Brine-Dependent Recovery Processes in Carbonate and Sandstone Petroleum Reservoirs: Review of Laboratory-Field Studies, Interfacial Mechanisms and Modeling Attempts
Abstract
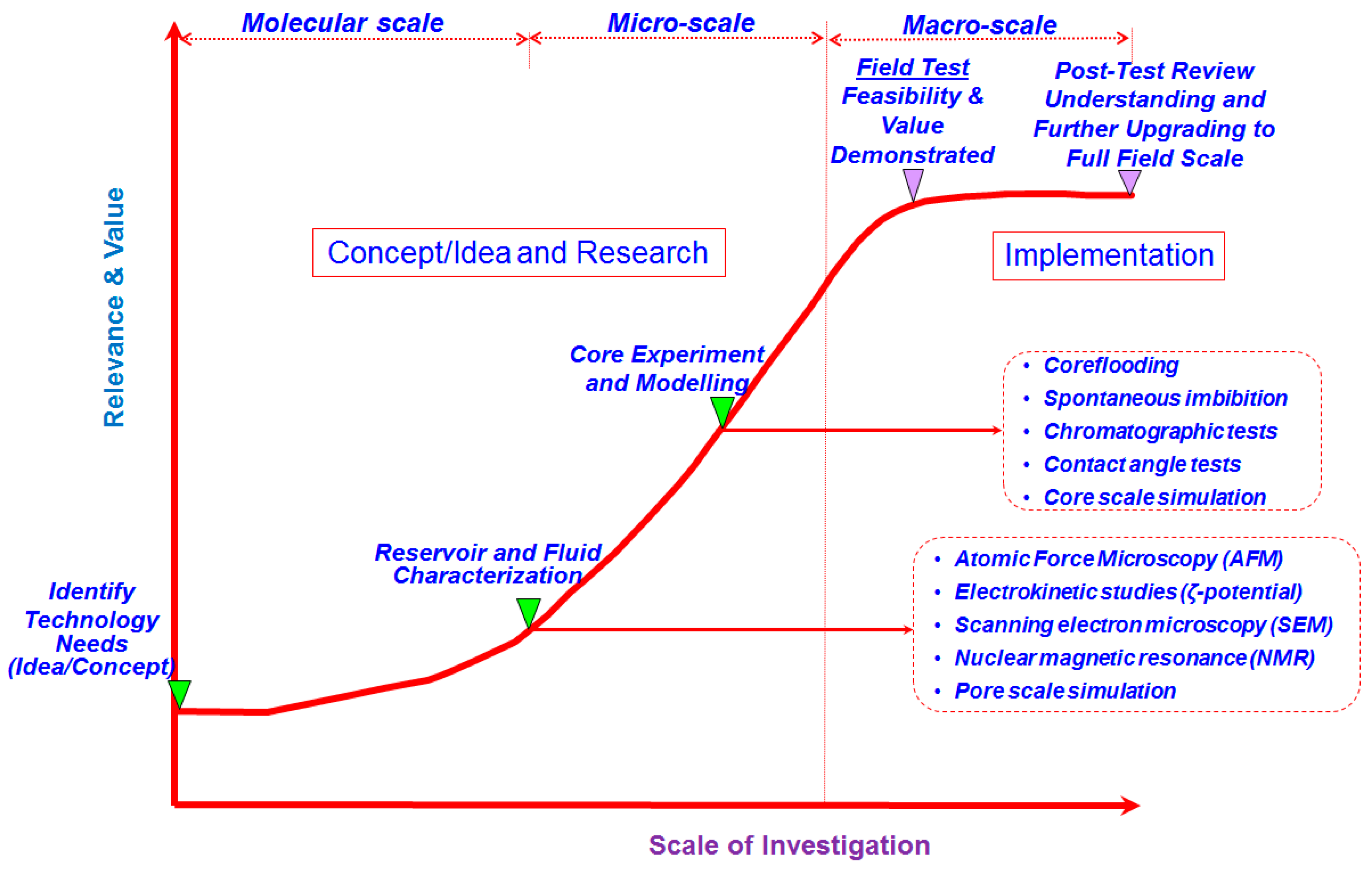
:1. Introduction
2. Laboratory Experimental Studies
2.1. Connate Water Content and Saturation
2.1.1. Sandstone Rocks
2.1.2. Carbonate Rocks
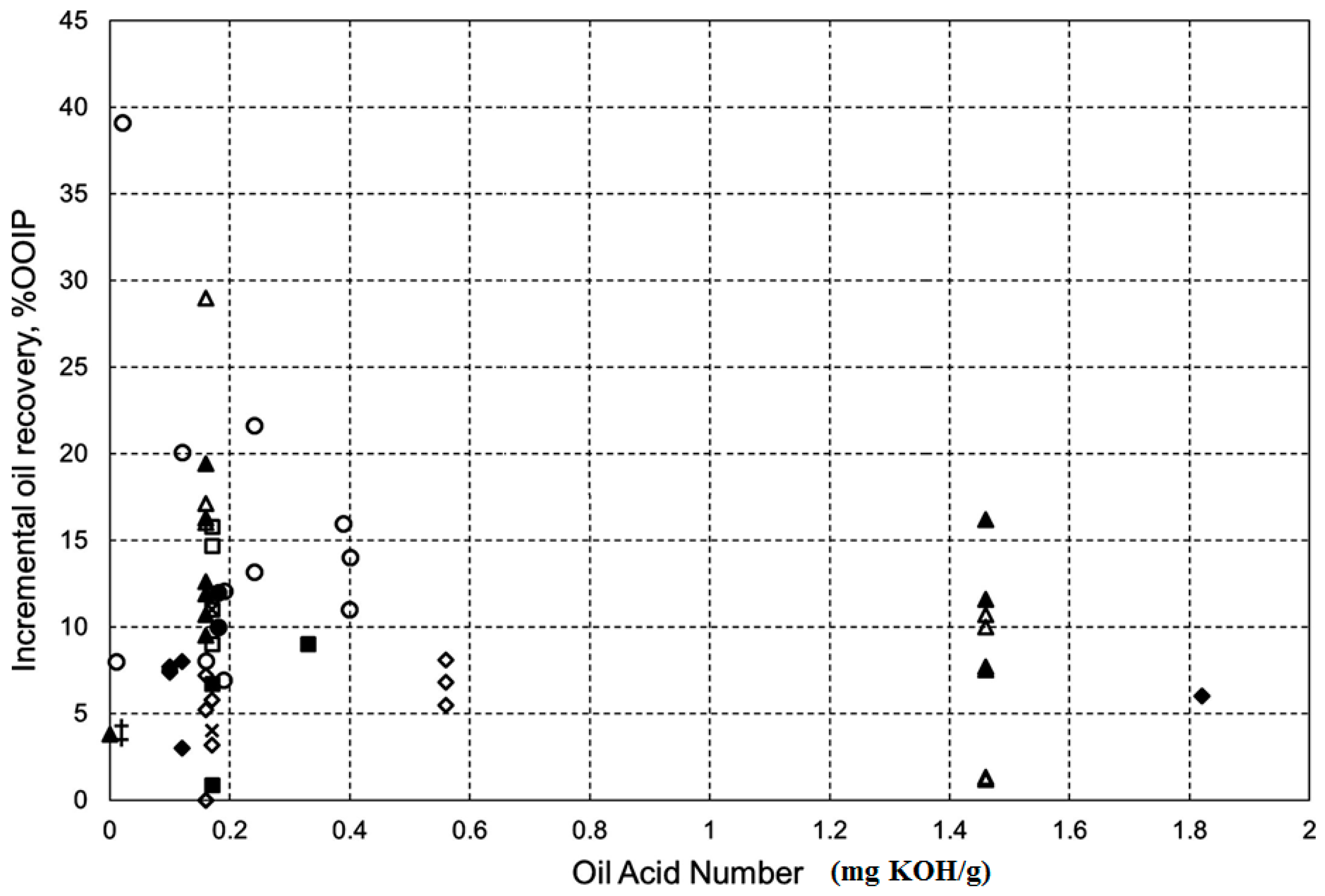
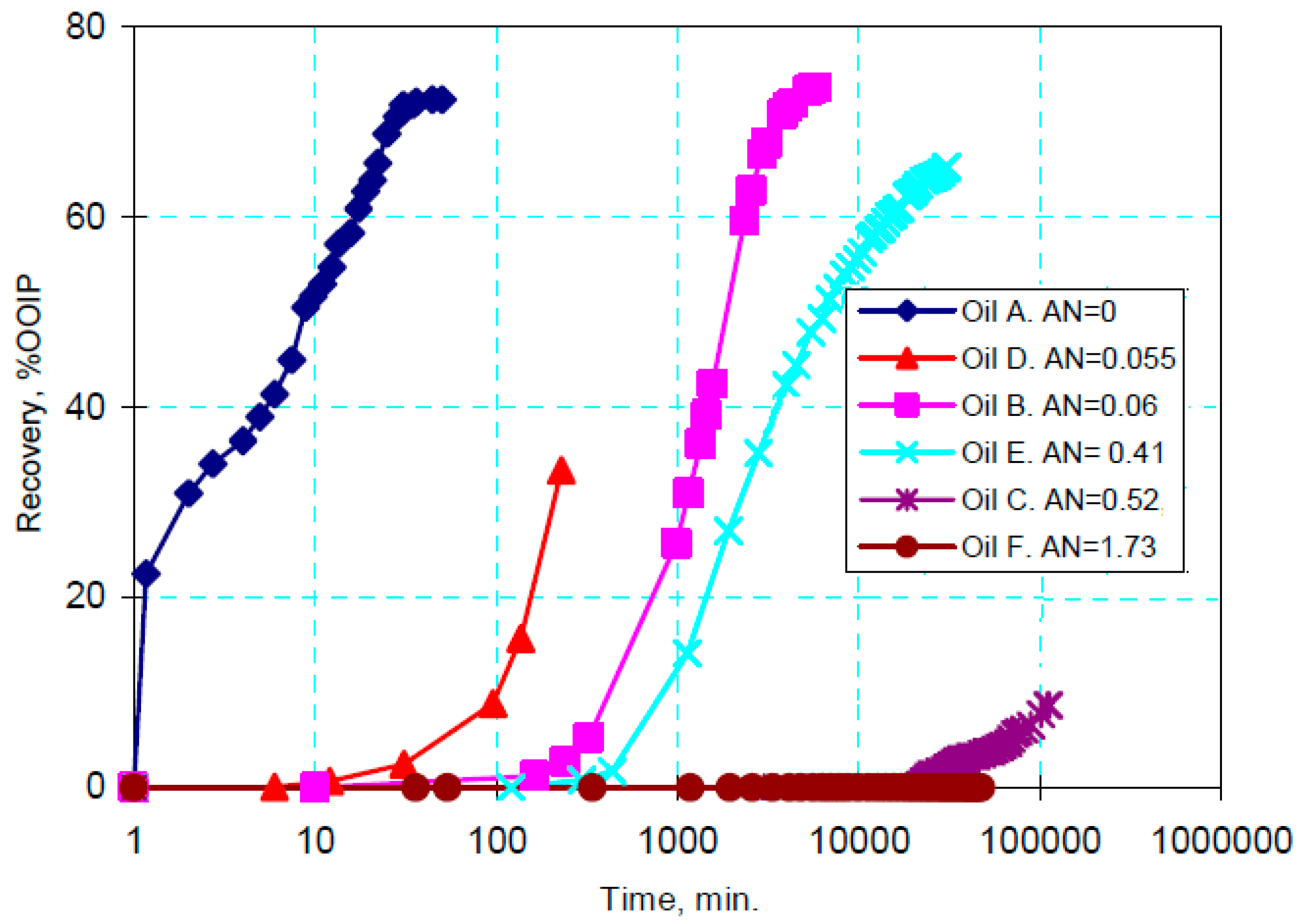
2.2. Crude Oil Composition
2.2.1. Sandstone Rocks
2.2.2. Carbonate Rocks
2.3. Rock Mineral Composition
2.3.1. Sandstone Rocks
2.3.2. Carbonate Rocks
2.4. Temperature and Pressure
2.4.1. Sandstone Rocks
2.4.2. Carbonate Rocks
2.5. Injected Brine Composition and Salinity
2.5.1. Sandstone Rocks
2.5.2. Carbonate Rocks
3. Field Application Studies
3.1. Sandstone Reservoirs
3.2. Carbonate Reservoirs
4. Proposed Underlying Recovery Mechanisms
4.1. Proposed Mechanisms in Sandstone Rocks
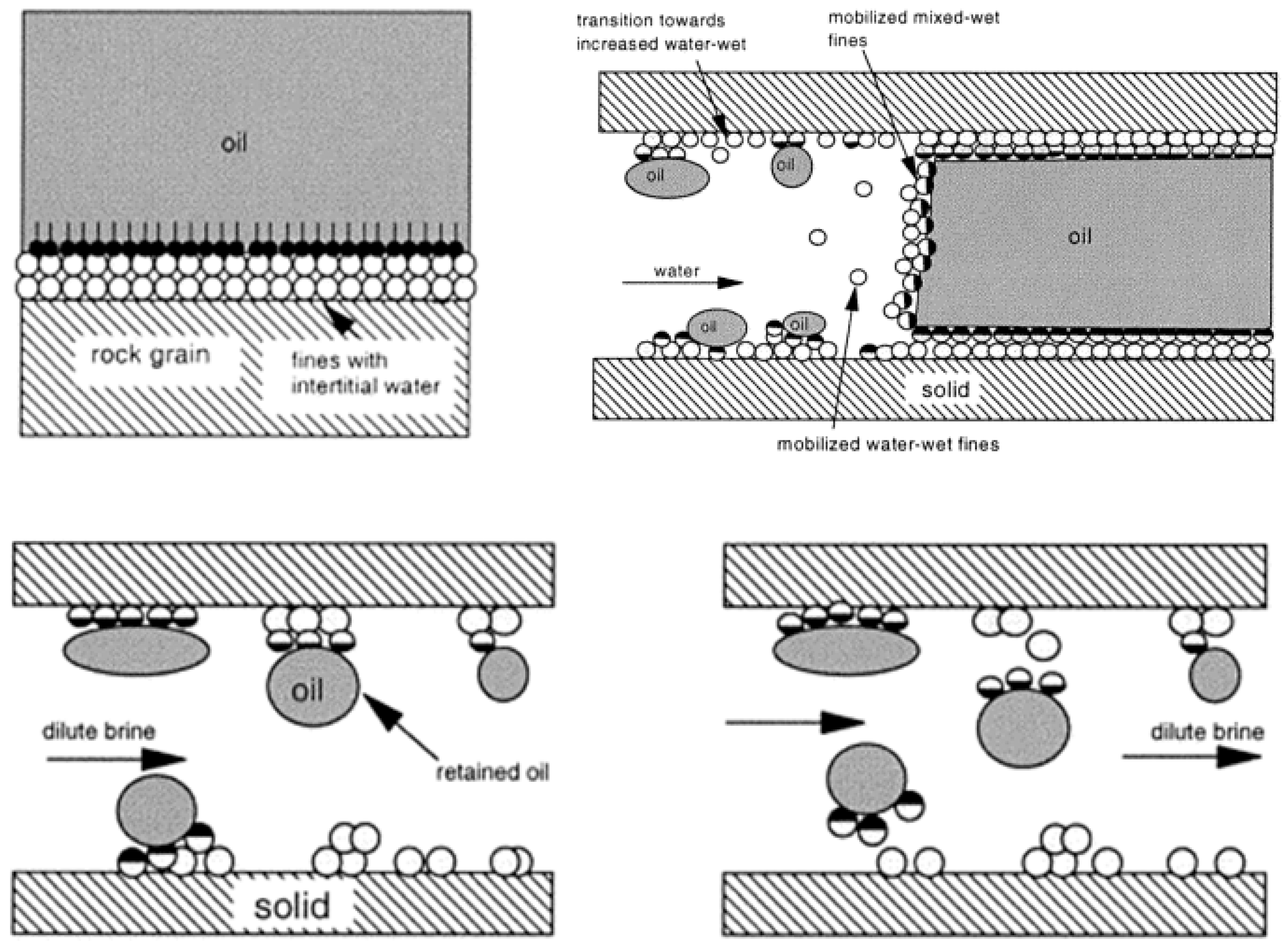
4.1.1. Swelling of Clay and Fines Mobilization
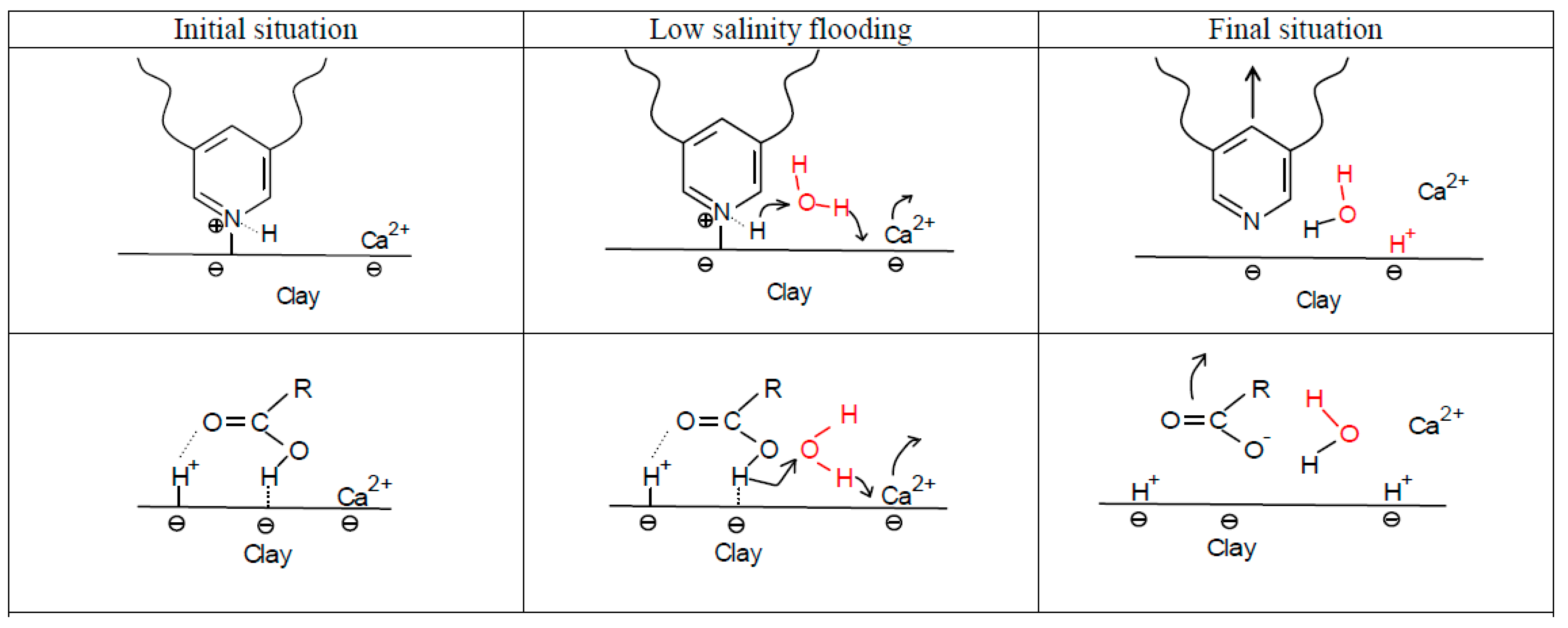
4.1.2. pH Gradient
4.1.3. Multi-Component Ionic Exchange (MIE)
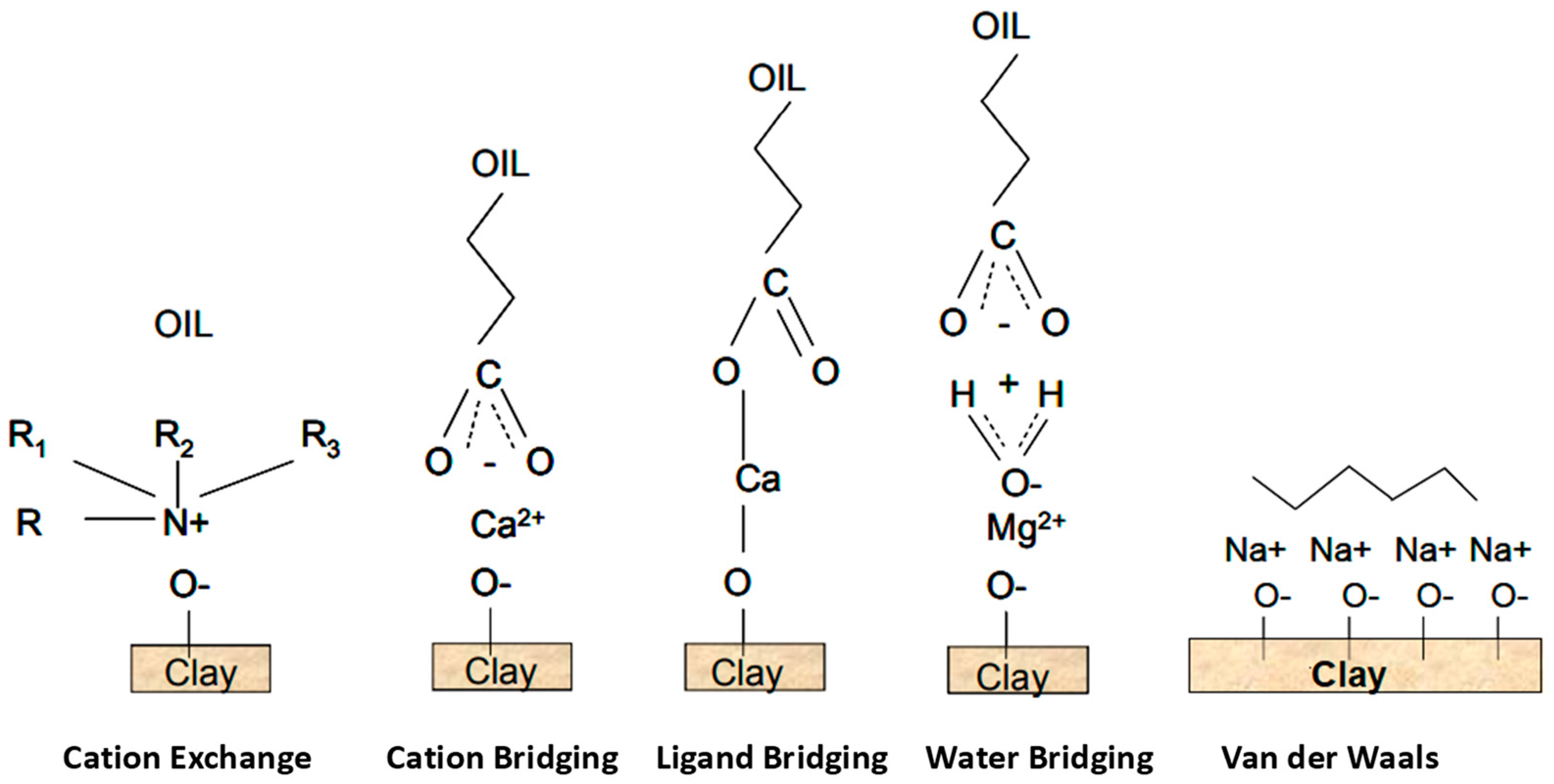
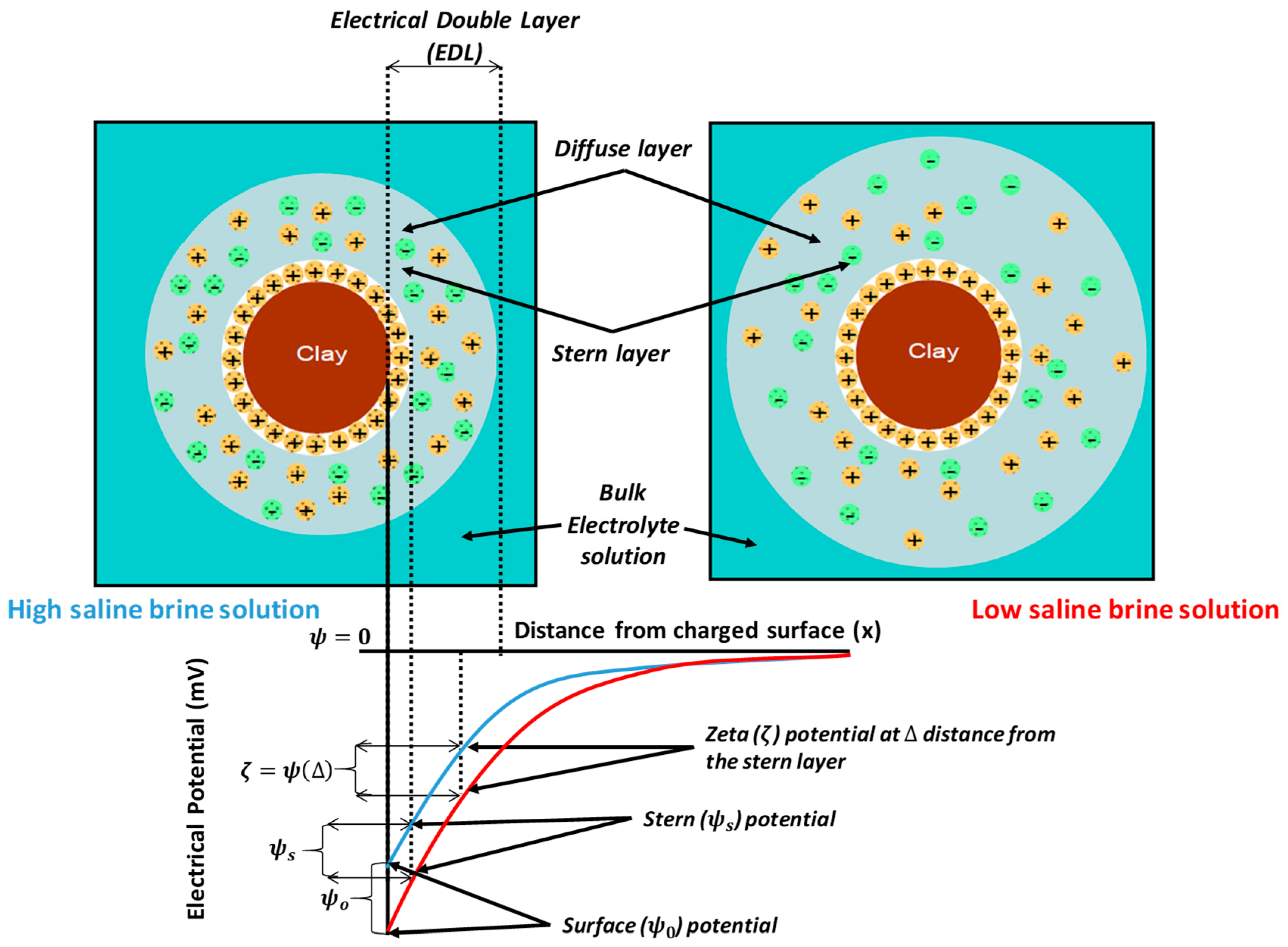
4.1.4. Electrical Interactions
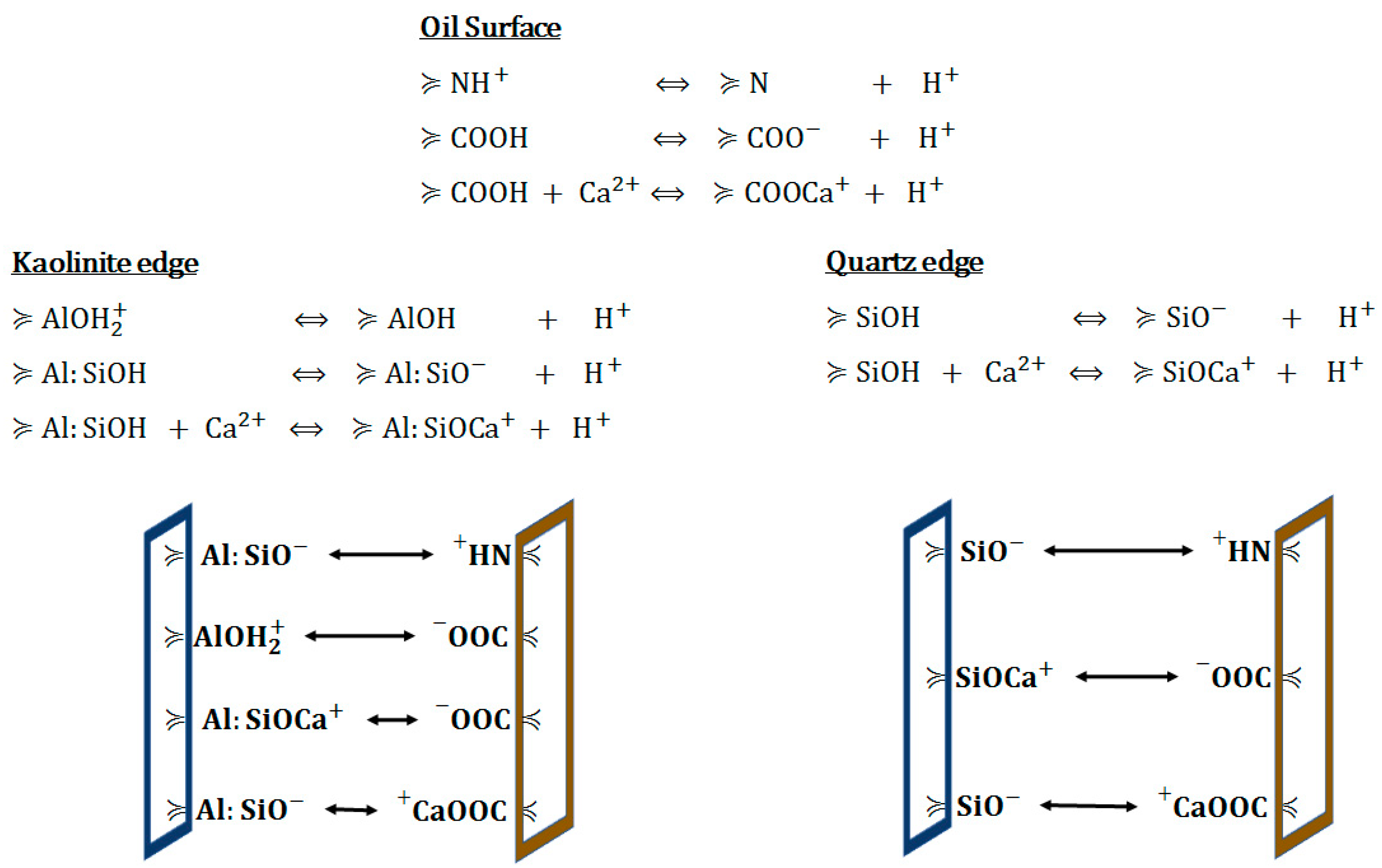
4.2. Proposed Mechanisms in Carbonate Rocks
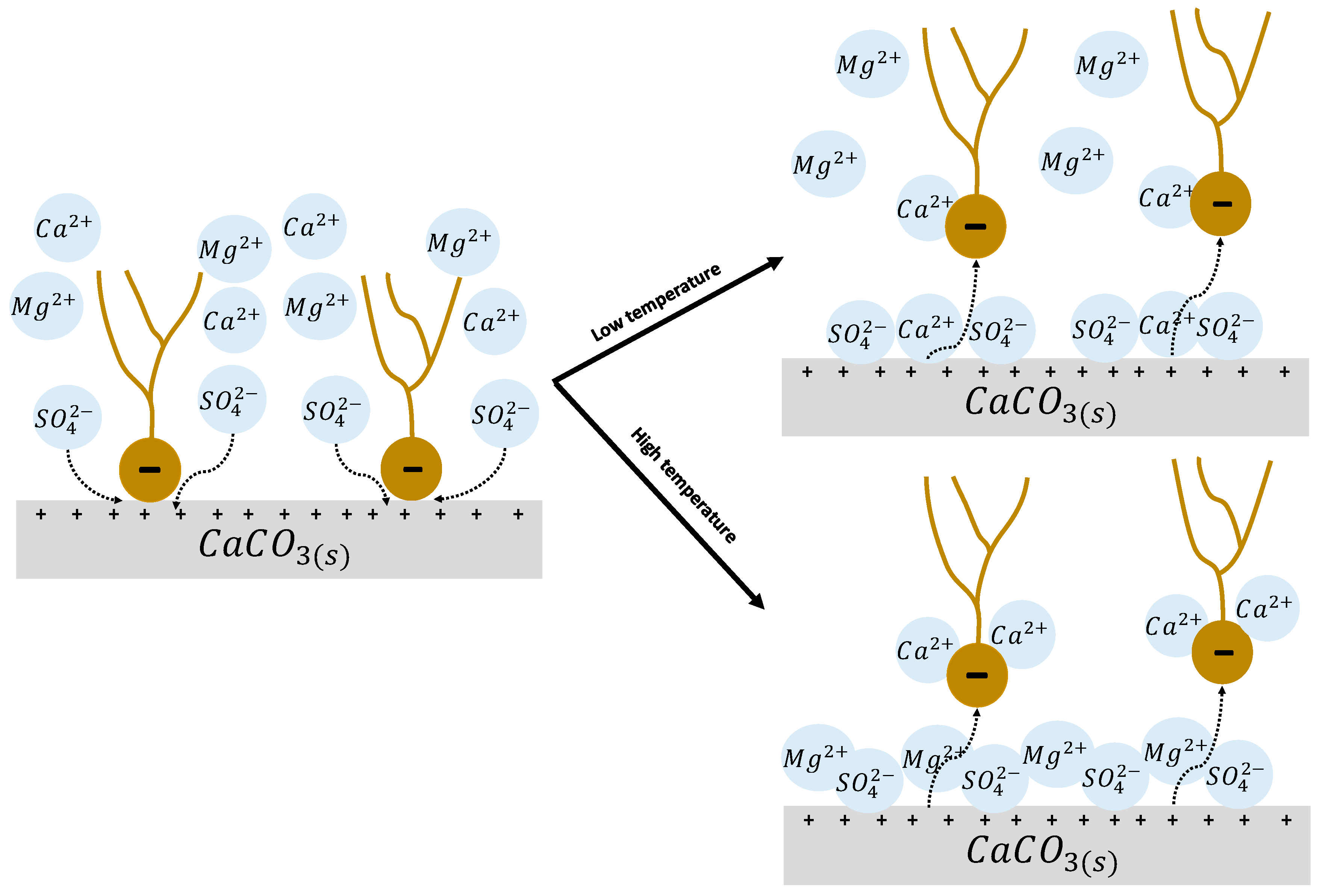
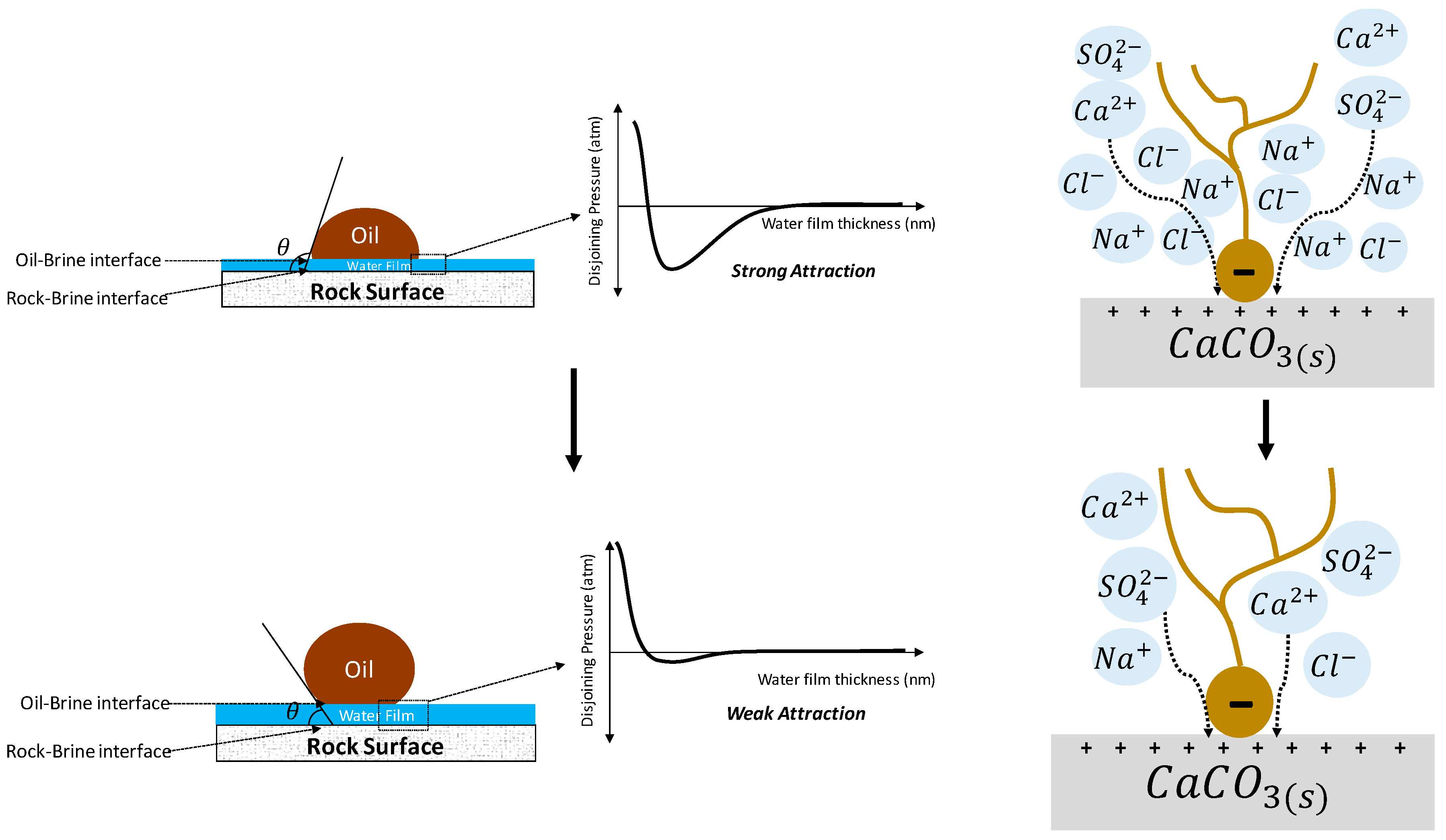
4.2.1. Rock Dissolution
4.2.2. Surface Ion Exchange
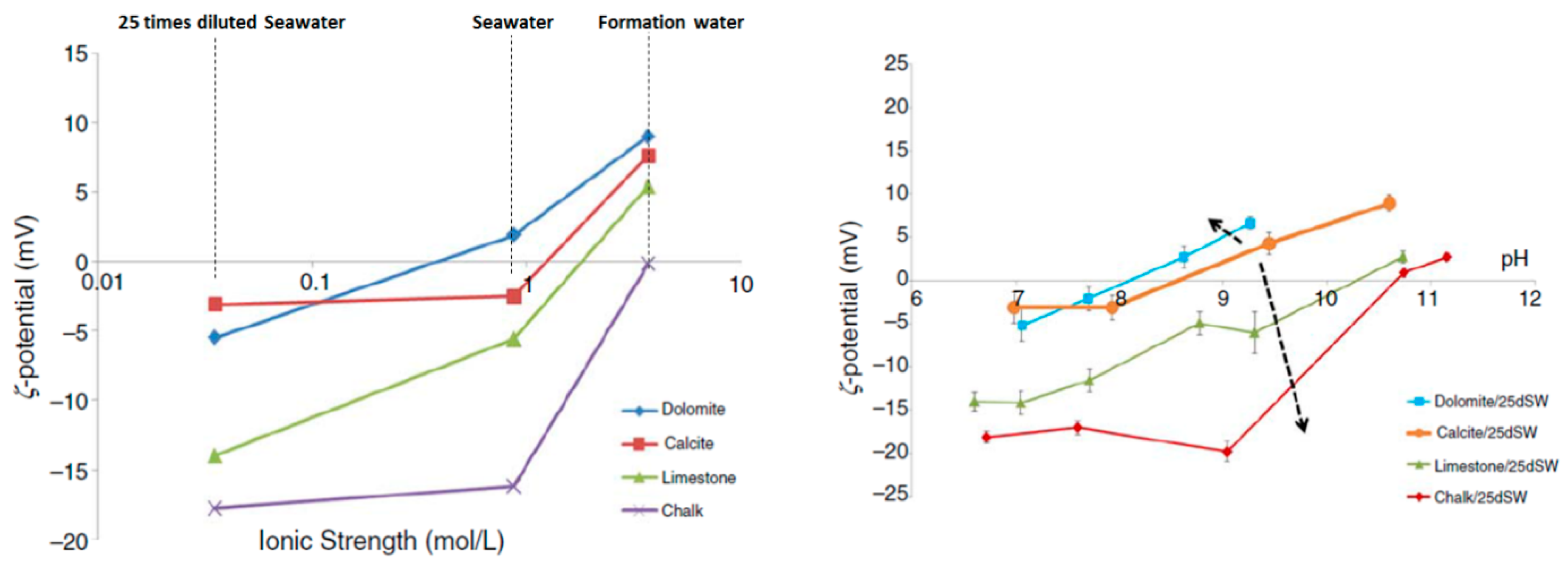
4.2.3. Electrical Interaction—DLE
5. Modeling of Brine-Dependent Recovery
5.1. Analytical Approach
5.2. Numerical Approach
5.2.1. Sandstone Rocks
5.2.2. Carbonate Rocks
