Physicochemical, Performance, Combustion and Emission Characteristics of Melaleuca Cajuputi Oil-Refined Palm Oil Hybrid Biofuel Blend
Abstract
:1. Introduction
Overview of Melaleuca Cajuputi Oil and Refined Palm Oil
2. Materials and Methods
2.1. Materials
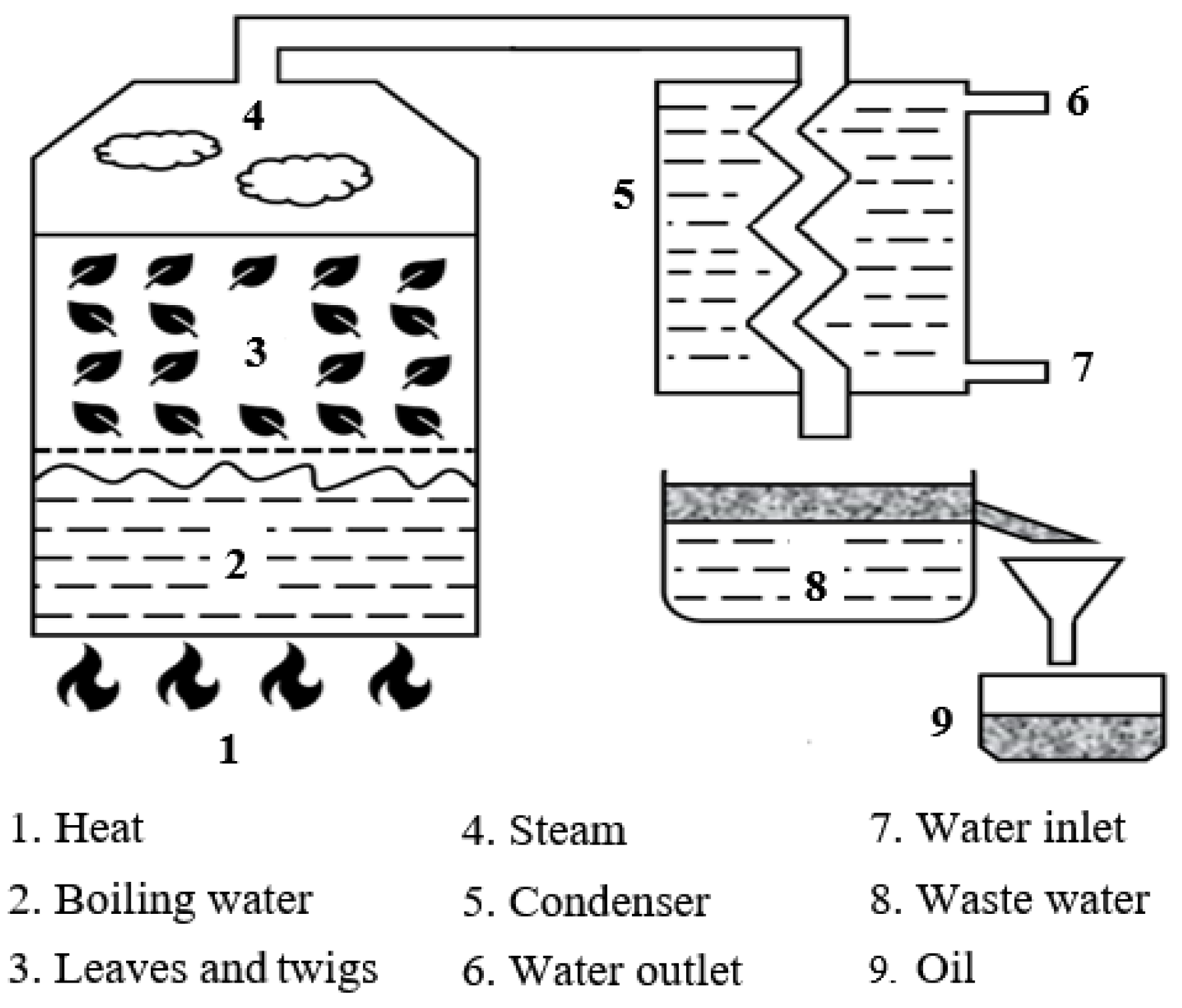
2.2. Hybrid Biofuel Preparation
