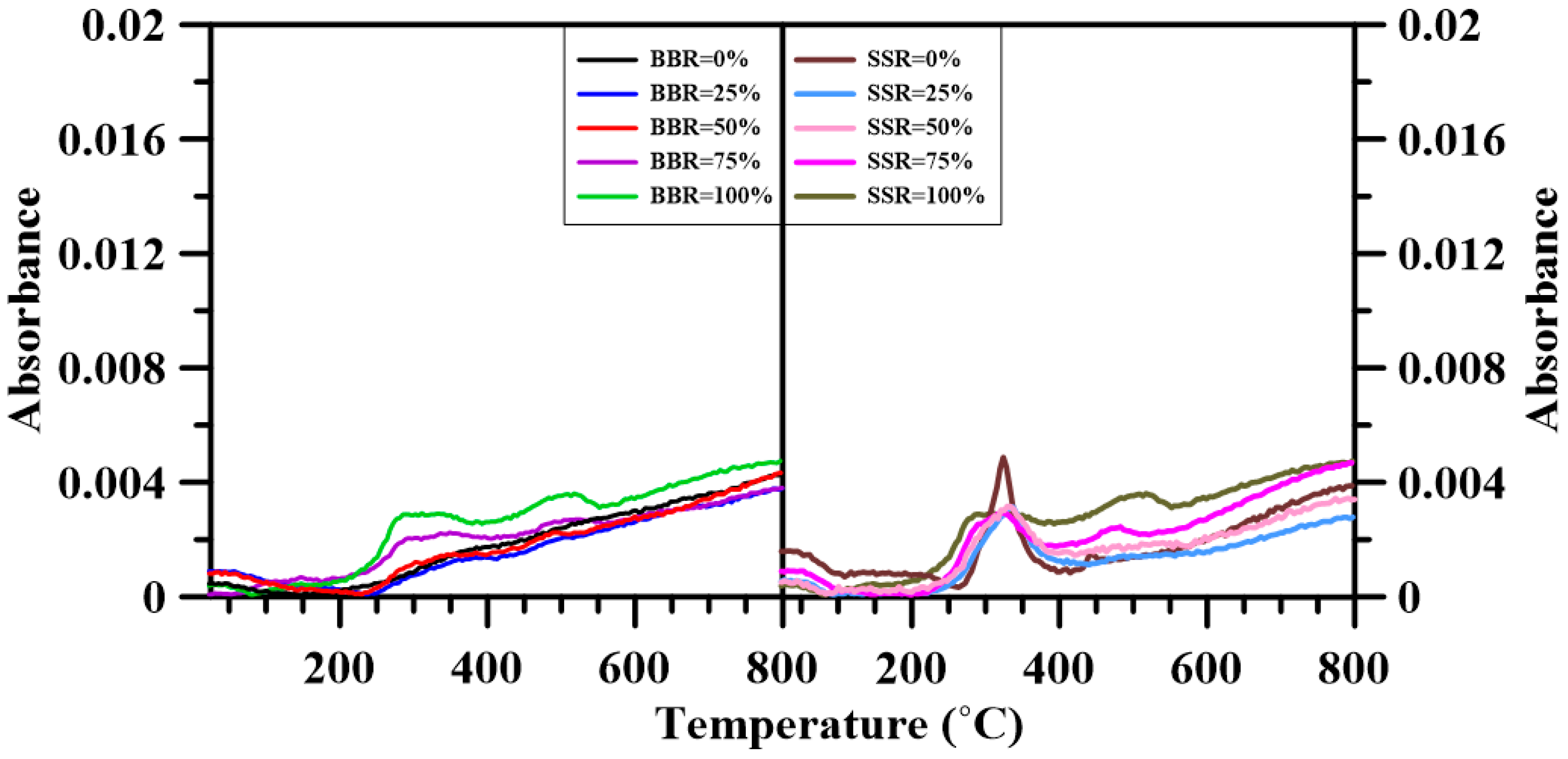
3.6. Fourier Transform Infrared Spectroscopy
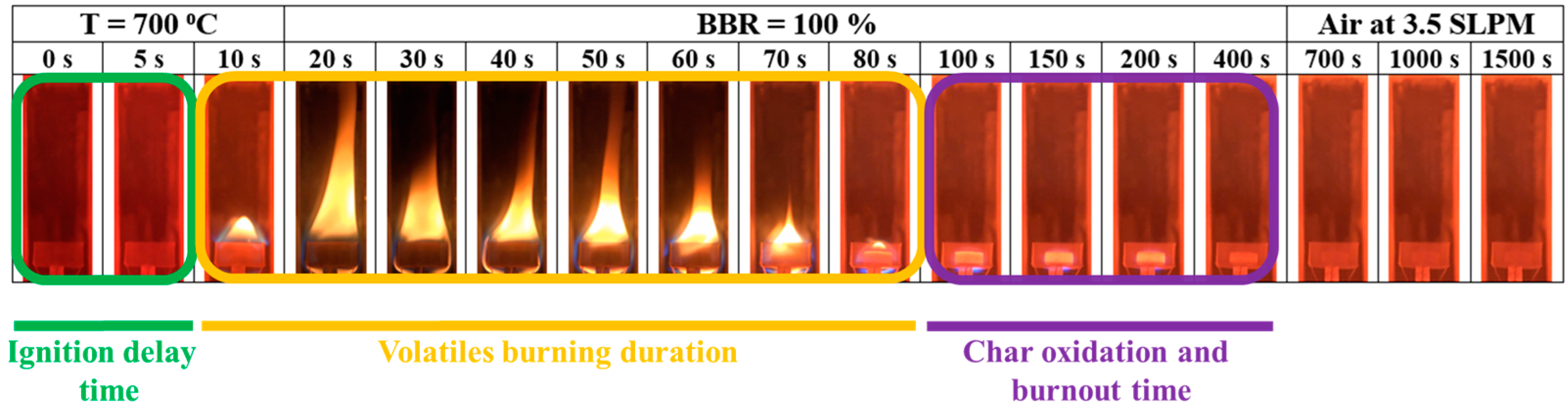
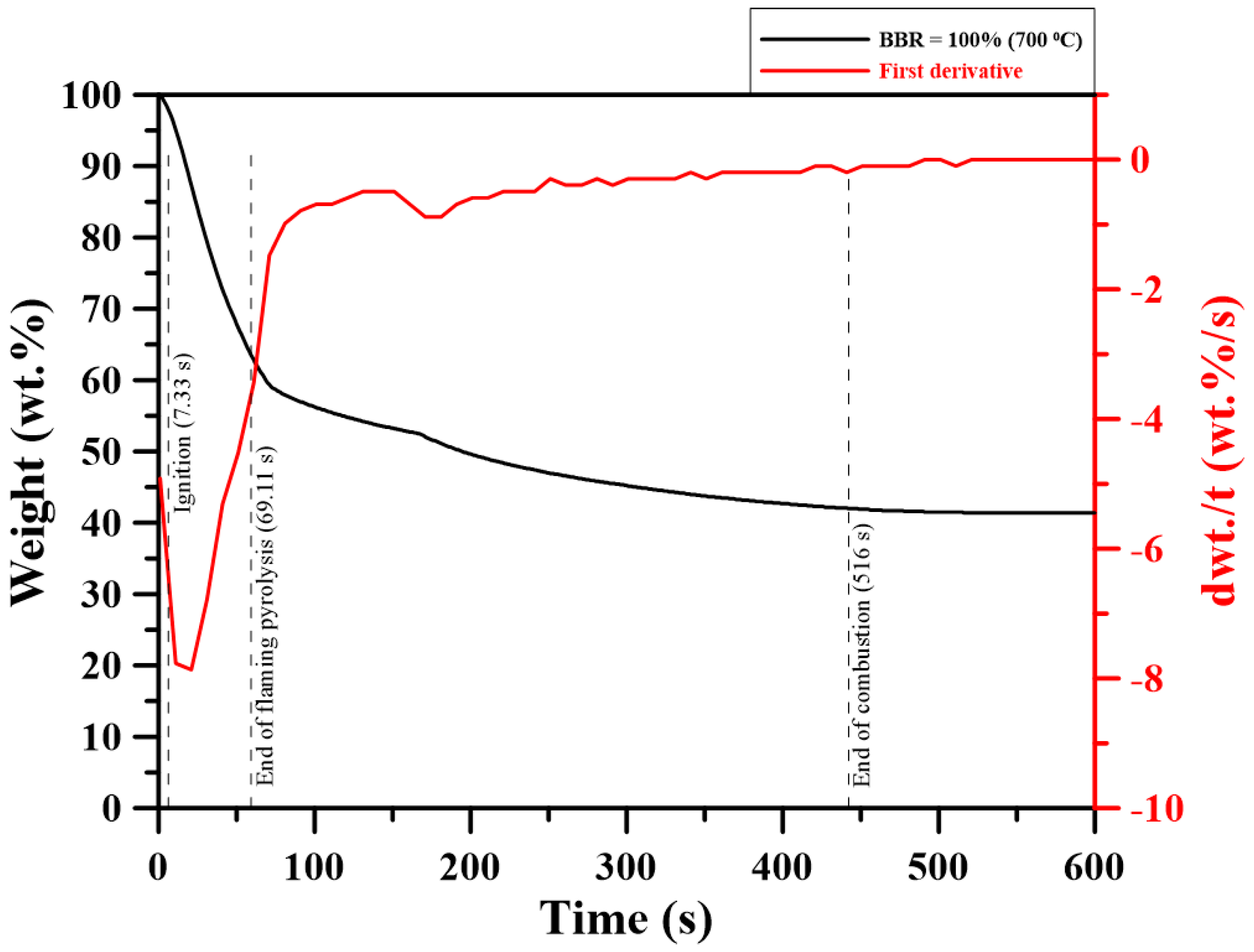
3.7. Single-Pellet Combustion
4. Conclusions
- Compared to both the shiitake substrate and Australian coal, dried sewage sludge has the lowest carbon content and the highest nitrogen and sulfur content. Furthermore, the sludge hydrogen and oxygen content levels are higher than those of Australian black coal, but lower than those of the shiitake substrate. Sewage sludge has the lowest HHV. However, on a dry and ash-free basis, its HHV is similar to that of shiitake. The proximate analysis results show that sewage sludge has the lowest fixed carbon content and the highest ash content on an as-received basis of the fuels. The shiitake substrate has the highest amount of volatiles.
- Both pure sludge and shiitake have higher flammability index and combustion characteristics index in the main decomposition stage compared to those of coal. Sludge addition to coal increased both indexes in stage 1 and a similar observation was made for the sludge-shiitake blends with shiitake addition to the sludge. In the final oxidation stage, adding sludge to the blends decreased their flammability index and combustion characteristics index, due to the low decomposition rate of the heavier and complex compounds in the sludge. For the sludge-shiitake blends, a 25 wt.% sludge addition to the sludge increases both indexes. The flammability index and the combustion characteristics index both decreased for SSR = 50%; and then increased with 75 wt.% shiitake addition to the sludge.
- Synergistic effects occurred for both the sludge-coal blends and sludge-shiitake blends. For all the blends, negative or low synergistic effects existed in the temperature range of 300 and 500 °C. For higher temperature, during the char oxidation stage, positive synergistic effects occurred. The addition of shiitake to the blends had positive synergy for all the blending ratios. However, when the BBR ≥ 50 wt.%, negative synergy occurred at high temperature.