6. Injection Water Issues and Remediation
7. Discussion
8. Concluding Remarks
- Brine-dependent recovery process has resulted in substantial improvement in oil recovery from laboratory to field scale studies, though the magnitude observed at the field scale is minimal compared to that observed in laboratory experiments.
- The improvement in recovery varies depending on brine content (connate and injected), rock mineralogy, oil type and structure, and temperature.
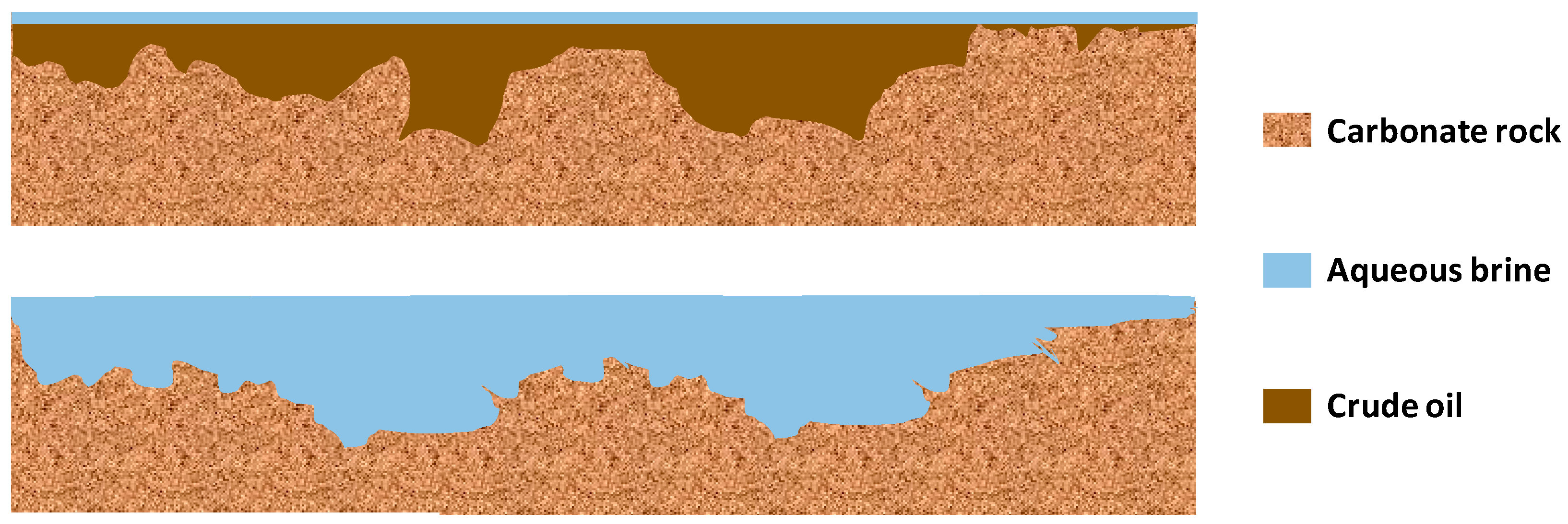
- Wettability alteration is the resultant effect of the recovery process, while the probable cause/mechanism is a combination of many proposed mechanisms.
- For a brine-dependent recovery process to be effective in carbonate rocks, the injected brine should contain PDIs depleted in NaCl, the rock should contain PDI-sourced minerals, and temperature should be high. For sandstone rocks, on the other hand, there is no temperature limitation but the optimum salinity of the injected brine should be less than 5000 ppm with modified heterovalent ion concentration.
- Various analytical and numerical models have been utilized to further interpret and predict the process performance, however, geochemical models comprising of surface sorption and complexation appear to give comparably better interpretation and prediction.
- Brine-dependent recovery process is a relatively inexpensive and environmental friendly process, particularly because of various emerging cost-effective water treatment technologies.
- Finally, the comparison between brine-dependent recovery process in sandstone and carbonate reservoir highlighted in this review potentially serves to highlight the need for relevant studies for the particular type of candidate reservoirs.
