Co-Digestion of Napier Grass with Food Waste and Napier Silage with Food Waste for Methane Production
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of the Mixture Ratios and Substrate Concentration for Methane Production
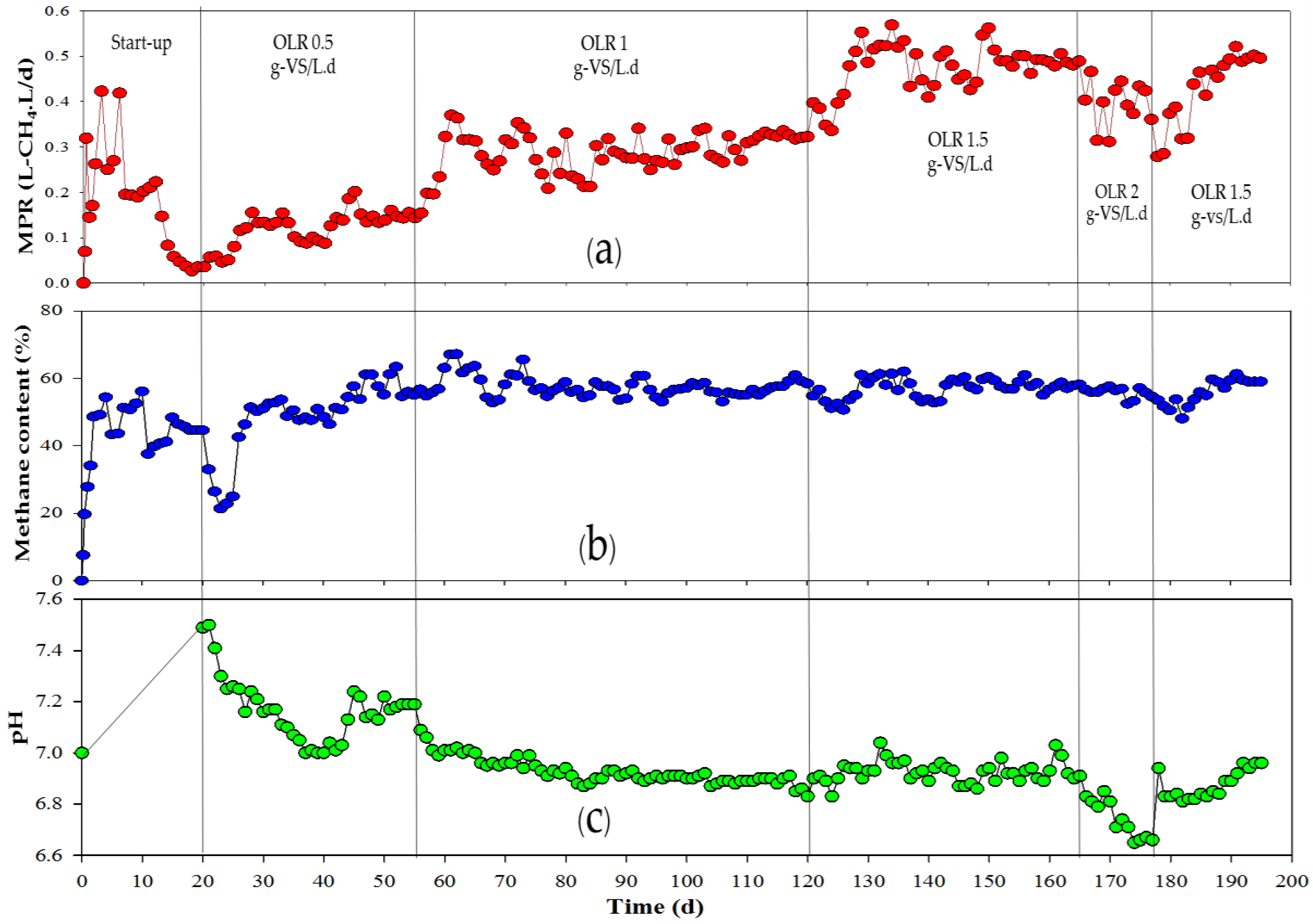
2.2. Repeated Batch Fermentation of Napier Grass Co-Digested with Food Waste
2.3. Energy Production and a Comparison with the Literature Values
2.4. Microbial Community
 CH4 + HCO3− ΔG = −31.0 kJ/mol
CH4 + HCO3− ΔG = −31.0 kJ/mol
 CH4 + 2H2O ΔG = −139 kJ/mol
CH4 + 2H2O ΔG = −139 kJ/mol
3. Material and Methods
3.1. Substrate
3.2. Inoculum
3.3. Optimization of the Mixture Ratios of Napier Grass and Napier Silage to Food Waste and Substrate Concentration for Methane Production
3.4. Repeated Batch Methane Production in the Horizontal Reactor
4. Analytical Methods
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| AD | anaerobic digestion |
| BA | basic anaerobic |
| C/N ratio | carbon to nitrogen ratio |
| COD | chemical oxygen demand |
| DGGE | denaturing gradient gel electrophoresis |
| Eq | equation |
| FW | food waste |
| G | grass |
| GC | gas chromatography |
| G:FW | co-digestion of grass with food waste |
| HPLC | high performance liquid chromatography |
| MP | methane production (mL-CH4/L) |
| MPRmax | maximum methane production rate (mL-CH4/L∙h) |
| MY | methane yield (mL-CH4/g-VSadded) |
| OLR | organic loading rate |
| PCR | polymerase chain reaction |
| rpm | revolutions per minute |
| S | silage |
| S:FW | co-digestion of silage with food waste |
| TCD | thermal conductivity detector |
| TS | total solid |
| VFAs | volatile fatty acids |
| VS | volatile solid |
References