- The results of the kinetic parameters showed that a reduction in both parameters occurred for BBR = 50% during the main oxidation stage. A similar observation was made for the sludge-shiitake blends. The catalytic effect of the sludge and the shiitake is pronounced in the final oxidation stage. A decrease in both the activation energy and the frequency factor occurred with sludge addition to the coal due to the catalytic effect of the inorganic materials in the sludge. A similar observation was made for the sludge-shiitake blends, as the lignin in the substrate catalytically promoted the reactions in the final oxidation stage. However, due to further reactions between the heavier and complex compounds in the samples, both the activation energy and the frequency factor increased with further sludge or shiitake addition to their respective blends (BBR = 75% and SSR = 25%).
- The FTIR results show that adding sludge to the sludge-coal blends decreased both CO2 and CO emissions. A decrease in the emitted CH4, NOx and SO2 was found for BBR = 25%. However, further addition of sludge to the blends increased their respective emissions. For the sludge-shiitake blends, both yielded values of NOx and SO2 decreased with shiitake addition. A 25 wt.% shiitake addition to the sludge (SSR = 75%) increased the amount of emitted CH4, CO and CO2. However, further shiitake addition decreased their respective yields.
- For single-pellet combustion experiments, adding sludge to coal decreased the ignition delay time of the pellet to a certain extent. However, adding sludge to shiitake increased the ignition delay time. Volatile combustion durations of the blends were longer than those of the individual fuels, with BBR = 25%. For other cases, they decreased with sludge addition to the blends. The volatile burning duration decreased with further sludge addition. Increasing the sludge ratio sharply decreased the total combustion time and increased the percentage of residual weight (ash) in the single-pellet combustion experiments.