- The areas that need further investigations are identified, and they include crude oil characterization beyond the current AN and BN characterization, effect of varying degree of mineralogy in sandstone rocks, development of semi-analytical models, generating the thermodynamic parameters to describe the surface sorption and complexation reactions for natural rocks
Funding
Acknowledgments
Conflicts of Interest
References
- Green, D.W.; Willhite, G.P. Enhanced Oil Recovery; Henry, L., Ed.; Doherty Memorial Fund of AIME, Society of Petroleum Engineers: Richardson, TX, USA, 1998. [Google Scholar]
- Alvarado, V.; Manrique, E. Enhanced oil recovery: An update review. Energies 2010, 3, 1529–1575. [Google Scholar] [CrossRef]
- Rod, S. Quantification of uncertainty in recovery efficiency predictions: Lessons learned from 250 mature carbonate fields. In Proceedings of the SPE Annual Technical Conference and Exhibition, Denver, CO, USA, 5–8 October 2003. [Google Scholar]
- Sheng, J. Enhanced Oil Recovery Field Case Studies; Gulf Professional Publishing: Houston, TX, USA, 2013. [Google Scholar]
- Muggeridge, A.; Cockin, A.; Webb, K.; Frampton, H.; Collins, I.; Moulds, T.; Salino, P. Recovery rates, enhanced oil recovery and technological limits. Phil. Trans. R. Soc. A 2014, 372, 20120320. [Google Scholar] [CrossRef] [PubMed]
- Kokal, S.; Al-Kaabi, A. Enhanced Oil Recovery: Challenges & Opportunities. Available online: https://www.world-petroleum.org/docs/docs/publications/2010yearbook/P64-69_Kokal-Al_Kaabi.pdf (accessed on 20 July 2018).