- Ashik, U.P.M.; Daud, W.M.A.W.; Hayashi, J. A review on methane transformation to hydrogen and nanocarbon: Relevance of catalyst characteristics and experimental parameters on yield. Renew. Sustain. Energy Rev. 2017, 76, 743–767. [Google Scholar] [CrossRef]
- Kamaruddin, M.H.; Osman, S.A.; Fawzi, M.; Mustaffa, N.; Tukiman, M.M. Predicting the performances of a CAMPRO engine retrofitted with liquefied petroleum gas (LPG) system using 1-dimensional software. MATEC Web Conf. 2017, 90. [Google Scholar] [CrossRef]
- Suksong, W.; Promnuan, K.; Seengenyoung, J.; Sompong, S. Anaerobic Co-Digestion of Palm Oil Mill Waste Residues with Sewage Sludge for Biogas Production. Energy Procedia 2017, 138, 789–794. [Google Scholar] [CrossRef]
- Saelor, S.; Kongjan, P.; O-Thong, S. Biogas Production from Anaerobic Co-digestion of Palm Oil Mill Effluent and Empty Fruit Bunches. Energy Procedia 2017, 138, 717–722. [Google Scholar] [CrossRef]
- Glanpracha, N.; Annachhatre, A.P. Anaerobic co-digestion of cyanide containing cassava pulp with pig manure. Bioresour. Technol. 2016, 214, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Prapinagsorn, W.; Sittijunda, S.; Reungsang, A. Co-digestion of napier grass and its silage with cow dung for methane production. Energies 2017, 10, 1654. [Google Scholar] [CrossRef]
- Amnuaycheewa, P.; Hengaroonprasan, R.; Rattanaporn, K.; Kirdponpattara, S.; Cheenkachorn, K.; Sriariyanun, M. Enhancing enzymatic hydrolysis and biogas production from rice straw by pretreatment with organic acids. Ind. Crops Prod. 2016, 87, 247–254. [Google Scholar] [CrossRef]
- Pu, Y.; Zhang, D.; Singh, P.M.; Ragauskas, A.J. The new forestry biofuels sector. Biofuels Bioprod. Biorefining 2008, 2, 58–73. [Google Scholar] [CrossRef]
- Department of Alternative Energy Development and Efficiency Napier Grass. Available online: http://weben.dede.go.th/webmax/content/napier-grass (accessed on 27 September 2018).