Author Contributions
Funding
Conflicts of Interest
References
- Environmental Protection Administration, R.O.C. Review of Sludge Treatment Status and Response Strategies; Environmental Protection Administration: Taipei, Taiwan, 2014.
- Lundin, M.; Olofsson, M.; Pettersson, G.; Zetterlund, H. Environmental and economic assessment of sewage sludge handling options. Resour. Conserv. Recycl. 2004, 41, 255–278. [Google Scholar] [CrossRef]
- Muchuweti, M.; Birkett, J.; Chinyanga, E.; Zvauya, R.; Scrimshaw, M.D.; Lester, J. Heavy metal content of vegetables irrigated with mixtures of wastewater and sewage sludge in Zimbabwe: Implications for human health. Agric. Ecosyst. Environ. 2006, 112, 41–48. [Google Scholar] [CrossRef]
- Werther, J.; Ogada, T. Sewage sludge combustion. Prog. Energy Combust. Sci. 1999, 25, 55–116. [Google Scholar] [CrossRef]
- European Union. Directive 2009/28/EC of the European Parliament and of the Council of 23 April 2009 on the promotion of the use of energy from renewable sources and amending and subsequently repealing Directives 2001/77/EC and 2003/30/EC. Off. J. Eur. Union 2009, 5, 2009. [Google Scholar]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G.; Morgan, T.J. An overview of the organic and inorganic phase composition of biomass. Fuel 2012, 94, 1–33. [Google Scholar] [CrossRef]
- García, G.; Arauzo, J.; Gonzalo, A.; Sánchez, J.L.; Ábrego, J. Influence of feedstock composition in fluidised bed co-gasification of mixtures of lignite, bituminous coal and sewage sludge. Chem. Eng. J. 2013, 222, 345–352. [Google Scholar] [CrossRef]
- Vamvuka, D.; Sfakiotakis, S.; Saxioni, S. Evaluation of urban wastes as promising co-fuels for energy production—A TG/MS study. Fuel 2015, 147, 170–183. [Google Scholar] [CrossRef]
- Cui, H.; Ninomiya, Y.; Masui, M.; Mizukoshi, H.; Sakano, T.; Kanaoka, C. Fundamental Behaviors in Combustion of Raw Sewage Sludge. Energy Fuels 2006, 20, 77–83. [Google Scholar] [CrossRef]
- Miller, R.; Bellan, J. Analysis of reaction products and conversion time in the pyrolysis of cellulose and wood particles. Combust. Sci. Technol. 1996, 119, 331–373. [Google Scholar] [CrossRef]
- Koppejan, J.; van Loo, S. The Handbook of Biomass Combustion and Co-Firing; Earthscan: London, UK, 2008. [Google Scholar]
- Rulkens, W. Sewage sludge as a biomass resource for the production of energy: Overview and assessment of the various options. Energy Fuels 2007, 22, 9–15. [Google Scholar] [CrossRef]
- Stasta, P.; Boran, J.; Bebar, L.; Stehlik, P.; Oral, J. Thermal processing of sewage sludge. Appl. Therm. Eng. 2006, 26, 1420–1426. [Google Scholar] [CrossRef]
- Roy, M.M.; Dutta, A.; Corscadden, K.; Havard, P.; Dickie, L. Review of biosolids management options and co-incineration of a biosolid-derived fuel. Waste Manag. 2011, 31, 2228–2235. [Google Scholar] [CrossRef] [PubMed]
- Otero, M.; Díez, C.; Calvo, L.F.; García, A.I.; Morán, A. Analysis of the co-combustion of sewage sludge and coal by TG-MS. Biomass Bioenergy 2002, 22, 319–329. [Google Scholar] [CrossRef]
- Ninomiya, Y.; Zhang, L.; Sakano, T.; Kanaoka, C.; Masui, M. Transformation of mineral and emission of particulate matters during co-combustion of coal with sewage sludge. Fuel 2004, 83, 751–764. [Google Scholar] [CrossRef]
- Folgueras, M.B.; Díaz, R.M.; Xiberta, J.; Prieto, I. Thermogravimetric analysis of the co-combustion of coal and sewage sludge. Fuel 2003, 82, 2051–2055. [Google Scholar] [CrossRef]
- Kijo-Kleczkowska, A.; Środa, K.; Kosowska-Golachowska, M.; Musiał, T.; Wolski, K. Experimental research of sewage sludge with coal and biomass co-combustion, in pellet form. Waste Manag. 2016, 53, 165–181. [Google Scholar] [CrossRef] [PubMed]
- Taiwan Agricultural Research Institute Council of Agriculture, Executive Yuan, Alternative Substrates for Growing Mushrooms. Available online: https://www.tari.gov.tw/english/form/index-1.asp?Parser=20,15,926,81,,,3007 (accessed on 22 July 2015).
- Taiwan Business TOPICS, Fungus among Us—The History of Mushrooms in Taiwan. Available online: https://topics.amcham.com.tw/2017/01/fungus-among-us-history-mushrooms-taiwan/ (accessed on 16 January 2017).