- Baptist, O.C.; Sweeney, S. The effect of clays on the permeability of reservoir sands to waters of different saline contents. Clay Clay Miner. 1954, 3, 505–515. [Google Scholar] [CrossRef]
- Bernard, G. Effect of floodwater salinity on recovery of oil from cores containing clays. In Proceedings of the SPE California Regional Meeting, Los Angeles, CA, USA, 26–27 October 1967. [Google Scholar]
- Martin, J. The Effects of Clay on the Displacement of Heavy Oil by Water. In Proceedings of the Venezuelan Annual Meeting, Caracas, Venezuela, 14–16 October 1959. [Google Scholar]
- Jadhunandan, P.P. Effects of Brine Composition, Crude Oil, and Aging Conditions on Wettability and Oil Recovery; Department of Petroleum Engineering, New Mexico Institute of Mining & Technology: Socorro, NM, USA, 1990. [Google Scholar]
- Jadhunandan, P.P.; Morrow, N.R. Effect of wettability on waterflood recovery for crude-oil/brine/rock systems. SPE Reserv. Eval. Eng. 1995, 10, 40–46. [Google Scholar] [CrossRef]
- Tang, G.Q.; Morrow, N.R. Salinity, temperature, oil composition, and oil recovery by waterflooding. SPE Reserv. Eval. Eng. 1997, 12, 269–276. [Google Scholar] [CrossRef]
- Tang, G.Q.; Morrow, N.R. Influence of brine composition and fines migration on crude oil/brine/rock interactions and oil recovery. J. Pet. Sci. Eng. 1999, 24, 99–111. [Google Scholar] [CrossRef]
- Yildiz, H.O.; Morrow, N.R. Effect of brine composition on recovery of Moutray crude oil by waterflooding. J. Pet. Sci. Eng. 1996, 14, 159–168. [Google Scholar] [CrossRef]
- Zhou, X.; Morrow, N.R.; Ma, S. Interrelationship of wettability, initial water saturation, aging time, and oil recovery by spontaneous imbibition and waterflooding. SPE J. 2000, 5, 199–207. [Google Scholar] [CrossRef]
- Sulak, R. Ekofisk field: The first 20 years. J. Pet. Technol. 1991, 43, 1265–1271. [Google Scholar] [CrossRef]
- Sylte, J.; Hallenbeck, L.; Thomas, L. Ekofisk formation pilot waterflood. In Proceedings of the SPE Annual Technical Conference and Exhibition, Houston, TX, USA, 2–5 October 1988. [Google Scholar]
- Hallenbeck, L.; Sylte, J.; Ebbs, D.; Thomas, L. Implementation of the Ekofisk field waterflood. SPE Form. Eval. 1991, 6, 284–290. [Google Scholar] [CrossRef]
- Hermansen, H.; Thomas, L.; Sylte, J.; Aasboe, B. Twenty five years of Ekofisk reservoir management. In Proceedings of the SPE Annual Technical Conference and Exhibition, San Antonio, TX, USA, 5–8 October 1997. [Google Scholar]
- Callegaro, C.; Masserano, F.; Bartosek, M.; Buscaglia, R.; Visintin, R.; Hartvig, S.K.; Huseby, O.K. Single Well Chemical Tracer Tests to Assess Low Salinity Water and Surfactant EOR Processes in West Africa. In Proceedings of the International Petroleum Technology Conference, Kuala Lumpur, Malaysia, 10–12 December 2014. [Google Scholar]
- McGuire, P.; Chatham, J.; Paskvan, F.; Sommer, D.; Carini, F. Low Salinity Oil Recovery: An Exciting New EOR Opportunity for Alaska’s North Slope. In Proceedings of the SPE Western Regional Meeting, Irvine, CA, USA, 30 March–1 April 2005. [Google Scholar]
- Yousef, A.; Liu, J.; Blanchard, G.; Al-Saleh, S.; Al-Zahrani, T.; Al-Zahrani, R.; Tammar, H.; Al-Mulhim, N. Smart Waterflooding: Industry’s First Field Test in Carbonate Reservoirs. In Proceedings of the SPE Annual Technical Conference and Exhibition, San Antonio, TX, USA, 8–10 October 2012. [Google Scholar]
- Awolayo, A.N.; Sarma, H.K.; AlSumaiti, A.M. An Experimental Investigation into the Impact of Sulfate Ions in Smart Water to Improve Oil Recovery in Carbonate Reservoirs. Transp. Porous Media 2015, 111, 649–668. [Google Scholar] [CrossRef]
- Lager, A.; Webb, K.J.; Black, C.J.J.; Singleton, M.; Sorbie, K.S. Low salinity oil recovery—An experimental investigation 1. Petrophysics 2008, 49. Available online: https://www.onepetro.org/journal-paper/SPWLA-2008-v49n1a2 (accessed on 20 August 2018).
- Yousef, A.A.; Al-Saleh, S.H.; Al-Kaabi, A.; Al-Jawfi, M.S. Laboratory investigation of the impact of injection-water salinity and ionic content on oil recovery from carbonate reservoirs. SPE Reserv. Eval. Eng. 2011, 14, 578–593. [Google Scholar] [CrossRef]
- Zhang, Y.; Sarma, H. Improving Waterflood Recovery Efficiency in Carbonate Reservoirs through Salinity Variations and Ionic Exchanges: A Promising Low-Cost “Smart-Waterflood” Approach. In Proceedings of the Abu Dhabi International Petroleum Conference and Exhibition, Abu Dhabi, UAE, 11–14 November 2012. [Google Scholar]
- Austad, T.; Strand, S.; Høgnesen, E.J.; Zhang, P. Seawater as IOR fluid in fractured chalk. In Proceedings of the SPE International Symposium on Oilfield Chemistry, The Woodlands, TX, USA, 2–4 February 2005. [Google Scholar]
- Fathi, S.J.; Austad, T.; Strand, S. “Smart Water” as a Wettability Modifier in Chalk: The Effect of Salinity and Ionic Composition. Energy Fuels 2010, 24, 2514–2519. [Google Scholar] [CrossRef] [Green Version]
- Pu, H.; Xie, X.; Yin, P.; Morrow, N.R. Low-salinity waterflooding and mineral dissolution. In Proceedings of the SPE Annual Technical Conference and Exhibition, Florence, Italy, 19−22 September 2010. [Google Scholar]
- Alameri, W.; Teklu, T.W.; Graves, R.M.; Kazemi, H.; AlSumaiti, A.M. Wettability alteration during low-salinity waterflooding in carbonate reservoir cores. In Proceedings of the SPE Asia Pacific Oil & Gas Conference and Exhibition, Adelaide, Australia, 14–16 October 2014. [Google Scholar]
- Høgnesen, E.; Strand, S.; Austad, T. Waterflooding of preferential oil-wet carbonates: Oil recovery related to reservoir temperature and brine composition. In Proceedings of the SPE Europec/EAGE Annual Conference, Madrid, Spain, 13–16 June 2005. [Google Scholar]
- Ligthelm, D.; Gronsveld, J.; Hofman, J.; Brussee, N.; Marcelis, F.; van der Linde, H. Novel Waterflooding Strategy By Manipulation Of Injection Brine Composition. In Proceedings of the EUROPEC/EAGE Conference and Exhibition, Amsterdam, The Netherlands, 8–11 June 2009. [Google Scholar]
- Loahardjo, N.; Xie, X.; Yin, P.; Morrow, N.R. Low salinity waterflooding of a reservoir rock. In Proceedings of the International Symposium of the Society of Core Analysts, Calgary, AB, Canada, 10–12 September 2007. [Google Scholar]
- Shariatpanahi, S.F.; Strand, S.; Austad, T. Evaluation of water-based enhanced oil recovery (EOR) by wettability alteration in a low-permeable fractured limestone oil reservoir. Energy Fuels 2010, 24, 5997–6008. [Google Scholar] [CrossRef]
- Sharma, M.M.; Filoco, P.R. Effect of Brine Salinity and Crude-Oil Properties on Oil Recovery and Residual Saturations. SPE J. 2000, 5, 293–300. [Google Scholar] [CrossRef]
- Skrettingland, K.; Holt, T.; Tweheyo, M.T.; Skjevrak, I. Snorre Low-Salinity-Water Injection--Coreflooding Experiments and Single-Well Field Pilot. SPE Reserv. Eval. Eng. 2011, 14, 182–192. [Google Scholar] [CrossRef]
- Zahid, A.; Shapiro, A.; Skauge, A. Experimental studies of low salinity water flooding in carbonate reservoirs: A new promising approach. In Proceedings of the SPE EOR Conference at Oil and Gas West Asia, Muscat, Oman, 16–18 April 2012. [Google Scholar]
- Winoto, W.; Loahardjo, N.; Xie, S.; Yin, P.; Morrow, N. Secondary and Tertiary Recovery of Crude Oil from Outcrop and Reservoir Rocks by Low Salinity Waterflooding. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 14–18 April 2012. [Google Scholar]
- Awolayo, A.N.; Sarma, H.K. Impact of Multi-ion Interactions on Oil Mobilization by Smart Waterflooding in Carbonate Reservoirs. J. Pet. Environ. Biotechnol. 2016, 7, 1–8. [Google Scholar] [CrossRef]
- Rivet, S.; Lake, L.W.; Pope, G.A. A coreflood investigation of low-salinity enhanced oil recovery. In Proceedings of the SPE Annual Technical Conference and Exhibition, Florence, Italy, 19–22 September 2010. [Google Scholar]
- Aksulu, H.; Håmsø, D.; Strand, S.; Puntervold, T.; Austad, T. Evaluation of low-salinity enhanced oil recovery effects in sandstone: Effects of the temperature and pH gradient. Energy Fuels 2012, 26, 3497–3503. [Google Scholar] [CrossRef]
- Rezaeidoust, A.; Puntervold, T.; Austad, T. A Discussion of the Low-Salinity EOR Potential for a North Sea Sandstone Field. In Proceedings of the SPE Annual Technical Conference and Exhibition, Florence, Italy, 19–22 September 2010; p. 12. [Google Scholar]
- Boussour, S.; Cissokho, M.; Cordier, P.; Bertin, H.J.; Hamon, G. Oil Recovery by Low-Salinity Brine Injection: Laboratory Results on Outcrop and Reservoir Cores. In Proceedings of the SPE Annual Technical Conference and Exhibition, New Orleans, LA, USA, 4–7 October 2009; p. 12. [Google Scholar]
- RezaeiDoust, A.; Puntervold, T.; Strand, S.; Austad, T. Smart water as wettability modifier in carbonate and sandstone: A discussion of similarities/differences in the chemical mechanisms. Energy Fuels 2009, 23, 4479–4485. [Google Scholar] [CrossRef]
- Austad, T.; Shariatpanahi, S.; Strand, S.; Black, C.J.J.; Webb, K.J. Conditions for a Low-Salinity Enhanced Oil Recovery (EOR) Effect in Carbonate Oil Reservoirs. Energy Fuels 2011, 26, 569–575. [Google Scholar] [CrossRef]
- Romanuka, J.; Hofman, J.; Ligthelm, D.; Suijkerbuijk, B.; Marcelis, F.; Oedai, S.; Brussee, N.; van der Linde, H.; Aksulu, H.; Austad, T. Low Salinity EOR in Carbonates. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 14–18 April 2012. [Google Scholar]
- Yousef, A.; Al-Saleh, S.; Al-Kaabi, A.; Al-Jawfi, M. Laboratory investigation of novel oil recovery method for carbonate reservoirs. In Proceedings of the Canadian Unconventional Resources and International Petroleum Conference, Calgary, AB, Canada, 19–21 October 2010. [Google Scholar]
- Yousef, A.; Al-Saleh, S.; Al-Jawfi, M. Improved/Enhanced Oil Recovery from Carbonate Reservoirs by Tuning Injection Water Salinity and Ionic Content. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 14–18 April 2012. [Google Scholar]
- Al Mahrouqi, D.; Vinogradov, J.; Jackson, M.D. Temperature dependence of the zeta potential in intact natural carbonates. Geophys. Res. Lett. 2016, 43. [Google Scholar] [CrossRef]
- Al Mahrouqi, D.; Vinogradov, J.; Jackson, M.D. Zeta potential of artificial and natural calcite in aqueous solution. Adv. Colloid Interface Sci. 2016, 240, 60–76. [Google Scholar] [CrossRef] [PubMed]
- Alroudhan, A.; Vinogradov, J.; Jackson, M. Zeta potential of intact natural limestone: Impact of potential-determining ions Ca2+, Mg2+ and SO42−. Colloids Surf. A Physicochem. Eng. Asp. 2016, 493, 83–98. [Google Scholar] [CrossRef]
- Austad, T.; RezaeiDoust, A.; Puntervold, T. Chemical mechanism of low salinity water flooding in sandstone reservoirs. In Proceedings of the SPE improved oil recovery symposium, Tulsa, OK, USA, 24–28 April 2010. [Google Scholar]
- Gupta, R.; Smith, P., Jr.; Willingham, T.; Lo Cascia, M.; Shyeh, J.; Harris, C. Enhanced Waterflood for Middle East Carbonate Cores–Impact of Injection Water Composition. In Proceedings of the SPE Middle East Oil and Gas Show and Conference, Manama, Bahrain, 25–28 September 2011. [Google Scholar]
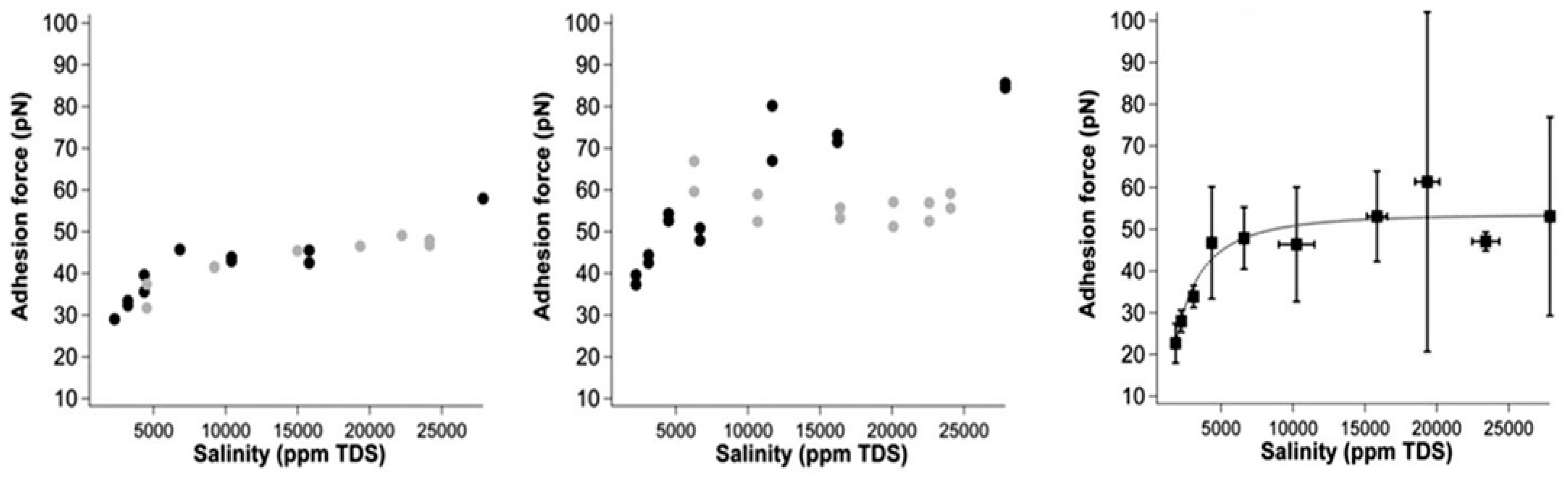
- Hilner, E.; Andersson, M.P.; Hassenkam, T.; Matthiesen, J.; Salino, P.; Stipp, S.L.S. The effect of ionic strength on oil adhesion in sandstone–the search for the low salinity mechanism. Sci. Rep. 2015, 5, 9933. [Google Scholar] [CrossRef] [PubMed]
- Mahani, H.; Keya, A.L.; Berg, S.; Bartels, W.-B.; Nasralla, R.; Rossen, W.R. Insights into the mechanism of wettability alteration by low-salinity flooding (LSF) in carbonates. Energy Fuels 2015, 29, 1352–1367. [Google Scholar] [CrossRef]
- Nasralla, R.A.; Nasr-El-Din, H.A. Impact of Electrical Surface Charges and Cation Exchange on Oil Recovery by Low Salinity Water. In Proceedings of the SPE Asia Pacific Oil and Gas Conference and Exhibition, Jakarta, Indonesia, 20–22 September 2011. [Google Scholar]
- Strand, S.; Høgnesen, E.J.; Austad, T. Wettability alteration of carbonates—Effects of potential determining ions (Ca2+ and SO42-) and temperature. Colloids Surf. A Physicochem. Eng. Asp. 2006, 275, 1–10. [Google Scholar] [CrossRef]
- Vinogradov, J.; Jaafar, M.; Jackson, M. Measurement of streaming potential coupling coefficient in sandstones saturated with natural and artificial brines at high salinity. J. Geophys. Res. Solid Earth 2010, 115. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Tweheyo, M.T.; Austad, T. Wettability alteration and improved oil recovery by spontaneous imbibition of seawater into chalk: Impact of the potential determining ions Ca2+, Mg2+, and SO42−. Colloids Surf. A Physicochem. Eng. Asp. 2007, 301, 199–208. [Google Scholar] [CrossRef]