2.3. Physicochemical Properties Analyses
2.4. Hybrid Biofuel Optimization and Selection
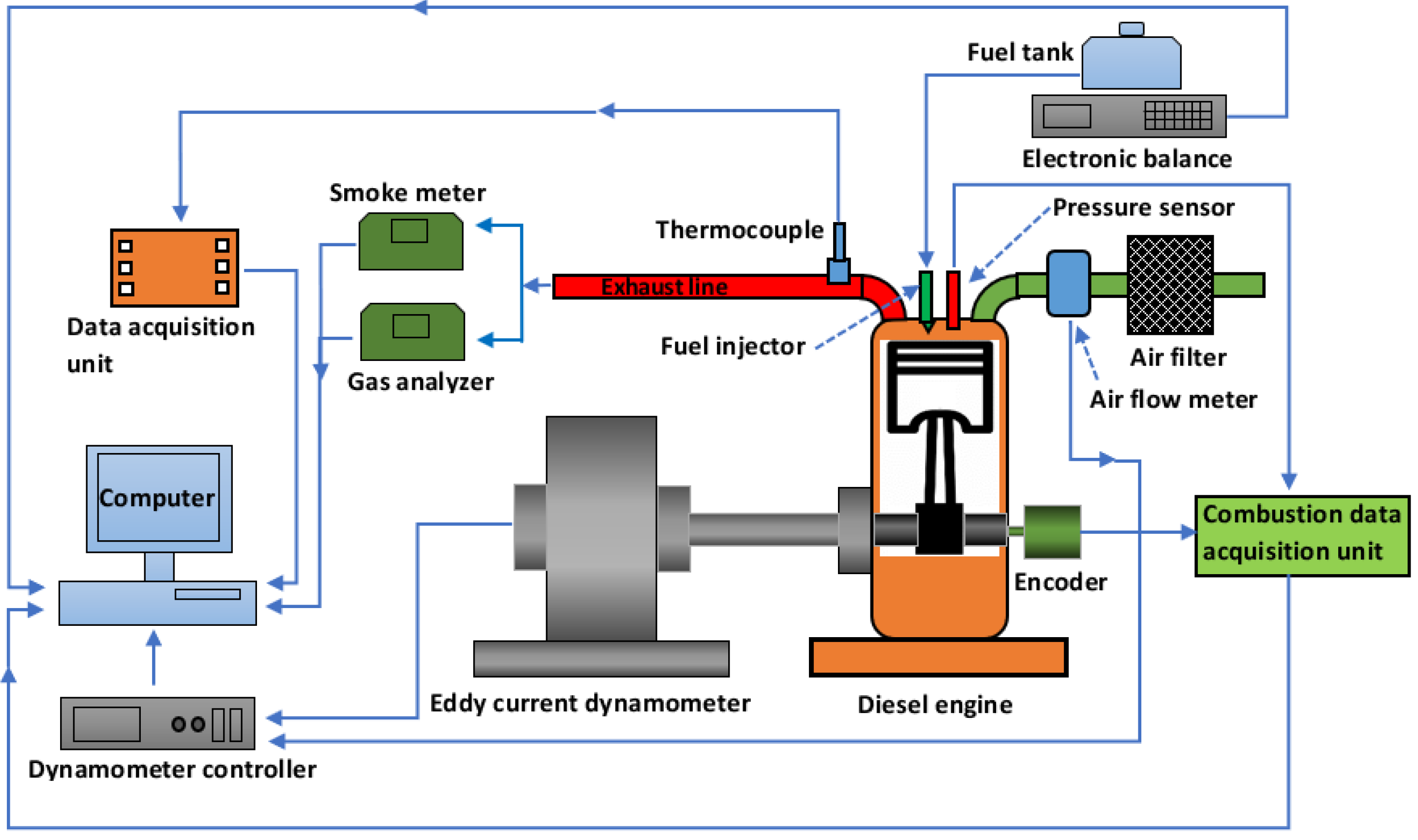
2.5. Experimental Setup and Measurement Procedure
3. Results
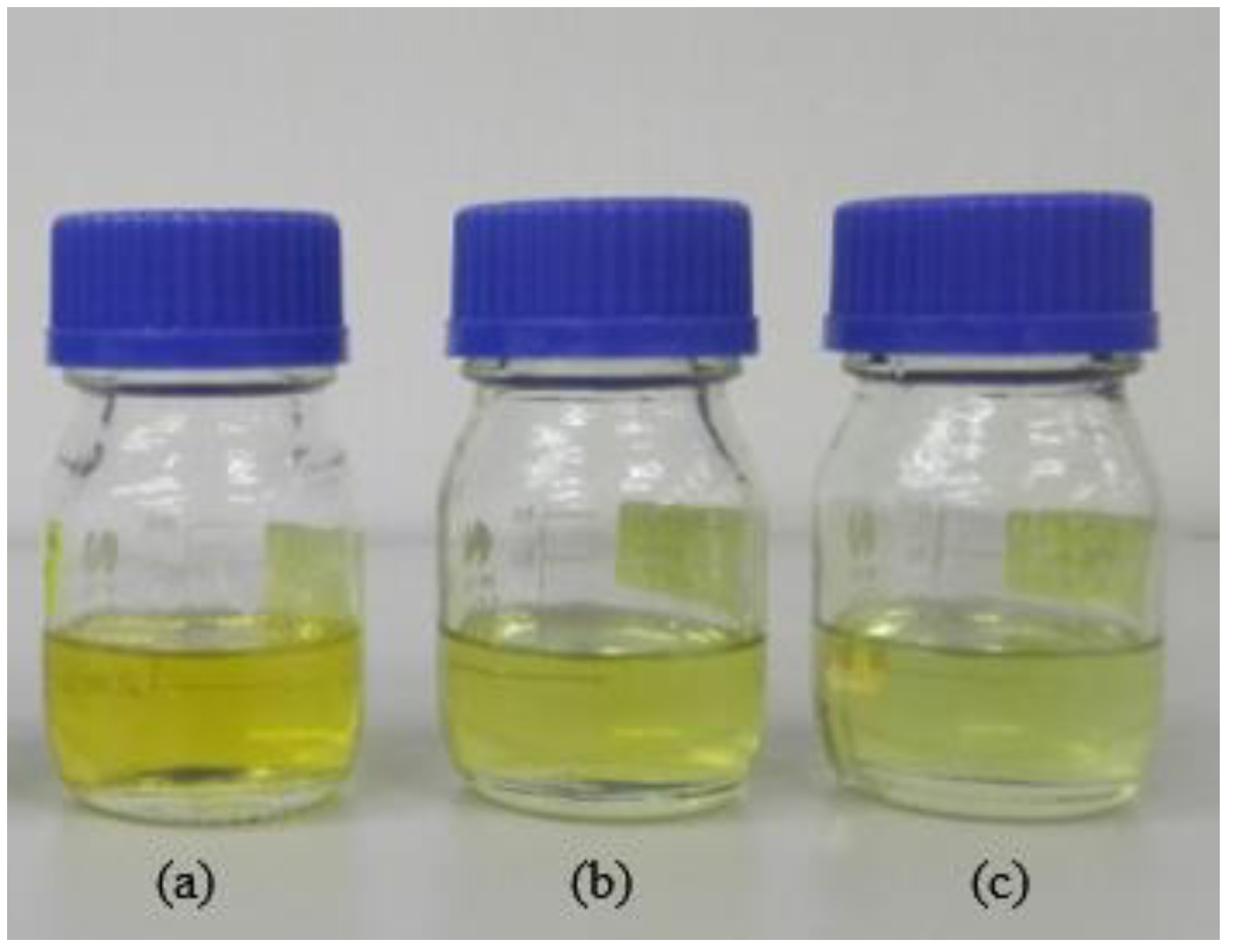
3.1. Gravitational Stability Test
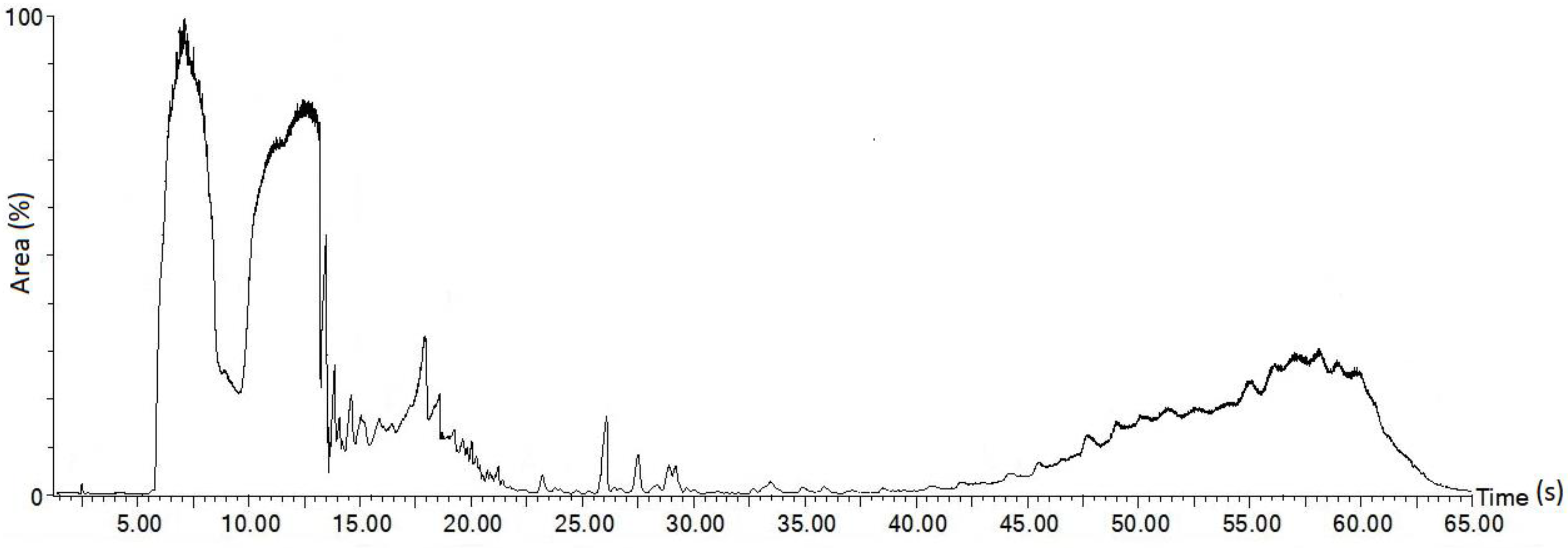