- Cassel, B.; Menard, K.; Shelton, C.; Earnest, C. Proximate Analysis of Coal and Coke Using the STA 8000 Simultaneous Thermal Analyzer; PerkinElmer Application Note; PerkinElmer: Waltham, MA, USA, 2012. [Google Scholar]
- Wu, Z.; Wang, S.; Zhao, J.; Chen, L.; Meng, H. Synergistic effect on thermal behavior during co-pyrolysis of lignocellulosic biomass model components blend with bituminous coal. Bioresour. Technol. 2014, 169, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Sadhukhan, A.K.; Gupta, P.; Goyal, T.; Saha, R.K. Modelling of pyrolysis of coal–biomass blends using thermogravimetric analysis. Bioresour. Technol. 2008, 99, 8022–8026. [Google Scholar] [CrossRef]
- Lu, J.J.; Chen, W.H. Investigation on the ignition and burnout temperatures of bamboo and sugarcane bagasse by thermogravimetric analysis. Appl. Energy 2015, 160, 49–57. [Google Scholar] [CrossRef]
- Chen, G.-B.; Li, Y.-H.; Lan, C.-H.; Lin, H.-T.; Chao, Y.-C. Micro-explosion and burning characteristics of a single droplet of pyrolytic oil from castor seeds. Appl. Therm. Eng. 2017, 114, 1053–1063. [Google Scholar] [CrossRef]
- Jin, Y.; Li, Y.; Liu, F. Combustion effects and emission characteristics of SO2, CO, NOx and heavy metals during co-combustion of coal and dewatered sludge. Front. Environ. Sci. Eng. 2016, 10, 201–210. [Google Scholar] [CrossRef]
- Coats, A.W.; Redfern, J. Kinetic parameters from thermogravimetric data. Nature 1964, 201, 68. [Google Scholar] [CrossRef]
- Gao, N.; Li, A.; Quan, C.; Du, L.; Duan, Y. TG–FTIR and Py–GC/MS analysis on pyrolysis and combustion of pine sawdust. J. Anal. Appl. Pyrolysis 2013, 100, 26–32. [Google Scholar] [CrossRef]
- Xu, T.; Huang, X. Study on combustion mechanism of asphalt binder by using TG–FTIR technique. Fuel 2010, 89, 2185–2190. [Google Scholar] [CrossRef]
- Oudghiri, F.; Allali, N.; Quiroga, J.M.; Rodríguez-Barroso, M.R. TG–FTIR analysis on pyrolysis and combustion of marine sediment. Infrared Phys. Technol. 2016, 78, 268–274. [Google Scholar] [CrossRef]
- Fan, C.; Yan, J.; Huang, Y.; Han, X.; Jiang, X. XRD and TG-FTIR study of the effect of mineral matrix on the pyrolysis and combustion of organic matter in shale char. Fuel 2015, 139, 502–510. [Google Scholar] [CrossRef]