- Al-Shalabi, E.W.; Sepehrnoori, K.; Delshad, M.; Pope, G. A Novel Method To Model Low-Salinity-Water Injection in Carbonate Oil Reservoirs. SPE J. 2014, 20, 1–154. [Google Scholar] [CrossRef]
- Andersen, P.Ø.; Evje, S.; Madland, M.V.; Hiorth, A. A geochemical model for interpretation of chalk core flooding experiments. Chem. Eng. Sci. 2012, 84, 218–241. [Google Scholar] [CrossRef]
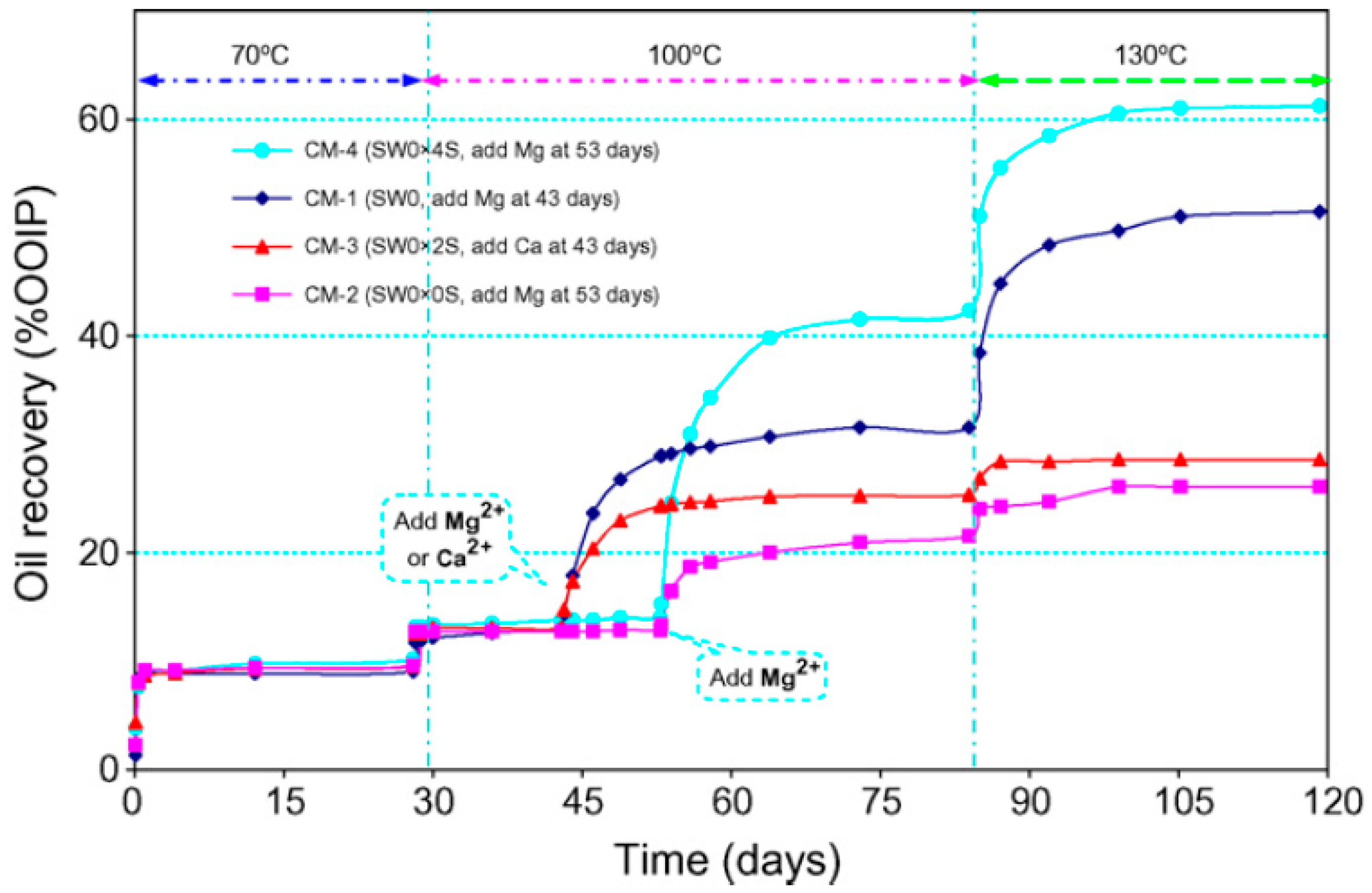
- Awolayo, A.N.; Sarma, H.K.; Nghiem, L.X. Modeling the characteristic thermodynamic interplay between potential determining ions during brine-dependent recovery process in carbonate rocks. Fuel 2018, 224, 701–717. [Google Scholar] [CrossRef]
- Dang, C.T.Q.; Nghiem, L.X.; Chen, Z.J.; Nguyen, Q.P. Modeling Low Salinity Waterflooding: Ion Exchange, Geochemistry and Wettability Alteration. In Proceedings of the SPE Annual Technical Conference and Exhibition, New Orleans, LA, USA, 30 September–2 October 2013. [Google Scholar]
- Evje, S.; Hiorth, A. A mathematical model for dynamic wettability alteration controlled by water-rock chemistry. Netw. Heterog. Media 2010, 5, 217–256. [Google Scholar] [CrossRef] [Green Version]
- Hiorth, A.; Cathles, L.; Madland, M. The impact of pore water chemistry on carbonate surface charge and oil wettability. Transp. Porous Media 2010, 85, 1–21. [Google Scholar] [CrossRef]
- Jerauld, G.R.; Webb, K.J.; Lin, C.-Y.; Seccombe, J.C. Modeling low-salinity waterflooding. SPE Reserv. Eval. Eng. 2008, 11. [Google Scholar] [CrossRef]
- Omekeh, A.V.; Friis, H.A.; Fjelde, I.; Evje, S. Modeling of Ion-Exchange and Solubility in Low Salinity Water Flooding. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 14–18 April 2012. [Google Scholar]
- Qiao, C.; Li, L.; Johns, R.T.; Xu, J. A Mechanistic Model for Wettability Alteration by Chemically Tuned Waterflooding in Carbonate Reservoirs. SPE J. 2015, 20, 767–783. [Google Scholar] [CrossRef]
- Venkatraman, A.; Hesse, M.A.; Lake, L.W.; Johns, R.T. Analytical solutions for flow in porous media with multicomponent cation exchange reactions. Water Resour. Res. 2014, 50, 5831–5847. [Google Scholar] [CrossRef] [Green Version]
- Zeinijahromi, A.; Nguyen, T.K.P.; Bedrikovetsky, P. Mathematical Model for Fines-Migration-Assisted Waterflooding With Induced Formation Damage. SPE J. 2013, 18, 518–533. [Google Scholar] [CrossRef]
- Adegbite, J.O.; Al-Shalabi, E.W.; Ghosh, B. Geochemical modeling of engineered water injection effect on oil recovery from carbonate cores. J. Pet. Sci. Eng. 2018, 170, 696–711. [Google Scholar] [CrossRef]
- Chandrasekhar, S.; Sharma, H.; Mohanty, K.K. Dependence of wettability on brine composition in high temperature carbonate rocks. Fuel 2018, 225, 573–587. [Google Scholar] [CrossRef]
- Awolayo, A.N.; Sarma, H.K. An analytical solution to interpret active ion transport during chemically-tuned waterflooding process in high-temperature carbonate rocks. Can. J. Chem. Eng. 2018. [Google Scholar] [CrossRef]
- Borazjani, S.; Behr, A.; Genolet, L.; Van Der Net, A.; Bedrikovetsky, P. Effects of fines migration on low-salinity waterflooding: Analytical modelling. Transp. Porous Media 2017, 116, 213–249. [Google Scholar] [CrossRef]
- Jackson, M.D.; Vinogradov, J.; Hamon, G.; Chamerois, M. Evidence, mechanisms and improved understanding of controlled salinity waterflooding part 1: Sandstones. Fuel 2016, 185, 772–793. [Google Scholar] [CrossRef] [Green Version]
- Sohal, M.A.; Thyne, G.; Søgaard, E.G. Review of recovery mechanisms of ionically modified waterflood in carbonate reservoirs. Energy Fuels 2016, 30, 1904–1914. [Google Scholar] [CrossRef]
- Sheng, J. Critical review of low-salinity waterflooding. J. Pet. Sci. Eng. 2014, 120, 216–224. [Google Scholar] [CrossRef]
- Al-Shalabi, E.W.; Sepehrnoori, K. A comprehensive review of low salinity/engineered water injections and their applications in sandstone and carbonate rocks. J. Pet. Sci. Eng. 2016, 139, 137–161. [Google Scholar] [CrossRef]
- Austad, T. Water-Based EOR in Carbonates and Sandstones: New Chemical Understanding of the EOR Potential Using “Smart Water”. In Enhanced Oil Recovery Field Case Studies, 1st ed.; Sheng, J., Ed.; Gulf Professional Publishing Elsevier: Waltham, MA, USA, 2013; pp. 301–335. [Google Scholar]
- Purswani, P.; Tawfik, M.S.; Karpyn, Z.T. Factors and mechanisms governing wettability alteration by chemically tuned waterflooding: A review. Energy Fuels 2017, 31, 7734–7745. [Google Scholar] [CrossRef]
- Afekare, D.A.; Radonjic, M. From mineral surfaces and coreflood experiments to reservoir implementations: Comprehensive review of low-salinity water flooding (LSWF). Energy Fuels 2017, 31, 13043–13062. [Google Scholar] [CrossRef]
- Myint, P.C.; Firoozabadi, A. Thin liquid films in improved oil recovery from low-salinity brine. Curr. Opin. Colloid Interface Sci. 2015, 20, 105–114. [Google Scholar] [CrossRef] [Green Version]
- Derkani, M.H.; Fletcher, A.J.; Abdallah, W.; Sauerer, B.; Anderson, J.; Zhang, Z.J. Low Salinity Waterflooding in Carbonate Reservoirs: Review of Interfacial Mechanisms. Colloids Interfaces 2018, 2, 20. [Google Scholar] [CrossRef]
- Buckley, S.E.; Leverett, M. Mechanism of fluid displacement in sands. Trans. AIME 1942, 146, 107–116. [Google Scholar] [CrossRef]
- Welge, H.J. A simplified method for computing oil recovery by gas or water drive. J. Pet. Technol. 1952, 4, 91–98. [Google Scholar] [CrossRef]
- Morrow, N.R.; Tang, G.-Q.; Valat, M.; Xie, X. Prospects of improved oil recovery related to wettability and brine composition. J. Pet. Sci. Eng. 1998, 20, 267–276. [Google Scholar] [CrossRef]
- Morrow, N.R. Wettability and Its Effect on Oil Recovery. J. Pet. Technol. 1990, 42, 1–476. [Google Scholar] [CrossRef]
- Masalmeh, S.K. Impact of capillary forces on residual oil saturation and flooding experiments for mixed to oil-wet carbonate reservoirs. In Proceedings of the Society of Core Analysts, Aberdeen, Scotland, UK, 27–30 August 2012. [Google Scholar]
- Shehata, A.M.; Alotaibi, M.B.; Nasr-El-Din, H.A. Waterflooding in carbonate reservoirs: Does the salinity matter? SPE Reserv. Eval. Eng. 2014, 17, 304–313. [Google Scholar] [CrossRef]
- Al Harrasi, A.; Al-maamari, R.S.; Masalmeh, S.K. Laboratory investigation of low salinity waterflooding for carbonate reservoirs. In Proceedings of the Abu Dhabi International Petroleum Conference and Exhibition, Abu Dhabi, UAE, 11–14 November 2012. [Google Scholar]
- Standnes, D.C.; Austad, T. Wettability alteration in chalk: 1. Preparation of core material and oil properties. J. Pet. Sci. Eng. 2000, 28, 111–121. [Google Scholar] [CrossRef]
- Xie, X.; Morrow, N.R. Oil recovery by spontaneous imbibition from weakly water-wet rocks. Petrophysics 2001, 42. [Google Scholar]
- Zhang, P.; Tweheyo, M.T.; Austad, T. Wettability alteration and improved oil recovery in chalk: The effect of calcium in the presence of sulfate. Energy Fuels 2006, 20, 2056–2062. [Google Scholar] [CrossRef]
- Al-Attar, H.H.; Mahmoud, M.Y.; Zekri, A.Y.; Almehaideb, R.; Ghannam, M. Low-salinity flooding in a selected carbonate reservoir: Experimental approach. J. Pet. Explor. Prod. Technol. 2013, 3, 139–149. [Google Scholar] [CrossRef]
- Alotaibi, M.B.; Azmy, R.; Nasr-El-Din, H.A. A comprehensive EOR study using low salinity water in sandstone reservoirs. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 24–28 April 2010. [Google Scholar]
- Awolayo, A.N.; Sarma, H.K.; Al-sumaiti, A.M. A Laboratory Study of Ionic Effect of Smart Water for Enhancing Oil Recovery in Carbonate Reservoirs. In Proceedings of the SPE EOR Conference at Oil and Gas West Asia, Muscat, Oman, 31 March–2 April 2014. [Google Scholar]
- Strand, S.; Austad, T.; Puntervold, T.; Høgnesen, E.J.; Olsen, M.; Barstad, S.M.F. “Smart Water” for Oil Recovery from Fractured Limestone: A Preliminary Study. Energy Fuels 2008, 22, 3126–3133. [Google Scholar] [CrossRef] [Green Version]
- Jackson, M.D.; Al-Mahrouqi, D.; Vinogradov, J. Zeta potential in oil-water-carbonate systems and its impact on oil recovery during controlled salinity water-flooding. Sci. Rep. 2016, 6, 37363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahani, H.; Keya, A.L.; Berg, S.; Nasralla, R. Electrokinetics of carbonate/brine interface in low-salinity waterflooding: Effect of brine salinity, composition, rock type, and pH on ζ-potential and a surface-complexation model. SPE J. 2017, 22, 53–68. [Google Scholar] [CrossRef]
- Xie, Q.; Liu, Y.; Wu, J.; Liu, Q. Ions tuning water flooding experiments and interpretation by thermodynamics of wettability. J. Pet. Sci. Eng. 2014, 124, 350–358. [Google Scholar] [CrossRef]
- Chen, Q.; Mercer, D.; Webb, K. NMR study on pore occupancy and wettability modification during low salinity waterflooding. In Proceedings of the 2010 International Symposium of Core Analysts, Halifax, NS, Canada, 4–7 October 2010; p. 12. [Google Scholar]
- Looyestijn, W.J.; Hofman, J. Wettability-Index Determination by Nuclear Magnetic Resonance. SPE Reserv. Eval. Eng. 2006, 9, 146–153. [Google Scholar] [CrossRef]
- Hassenkam, T.; Mitchell, A.C.; Pedersen, C.S.; Skovbjerg, L.; Bovet, N.; Stipp, S.L.S. The low salinity effect observed on sandstone model surfaces. Colloids Surfaces A Physicochem. Eng. Asp. 2012, 403, 79–86. [Google Scholar] [CrossRef]
- Lebedeva, E.; Senden, T.J.; Knackstedt, M.; Morrow, N. Improved Oil Recovery from Tensleep Sandstone–Studies of Brine-Rock Interactions by Micro-CT and AFM. In Proceedings of the IOR 2009-15th European Symposium on Improved Oil Recovery, Paris, France, 27–29 April 2009. [Google Scholar]
- Sarma, H.K. SPE Training Course on Chemical Enhanced Oil Recovery Methods; Unpublished: Kuala Lumpur, Malaysia, 2015. [Google Scholar]
- Strand, S.; Standnes, D.C.; Austad, T. New wettability test for chalk based on chromatographic separation of SCN− and SO42−. J. Pet. Sci. Eng. 2006, 52, 187–197. [Google Scholar] [CrossRef]
- Anderson, W. Wettability literature survey-part 1: Rock/oil/brine interactions and the effects of core handling on wettability. J. Pet. Technol. 1986, 38, 1125–1144. [Google Scholar] [CrossRef]
- Ding, H.; Rahman, S. Experimental and theoretical study of wettability alteration during low salinity water flooding-an state of the art review. Colloids Surf. A Physicochem. Eng. Asp. 2017, 520, 622–639. [Google Scholar] [CrossRef]
- Israelachvili, J.N. Intermolecular and Surface Forces: Revised, 3rd ed.; Academic Press: New York, NY, USA, 2011. [Google Scholar]
- Okasha, T.M.; Alshiwaish, A. Effect of brine salinity on interfacial tension in Arab-D carbonate reservoir, Saudi Arabia. In Proceedings of the SPE Middle East Oil and Gas Show and Conference, Manama, Bahrain, 15–18 March 2009. [Google Scholar]
- Meng, W.; Haroun, M.R.; Sarma, H.K.; Adeoye, J.T.; Aras, P.; Punjabi, S.; Rahman, M.M.; Al Kobaisi, M. A Novel Approach of Using Phosphate-spiked Smart Brines to Alter Wettability in Mixed Oil-wet Carbonate Reservoirs. In Proceedings of the Abu Dhabi International Petroleum Exhibition and Conference, Abu Dhabi, UAE, 9–12 November 2015. [Google Scholar]
- Lashkarbolooki, M.; Ayatollahi, S.; Riazi, M. Effect of salinity, resin, and asphaltene on the surface properties of acidic crude oil/smart water/rock system. Energy Fuels 2014, 28, 6820–6829. [Google Scholar] [CrossRef]
- Shariatpanahi, S.F.; Strand, S.; Austad, T. Initial wetting properties of carbonate oil reservoirs: Effect of the temperature and presence of sulfate in formation water. Energy Fuels 2011, 25, 3021–3028. [Google Scholar] [CrossRef]
- Suijkerbuijk, B.; Hofman, J.; Ligthelm, D.; Romanuka, J.; Brussee, N.; van der Linde, H.; Marcelis, F. Fundamental investigations into wettability and low salinity flooding by parameter isolation. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 14–18 April 2012. [Google Scholar]
- Lindlof, J.C.; Stoffer, K.G. A Case Study of Seawater Injection Incompatibility. SPE J. 1983, 35, 1256–1262. [Google Scholar] [CrossRef]
- Zhang, Y.; Morrow, N. Comparison of secondary and tertiary recovery with change in injection brine composition for crude-oil/sandstone combinations. In Proceedings of the SPE/DOE Symposium on Improved Oil Recovery, Tulsa, OK, USA, 22–26 April 2006. [Google Scholar]
- Shehata, A.M.; Nasr-El-Din, H.A. Laboratory Investigations To Determine the Effect of Connate-Water Composition on Low-Salinity Waterflooding in Sandstone Reservoirs. SPE Reserv. Eval. Eng. 2017, 20, 59–76. [Google Scholar] [CrossRef]
- Cissokho, M.; Bertin, H.; Boussour, S.; Cordier, P.; Hamon, G. Low salinity oil recovery on clayey sandstone: Experimental study. Petrophysics 2010, 51. Available online: https://www.onepetro.org/journal-paper/SPWLA-2010-v51n5a2 (accessed on 2 September 2018).