- Ambye-Jensen, M.; Johansen, K.S.; Didion, T.; Kádár, Z.; Schmidt, J.E.; Meyer, A.S. Ensiling as biological pretreatment of grass (Festulolium Hykor): The effect of composition, dry matter, and inocula on cellulose convertibility. Biomass Bioenergy 2013, 58, 303–312. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.C.; Nissilä, M.E.; Wu, S.Y.; Lin, C.Y.; Puhakka, J.A. Silage as source of bacteria and electrons for dark fermentative hydrogen production. Int. J. Hydrog. Energy 2012, 37, 15518–15524. [Google Scholar] [CrossRef]
- Aryal, N.; Kvist, T.; Ammam, F.; Pant, D.; Ottosen, L.D.M. An overview of microbial biogas enrichment. Bioresour. Technol. 2018, 264, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Chandra, R.; Takeuchi, H.; Hasegawa, T. Methane production from lignocellulosic agricultural crop wastes: A review in context to second generation of biofuel production. Renew. Sustain. Energy Rev. 2012, 16, 1462–1476. [Google Scholar] [CrossRef]
- Paritosh, K.; Kushwaha, S.K.; Yadav, M.; Pareek, N.; Chawade, A.; Vivekanand, V. Food Waste to Energy: An Overview of Sustainable Approaches for Food Waste Management and Nutrient Recycling. Biomed Res. Int. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Kiran, E.U.; Trzcinski, A.P.; Ng, W.J.; Liu, Y. Bioconversion of food waste to energy: A review. Fuel 2014, 134, 389–399. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, Y.; Wang, D.; Chen, F.; Li, X.; Zeng, G.; Yang, Q. Potential impact of salinity on methane production from food waste anaerobic digestion. Waste Manag. 2017, 67, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Liu, G.; Yan, B.; Shan, R.; Wang, J.; Li, T.; Xu, W. Experimental study of co-digestion of food waste and tall fescue for bio-gas production. Renew. Energy 2016, 88, 273–279. [Google Scholar] [CrossRef]
- Kovács, E.; Wirth, R.; Maróti, G.; Bagi, Z.; Nagy, K.; Minárovits, J.; Rákhely, G.; Kovács, K.L. Augmented biogas production from protein-rich substrates and associated metagenomic changes. Bioresour. Technol. 2015, 178, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Angelidaki, I.; Sanders, W. Assessment of the anaerobic biodegradability of macropollutants. Rev. Environ. Sci. Biotechnol. 2004, 3, 117–129. [Google Scholar] [CrossRef] [Green Version]
- Deublein, D.; Steinhauser, A. Biogas from Waste and Renewable Resources: An Introduction; Deublein, D., Steinhauser, A., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2008; ISBN 9783527621705. [Google Scholar]
- Callaghan, F.J.; Wase, D.A.J.; Thayanithy, K.; Forster, C.F. Continuous co-digestion of cattle slurry with fruit and vegetable wastes and chicken manure. Biomass Bioenergy 2002, 22, 71–77. [Google Scholar] [CrossRef]
- Prapinagsorn, W.; Sittijunda, S.; Reungsang, A. Co-digestion of napier grass and its silage with cow dung for bio-hydrogen and methane production by two-stage anaerobic digestion process. Energies 2018, 11. [Google Scholar] [CrossRef]
- González, L.M.L.; Reyes, I.P.; Romero, O.R. Anaerobic co-digestion of sugarcane press mud with vinasse on methane yield. Waste Manag. 2017, 68, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.M.S.; Mahler, C.F.; Oliveira, L.B.; Bassin, J.P. Hydrogen and methane production in a two-stage anaerobic digestion system by co-digestion of food waste, sewage sludge and glycerol. Waste Manag. 2018, 76, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Schievano, A.; Tenca, A.; Lonati, S.; Manzini, E.; Adani, F. Can two-stage instead of one-stage anaerobic digestion really increase energy recovery from biomass? Appl. Energy 2014, 124, 335–342. [Google Scholar] [CrossRef]