- Mau, V.; Gross, A. Energy conversion and gas emissions from production and combustion of poultry-litter-derived hydrochar and biochar. Appl. Energy 2018, 213, 510–519. [Google Scholar] [CrossRef]
- Wang, C.; Wu, Y.; Liu, Q.; Yang, H.; Wang, F. Analysis of the behaviour of pollutant gas emissions during wheat straw/coal cofiring by TG–FTIR. Fuel Process. Technol. 2011, 92, 1037–1041. [Google Scholar] [CrossRef]
- Yao, Z.; Ma, X.; Wu, Z.; Yao, T. TGA–FTIR analysis of co-pyrolysis characteristics of hydrochar and paper sludge. J. Anal. Appl. Pyrolysis 2017, 123, 40–48. [Google Scholar] [CrossRef]
- Law, C.K. Combustion Physics; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Nassar, M.M. Thermal Analysis Kinetics of Bagasse and Rice Straw. Energy Sources 1998, 20, 831–837. [Google Scholar] [CrossRef]
- Robinson, C.; Smith, D.B. The auto-ignition temperature of methane. J. Hazard. Mater. 1984, 8, 199–203. [Google Scholar] [CrossRef]
- Mc Dougall, I. Chapter 4—Ferroalloys Processing Equipment A2—Gasik, Michael. In Handbook of Ferroalloys; Butterworth-Heinemann: Oxford, UK, 2013; pp. 83–138. [Google Scholar]

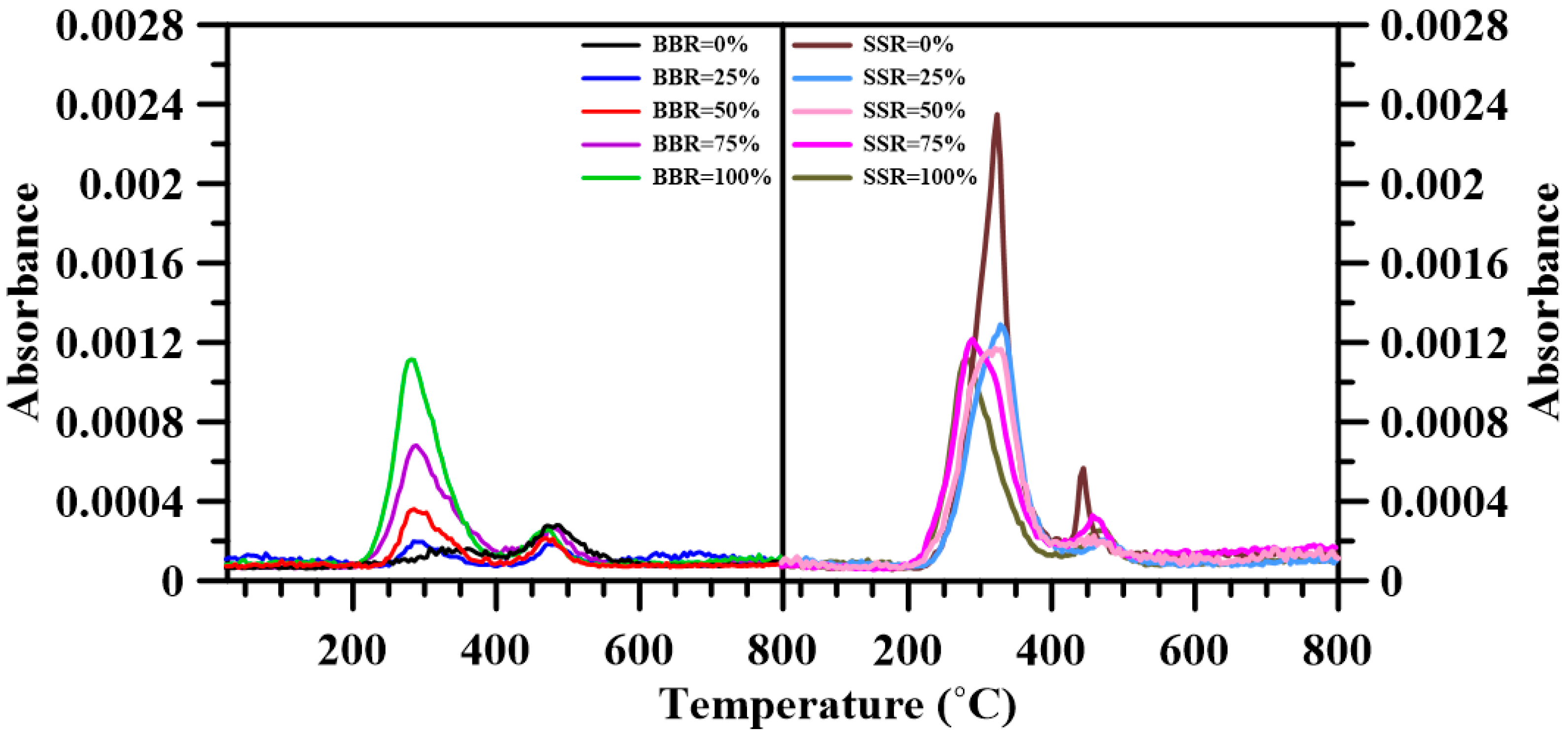
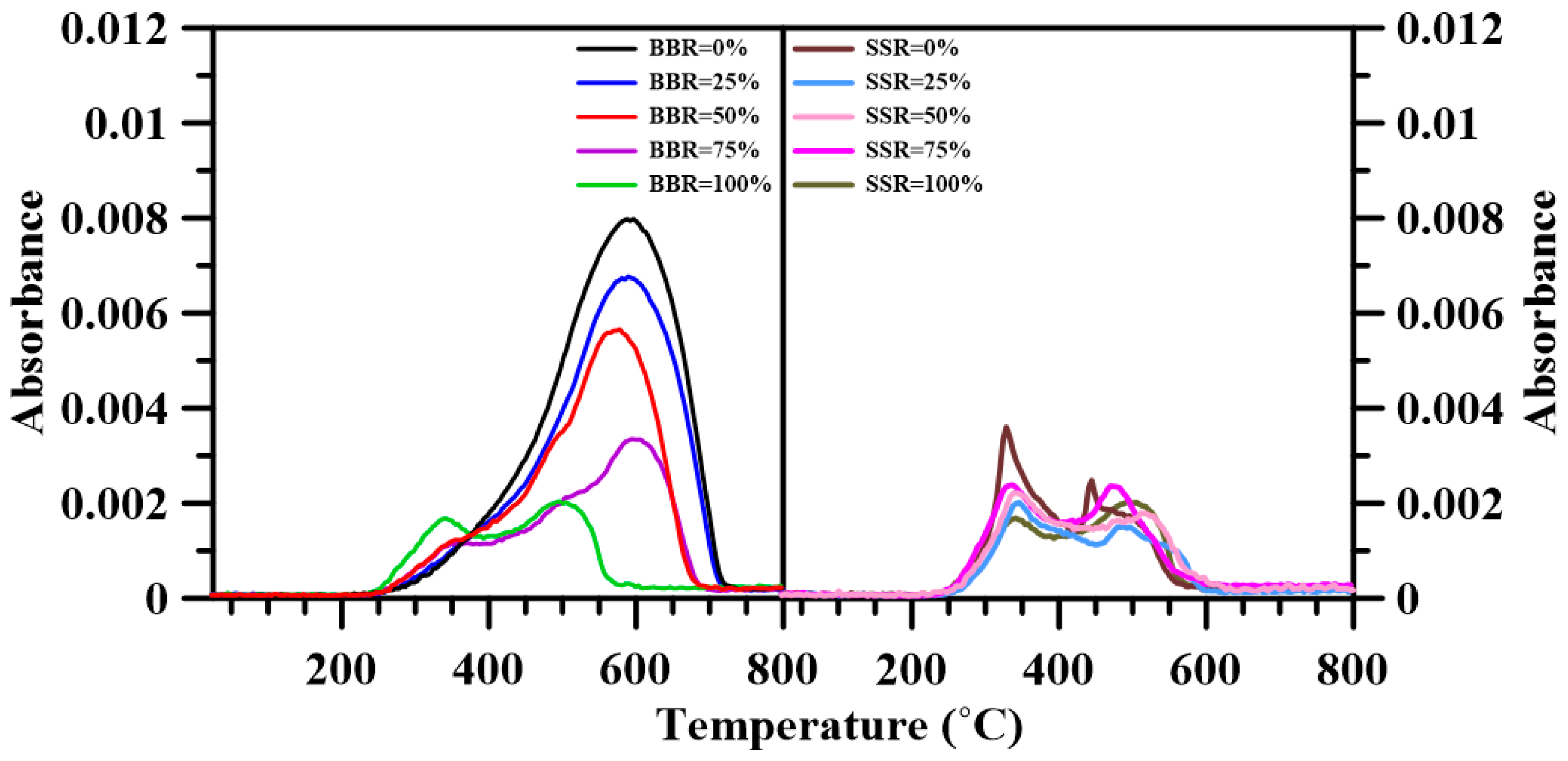
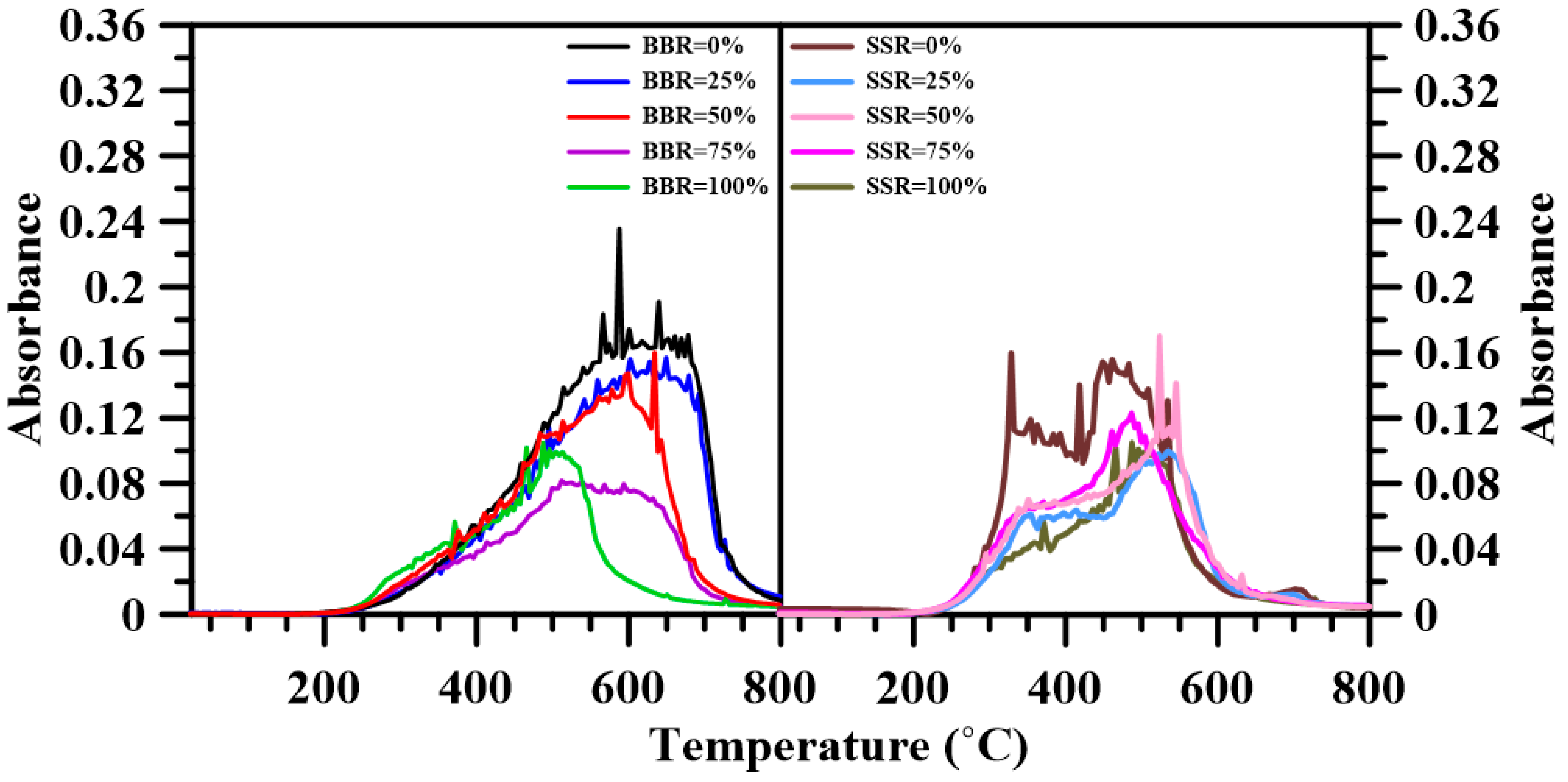
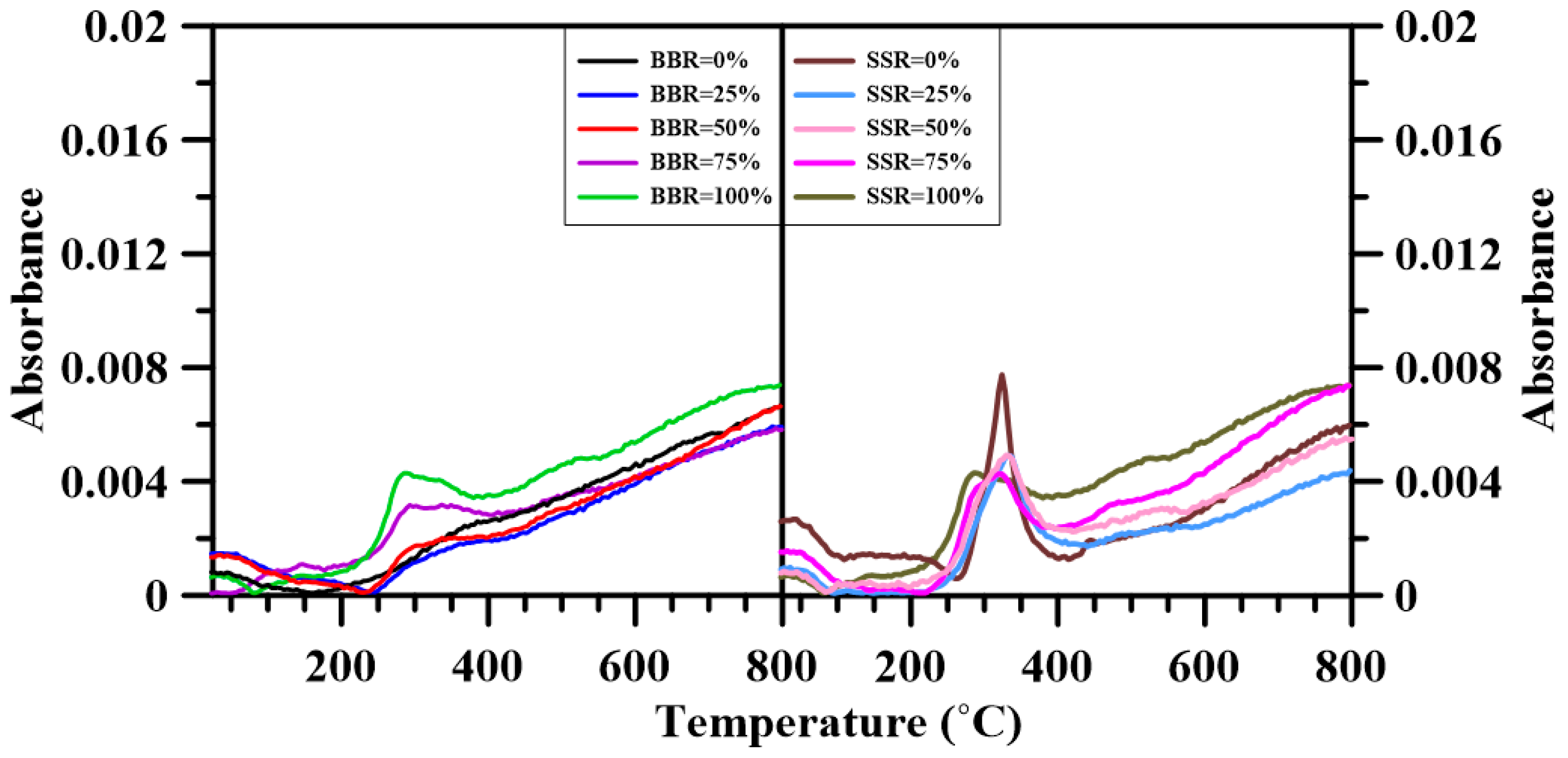
| Gas Species | Wavenumber Range (cm−1) | Functional Groups | Ref. |
|---|---|---|---|
| CH4 | 3000–2700 | C-H | [28] |
| CO2 | 2400–2240 | C=O | [29,30,31] |
| CO | 2240–2060 | C-O | [29,30,31] |
| NOx | 1795–1520 | -NO2 and -NO | [32,33] |
| SO2 | 1374–1342 | S=O | [29,33] |
| Sample | Ultimate Analysis (wt.%) | ||||
| C | H | O | N | S | |
| DSSar | 28.40 | 5.29 | 25.58 | 4.65 | 2.66 |
| Shiitakear | 40.49 | 5.95 | 29.03 | 3.23 | 0.38 |
| Australian coal | 73.10 | 4.30 | 5.27 | 1.65 | 0.48 |
| Sample | Proximate Analysis (wt.%) | HHV (MJ·kg−1) | |||
| M | VM | ASH | FC * | ||
| DSSar | 8.07 | 48.90 | 39.61 | 3.42 | 11.37 |
| DSSdaf | - | 91.50 | - | 8.50 | 21.67 |
| Shiitakear | 13.28 | 69.05 | 13.52 | 4.15 | 15.48 |
| Shiitakedaf | - | 92.83 | - | 7.17 | 21.66 |
| Australian coal | 1.96 | 30.77 | 17.18 | 50.09 | 26.10 |
| BBR % | Ti | Tb | DTGmax | DTGmean | ||||||||
| Ti1 | Ti2 | Tb1 | Tb2 | Stage 1 | Stage 2 | Stage 1 | Stage 2 | C1 | C2 | S1 | S2 | |
| 0 | - | 438.29 | - | 709.15 | - | 6.33 | - | 5.15 | - | 3.30 | - | 2.39 |