3.2. Chemical Constituent of Melaleuca Cajuputi Oil
3.3. Basic Physicochemical Properties of RPO-MCO Blends
3.4. Optimization of Hybrid Biofuel
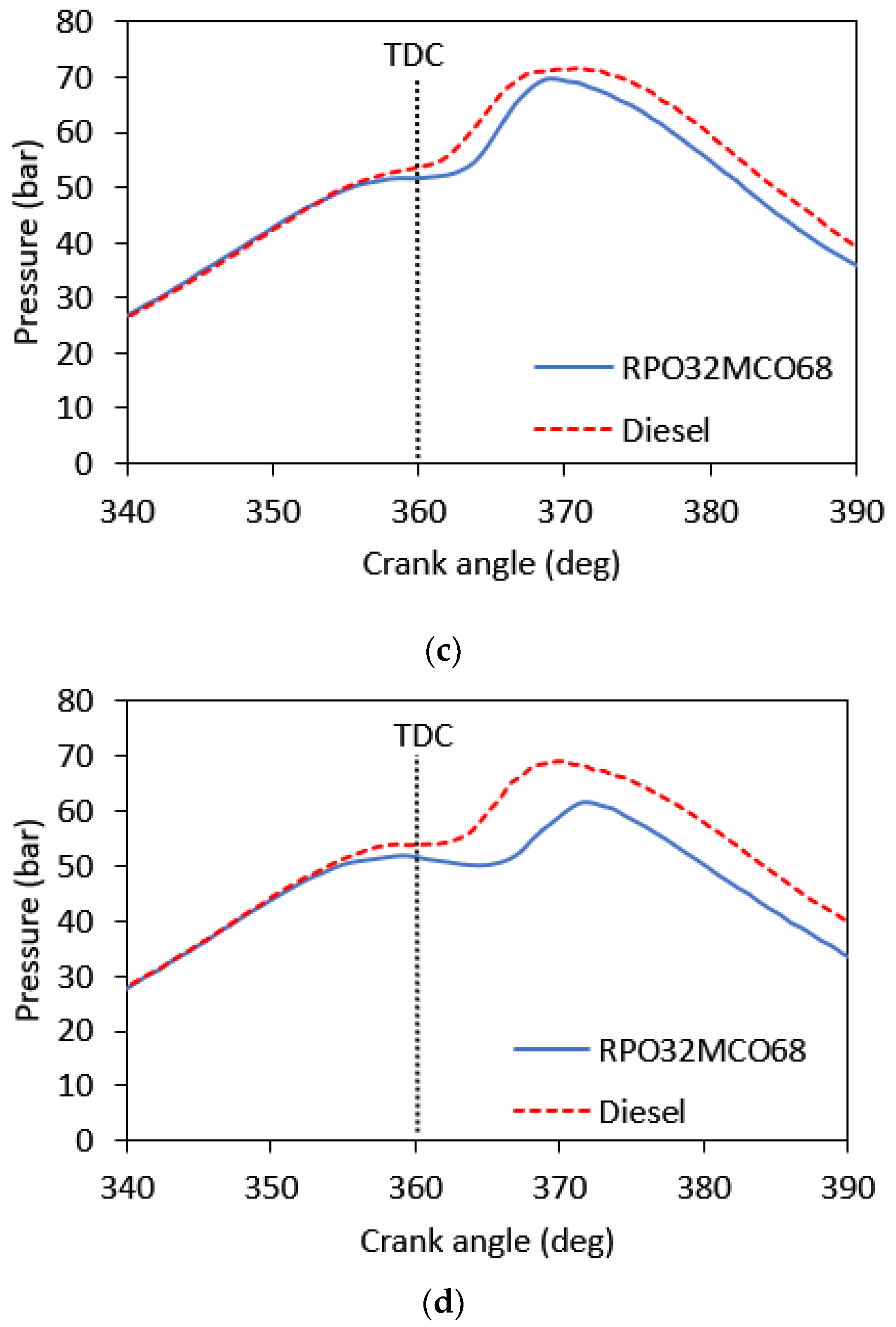
3.5. Combustion Analysis
3.6. Performance Analysis
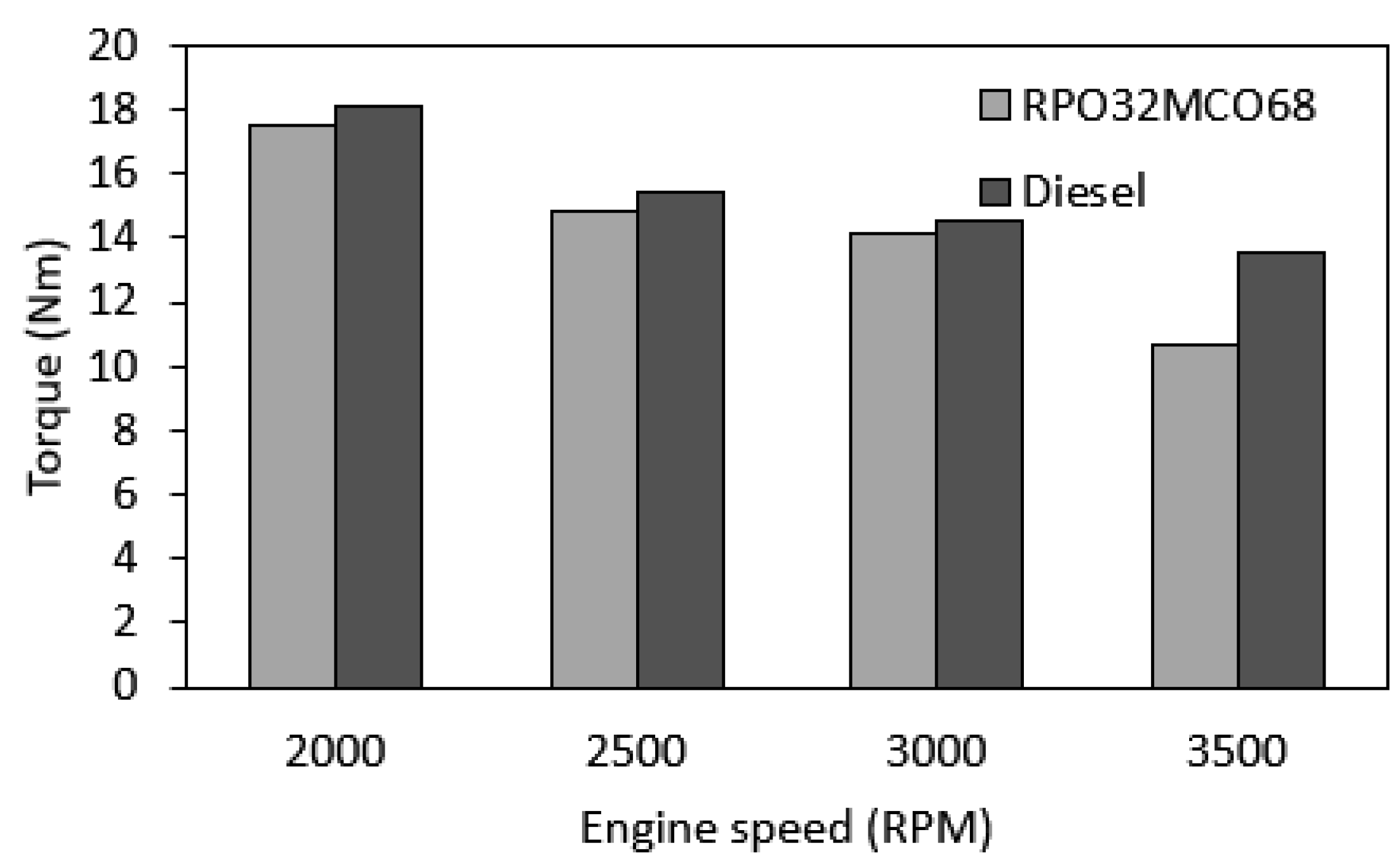
3.6.1. Engine Torque
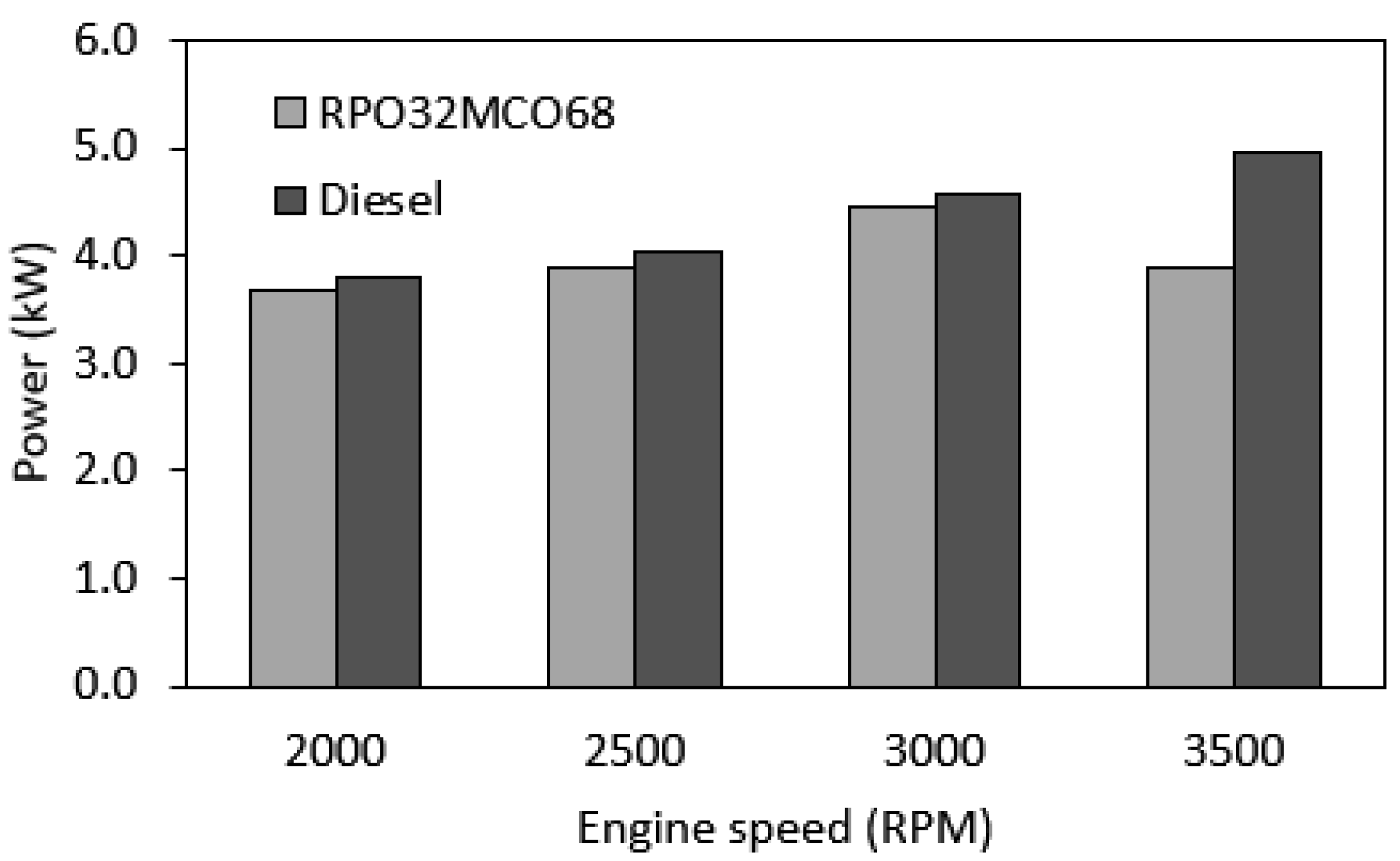