- Arreola-Vargas, J.; Flores-Larios, A.; González-Álvarez, V.; Corona-González, R.I.; Méndez-Acosta, H.O. Single and two-stage anaerobic digestion for hydrogen and methane production from acid and enzymatic hydrolysates of Agave tequilana bagasse. Int. J. Hydrog. Energy 2016, 41, 897–904. [Google Scholar] [CrossRef]
- Nualsri, C.; Kongjan, P.; Reungsang, A. Direct integration of CSTR-UASB reactors for two-stage hydrogen and methane production from sugarcane syrup. Int. J. Hydrog. Energy 2016, 41, 17884–17895. [Google Scholar] [CrossRef]
- Yong, Z.; Dong, Y.; Zhang, X.; Tan, T. Anaerobic co-digestion of food waste and straw for biogas production. Renew. Energy 2015, 78, 527–530. [Google Scholar] [CrossRef]
- Dareioti, M.A.; Kornaros, M. Anaerobic mesophilic co-digestion of ensiled sorghum, cheese whey and liquid cow manure in a two-stage CSTR system: Effect of hydraulic retention time. Bioresour. Technol. 2015, 175, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Mamimin, C.; Singkhala, A.; Kongjan, P.; Suraraksa, B.; Prasertsan, P.; Imai, T.; O-Thong, S. Two-stage thermophilic fermentation and mesophilic methanogen process for biohythane production from palm oil mill effluent. Int. J. Hydrog. Energy 2015, 40, 6319–6328. [Google Scholar] [CrossRef]
- Monlau, F.; Kaparaju, P.; Trably, E.; Steyer, J.P.; Carrere, H. Alkaline pretreatment to enhance one-stage CH4 and two-stage H2/CH4 production from sunflower stalks: Mass, energy and economical balances. Chem. Eng. J. 2015, 260, 377–385. [Google Scholar] [CrossRef]
- Maspolim, Y.; Zhou, Y.; Guo, C.; Xiao, K.; Ng, W.J. Comparison of single-stage and two-phase anaerobic sludge digestion systems—Performance and microbial community dynamics. Chemosphere 2015, 140, 54–62. [Google Scholar] [CrossRef] [PubMed]
- dos Reis, C.M.; Carosia, M.F.; Sakamoto, I.K.; Amâncio Varesche, M.B.; Silva, E.L. Evaluation of hydrogen and methane production from sugarcane vinasse in an anaerobic fluidized bed reactor. Int. J. Hydrog. Energy 2015, 40, 8498–8509. [Google Scholar] [CrossRef]
- Venkiteshwaran, K.; Bocher, B.; Maki, J.; Zitomer, D. Relating Anaerobic Digestion Microbial Community and Process Function. Microbiol. Insights 2016, 8, 37. [Google Scholar] [CrossRef] [PubMed]
- Demirel, B.; Scherer, P. The roles of acetotrophic and hydrogenotrophic methanogens during anaerobic conversion of biomass to methane: A review. Rev. Environ. Sci. Biotechnol. 2008, 7, 173–190. [Google Scholar] [CrossRef]
- Owen, W.F.; Stuckey, D.C.; Healy, J.B.; Young, L.Y.; McCarty, P.L. Bioassay for monitoring biochemical methane potential and anaerobic toxicity. Water Res. 1979, 13, 485–492. [Google Scholar] [CrossRef]
- American Public Health Association Standard Methods for the Examination of Water and Wastewater, 19th ed.; American Public Health Association: Washington, WA, USA, 1995; ISBN 0875532292 (Supplement) 0875532233.
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass: Laboratory Analytical Procedure (LAP). Available online: http://www.academia.edu/download/34289991/Determination-Structural-Carbohydrates-L....pdf (accessed on 20 August 2018).