| 25 | 247.30 | 458.42 | 306.90 | 693.62 | 1.13 | 5.68 | 0.96 | 4.68 | 1.85 | 2.70 | 0.58 | 1.82 |
| 50 | 240.97 | 462.41 | 357.28 | 665.57 | 2.11 | 4.74 | 1.77 | 3.93 | 3.64 | 2.22 | 1.81 | 1.31 |
| 75 | 238.31 | 446.67 | 367.44 | 629.45 | 3.25 | 4.29 | 2.51 | 2.95 | 5.72 | 2.15 | 3.91 | 1.01 |
| 100 | 239.40 | 439.32 | 368.57 | 633.29 | 4.80 | 3.94 | 3.48 | 1.65 | 8.37 | 2.04 | 7.90 | 0.53 |
| SSR % | Ti | Tb | DTGmax | DTGmean | ||||||||
| Ti1 | Ti2 | Tb1 | Tb2 | Stage 1 | Stage 2 | Stage 1 | Stage 2 | C1 | C2 | S1 | S2 | |
| 0 | 277.43 | 426.59 | 404 | 680.82 | 14.86 | 6.48 | 7.02 | 1.83 | 19.30 | 3.56 | 33.56 | 0.96 |
| 25 | 272.38 | 457.96 | 417.81 | 666.85 | 10.84 | 4.80 | 5.56 | 1.68 | 14.61 | 2.29 | 19.42 | 0.58 |
| 50 | 266.82 | 469.67 | 413.85 | 688.17 | 6.95 | 3.52 | 4.49 | 1.67 | 9.76 | 1.59 | 10.59 | 0.39 |
| 75 | 253.93 | 436.20 | 379.99 | 620.62 | 5.88 | 4.14 | 4.33 | 1.89 | 9.12 | 2.18 | 10.39 | 0.66 |
| 100 | 239.40 | 439.32 | 368.57 | 633.29 | 4.80 | 3.94 | 3.48 | 1.65 | 8.37 | 2.04 | 7.90 | 0.53 |
| BBR % | Temperature Range (°C) | E (kJ·mol−1) | A (s−1) | N | R2 |
| 0 | - | - | - | - | - |
| 278–730 | 105.74 | 1.06 × 1013 | 1.20 | 0.971 | |
| 25 | 210–308 | 85.68 | 2.80 × 1012 | 1.50 | 0.991 |
| 309–714 | 59.27 | 4.93 × 109 | 1.20 | 0.994 | |
| 50 | 203–359 | 75.18 | 4.83 × 1011 | 1.50 | 0.969 |
| 360–692 | 54.70 | 3.48 × 109 | 1.20 | 0.993 | |
| 75 | 202–369 | 80.90 | 3.83 × 1012 | 1.50 | 0.950 |
| 370–673 | 70.89 | 1.66 × 1011 | 1.20 | 0.964 | |
| 100 | 200–370 | 80.42 | 6.18 × 1012 | 1.50 | 0.960 |
| 371–624 | 64.89 | 1.07 × 1011 | 1.20 | 0.981 | |
| SSR % | Temperature Range (°C) | E (kJ·mol−1) | A (s−1) | N | R2 |
| 0 | 206–408 | 97.07 | 1.46 × 1014 | 1.50 | 0.972 |
| 408–579 | 62.93 | 8.23 × 1010 | 1.20 | 0.989 | |
| 25 | 206–432 | 87.00 | 1.55 × 1013 | 1.50 | 0.956 |
| 432–562 | 75.30 | 5.97 × 1011 | 1.20 | 0.980 | |
| 50 | 200–417 | 79.25 | 2.98 × 1012 | 1.50 | 0.977 |
| 417–632 | 61.80 | 4.38 × 1010 | 1.20 | 0.983 | |
| 75 | 194–382 | 84.33 | 1.25 × 1013 | 1.50 | 0.982 |
| 383–648 | 61.83 | 6.05 × 1010 | 1.20 | 0.994 | |
| 100 | 200–370 | 80.42 | 6.18 × 1012 | 1.50 | 0.960 |
| 371–624 | 64.89 | 1.07 × 1011 | 1.20 | 0.981 |
| BBR % | CH4 | CO | CO2 | NOx | SO2 |
| 0% | 0.010 | 0.186 | 5.035 | 0.275 | 0.180 |
| 25% | 0.009 | 0.146 | 4.114 | 0.228 | 0.150 |