3.6.2. Engine Brake Power
3.6.3. Brake Specific Fuel Consumption
3.7. Emissions Analysis
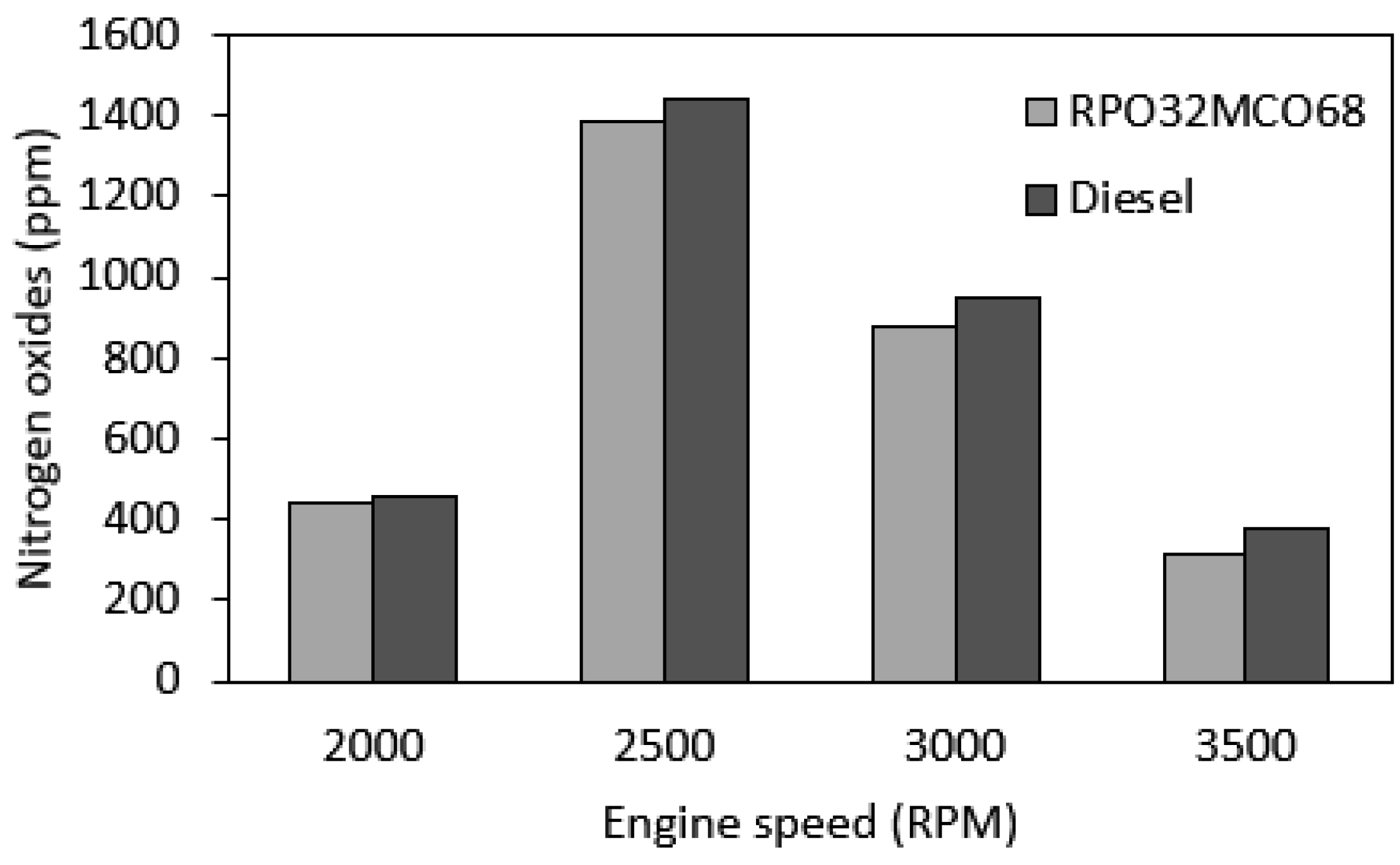
3.7.1. NOx Emission
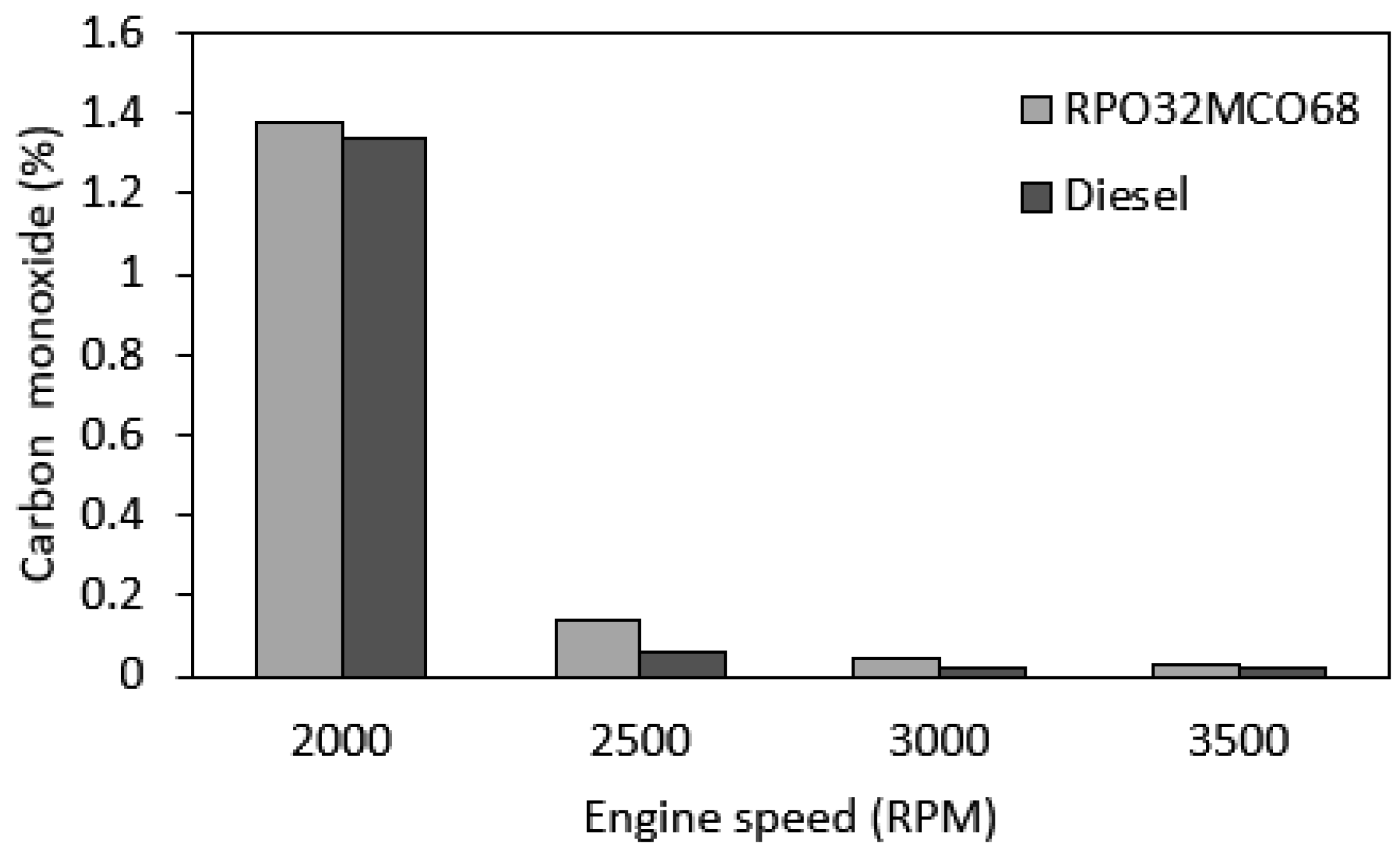
3.7.2. CO Emission
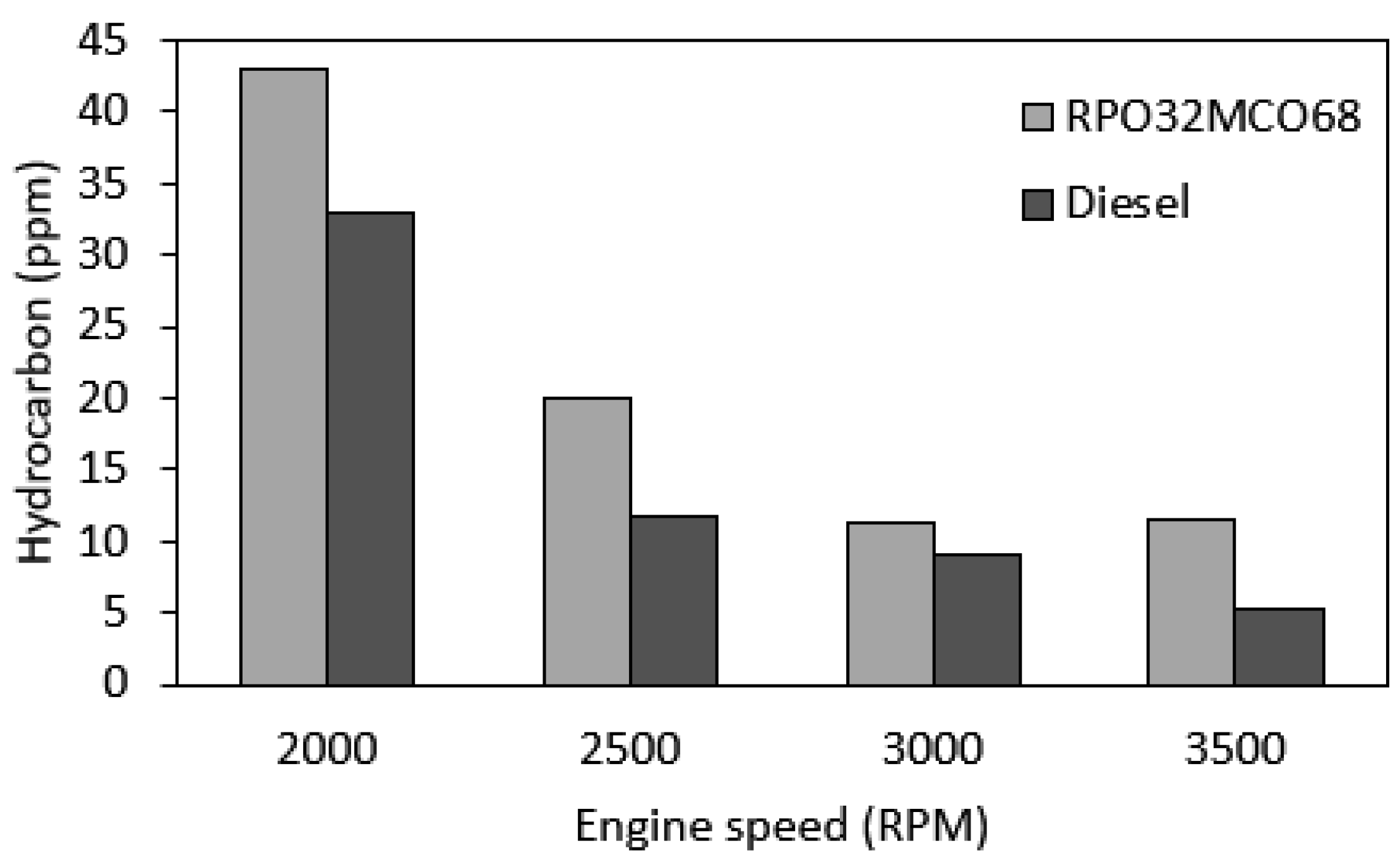
3.7.3. HC Emission
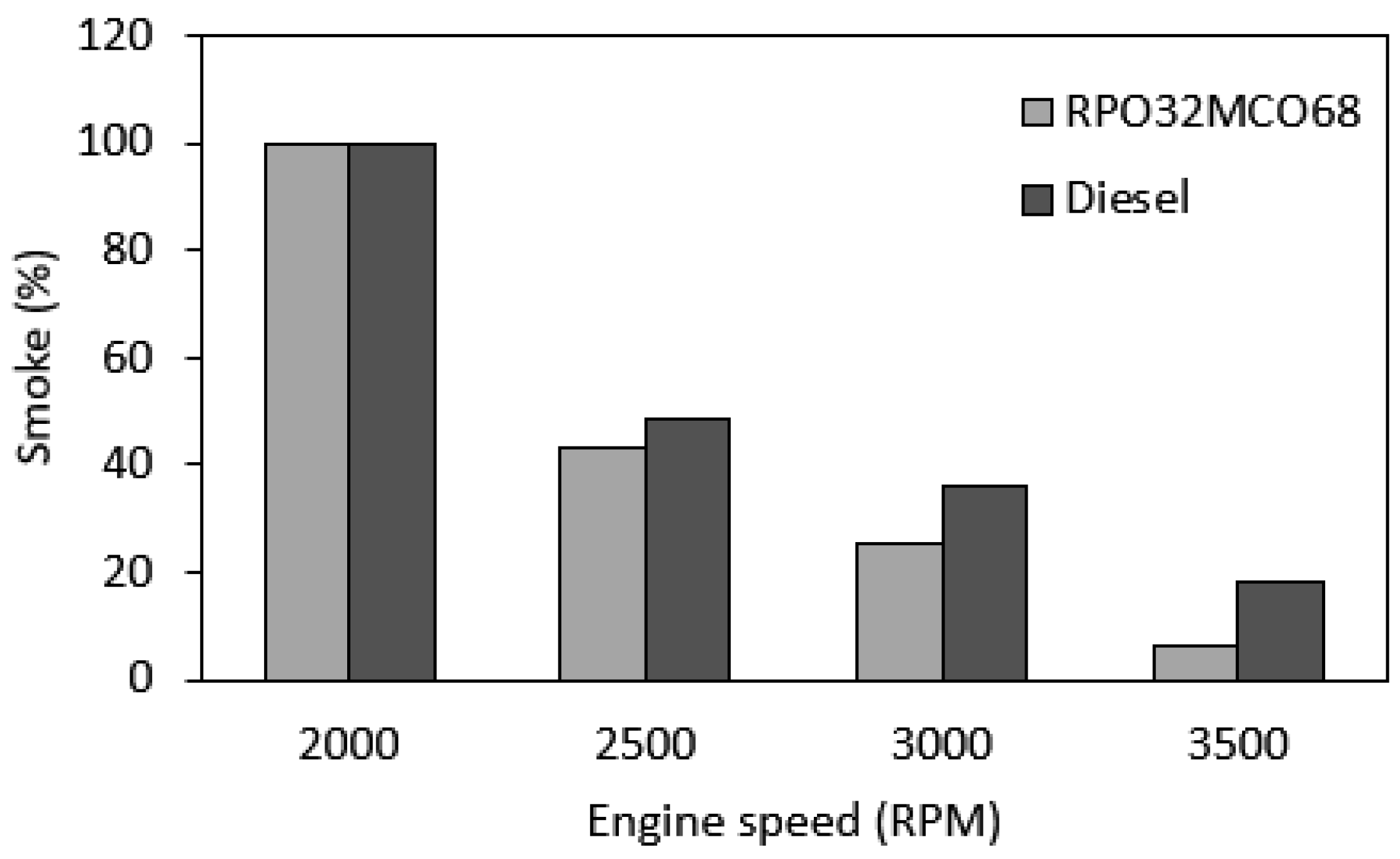
3.7.4. Smoke Emission
4. Conclusions
- It was found that MCO is mainly composed of 64.8% hydrocarbons (HC) and 34.6% oxygenated products. Higher percentage of HC contribute to high calorific value (CV) of MCO which indicated it as a potential source of biofuel. The presence of oxygenated products promote better combustion efficiency and lower smoke opacity.