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rerbers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.K.; Suh, W.I.; Farooq, W.; Moon, M.; Shrivastav, A.; Park, M.S.; Yang, J.W. Rapid quantification of microalgal lipids in aqueous medium by a simple colorimetric method. Bioresour. Technol. 2014, 155, 330–333. [Google Scholar] [CrossRef] [PubMed]
- Laocharoen, S.; Reungsang, A.; Plangklang, P. Bioaugmentation of Lactobacillus delbrueckii ssp. bulgaricus TISTR 895 to enhance bio-hydrogen production of Rhodobacter sphaeroides KKU-PS5. Biotechnol. Biofuels 2015, 8, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Kongjan, P.; O-Thong, S.; Kotay, M.; Min, B.; Angelidaki, I. Biohydrogen production from wheat straw hydrolysate by dark fermentation using extreme thermophilic mixed culture. Biotechnol. Bioeng. 2010, 105, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic. Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [PubMed]
- Rutz, D.; Janssen, R. Biofuel Technology Handbook; WIP Renewable Energies: München, Germany, 2007; ISBN 978-1-57444-703-3. [Google Scholar]


| Ratio (g-VS/g-VS/) | C/N ratio | MPRmax (mL-CH4/L∙h) | MP (mL-CH4/L) | MY* (mL-CH4/g-VS) | Final pH |
|---|---|---|---|---|---|
| G:FW | |||||
| 4.75:0.25 | 51.92 | 3.0 ± 0.1 | 1229.6 ± 13.8 | 246e ± 2.8 | 7.47 ± 0.01 |
| 4.5:0.5 | 46.83 | 3.2 ± 0.5 | 1291.8 ± 52.4 | 258e ± 10.5 | 7.47 ± 0.01 |
| 4.25:0.75 | 42.58 | 2.7 ± 0.2 | 1478.5 ± 116.2 | 296d ± 23.2 | 7.46 ± 0.01 |
| 4:1 | 38.99 | 2.8 ± 0.2 | 1596.2 ± 129.7 | 319cd ± 25.9 | 7.45 ± 0.01 |
| 3:2 | 28.87 | 5.9 ± 0.7 | 1666.1 ± 127.7 | 333bc ± 25.5 | 7.44 ± 0.02 |
| 2.5:2.5 | 25.40 | 3.5 ± 0.7 | 1664.8 ± 15.6 | 333bc ± 15.6 | 7.45 ± 0.01 |
| 2:3 | 22.60 | 7.3 ± 0.2 | 1802.6 ± 57.2 | 361b ± 11.4 | 7.45 ± 0.01 |
| 1:4 | 18.35 | 8.3 ± 0.3 | 2053.7 ± 8.8 | 411a ± 1.8 | 7.46 ± 0.01 |
| S:FW | |||||
| 4.75:0.25 | 42.91 | 2.3 ± 0.6 | 1000.6 ± 2.5 | 200f ± 0.5 | 7.41 ± 0.01 |
| 4.5:0.5 | 39.50 | 2.3 ± 0.1 | 1294.7 ± 3.1 | 259e ± 0.6 | 7.44 ± 0.01 |
| 4.25:0.75 | 36.56 | 1.7 ± 0.1 | 1570.4 ± 11.8 | 314d ± 2.4 | 7.43 ± 0.01 |
| 4:1 | 34.00 | 2.5 ± 0.4 | 1610.6 ± 89.5 | 322cd ± 17.9 | 7.43 ± 0.02 |
| 3:2 | 26.41 | 3.2 ± 0.1 | 1808.2 ± 17.5 | 362b ± 3.5 | 7.41 ± 0.03 |
| 2.5:2.5 | 23.68 | 3.1 ± 0.3 | 1701.7 ± 3.6 | 340c ± 0.7 | 7.43 ± 0.01 |
| 2:3 | 21.42 | 4.1 ± 0.2 | 1660.3 ± 93.8 | 332cd ± 18.8 | 7.43 ± 0.01 |
| 1:4 | 17.89 | 5.9 ± 0.5 | 1856.0 ± 66.0 | 371b ± 13.2 | 7.45 ± 0.02 |
| G | 58.15 | 1.9 ± 0.2 | 1200.2 ± 84.0 | 240e ± 16.8 | 7.46 ± 0.01 |
| S | 46.93 | 2.1 ± 0.5 | 964.9 ± 9.6 | 193f ± 5.9 | 7.44 ± 0.03 |
| FW | 15.26 | 7.3 ± 0.3 | 2052.0 ± 8.3 | 410a ± 5.7 | 7.49 ± 0.02 |
| Substrate Concentrations (g-VS/L) | Lag Time (d) | MPRmax (mL-CH4/L∙d) | MY* (mL-CH4/g-VS) | Final pH |