| 50% | 0.010 | 0.124 | 3.757 | 0.271 | 0.180 |
| 75% | 0.013 | 0.078 | 2.324 | 0.268 | 0.180 |
| 100% | 0.017 | 0.052 | 2.042 | 0.337 | 0.227 |
| SSR % | CH4 | CO | CO2 | NOx | SO2 |
| 0% | 0.023 | 0.060 | 3.492 | 0.259 | 0.166 |
| 25% | 0.018 | 0.049 | 2.466 | 0.203 | 0.131 |
| 50% | 0.019 | 0.055 | 2.598 | 0.240 | 0.152 |
| 75% | 0.021 | 0.061 | 2.639 | 0.296 | 0.195 |
| 100% | 0.017 | 0.052 | 2.042 | 0.337 | 0.227 |
| BBR % | Single Pellet Combustion Characterization at 600 °C | HHV (MJ·kg−1) | |||
| tid (s) | tf (s) | ttot (s) | Ash (wt.%) | ||
| 0 | Indistinct | Indistinct | 1611 ± 44.69 | 19.63 ± 0.87 | 26.10 ± 0.05 |
| 25 | Indistinct | Indistinct | 1371.80 ± 38.71 | 24.01 ± 0.79 | 22.48 ± 0.06 |
| 50 | Indistinct | Indistinct | 1174.20 ± 41.67 | 30.53 ± 0.19 | 18.64 ± 0.18 |
| 75 | Indistinct | Indistinct | 904.50 ± 27.93 | 36.46 ± 0.32 | 15.17 ± 0.03 |
| 100 | 26.27 ± 4.82 | 73.92 ± 2.07 | 615.6 ± 39.60 | 41.86 ± 0.82 | 11.91 ± 0.05 |
| BBR % | Single Pellet Combustion Characterization at 700 °C | HHV (MJ·kg−1) | |||
| tid (s) | tf (s) | ttot (s) | Ash (wt.%) | ||
| 0 | 9.04 ± 0.66 | 75.94 ± 1.83 | 1634.80 ± 41.89 | 18.23 ± 1.22 | 26.10 ± 0.05 |
| 25 | 7.22 ± 0.49 | 83.89 ± 2.38 | 1517.40 ± 100.34 | 23.94 ± 0.23 | 22.48 ± 0.06 |
| 50 | 6.71 ± 0.28 | 82.23 ± 0.10 | 1179.80 ± 82.01 | 30.19 ± 0.58 | 18.64 ± 0.18 |
| 75 | 7.14 ± 0.42 | 80.62 ± 2.06 | 894 ± 22.77 | 34.96 ± 0.94 | 15.17 ± 0.03 |
| 100 | 7.60 ± 0.39 | 69.03 ± 0.48 | 519.40 ± 19.70 | 41.76 ± 1.14 | 11.91 ± 0.05 |
| BBR % | Single Pellet Combustion Characterization at 800 °C | HHV (MJ·kg−1) | |||
| tid (s) | tf (s) | ttot (s) | Ash (wt.%) | ||
| 0 | 3.67 ± 0.32 | 73.30 ± 1.20 | 1534.60 ± 29.12 | 17.58 ± 0.51 | 26.10 ± 0.05 |
| 25 | 2.75 ± 0.21 | 76.88 ± 1.59 | 1223.40 ± 36.59 | 23.28 ± 0.46 | 22.48 ± 0.06 |
| 50 | 2.47 ± 0.18 | 75.88 ± 0.96 | 1031.80 ± 27.68 | 29.12 ± 1.08 | 18.64 ± 0.18 |
| 75 | 3.04 ± 0.19 | 68.47 ± 0.54 | 783.60 ± 41.21 | 34.39 ± 0.44 | 15.17 ± 0.03 |
| 100 | 3.47 ± 0.07 | 61.49 ± 2.09 | 458 ± 21.62 | 40.90 ± 1.15 | 11.91 ± 0.05 |
| SSR % | Single Pellet Combustion Characterization at 600 °C | HHV (MJ·kg−1) | |||
| tid (s) | tf (s) | ttot (s) | Ash (wt.%) | ||
| 0 | 19.52 ± 3.61 | 86.89 ± 3.01 | 689.6 ± 12.92 | 13.43 ± 1.06 | 16.65 ± 0.06 |