- The blend of high viscosity RPO with low viscosity MCO successfully reduced the viscosity and density of hybrid biofuel. The higher the fraction of MCO, the lower the viscosity and density of the blends. CV was increased with the increase of MCO in the blends.
- The key properties of optimum hybrid biofuel (RPO32MCO68) obtained viscosity, density, and CV of 5.45 mm2/s, 880.20 kg/m3, and 42.1 MJ/kg respectively. These key properties were in accordance with the ASTM 6751/EN 14214 standards and demonstrated comparable properties to those of baseline diesel fuel.
- At the entire range of speeds, in-cylinder peak pressure, brake torque, and brake power for the optimum hybrid biofuel blend were slightly lower than those of baseline diesel fuel. The largest drop in peak pressure, brake torque, and brake power are 10.7%, 21.4%, and 20.6%, respectively.
- Notably, NOx emission and smoke opacity were decreased as compared to diesel fuel across the speed range. The largest NOx reduction was 17.3% and the smoke opacity reduction was 64.5% at maximum engine speed. Meanwhile, CO emission was found similar in comparison to diesel fuel. BSFC and HC emissions were found to be slightly higher than those of baseline diesel fuel.
- Overall, this study has shown that the RPO32MCO68 hybrid biofuel blend has successfully run a diesel engine with comparable engine performance and exhaust emissions to those of diesel fuel. This suggested that the blend is marked as a potential new source of biofuel.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barnwal, B.K.; Sharma, M.P. Prospects of biodiesel production from vegetable oils in India. Renew. Sustain. Energy Rev. 2005, 9, 363–378. [Google Scholar] [CrossRef]
- Ashraful, A.M.; Masjuki, H.H.; Kalam, M.A.; Rizwanul Fattah, I.M.; Imtenan, S.; Shahir, S.A.; Mobarak, H.M. Production and comparison of fuel properties, engine performance, and emission characteristics of biodiesel from various non-edible vegetable oils: A review. Energy Convers. Manag. 2014, 80, 202–228. [Google Scholar] [CrossRef]
- Hellier, P.; Ladommatos, N.; Yusaf, T. The influence of straight vegetable oil fatty acid composition on compression ignition combustion and emissions. Fuel 2015, 143, 131–143. [Google Scholar] [CrossRef]
- Ndayishimiye, P.; Tazerout, M. Use of palm oil-based biofuel in the internal combustion engines: Performance and emissions characteristics. Energy 2011, 36, 1790–1796. [Google Scholar] [CrossRef]
- Emberger, P.; Hebecker, D.; Pickel, P.; Remmele, E.; Thuneke, K. Emission behaviour of vegetable oil fuel compatible tractors fuelled with different pure vegetable oils. Fuel 2016, 167, 257–270. [Google Scholar] [CrossRef]
- Li, Q.; Backes, F.; Wachtmeister, G. Application of canola oil operation in a diesel engine with common rail system. Fuel 2015, 159, 141–149. [Google Scholar] [CrossRef]
- Yusop, A.F.; Mamat, R.; Yusaf, T.; Najafi, G.; Yasin, M.H.M.; Khathri, A.M. Analysis of particulate matter (pm) emissions in diesel engines using palm oil biodiesel blended with diesel fuel. Energies 2018, 11, 1039. [Google Scholar] [CrossRef]
- Ramadhas, A.S.; Jayaraj, S.; Muraleedharan, C. Characterization and effect of using rubber seed oil as fuel in the compression ignition engines. Renew. Energy 2005, 30, 795–803. [Google Scholar] [CrossRef]
- Sidibé, S.S.; Blin, J.; Vaitilingom, G.; Azoumah, Y. Use of crude filtered vegetable oil as a fuel in diesel engines state of the art: Literature review. Renew. Sustain. Energy Rev. 2010, 14, 2748–2759. [Google Scholar] [CrossRef]
- Deepanraj, B.; Srinivas, M.; Arun, N.; Sankaranarayanan, G.; Abdul Salam, P. Comparison of jatropha and karanja biofuels on their combustion characteristics. Int. J. Green Energy 2017, 14, 1231–1237. [Google Scholar] [CrossRef]
- Chauhan, B.S.; Singh, R.K.; Cho, H.M.; Lim, H.C. Practice of diesel fuel blends using alternative fuels: A review. Renew. Sustain. Energy Rev. 2016, 59, 1358–1368. [Google Scholar] [CrossRef]
- Santhosh, M.; Padmanaban, K.P. Experimental investigation on engine performances, combustion characteristics and emission of exhaust gases of VCR engine fuelled with cottonseed oil methyl ester blended with diesel. Int. J. Green Energy 2014, 13, 1534–1545. [Google Scholar] [CrossRef]
- Adam, I.; Abdul Aziz, A.; Heikal, M.; Yusup, S.; Firmansyah; Ahmad, A.; Zainal Abidin, E. Performance and emission analysis of rubber seed, palm, and their combined blend in a multi-cylinder diesel engine. Energies 2018, 11, 1522. [Google Scholar] [CrossRef]
- Rahman, M.; Rasul, M.; Hassan, N.; Hyde, J. Prospects of biodiesel production from macadamia oil as an alternative fuel for diesel engines. Energies 2016, 9, 403. [Google Scholar] [CrossRef]
- Rahman, M.; Rasul, M.; Hassan, N. Study on the tribological characteristics of Australian native first generation and second generation biodiesel fuel. Energies 2017, 10, 55. [Google Scholar] [CrossRef]
- Gui, M.M.; Lee, K.T.; Bhatia, S. Feasibility of edible oil vs. non-edible oil vs. waste edible oil as biodiesel feedstock. Energy 2008, 33, 1646–1653. [Google Scholar] [CrossRef]
- Rahman, S.M.A.; Nabi, M.N.; Van, T.C.; Suara, K.; Jafari, M.; Dowell, A.; Islam, M.A.; Marchese, A.J.; Tryner, J.; Hossain, M.F.; et al. Performance and Combustion Characteristics Analysis of Multi-Cylinder CI Engine Using Essential Oil Blends. Energies 2018, 11, 738. [Google Scholar] [CrossRef]
- Kasiraman, G.; Nagalingam, B.; Balakrishnan, M. Performance, Emission and combustion improvements in a direct injection diesel engine using cashew nut shell oil as fuel with camphor oil blending. Energy 2012, 47, 116–124. [Google Scholar] [CrossRef]