- Buckley, J.S.; Liu, Y.; Monsterleet, S. Mechanisms of wetting alteration by crude oils. SPE J. 1998, 3, 54–61. [Google Scholar] [CrossRef]
- Fjelde, I.; Omekeh, A.V.; Sokama-Neuyam, Y.A. Low salinity water flooding: Effect of crude oil composition. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 12–16 April 2014. [Google Scholar]
- Agbalaka, C.C.; Dandekar, A.Y.; Patil, S.L.; Khataniar, S.; Hemsath, J.R. Coreflooding studies to evaluate the impact of salinity and wettability on oil recovery efficiency. Transp. Porous Media 2009, 76, 77–94. [Google Scholar] [CrossRef]
- Li, Y. Oil recovery by low salinity water injection into a reservoir: A new study of tertiary oil recovery mechanism. Transp. Porous Media 2011, 90, 333–362. [Google Scholar] [CrossRef]
- Gamage, P.H.S.; Thyne, G.D. Comparison of oil recovery by low salinity waterflooding in secondary and tertiary recovery modes. In Proceedings of the SPE Annual Technical Conference and Exhibition, Denver, CO, USA, 30 October–2 November 2011. [Google Scholar]
- RezaeiDoust, A.; Puntervold, T.; Austad, T. Chemical Verification of the EOR Mechanism by Using Low Saline/Smart Water in Sandstone. Energy Fuels 2011, 25, 2151–2162. [Google Scholar] [CrossRef]
- Burgos, W.D.; Pisutpaisal, N.; Mazzarese, M.C.; Chorover, J. Adsorption of quinoline to kaolinite and montmorillonite. Environ. Eng. Sci. 2002, 19, 59–68. [Google Scholar] [CrossRef]
- Madsen, L.; Ida, L. Adsorption of carboxylic acids on reservoir minerals from organic and aqueous phase. SPE Reserv. Eval. Eng. 1998, 1, 47–51. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, X.; Morrow, N. Waterflood performance by injection of brine with different salinity for reservoir cores. In Proceedings of the SPE Annual Technical Conference and Exhibition, Anaheim, CA, USA, 11–14 November 2007. [Google Scholar]
- Viksund, B.G.; Morrow, N.R.; Ma, S.; Wang, W.; Graue, A. Initial water saturation and oil recovery from chalk and sandstone by spontaneous imbibition. In Proceedings of the International Symposium of Society of Core Analysts, The Hague, The Netherlands, 26–30 August 1998. [Google Scholar]
- Puntervold, T.; Strand, S.; Austad, T. Water flooding of carbonate reservoirs: Effects of a model base and natural crude oil bases on chalk wettability. Energy Fuels 2007, 21, 1606–1616. [Google Scholar] [CrossRef]
- Puntervold, T.; Strand, S.; Austad, T. New method to prepare outcrop chalk cores for wettability and oil recovery studies at low initial water saturation. Energy Fuels 2007, 21, 3425–3430. [Google Scholar] [CrossRef]
- Fernø, M.A.; Grønsdal, R.; Åsheim, J.; Nyheim, A.; Berge, M.; Graue, A. Use of sulfate for water based enhanced oil recovery during spontaneous imbibition in chalk. Energy Fuels 2011, 25, 1697–1706. [Google Scholar] [CrossRef]
- Shariatpanahi, S.F.; Hopkins, P.; Aksulu, H.; Strand, S.; Puntervold, T.; Austad, T. Water based EOR by wettability alteration in dolomite. Energy Fuels 2016, 30, 180–187. [Google Scholar] [CrossRef]
- Dubey, S.T.; Doe, P.H. Base number and wetting properties of crude oils. SPE Reserv. Eval. Eng. 1993, 8, 195–200. [Google Scholar] [CrossRef]
- Denekas, M.O.; Mattax, C.C.; Davis, G.T. Effects of Crude Oil Components on Rock Wettability. PE Trans. AIME 1959, 216, 330–333. [Google Scholar]
- Cuiec, L. Rock/Crude-Oil Interactions and Wettability: An Attempt To Understand Their Interrelation. In Proceedings of the SPE Annual Technical Conference and Exhibition, Houston, TX, USA, 16–19 September 1984. [Google Scholar]
- Ehrlich, R. Wettability alteration during displacement of oil by water from petroleum reservoir rock. In Proceedings of the 48th National Colloid Symposium ACS preprints, Austin, TX, USA, 24–26 June 1974. [Google Scholar]
- Block, A.; Simms, B.B. Desorption and exchange of adsorbed octadecylamine and stearic acid on steel and glass. J. Colloid Interface Sci. 1967, 25, 514–518. [Google Scholar] [CrossRef]
- Strassner, J.E. Effect of pH on interfacial films and stability of crude oil-water emulsions. J. Pet. Technol. 1968, 20, 303–312. [Google Scholar] [CrossRef]
- McCaffery, F.G.; Mungan, N. Contact angle and interfacial tension studies of some hydrocarbon-water-solid systems. J. Can. Pet. Technol. 1970, 9. [Google Scholar] [CrossRef]
- Marsden, S.S.; Nikias, P.A. The Wettability of the Bradford Sand. Prod. Mon. 1962, 26, 2–5. [Google Scholar]
- Basu, S.; Sharma, M.M. Investigating the role of crude-oil components on wettability alteration using atomic force microscopy. In Proceedings of the International Symposium on Oilfield Chemistry, Houston, TX, USA, 18–21 February 1997. [Google Scholar]
- Dubey, S.; Waxman, M. Asphaltene adsorption and desorption from mineral surfaces. SPE Reserv. Eval. Eng. 1991, 6, 389–395. [Google Scholar] [CrossRef]
- Akhlaq, M.S.; Kessel, D.; Dornow, W. Separation and chemical characterization of wetting crude oil compounds. J. Colloid Interface Sci. 1996, 180, 309–314. [Google Scholar] [CrossRef]
- Czarnecka, E.; Gillott, J. Formation and characterization of clay complexes with bitumen from Athabasca oil sand. Clays Clay Miner. 1980, 28, 197–203. [Google Scholar] [CrossRef]
- Collins, S.H.; Melrose, J.C. Adsorption of asphaltenes and water on reservoir rock minerals. In Proceedings of the SPE Oilfield and Geothermal Chemistry Symposium, Denver, CO, USA, 1–3 June 1983. [Google Scholar]
- Johansen, R.T.; Dunning, H.N. Relative Wetting Tendencies of Crude Oils by Capillarimetric Method; Bureau of Mines, Bartlesville Petroleum Research Center: Bartlesville, OK, USA, 1961. [Google Scholar]
- Buckley, J.S.; Wang, J. Crude oil and asphaltene characterization for prediction of wetting alteration. J. Pet. Sci. Eng. 2002, 33, 195–202. [Google Scholar] [CrossRef]
- Buckley, J.S.; Liu, Y. Some mechanisms of crude oil/brine/solid interactions. J. Pet. Sci. Eng. 1998, 20, 155–160. [Google Scholar] [CrossRef]
- Buckley, J.S.; Liu, Y.; Xie, X.; Morrow, N.R. Asphaltenes and crude oil wetting-the effect of oil composition. SPE J. 1997, 2, 107–119. [Google Scholar] [CrossRef]
- Skauge, A.; Standal, S.; Boe, S.O.; Skauge, T.; Blokhus, A.M. Effects of Organic Acids and Bases, and Oil Composition on Wettability. In Proceedings of the SPE Annual Technical Conference and Exhibition, Houston, TX, USA, 3–6 October 1999. [Google Scholar]
- Hoeiland, S.; Barth, T.; Blokhus, A.M.; Skauge, A. The effect of crude oil acid fractions on wettability as studied by interfacial tension and contact angles. J. Pet. Sci. Eng. 2001, 30, 91–103. [Google Scholar] [CrossRef]
- Mwangi, P.; Brady, P.V.; Radonjic, M.; Thyne, G. The effect of organic acids on wettability of sandstone and carbonate rocks. J. Pet. Sci. Eng. 2018, 165, 428–435. [Google Scholar] [CrossRef]
- Lowe, A.C.; Phillips, M.C.; Riddiford, A.C. On the wetting of carbonate surfaces by oil and water. J. Can. Pet. Technol. 1973, 12. [Google Scholar] [CrossRef]
- Benner, F.C.; Bartel, F.E. The effect of polar impurities upon capillary and surface phenomena in petroleum production. In Proceedings of the Drilling and production practice, New York, NY, USA, 1 January 1941. [Google Scholar]
- Morrow, N.R.; Cram, P.J.; McCaffery, F.G. Displacement Studies in Dolomite With Wettability Control by Octanoic Acid. Soc. Pet. Eng. J. 1973, 13, 221–232. [Google Scholar] [CrossRef]
- Shimoyama, A.; Johns, W.D. Formation of alkanes from fatty acids in the presence of CaCO3. Geochim. Cosmochim. Acta 1972, 36, 87–91. [Google Scholar] [CrossRef]
- Zhang, P.; Austad, T. The relative effects of acid number and temperature on chalk wettability. In Proceedings of the SPE International Symposium on Oilfield Chemistry, The Woodlands, TX, USA, 2–4 February 2005. [Google Scholar]
- Zhang, P.; Austad, T. Waterflooding in chalk: Relationship between oil recovery, new wettability index, brine composition and cationic wettability modifier. In Proceedings of the SPE Europec/EAGE Annual Conference, Madrid, Spain, 13–16 June 2005; p. 7. [Google Scholar]
- Thomas, M.M.; Clouse, J.A.; Longo, J.M. Adsorption of organic compounds on carbonate minerals: 1. Model compounds and their influence on mineral wettability. Chem. Geol. 1993, 109, 201–213. [Google Scholar] [CrossRef]
- Mwangi, P.; Thyne, G.; Rao, D. Extensive Experimental Wettability Study in Sandstone and Carbonate-Oil-Brine Systems: Part 1–Screening Tool Development. In Proceedings of the International Symposium of the Society of Core Analysts, Napa Valley, CA, USA, 16–19 September 2013. [Google Scholar]
- Gomari, K.R.; Hamouda, A. Effect of fatty acids, water composition and pH on the wettability alteration of calcite surface. J. Pet. Sci. Eng. 2006, 50, 140–150. [Google Scholar] [CrossRef]
- Fathi, S.J.; Austad, T.; Strand, S.; Puntervold, T. Wettability alteration in carbonates: The effect of water-soluble carboxylic acids in crude oil. Energy Fuels 2010, 24, 2974–2979. [Google Scholar] [CrossRef] [Green Version]
- Fathi, S.J.; Austad, T.; Strand, S. Effect of water-extractable carboxylic acids in crude oil on wettability in carbonates. Energy Fuels 2011, 25, 2587–2592. [Google Scholar] [CrossRef] [Green Version]
- Carroll, D. Ion exchange in clays and other minerals. Geol. Soc. Am. Bull. 1959, 70, 749–779. [Google Scholar] [CrossRef]
- Hughes, R.V.; Pfister, R.J. Advantages of Brines in Secondary Recovery of Petroleum by Water-flooding. SPE J. Pap. 1947, 170, 187–201. [Google Scholar] [CrossRef]
- Smith, K.W. Brines as flooding liquids. In Proceedings of the 7th Annual Technical Meetings of the Bradford District Research Group, State College, PA, USA, 6–7 November 1942. [Google Scholar]
- Reiter, P.K. A Water-Sensitive Sandstone Flood Using Low Salinity Water; University of Oklahoma: Norman, OK, USA, 1961. [Google Scholar]
- Wickramathilaka, S.; Howard, J.; Morrow, N.; Buckley, J. An experimental study of low salinity waterflooding and spontaneous imbibition. In Proceedings of the IOR 2011 16th European Symposium on Improved Oil Recovery, Cambridge, UK, 12–14 April 2011. [Google Scholar]
- Appelo, C.A.J.; Postma, D. Geochemistry, Groundwater and Pollution, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2005; p. 668. [Google Scholar]
- Law, S.; McDonald, A.; Fellows, S.; Reed, J.; Sutcliffe, P.G. Influence of Clay Content and Type on Oil Recovery Under Low Salinity Waterflooding in North Sea Reservoirs. In Proceedings of the SPE Offshore Europe Conference and Exhibition, Aberdeen, Scotland, UK, 8–11 September 2015. [Google Scholar]
- Tong, Z.; Yin, P.; Morrow, N.R.; Brabec, D.J. Improvement of Water Flood Performance of Low-Permeability Sandstone Reservoirs. In Proceedings of the AAPG Rocky Mountain Section Annual Meeting, Billings, MT, USA, 10–13 June 2006. [Google Scholar]
- Lager, A.; Webb, K.J.; Black, C.J.J. Impact of brine chemistry on oil recovery. In Proceedings of the 14th European Symposium on Improved Oil Recovery, Cairo, Egypt, 22–24 April 2007. [Google Scholar]
- Seccombe, J.C.; Lager, A.; Webb, K.J.; Jerauld, G.; Fueg, E. Improving wateflood recovery: LoSalTM EOR field evaluation. In Proceedings of the SPE Symposium on Improved Oil Recovery, Tulsa, OK, USA, 20–23 April 2008. [Google Scholar]
- Zhou, Z.; Gunter, W.D. The nature of the surface charge of kaolinite. Clays Clay Miner. 1992, 40, 365–368. [Google Scholar] [CrossRef]
- Lebedeva, E.V.; Fogden, A. Micro-CT and wettability analysis of oil recovery from sand packs and the effect of waterflood salinity and kaolinite. Energy Fuels 2011, 25, 5683–5694. [Google Scholar] [CrossRef]
- Pu, H.; Xie, X.; Yin, P.; Morrow, N. Application of coalbed methane water to oil recovery by low salinity waterflooding. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 19–23 April 2008; pp. 19–23. [Google Scholar]
- Hassenkam, T.; Pedersen, C.S.; Dalby, K.; Austad, T.; Stipp, S.L.S. Pore scale observation of low salinity effects on outcrop and oil reservoir sandstone. Colloids Surf. A Physicochem. Eng. Asp. 2011, 390, 179–188. [Google Scholar] [CrossRef]
- Lee, S.Y.; Webb, K.J.; Collins, I.; Lager, A.; Clarke, S.; Sullivan, M.; Routh, A.; Wang, X. Low Salinity Oil Recovery: Increasing Understanding of the Underlying Mechanisms. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 24–28 April 2010; p. 11. [Google Scholar]
- Brady, P.V.; Morrow, N.R.; Fogden, A.; Deniz, V.; Loahardjo, N. Electrostatics and the low salinity effect in sandstone reservoirs. Energy Fuels 2015, 29, 666–677. [Google Scholar] [CrossRef]
- Farooq, U.; Tweheyo, M.T.; Sjøblom, J.; Øye, G. Surface characterization of model, outcrop, and reservoir samples in low salinity aqueous solutions. J. Dispers. Sci. Technol. 2011, 32, 519–531. [Google Scholar] [CrossRef]
- Awolayo, A.; Sarma, H.; Nghiem, L. Thermodynamic Modeling of Brine Dilution-Dependent Recovery in Carbonate Rocks with Different Mineralogical Content. Energy Fuels 2018, 32, 8921–8943. [Google Scholar] [CrossRef]
- Mazzullo, S.; Chilingarian, G.V.; Bissell, H.J. Carbonate rock classifications. Dev. Pet. Sci. 1992, 30, 59–108. [Google Scholar] [CrossRef]
- Megawati, M.; Hiorth, A.; Madland, M. The impact of surface charge on the mechanical behavior of high-porosity chalk. Rock Mech. Rock Eng. 2013, 46, 1073–1090. [Google Scholar] [CrossRef]