|---|---|---|---|---|
| Napier grass to food waste ratio of 1:4 (g-VS/g-VS) | ||||
| 10 | 0.1 ± 0.0 | 244.3 ± 12.5 | 416a ± 19.1 | 7.42 ± 0.01 |
| 15 | 1.6 ± 0.6 | 305.6 ± 11.3 | 403ba ± 11.5 | 7.47 ± 0.04 |
| 20 | 7.3 ± 1.0 | 430.5 ± 7.4 | 400ba ± 15.0 | 7.53 ± 0.01 |
| 25 | 14.3 ± 0.2 | 411.0 ± 22.1 | 398ba ± 2.0 | 7.54 ± 0.08 |
| 30 | 20.3 ± 0.2 | 445.8 ± 42.0 | 379c ± 7.1 | 7.65 ± 0.04 |
| 35 | 26.5 ± 1.2 | 419.7 ± 3.7 | 377c ± 1.4 | 7.46 ± 0.02 |
| 40 | 32.5 ± 1.7 | 396.1 ± 19.3 | 385cb ± 4.0 | 7.46 ± 0.03 |
| 45 | 45.9 ± 5.1 | 350.7 ± 32.6 | 352d ± 5.0 | 7.44 ± 0.02 |
| 50 | 56.6 ± 4.9 | 454.2 ± 23.9 | 374c ± 6.9 | 7.43 ± 0.03 |
| Napier silage to food waste ratio of 3:2 (g-VS/g-VS) | ||||
| 10 | 0 | 203.7 ± 18.6 | 314d ± 4.0 | 7.30 ± 0.02 |
| 15 | 0 | 227.1 ± 23.9 | 324dc ± 12.9 | 7.28 ± 0.01 |
| 20 | 0.4 ± 0.3 | 256.7 ± 14.7 | 353a ± 5.8 | 7.30 ± 0.02 |
| 25 | 3.3 ± 0.4 | 313.8 ± 15.1 | 349a ± 2.5 | 7.33 ± 0.02 |
| 30 | 6.9 ± 0.8 | 339.9 ± 22.6 | 353a ± 8.2 | 7.38 ± 0.01 |
| 35 | 12.1 ± 0.4 | 346.8 ± 41.5 | 339ba ± 8.2 | 7.21 ± 0.02 |
| 40 | 15.5 ± 1.1 | 380.8 ± 48.1 | 348a ± 6.1 | 7.27 ± 0.02 |
| 45 | 20.1 ± 2.7 | 378.5 ± 34.1 | 342ba ± 2.8 | 7.27 ± 0.03 |
| 50 | 25.4 ± 0.7 | 406.8 ± 9.1 | 331cb ± 7.1 | 7.27 ± 0.03 |
| Parameters | OLR (g-VS/L·d) | |||
|---|---|---|---|---|
| 0.5 | 1 | 1.5 | 2 | |
| MPR (L-CH4/L·d) | 0.15 ± 0.01 | 0.32 ± 0.01 | 0.50 ± 0.02 | 0.40 ± 0.04 |
| MY (L-CH4/g-VS) | 0.29 ± 0.02 | 0.32 ± 0.01 | 0.33± 0.01 | 0.20 ± 0.02 |
| Methane content (%) | 57.8 ± 3.30 | 57.6 ± 1.80 | 58.1 ± 0.03 | 54.6 ± 1.84 |
| Alkalinity (mg-CaCO3/L) | 2537 ± 21 | 2020 ± 48 | 2063 ± 38 | 1777 ± 28 |
| VFAs/alkalinity ratio | 0.045 ± 0.006 | 0.058 ± 0.002 | 0.063 ± 0.001 | 0.353 ± 0.026 |
| Final pH | 7.17 ± 0.04 | 6.88 ± 0.03 | 6.93 ± 0.04 | 6.66 ± 0.01 |
| Substrate | Inoculum | Temperature (°C) | Energy yield (kJ/g-VS) | Reference |
|---|---|---|---|---|
| Batch mode: | ||||
| Food waste, sewage sludge and raw glycerol | Anaerobic sludge | 35 | 12.3 | [24] |
| Pre-treated solid residue of grass with cow dung | Anaerobic sludge | 30 ± 2 | 13.3 | [22] |
| Pre-treated solid residue of Silage with cow dung | Anaerobic sludge | 30 ± 2 | 13.4 | |
| Grass with cow dung | Anaerobic sludge | 30 ± 2 | 6.5 | [6] |
| Silage with cow dung | Anaerobic sludge | 30 ± 2 | 7.5 | |
| Agave tequilana bagasse hydrolysate | Anaerobic granular sludge | 37 | 5.84a | [26] |
| Food waste with straw | Granular sludge | 7.04 | 14.1 | [28] |
| Maize silage with swine manure | Thermophilic anaerobic sludge | 55 | 15.2 | [25] |
| Napier grass with food waste | Anaerobic granular sludge | 30 ± 3 | 14.5 | The current study |