| 25 | 27.10 ± 0.96 | 77.64 ± 0.65 | 646.40 ± 19.74 | 22.95 ± 0.49 | 15.20 ± 0.01 |
| 50 | 34.94 ± 0.51 | 68.53 ± 1.00 | 618.40 ± 22.42 | 29.82 ± 0.63 | 14.03 ± 0.06 |
| 75 | 33.47 ± 6.22 | 67.12 ± 5.53 | 597.40 ± 29.21 | 37.40 ± 0.28 | 12.69 ± 0.02 |
| 100 | 26.27 ± 4.82 | 73.92 ± 2.07 | 615.6 ± 39.60 | 41.86 ± 0.82 | 11.91 ± 0.05 |
| SSR % | Single Pellet Combustion Characterization at 700 °C | HHV (MJ·kg−1) | |||
| tid (s) | tf (s) | tid (s) | Ash (wt.%) | ||
| 0 | 5.36 ± 0.59 | 84.91 ± 1.65 | 602.20 ± 21.01 | 13.70 ± 0.61 | 16.65 ± 0.06 |
| 25 | 5.48 ± 0.44 | 79.88 ± 2.43 | 599.40 ± 33.28 | 22 ± 0.43 | 15.20 ± 0.01 |
| 50 | 6.69 ± 0.54 | 78.76 ± 1.06 | 578.20 ± 30.72 | 28.53 ± 1.08 | 14.03 ± 0.06 |
| 75 | 7.54 ± 0.51 | 73.72 ± 1.06 | 572.80 ±13.14 | 36.42 ± 1.10 | 12.69 ± 0.02 |
| 100 | 7.60 ± 0.39 | 69.03 ± 0.48 | 519.40 ± 19.70 | 41.76 ± 1.14 | 11.91 ± 0.05 |
| SSR % | Single Pellet Combustion Characterization at 800 °C | HHV (MJ·kg−1) tf (s) | |||
| tid (s) | tf (s) | ttot (s) | tid (s) | ||
| 0 | 2.16 ± 0.18 | 77.53 ± 1.20 | 0 | 2.16 ± 0.18 | 77.53 ± 1.20 |
| 25 | 2.27 ± 0.50 | 76.60 ± 1.21 | 25 | 2.27 ± 0.50 | 76.60 ± 1.21 |
| 50 | 3.00 ± 0.04 | 72.41 ± 0.07 | 50 | 3.00 ± 0.04 | 72.41 ± 0.07 |
| 75 | 3.03 ± 0.34 | 67.17 ± 1.25 | 75 | 3.03 ± 0.34 | 67.17 ± 1.25 |
| 100 | 3.47 ± 0.07 | 61.49 ± 2.03 | 100 | 3.47 ± 0.07 | 61.49 ± 2.03 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, G.-B.; Chatelier, S.; Lin, H.-T.; Wu, F.-H.; Lin, T.-H. A Study of Sewage Sludge Co-Combustion with Australian Black Coal and Shiitake Substrate. Energies 2018, 11, 3436. https://doi.org/10.3390/en11123436
Chen G-B, Chatelier S, Lin H-T, Wu F-H, Lin T-H. A Study of Sewage Sludge Co-Combustion with Australian Black Coal and Shiitake Substrate. Energies. 2018; 11(12):3436. https://doi.org/10.3390/en11123436
Chicago/Turabian StyleChen, Guan-Bang, Samuel Chatelier, Hsien-Tsung Lin, Fang-Hsien Wu, and Ta-Hui Lin. 2018. "A Study of Sewage Sludge Co-Combustion with Australian Black Coal and Shiitake Substrate" Energies 11, no. 12: 3436. https://doi.org/10.3390/en11123436
APA StyleChen, G.-B., Chatelier, S., Lin, H.-T., Wu, F.-H., & Lin, T.-H. (2018). A Study of Sewage Sludge Co-Combustion with Australian Black Coal and Shiitake Substrate. Energies, 11(12), 3436. https://doi.org/10.3390/en11123436