- Vallinayagam, R.; Vedharaj, S.; Yang, W.M.; Raghavan, V.; Saravanan, C.G.; Lee, P.S.; Chua, K.J.E.; Chou, S.K. Investigation of evaporation and engine characteristics of pine oil biofuel fumigated in the inlet manifold of a diesel engine. Appl. Energy 2014, 115, 514–524. [Google Scholar] [CrossRef]
- Hoki, M.; Takeda, S.; Ito, N.; Yazawa, H. Engine Performance by Eucalyptus Oil Blended Fuel; Bulletin of the Faculty of Agriculture; Mie University: Mie, Japan, December 1981. [Google Scholar]
- Poola, R.B.; Nagalingam, B.; Gopalakrishnan, K.V. Performance studies with biomass-derived high-octane fuel additives in a two-stroke spark-ignition engine. Biomass Bioenergy 1994, 6, 369–379. [Google Scholar] [CrossRef]
- Devan, P.K.; Mahalakshmi, N.V. A study of the performance, emission and combustion characteristics of a compression ignition engine using methyl ester of paradise oil–eucalyptus oil blends. Appl. Energy 2009, 86, 675–680. [Google Scholar] [CrossRef]
- Vallinayagam, R.; Vedharaj, S.; Yang, W.M.; Lee, P.S.; Chua, K.J.E.; Chou, S.K. Pine oil–biodiesel blends: A double biofuel strategy to completely eliminate the use of diesel in a diesel engine. Appl. Energy 2014, 130, 466–473. [Google Scholar] [CrossRef]
- Kommana, S.; Banoth, B.N.; Kadavakollu, K.R. Performance and emission of VCR-CI engine with palm kernel and eucalyptus blends. Perspect. Sci. 2016, 8, 195–197. [Google Scholar] [CrossRef]
- Sakasegawa, M.; Hori, K.; Yatagai, M. Composition and antitermite activities of essential oils from melaleuca species. J. Wood Sci. 2003, 49, 181–187. [Google Scholar] [CrossRef]
- Southwell, I.; Lowe, R. Tea Tree: The Genus Melaleuca; Harwood Academic Publishers: Amsterdam, The Netherland, 1999. [Google Scholar]
- Schimleck, L.R.; Doran, J.C.; Rimbawanto, A. Near infrared spectroscopy for cost effective screening of foliar oil characteristics in a melaleuca cajuputi breeding population. J. Agric. Food Chem. 2003, 51, 2433–2437. [Google Scholar] [CrossRef] [PubMed]
- Oyen, L.P.A.; Dung, N.X. Plant Resources of South East Asia 19: Essential-Oil Plants; Backhuys Publishers: Leiden, The Netherlands, 1999. [Google Scholar]
- Jajaei, S.M.; Daud, W.R.W.; Markom, M.; Zakaria, Z.; Presti, M.L.; Costa, R.; Mondello, L.; Santi, L. Extraction of Melaleuca cajuputi using supercritical fluid extraction and solvent extraction. J. Essent. Oil Res. 2010, 22, 205–210. [Google Scholar] [CrossRef]
- Silva, C.J.; Barbosa, L.C.A.; Maltha, C.R.A.; Pinheiro, A.L.; Ismail, F.M.D. Comparative study of the essential oils of seven Melaleuca (Myrtaceae) species grown in Brazil. Flavour Fragr. J. 2007, 22, 474–478. [Google Scholar] [CrossRef]
- Mat Yasin, M.H.; Mamat, R.; Najafi, G.; Ali, O.M.; Yusop, A.F. Potentials of palm oil as new feedstock oil for a global alternative fuel: A review. Renew. Sustain. Energy Rev. 2017, 79, 1034–1049. [Google Scholar] [CrossRef]
- Ong, H.C.; Mahlia, T.M.I.; Masjuki, H.H.; Honnery, D. Life cycle cost and sensitivity analysis of palm biodiesel production. Fuel 2012, 98, 131–139. [Google Scholar] [CrossRef]
- Reham, S.S.; Masjuki, H.H.; Kalam, M.A.; Shancita, I.; Rizwanul Fattah, I.M.; Ruhul, A.M. Study on stability, fuel properties, engine combustion, performance and emission characteristics of biofuel emulsion. Renew. Sustain. Energy Rev. 2015, 52, 1566–1579. [Google Scholar] [CrossRef]
- Giuliano Albo, P.A.; Lago, S.; Wolf, H.; Pagel, R.; Glen, N.; Clerck, M.; Ballereau, P. Density, viscosity and specific heat capacity of diesel blends with rapeseed and soybean oil methyl ester. Biomass Bioenergy 2017, 96, 87–95. [Google Scholar] [CrossRef]
- Melo-Espinosa, E.A.; Piloto-Rodríguez, R.; Goyos-Pérez, L.; Sierens, R.; Verhelst, S. Emulsification of animal fats and vegetable oils for their use as a diesel engine fuel: An overview. Renew. Sustain. Energy Rev. 2015, 47, 623–633. [Google Scholar] [CrossRef]
- NguyenThi, T.; Bazile, J.-P.; Bessières, D. Density measurements of waste cooking oil biodiesel and diesel blends over extended pressure and temperature ranges. Energies 2018, 11, 1212. [Google Scholar] [CrossRef]
- Motl, O.; Hodačová, J.; Ubik, K. Composition of Vietnamese cajuput essential oil. Flavour Fragr. J. 1990, 5, 39–42. [Google Scholar] [CrossRef]
- Torri, I.D.V.; Paasikallio, V.; Faccini, C.S.; Huff, R.; Caramão, E.B.; Sacon, V.; Oasmaa, A.; Zini, C.A. Bio-oil production of softwood and hardwood forest industry residues through fast and intermediate pyrolysis and its chromatographic characterization. Bioresour. Technol. 2016, 200, 680–690. [Google Scholar] [CrossRef] [PubMed]
- Kommana, S.; Naik Banoth, B.; Radha Kadavakollu, K. Eucalyptus-palm kernel oil blends: A complete elimination of diesel in a 4-stroke vcr diesel engine. J. Combust. 2015, 2015, 182879. [Google Scholar] [CrossRef]
- Pinzi, S.; Garcia, I.L.; Lopez-Gimenez, F.J.; Luque de Castro, M.D.; Dorado, G.; Dorado, M.P. The ideal vegetable oil-based biodiesel composition: A review of social, economical and technical implications. Energy Fuels 2009, 23, 2325–2341. [Google Scholar] [CrossRef]
- Laza, T.; Bereczky, Á. Basic fuel properties of rapeseed oil-higher alcohols blends. Fuel 2011, 90, 803–810. [Google Scholar] [CrossRef]
- Meher, L.C.; Vidya Sagar, D.; Naik, S.N. Technical aspects of biodiesel production by transesterification—A review. Renew. Sustain. Energy Rev. 2006, 10, 248–268. [Google Scholar] [CrossRef]
- Wang, Y.D.; Al-Shemmeri, T.; Eames, P.; McMullan, J.; Hewitt, N.; Huang, Y.; Rezvani, S. An experimental investigation of the performance and gaseous exhaust emissions of a diesel engine using blends of a vegetable oil. Appl. Therm. Eng. 2006, 26, 1684–1691. [Google Scholar] [CrossRef]
- Rakopoulos, D.C.; Rakopoulos, C.D.; Giakoumis, E.G.; Dimaratos, A.M.; Founti, M.A. Comparative environmental behavior of bus engine operating on blends of diesel fuel with four straight vegetable oils of Greek origin: Sunflower, cottonseed, corn and olive. Fuel 2011, 90, 3439–3446. [Google Scholar] [CrossRef]