| Napier silage with food waste | Anaerobic granular sludge | 30 ± 3 | 12.7 | The current study |
| Continuous, semi-continuous and repeated batch mode: | ||||
| Sugarcane press mud with vinasse | Anaerobic sludge | 35 | 13.1 | [23] |
| Sugarcane syrup | Anaerobic sludge | 30 ± 2 | 9.76a | [27] |
| Ensiled sorghum, cheese whey and liquid cow manure | Anaerobic sludge | 37 | 8.0b | [29] |
| Palm oil mill effluent | Anaerobic sludge | 28–34 | 8.2a | [30] |
| Sun flower stalk | Granular sludge | 35 | 6.9c | [31] |
| Napier grass with food waste at the optimum OLR | Anaerobic granular sludge | 30 ± 3 | 11.9 | The current study |
| Substrate | Napier Grass | Napier Silage | Food Waste |
|---|---|---|---|
| Total solids (g/kg) | 311.02 ± 1.4 | 405.40 ± 1.7 | 194.74 ± 0.6 |
| Volatile solids (g/kg) | 288.75 ± 1.2 | 379.74 ± 1.8 | 179.43 ± 0.7 |
| Total COD (g/kg) | na | na | 171.77 ± 2.9 |
| Total sugars (g/kg) | 30.9 ± 3.40 | na | 77.06 ± 6.6 |
| Proteins (g/kg) | 27 ± 2.97 | na | 64.74 ± 0.8 |
| Lipids (g/kg) | 14.8 ± 1.63 | na | 28.71 ± 1.81 |
| Lignin (%) | 31.74 ± 1.0 | 35.18 ± 0.7 | na |
| Moisture (%) | 68.89 ± 0.1 | 59.46 ± 0.2 | 80.53 ± 0.1 |
| Ash (g/kg) | 22.27 ± 0.2 | 25.66 ± 0.3 | 15.32 ± 0.1 |
| Organic carbon (%) | 45.36 | 43.18 | 38.16 |
| Total kjeldahl nitrogen (%) | 0.78 | 0.92 | 2.50 |
| Stock | Composition and Quantity (g/L) | Used (mL/L) |
|---|---|---|
| A | NH4Cl, 100; NaCl, 10; MgCl2.6H2O, 10; CaCl2.2H2O, 5 | 10 |
| B | K2HPO4.3H2O, 200 | 2 |
| C | NaHCO3, 52 | 50 |
| D | FeCl2.4H2O, 2; H3BO3, 0.05; ZnCl2, 0.05; CuCl2.2H2O, 0.038; MnCl2.4H2O, 0.05; (NH4)6Mo7O24.4H2O, 0.05; AlCl3.6H2O, 0.05; CoCl2.6H2O, 0.05; NiCl2.6H2O, 0.092; EDTA, 0.5; concentrated HCl, 1 mL; Na2SeO3.5H2O, 0.1 | 1 |
| E | Yeast extract, 100 | 1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boonpiyo, S.; Sittijunda, S.; Reungsang, A. Co-Digestion of Napier Grass with Food Waste and Napier Silage with Food Waste for Methane Production. Energies 2018, 11, 3200. https://doi.org/10.3390/en11113200
Boonpiyo S, Sittijunda S, Reungsang A. Co-Digestion of Napier Grass with Food Waste and Napier Silage with Food Waste for Methane Production. Energies. 2018; 11(11):3200. https://doi.org/10.3390/en11113200
Chicago/Turabian StyleBoonpiyo, Suriyan, Sureewan Sittijunda, and Alissara Reungsang. 2018. "Co-Digestion of Napier Grass with Food Waste and Napier Silage with Food Waste for Methane Production" Energies 11, no. 11: 3200. https://doi.org/10.3390/en11113200
APA StyleBoonpiyo, S., Sittijunda, S., & Reungsang, A. (2018). Co-Digestion of Napier Grass with Food Waste and Napier Silage with Food Waste for Methane Production. Energies, 11(11), 3200. https://doi.org/10.3390/en11113200