- Korsnes, R.I.; Madland, M.V.; Austad, T.; Haver, S.; Røsland, G. The effects of temperature on the water weakening of chalk by seawater. J. Pet. Sci. Eng. 2008, 60, 183–193. [Google Scholar] [CrossRef]
- Korsnes, R.I.; Strand, S.; Hoff, Ø.; Pedersen, T.; Madland, M.V.; Austad, T. Does the chemical interaction between seawater and chalk affect the mechanical properties of chalk. In Proceedings of the International Symposium of the International Society for Rock Mechanics, Liège, Belgium, 9–12 May 2006; pp. 427–434. [Google Scholar]
- Awolayo, A.N.; Sarma, H.K.; AlSumaiti, A.M. An Experimental Study of Smart Waterflooding on Fractured Carbonate Reservoirs. In Proceedings of the ASME 2014 33rd International Conference on Ocean, Offshore and Arctic Engineering, San Francisco, CA, USA, 8–12 June 2014; p. V005T011A024. [Google Scholar]
- Pierre, A.; Lamarche, J.M.; Mercier, R.; Foissy, A.; Persello, J. Calcium as potential determining ion in aqueous calcite suspensions. J. Dispers. Sci. Technol. 1990, 11, 611–635. [Google Scholar] [CrossRef]
- Zhang, P.; Austad, T. Wettability and oil recovery from carbonates: Effects of temperature and potential determining ions. Colloids Surf. A Physicochem. Eng. Asp. 2006, 279, 179–187. [Google Scholar] [CrossRef]
- Austad, T.; Strand, S.; Madland, M.V.; Puntervold, T.; Korsnes, R.I. Seawater in Chalk: An EOR and Compaction Fluid. SPE J. Pap. 2008, 11, 648–654. [Google Scholar] [CrossRef]
- Strand, S.; Standnes, D.C.; Austad, T. Spontaneous imbibition of aqueous surfactant solutions into neutral to oil-wet carbonate cores: Effects of brine salinity and composition. Energy Fuels 2003, 17, 1133–1144. [Google Scholar] [CrossRef]
- Fathi, S.J.; Austad, T.; Strand, S. Water-based enhanced oil recovery (EOR) by “smart water”: Optimal ionic composition for EOR in carbonates. Energy Fuels 2011, 25, 5173–5179. [Google Scholar] [CrossRef] [Green Version]
- Kasha, A.; Al-Hashim, H.; Abdallah, W.; Taherian, R.; Sauerer, B. Effect of Ca2+, Mg2+ and SO42− ions on the zeta potential of calcite and dolomite particles aged with stearic acid. Colloids Surf. A Physicochem. Eng. Asp. 2015, 482, 290–299. [Google Scholar] [CrossRef]
- Ravari, R.R.; Strand, S.; Austad, T. Care must be taken to use outcrop limestone cores to mimic reservoir core material in SCAL linked to wettability alteration. In Proceedings of the 11th Intenational Symposium on Reservoir Wettability, Calgary, CA, USA, 7–9 September 2010. [Google Scholar]
- Vdović, N.; Bišćan, J. Electrokinetics of natural and synthetic calcite suspensions. Colloids Surf. A Physicochem. Eng. Asp. 1998, 137, 7–14. [Google Scholar] [CrossRef]
- Karimi, M.; Al-Maamari, R.S.; Ayatollahi, S.; Mehranbod, N. Mechanistic study of wettability alteration of oil-wet calcite: The effect of magnesium ions in the presence and absence of cationic surfactant. Colloids Surf. A Physicochem. Eng. Asp. 2015, 482, 403–415. [Google Scholar] [CrossRef]
- Dias, H.P.; Gonçalves, G.R.; Freitas, J.C.; Gomes, A.O.; de Castro, E.V.; Vaz, B.G.; Aquije, G.M.; Romão, W. Catalytic decarboxylation of naphthenic acids in crude oils. Fuel 2015, 158, 113–121. [Google Scholar] [CrossRef]
- Shimoyama, A.; Johns, W.D. Catalytic conversion of fatty acids to petroleum-like paraffins and their maturation. Nat. Phys. Sci. 1971, 232, 140–144. [Google Scholar] [CrossRef]
- Jurg, J.; Eisma, E. Petroleum hydrocarbons: Generation from fatty acid. Science 1964, 144, 1451–1452. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.-M.; Torsaeter, O.; Xie, X.; Morrow, N. The effect of crude-oil aging time and temperature on the rate of water imbibition and long-term recovery by imbibition. SPE Form. Eval. 1995, 10, 259–266. [Google Scholar] [CrossRef]
- Gamage, P.; Thyne, G. Systematic investigation of the effect of temperature during aging and low salinity flooding of Berea sandstone and Minn. In Proceedings of the IOR 2011 16th European Symposium on Improved Oil Recovery, Cambridge, UK, 12–14 April 2011. [Google Scholar]
- Vinogradov, J.; Jackson, M.D. Zeta potential in intact natural sandstones at elevated temperatures. Geophys. Res. Lett. 2015, 42, 6287–6294. [Google Scholar] [CrossRef] [Green Version]
- Saunders, J.; Jackson, M.; Gulamali, M.; Vinogradov, J.; Pain, C. Streaming potentials at hydrocarbon reservoir conditions. Geophysics 2012, 77, E77–E90. [Google Scholar] [CrossRef] [Green Version]
- Jaafar, M.Z.; Vinogradov, J.; Jackson, M.D. Measurement of streaming potential coupling coefficient in sandstones saturated with high salinity NaCl brine. Geophys. Res. Lett. 2009, 36. [Google Scholar] [CrossRef] [Green Version]
- Revil, A.; Pezard, P.A.; Glover, P.W.J. Streaming potential in porous media: 1. Theory of the zeta potential. J. Geophys. Res. Solid Earth 1999, 104, 20021–20031. [Google Scholar] [CrossRef] [Green Version]
- Rao, D.N. Wettability Effects in Thermal Recovery Operations. SPE Reserv. Eval. Eng. 1999, 2, 420–430. [Google Scholar] [CrossRef]
- Heidari, M.A.; Habibi, A.; Ayatollahi, S.; Masihi, M.; Ashoorian, S. Effect of time and temperature on crude oil aging to do a right surfactant flooding with a new approach. In Proceedings of the Offshore Technology Conference-Asia, Kuala Lumpur, Malaysia, 25–28 March 2014. [Google Scholar]
- Mahani, H.; Menezes, R.; Berg, S.; Fadili, A.; Nasralla, R.; Voskov, D.; Joekar-Niasar, V. Insights into the impact of temperature on the wettability alteration by low salinity in carbonate rocks. Energy Fuels 2017, 31, 7839–7853. [Google Scholar] [CrossRef]
- Tang, G.Q.; Morrow, N.R. Oil recovery by waterflooding and imbibition—Invading brine cation valency and salinity. In Proceedings of the International Symposium of the Society of Core Analysts, Golden, CO, USA, 1–4 August 1999. [Google Scholar]
- Webb, K.; Black, C.; Edmonds, I. Low salinity oil recovery—The role of reservoir condition corefloods. In Proceedings of the 13th EAGE Symposium on Improved Oil Recovery, Budapest, Hungary, 25–27 April 2005; pp. 25–27. [Google Scholar]
- Webb, K.J.; Black, C.J.J.; Al-Ajeel, H. Low Salinity Oil Recovery-Log-Inject-Log. In Proceedings of the SPE/DOE Symposium on Improved Oil Recovery, Tulsa, OK, USA, 17–21 April 2003. [Google Scholar]
- Wideroee, H.C.; Rueslaatten, H.; Boassen, T.; Crescente, C.M.; Raphaug, M.; Soerland, G.H.; Urkedal, H. Investigation of low salinity water flooding by NMR and CryoESEM. In Proceedings of the 2010 International Symposium of the Society of Core Analysts, Halifax, Nova Scotia, 4–7 October 2010; p. 12. [Google Scholar]
- Matthiesen, J.; Bovet, N.; Hilner, E.; Andersson, M.P.; Schmidt, D.; Webb, K.; Dalby, K.N.; Hassenkam, T.; Crouch, J.; Collins, I. How naturally adsorbed material on minerals affects low salinity enhanced oil recovery. Energy Fuels 2014, 28, 4849–4858. [Google Scholar] [CrossRef]
- Webb, K.; Black, C.; Tjetland, G. A laboratory study investigating methods for improving oil recovery in carbonates. In Proceedings of the International Petroleum Technology Conference, Doha, Qatar, 21–23 November 2005. [Google Scholar]
- Alshakhs, M.J.; Kovscek, A.R. Understanding the role of brine ionic composition on oil recovery by assessment of wettability from colloidal forces. Adv. Colloid Interface Sci. 2016, 233, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekhar, S.; Mohanty, K. Wettability Alteration with Brine Composition in High Temperature Carbonate Reservoirs. In Proceedings of the SPE Annual Technical Conference and Exhibition, New Orleans, LA, USA, 30 September–2 October 2013. [Google Scholar]
- Vo, L.T.; Gupta, R.; Hehmeyer, O.J. Ion Chromatography Analysis of Advanced Ion Management Carbonate Coreflood Experiments. In Proceedings of the Abu Dhabi International Petroleum Conference and Exhibition, Abu Dhabi, UAE, 11–14 November 2012. [Google Scholar]
- Nyström, R.; Lindén, M.; Rosenholm, J.B. The Influence of Na+, Ca2+, Ba2+, and La3+ on the ζ-Potential and the Yield Stress of Calcite Dispersions. J. Colloid Interface Sci. 2001, 242, 259–263. [Google Scholar] [CrossRef]
- Seccombe, J.; Lager, A.; Jerauld, G.; Jhaveri, B.; Buikema, T.; Bassler, S.; Denis, J.; Webb, K.; Cockin, A.; Fueg, E. Demonstration of low-salinity EOR at interwell scale, Endicott field, Alaska. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 24–28 April 2010. [Google Scholar]
- Lager, A.; Webb, K.J.; Collins, I.; Richmond, D. LoSal enhanced oil recovery: Evidence of enhanced oil recovery at the reservoir scale. In Proceedings of the SPE/DOE Symposium on Improved Oil Recovery, Tulsa, OK, USA, 20–23 April 2008. [Google Scholar]
- Robertson, E. Low-Salinity Waterflooding to Improve Oil Recovery-Historical Field Evidence. In Proceedings of the SPE Annual Technical Conference and Exhibition, Anaheim, CA, USA, 11–14 November 2007. [Google Scholar]
- Thyne, G.D.; Gamage, S.; Hasanka, P. Evaluation of the effect of low salinity waterflooding for 26 fields in Wyoming. In Proceedings of the SPE Annual Technical Conference and Exhibition, Denver, CO, USA, 30 October–2 November 2011. [Google Scholar]
- Vledder, P.; Gonzalez, I.; Carrera Fonseca, J.; Wells, T.; Ligthelm, D. Low Salinity Water Flooding: Proof of Wettability Alteration On A Field Wide Scale. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 24–28 April 2010. [Google Scholar]
- Mahani, H.; Sorop, T.; Ligthelm, D.J.; Brooks, D.; Vledder, P.; Mozahem, F.; Ali, Y. Analysis of field responses to low-salinity waterflooding in secondary and tertiary mode in Syria. In Proceedings of the SPE EUROPEC/EAGE Annual Conference and Exhibition, Vienna, Austria, 23–26 May 2011. [Google Scholar]
- Hegre, E. Low Salinity Water Injection-Case Study Heidrun Field; FORCE Seminar: Stavanger, Norway, 2008; Volume 15. [Google Scholar]
- Abdulla, F.; Hashem, H.S.; Abdulraheem, B.; Al-Nnaqi, M.; Al-Qattan, A.; John, H.; Cunningham, P.R.P.; Briggs, P.J.; Thawer, R. First EOR Trial using Low Salinity Water Injection in the Greater Burgan Field, Kuwait. In Proceedings of the SPE Middle East Oil and Gas Show and Conference, Manama, Bahrain, 10–13 March 2013. [Google Scholar]
- Al-Qattan, A.; Sanaseeri, A.; Al-Saleh, Z.; Singh, B.B.; Al-Kaaoud, H.; Delshad, M.; Hernandez, R.; Winoto, W.; Badham, S.; Bouma, C.; et al. Low Salinity Waterflood and Low Salinity Polymer Injection in the Wara Reservoir of the Greater Burgan Field. In Proceedings of the SPE EOR Conference at Oil and Gas West Asia, Muscat, Oman, 25–28 March 2018. [Google Scholar]
- Rotondi, M.; Callegaro, C.; Masserano, F.; Bartosek, M. Low Salinity Water Injection: Eni’s Experience. In Proceedings of the Abu Dhabi International Petroleum Exhibition and Conference, Abu Dhabi, UAE, 10–13 November 2014. [Google Scholar]
- Callegaro, C.; Bartosek, M.; Nobili, M.; Masserano, F.; Pollero, M.; Baz, D.M.M.; Kortam, M.M. Design and Implementation of Low Salinity Waterflood in a North African Brown Field. In Proceedings of the Abu Dhabi International Petroleum Exhibition and Conference, Abu Dhabi, UAE, 9–12 November 2015. [Google Scholar]
- Zeinijahromi, A.; Ahmetgareev, V.; Bedrikovetsky, P. Case Study of 25 Years of Low Salinity Water Injection. In Proceedings of the SPE/IATMI Asia Pacific Oil & Gas Conference and Exhibition, Bali, Indonesia, 20–22 October 2015. [Google Scholar]
- Zeinijahromi, A.; Ahmetgareev, V.; Badalyan, A.; Khisamov, R.; Bedrikovetsky, P. Case study of low salinity water injection in Zichebashskoe field. J. Pet. Sci. Res. 2015. [Google Scholar] [CrossRef]
- Akhmetgareev, V.; Khisamov, R. 40 Years of Low-Salinity Waterflooding in Pervomaiskoye Field, Russia: Incremental Oil. In Proceedings of the SPE European Formation Damage Conference and Exhibition, Budapest, Hungary, 3–5 June 2015. [Google Scholar]
- Robbana, E.; Buikema, T.A.; Mair, C.; Williams, D.; Mercer, D.J.; Webb, K.J.; Hewson, A.; Reddick, C.E. Low Salinity Enhanced Oil Recovery—Laboratory to Day One Field Implementation—LoSal EOR into the Clair Ridge Project. In Proceedings of the Abu Dhabi International Petroleum Conference and Exhibition, Abu Dhabi, UAE, 11–14 November 2012. [Google Scholar]
- Erke, S.I.; Volokitin, Y.E.; Edelman, I.Y.; Karpan, V.M.; Nasralla, R.A.; Bondar, M.Y.; Mikhaylenko, E.E.; Evseeva, M. Low Salinity Flooding Trial at West Salym Field. In Proceedings of the SPE Improved Oil Recovery Conference, Tulsa, OK, USA, 11–13 April 2016. [Google Scholar]
- Suijkerbuijk, B.M.J.M.; Sorop, T.G.; Parker, A.R.; Masalmeh, S.K.; Chmuzh, I.V.; Karpan, V.M.; Volokitin, Y.E.; Skripkin, A.G. Low Salinity Waterflooding at West-Salym: Laboratory Experiments and Field Forecasts. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 12–16 April 2014. [Google Scholar]
- Thomas, L.; Dixon, T.; Evans, C.; Vienot, M. Ekofisk waterflood pilot. J. Pet. Technol. 1987, 39, 221–232. [Google Scholar] [CrossRef]
- Hermansen, H.; Landa, G.; Sylte, J.; Thomas, L. Experiences after 10 years of waterflooding the Ekofisk Field, Norway. J. Pet. Sci. Eng. 2000, 26, 11–18. [Google Scholar] [CrossRef]