- Mohan, B.; Yang, W.; Raman, V.; Sivasankaralingam, V.; Chou, S.K. Optimization of biodiesel fueled engine to meet emission standards through varying nozzle opening pressure and static injection timing. Appl. Energy 2014, 130, 450–457. [Google Scholar] [CrossRef]






| Model/Make | Yanmar L70N |
|---|---|
| Fuel injection system | Direct injection |
| Maximum output (kW) | 4.9 |
| Rated speed (rpm) | 3600 |
| Displacement (litre) | 0.320 |
| Bore × stroke (mm) | 78 × 67 |
| Cylinder | single |
| Compression ratio | 20:1 |
| Cooling system | Forced air |
| Lubrication system | Forced lubricating |
| No. | Compound | Chemical Class | Molecular Formula | Composition (%) |
|---|---|---|---|---|
| 1 | α-Pinene | hydrocarbon | C10H16 | 26.12 |
| 2 | 1,8-Cineole | oxygenated product (ether) | C10H18O | 27.66 |
| 3 | γ-Terpinene | hydrocarbon | C10H16 | 1.23 |
| 4 | Terpinolene | hydrocarbon | C10H16 | 0.38 |
| 5 | Undecane | hydrocarbon | C11H24 | 0.15 |
| 6 | Trans-decalin, 2-methyl- | hydrocarbon | C11H20 | 0.31 |
| 7 | Trans-9-methyldecalin | hydrocarbon | C11H20 | 0.40 |
| 8 | 1-Isopropenyl-4-methyl-1,2-cyclohexanediol | oxygenated product (alcohol) | C10H18O2 | 0.53 |
| 9 | Naphthalene, decahydro-2,3-dimethyl- | hydrocarbon | C12H22 | 0.35 |
| 10 | 1-Menthol | oxygenated product (alcohol) | C10H20O | 2.49 |
| 11 | α-Terpineol | oxygenated product (alcohol) | C10H18O | 0.87 |
| 12 | Verbenol | oxygenated product (alcohol) | C10H16O | 0.36 |
| 13 | Cyclohexane, (4-methylpentyl)- | hydrocarbon | C12H24 | 0.37 |
| 14 | 6-Methyltridecane | hydrocarbon | C14H30 | 0.24 |
| 15 | Undecane, 2,3-dimethyl- | hydrocarbon | C13H28 | 0.11 |
| 16 | Dodecane, 2-methyl-6-propyl- | hydrocarbon | C16H34 | 0.13 |
| 17 | Undecane, 2,9-dimethyl- | hydrocarbon | C13H28 | 0.11 |
| 18 | Tridecane | hydrocarbon | C13H28 | 0.08 |
| 19 | α-Terpinyl acetate | oxygenated product (acetate) | C12H20O2 | 0.10 |
| 20 | Ylangene | hydrocarbon | C15H24 | 0.02 |
| 21 | β-Caryophyllene | hydrocarbon | C15H24 | 0.44 |
| 22 | α-Caryophyllene | hydrocarbon | C15H24 | 0.24 |
| 23 | Longifolene-(v4) | hydrocarbon | C15H24 | 0.07 |
| 24 | β-Selinene | hydrocarbon | C15H24 | 0.20 |
| 25 | α-Selinene | hydrocarbon | C15H24 | 0.17 |
| 26 | α-Bergamotene | hydrocarbons | C15H24 | 0.03 |
| 27 | δ-Cadinene | hydrocarbon | C15H24 | 0.03 |
| 28 | Caryophyllene oxide | oxygenated product | C15H24O | 0.03 |
| 29 | Viridiflorol | oxygenated product (alcohol) | C15H26O | 0.17 |
| 30 | β-Eudesmol | oxygenated product (alcohol) | C15H26O | 0.06 |
| 31 | Sulfurous acid, butyl heptadecyl ester | others | C21H44O3S | 0.16 |
| 32 | Tetradecane, 2,6,10-trimethyl- | hydrocarbon | C17H36 | 1.50 |
| 33 | 1-Hexadecanol, 2-methyl- | Oxygenated product (alcohol) | C17H36O | 2.32 |
| 34 | 17-Pentatriacontene | hydrocarbon | C35H70 | 6.33 |
| 35 | Tetratetracontane | hydrocarbon | C44H90 | 25.85 |
| Class compositions | ||||
| Hydrocarbons | 64.8 | |||
| Oxygenated products | 34.6 | |||
| Others | 0.2 | |||
| Total composition | 99.6 | |||
| Property | ASTM D6751 | EN 14214 | Diesel | RPO25 MCO75 | RPO50 MCO50 | RPO75 MCO25 | RPO32 MCO68 | RPO | MCO |
|---|---|---|---|---|---|---|---|---|---|
| Kinematic viscosity at 40 °C (mm2/s) | 1.9–6.0 | 3.5–5.0 | 3.4 | 4.6 | 9.6 | 19.0 | 5.45 | 42 | 2.2 |
| Density (kg/m3) | 880 | 860–900 | 835 | 878.2 | 887.1 | 895.98 | 880.20 | 905.5 | 871.4 |
| Calorific value (MJ/kg) | - | 35 | 44.8 | 42.3 | 41.5 | 40.8 | 42.1 | 40.3 | 43.2 |
| Flash point (°C) | 100–170 | >120 | 50–55 | 51–52 | 53–55 | 65–67 | 52–53 | 210–215 | 50–51 |
| Boiling point (°C) | - | - | 150–343 | 134 | 141 | 189 | 135 | >250 | 121 |
| Water content (vol. %) | Max. 0.05 | - | - | 0.31 | 0.33 | 0.43 | 0.32 | 0.56 | 0.28 |
| Acid value (mg KOH/g) | Max. 0.5 | Max. 0.5 | - | 0.55 | 0.69 | 0.89 | 0.60 | 7.28 | 5.52 |
| Fatty Acid | Mol. Formula | Composition (%) |
|---|---|---|
| Lauric | C12H24O2 | 2.4 |
| Myristic | C14H284O2 | 1.2 |
| Palmitic | C16H32O2 | 32.6 |
| Stearic | C18H36O2 | 2.9 |
| Oleic | C18H34O2 | 49 |
| Linoleic | C18H32O2 | 11.9 |
| Blend (vol. %) | Kinematic Viscosity (mm2/s) | Discrepancy (%) | Density (kg/m3) | Discrepancy (%) | ||
|---|---|---|---|---|---|---|
| Measured | Calculated | Measured | Calculated | |||
| RPO25MCO75 | 4.60 | 4.70 | 2.2 | 878.20 | 879.04 | 0.10 |
| RPO50MCO50 | 9.60 | 9.70 | 1.0 | 887.10 | 887.64 | 0.10 |
| RPO75MCO25 | 19.0 | 19.90 | 4.7 | 895.98 | 896.23 | 0.03 |
| RPO32MCO68 | 5.45 | 5.70 | 4.6 | 880.20 | 881.44 | 0.14 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Che Mat, S.; Idroas, M.Y.; Teoh, Y.H.; Hamid, M.F. Physicochemical, Performance, Combustion and Emission Characteristics of Melaleuca Cajuputi Oil-Refined Palm Oil Hybrid Biofuel Blend. Energies 2018, 11, 3146. https://doi.org/10.3390/en11113146
Che Mat S, Idroas MY, Teoh YH, Hamid MF. Physicochemical, Performance, Combustion and Emission Characteristics of Melaleuca Cajuputi Oil-Refined Palm Oil Hybrid Biofuel Blend. Energies. 2018; 11(11):3146. https://doi.org/10.3390/en11113146
Chicago/Turabian StyleChe Mat, Sharzali, Mohamad Yusof Idroas, Yew Heng Teoh, and Mohd Fadzli Hamid. 2018. "Physicochemical, Performance, Combustion and Emission Characteristics of Melaleuca Cajuputi Oil-Refined Palm Oil Hybrid Biofuel Blend" Energies 11, no. 11: 3146. https://doi.org/10.3390/en11113146
APA StyleChe Mat, S., Idroas, M. Y., Teoh, Y. H., & Hamid, M. F. (2018). Physicochemical, Performance, Combustion and Emission Characteristics of Melaleuca Cajuputi Oil-Refined Palm Oil Hybrid Biofuel Blend. Energies, 11(11), 3146. https://doi.org/10.3390/en11113146