- Barkved, O.; Heavey, P.; Kjelstadli, R.; Kleppan, T.; Kristiansen, T.G. Valhall field-still on plateau after 20 years of production. In Proceedings of the Offshore Europe, Aberdeen, UK, 2–5 September 2003. [Google Scholar]
- Griffin, T.A.; Best, K.D.; Thingvoll, T.T.; Stockden, I.L.; Tjetland, G. Monitoring Waterflood Performance in a Depleted Fractured Chalk Reservoir. In Proceedings of the Offshore Europe, Scotland, UK, 4–7 September 2007. [Google Scholar]
- Tjetland, G.; Kristiansen, T.G.; Buer, K. Reservoir management aspects of early waterflood response after 25 years of depletion in the Valhall field. In Proceedings of the International Petroleum Technology Conference, Dubai, UAE, 4–6 December 2007. [Google Scholar]
- Al-adasani, A.; Bai, B.; Wu, Y.-S. Investigating low-salinity waterflooding recovery mechanisms in sandstone reservoirs. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 14–18 April 2012. [Google Scholar]
- Lever, A.; Dawe, R.A. Water-sensitivity and migration of fines in the hopeman sandstone. J. Pet. Geol. 1984, 7, 97–107. [Google Scholar] [CrossRef]
- Fogden, A.; Kumar, M.; Morrow, N.R.; Buckley, J.S. Mobilization of fine particles during flooding of sandstones and possible relations to enhanced oil recovery. Energy Fuels 2011, 25, 1605–1616. [Google Scholar] [CrossRef]
- Hussein, F.; Zeinijahromi, A.; Bedrikovetsky, P. Improved Oil Recovery with Waterflooding by Mobilising Fines (laboratory-based incremental recovery). In Proceedings of the North Africa Technical Conference and Exhibition, Cairo, Egypt, 20–22 February 2012. [Google Scholar]
- Sarkar, A.K.; Sharma, M.M. Fines migration in two-phase flow. J. Pet. Technol. 1990, 42, 646–652. [Google Scholar] [CrossRef]
- Alagic, E.; Skauge, A. Combined low salinity brine injection and surfactant flooding in mixed−wet sandstone cores. Energy Fuels 2010, 24, 3551–3559. [Google Scholar] [CrossRef]
- Ashraf, A.; Hadia, N.; Torsaeter, O.; Tweheyo, M.T. Laboratory Investigation of Low Salinity Waterflooding as Secondary Recovery Process: Effect of Wettability. In Proceedings of the SPE Oil and Gas India Conference and Exhibition, Mumbai, India, 20–22 January 2010; p. 12. [Google Scholar]
- Al-Sarihi, A.; Zeinijahromi, A.; Genolet, L.; Behr, A.; Kowollik, P.; Bedrikovetsky, P. Effects of Fines Migration on Residual Oil during Low-Salinity Waterflooding. Energy Fuels 2018, 32, 8296–8309. [Google Scholar] [CrossRef]
- Alhuraishawy, A.K.; Bai, B.; Wei, M.; Geng, J.; Pu, J. Mineral dissolution and fine migration effect on oil recovery factor by low-salinity water flooding in low-permeability sandstone reservoir. Fuel 2018, 220, 898–907. [Google Scholar] [CrossRef]
- Nasralla, R.A.; Nasr-El-Din, H.A. Double-Layer Expansion: Is It a Primary Mechanism of Improved Oil Recovery by Low-Salinity Waterflooding? SPE Reserv. Eval. Eng. 2014, 17, 49–59. [Google Scholar] [CrossRef]
- Al-Saedi, H.N.; Alhuraishawy, A.K.; Flori, R.; Brady, P.V. Sequential injection mode of high-salinity/low-salinity water in sandstone reservoirs: Oil recovery and surface reactivity tests. J. Pet. Explor. Prod. Technol. 2018. [Google Scholar] [CrossRef]
- Omekeh, A.V.; Evje, S.; Fjelde, I.; Friis, H.A. Experimental and modeling investigation of ion exchange during low salinity waterflooding. In Proceedings of the International Symposium of the Society of Core Analysts, Austin, TX, USA, 18–21 September 2011; pp. 18–21. [Google Scholar]
- Fjelde, I.; Asen, S.M.; Omekeh, A.V. Low salinity water flooding experiments and interpretation by simulations. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 14–18 April 2012. [Google Scholar]
- Kadeethum, T.; Awolayo, A.N.; Sarma, H.K.; Maini, B.; Jaruwattanasakul, C. Uncertainty Analysis of Smart Waterflood Recovery Performance in Clastic Reservoirs. Adv. Pet. Explor. Dev. 2017, 14, 18–32. [Google Scholar] [CrossRef]
- Takahashi, S.; Kovscek, A.R. Wettability estimation of low-permeability, siliceous shale using surface forces. J. Pet. Sci. Eng. 2010, 75, 33–43. [Google Scholar] [CrossRef]
- Buckley, J.S.; Takamura, K.; Morrow, N.R. Influence of electrical surface charges on the wetting properties of crude oils. SPE Reserv. Eval. Eng. 1989, 4, 332–340. [Google Scholar] [CrossRef]
- Valluri, M.K.; Alvarez, J.O.; Schechter, D.S. Study of the Rock/Fluid Interactions of Sodium and Calcium Brines with Ultra-Tight Rock Surfaces and their Impact on Improving Oil Recovery by Spontaneous Imbibition. In Proceedings of the SPE Low Perm Symposium, Denver, CO, USA, 5–6 May 2016; p. 17. [Google Scholar]
- Brady, P.V.; Krumhansl, J.L. A surface complexation model of oil–brine–sandstone interfaces at 100 °C: Low salinity waterflooding. J. Pet. Sci. Eng. 2012, 81, 171–176. [Google Scholar] [CrossRef]
- Brady, P.V.; Krumhansl, J.L.; Mariner, P.E. Surface Complexation Modeling for Improved Oil Recovery. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 14–18 April 2012. [Google Scholar]
- Xie, Q.; Saeedi, A.; Pooryousefy, E.; Liu, Y. Extended DLVO-based estimates of surface force in low salinity water flooding. J. Mol. Liq. 2016, 221, 658–665. [Google Scholar] [CrossRef]
- Karoussi, O.; Hamouda, A.A. Imbibition of sulfate and magnesium ions into carbonate rocks at elevated temperatures and their influence on wettability alteration and oil recovery. Energy Fuels 2007, 21, 2138–2146. [Google Scholar] [CrossRef]
- Austad, T.; Strand, S.; Puntervold, T. Is wettability alteration of carbonates by seawater caused by rock dissolution. In Proceedings of the International Symposium of the Society of Core Analysts, Noordwijk, The Netherlands, 27–30 September 2009. [Google Scholar]
- Den Ouden, L.; Nasralla, R.; Guo, H.; Bruining, H.; van Kruijsdijk, C. Calcite dissolution behaviour during low salinity water flooding in carbonate rock. In Proceedings of the IOR 2015 18th European Symposium on Improved Oil Recovery, Dresden, Germany, 14–16 April 2015. [Google Scholar]
- Nasralla, R.A.; Snippe, J.R.; Farajzadeh, R. Coupled Geochemical-Reservoir Model to Understand the Interaction Between Low Salinity Brines and Carbonate Rock. In Proceedings of the SPE Asia Pacific Enhanced Oil Recovery Conference, Kuala Lumpur, Malaysia, 11–13 August 2015. [Google Scholar]
- Awolayo, A.N.; Sarma, H.K.; AlSumaiti, A.M. Impact of Ionic Exchanges between Active and Non-active Ions on Displacement Efficiency in Smart Waterflood Application. In Proceedings of the 76th EAGE Conference and Exhibition 2014, Amsterdam, The Netherlands, 16–19 June 2014. [Google Scholar]
- Chandrasekhar, S.; Sharma, H.; Mohanty, K.K. Wettability Alteration with Brine Composition in High Temperature Carbonate Rocks. In Proceedings of the SPE Annual Technical Conference and Exhibition, Dubai, UAE, 26–28 September 2016. [Google Scholar]
- Takamura, K.; Chow, R.S. The electric properties of the bitumen/water interface Part II. Application of the ionizable surface-group model. Colloids Surf. 1985, 15, 35–48. [Google Scholar] [CrossRef]
- Brady, P.V.; Thyne, G. Functional wettability in carbonate reservoirs. Energy Fuels 2016, 30, 9217–9225. [Google Scholar] [CrossRef]
- Goldberg, S.; Forster, H.S. Boron sorption on calcareous soils and reference calcites. Soil Sci. 1991, 152, 304–310. [Google Scholar] [CrossRef]
- Sø, H.U.; Postma, D.; Jakobsen, R.; Larsen, F. Sorption of phosphate onto calcite; results from batch experiments and surface complexation modeling. Geochim. Cosmochim. Acta 2011, 75, 2911–2923. [Google Scholar] [CrossRef]
- Lemon, P.; Zeinijahromi, A.; Bedrikovetsky, P.; Shahin, I. Effects of injected-water salinity on waterflood sweep efficiency through induced fines migration. J. Can. Pet. Technol. 2011, 50, 82–94. [Google Scholar] [CrossRef]
- Rahbar, M.; Ayatollahi, S.; Ghatee, M.H. The Roles of Nano-Scale Intermolecular Forces on the Film Stability during Wettability Alteration Process of the Oil Reservoir Rocks. In Proceedings of the Trinidad and Tobago Energy Resources Conference, Port of Spain, Trinidad, 27–30 June 2010; p. 10. [Google Scholar]
- Awolayo, A.; Sarma, H.; Nghiem, L.; Emre, G. A Geochemical Model for Investigation of Wettability Alteration during Brine-Dependent Flooding in Carbonate Reservoirs. In Proceedings of the SPE Abu Dhabi International Petroleum Exhibition & Conference, Abu Dhabi, UAE, 13–16 November 2017. [Google Scholar]
- Tripathi, I.; Mohanty, K. Instability due to wettability alteration in displacements through porous media. Chem. Eng. Sci. 2008, 63, 5366–5374. [Google Scholar] [CrossRef]
- Wu, Y.-S.; Bai, B. Efficient simulation for low salinity waterflooding in porous and fractured reservoirs. In Proceedings of the SPE Reservoir Simulation Symposium, The Woodlands, TX, USA, 2–4 February 2009. [Google Scholar]
- Omekeh, A.V.; Evje, S.; Friis, H.A. Modeling of low salinity effects in sandstone oil rocks. Int. J. Numer. Anal. Model. 2012, 1, 1–18. [Google Scholar]
- Dang, C.T.Q.; Nghiem, L.X.; Chen, Z.; Nguyen, N.T.B.; Nguyen, Q.P. CO2 Low Salinity Water Alternating Gas: A New Promising Approach for Enhanced Oil Recovery. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 12–16 April 2014. [Google Scholar]
- Korrani, A.K.N.; Jerauld, G.R.; Sepehrnoori, K. Coupled Geochemical-Based Modeling of Low Salinity Waterflooding. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 12–16 April 2014. [Google Scholar]
- Elakneswaran, Y.; Shimokawara, M.; Nawa, T.; Takahashi, S. Surface Complexation and Equilibrium Modelling for Low Salinity Waterflooding in Sandstone Reservoirs. In Proceedings of the Abu Dhabi International Petroleum Exhibition & Conference, Abu Dhabi, UAE, 13 November 2017; p. 15. [Google Scholar]
- Erzuah, S.; Fjelde, I.; Omekeh, A.V. Wettability Estimation by Surface Complexation Simulations. In Proceedings of the SPE Europec featured at 79th EAGE Conference and Exhibition, Paris, France, 12–15 June 2017; p. 17. [Google Scholar]
- Lima, S.A.; Murad, M.A.; Domingues, R. A New Multiscale Computational Model for Low Salinity Alkaline Waterflooding in Clay-Bearing Sandstones. In Proceedings of the SPE Reservoir Simulation Conference, Montgomery, TX, USA, 20–22 February 2017; p. 17. [Google Scholar]
- Korrani, A.K.N.; Jerauld, G.R. Modeling Wettability Change in Sandstones and Carbonates Using a Surface-Complexation-Based Method. In Proceedings of the SPE Improved Oil Recovery Conference, Tulsa, OK, USA, 14–18 April 2018; p. 27. [Google Scholar]
- Yu, L.; Evje, S.; Kleppe, H.; Kårstad, T.; Fjelde, I.; Skjaeveland, S. Spontaneous imbibition of seawater into preferentially oil-wet chalk cores—Experiments and simulations. J. Pet. Sci. Eng. 2009, 66, 171–179. [Google Scholar] [CrossRef]
- Evje, S.; Hiorth, A.; Madland, M.V.; Korsnes, R.I. A mathematical model relevant for weakening of chalk reservoirs due to chemical reactions. Netw. Heterog. Media 2009, 4, 755–788. [Google Scholar] [CrossRef] [Green Version]
- Andersen, P.; Evje, S. A Mathematical Model for Interpretation of Brine-Dependent Spontaneous Imbibition Experiments. In Proceedings of the ECMOR XIII-13th European Conference on the Mathematics of Oil Recovery, Biarritz, France, 10–13 September 2012. [Google Scholar]
- Al-Shalabi, E.W.; Delshad, M.; Sepehrnoori, K. Does the Double Layer Expansion Mechanism Contribute to the LSWI Effect on Hydrocarbon Recovery from Carbonate Rocks? In Proceedings of the SPE Reservoir Characterization and Simulation Conference and Exhibition, Abu Dhabi, UAE, 16–18 September 2013. [Google Scholar]
- Al-Shalabi, E.W.; Sepehrnoori, K.; Pope, G. Mechanistic Modeling of Oil Recovery Due to Low Salinity Water Injection in Oil Reservoirs. In Proceedings of the SPE Middle East Oil & Gas Show and Conference, Manama, Bahrain, 8–11 March 2015. [Google Scholar]
- Al-Shalabi, E.W.; Sepehrnoori, K.; Pope, G. Geochemical Interpretation of Low-Salinity-Water Injection in Carbonate Oil Reservoirs. SPE J. 2015, 20, 211–212. [Google Scholar] [CrossRef]
- Korrani, A.K.N.; Fu, W.; Sanaei, A.; Sepehrnoori, K. Mechanistic Modeling of Modified Salinity Waterflooding in Carbonate Reservoirs. In Proceedings of the SPE Annual Technical Conference and Exhibition, Houston, TX, USA, 28–30 September 2015. [Google Scholar]
- Qiao, C.; Johns, R.T.; Li, L. Modeling Low Salinity Waterflooding in Chalk and Limestone Reservoirs. Energy Fuels 2016, 30, 884–895. [Google Scholar] [CrossRef]
- Eftekhari, A.A.; Thomsen, K.; Stenby, E.H.; Nick, H.M. Thermodynamic Analysis of Chalk–Brine–Oil Interactions. Energy Fuels 2017, 31, 11773–11782. [Google Scholar] [CrossRef]
- Abdou, M.; Carnegie, A.; Mathews, S.G.; McCarthy, K.; O’Keefe, M.; Raghuraman, B.; Wei, W.; Xian, C. Finding value in formation water. Oilfield Rev. 2011, 23, 24–35. [Google Scholar]
- Crabtree, M.; Eslinger, D.; Fletcher, P.; Miller, M.; Johnson, A.; King, G. Fighting scale—Removal and prevention. Oilfield Rev. 1999, 11, 30–45. [Google Scholar]
- Vetter, O.J.; Farone, W.A.; Veith, E.; Lankford, S. Calcium carbonate scale considerations: A practical approach. In Proceedings of the SPE Production Technology Symposium, Lubbock, TX, USA, 16–17 November 1987. [Google Scholar]
- Puntervold, T.; Strand, S.; Austad, T. Coinjection of seawater and produced water to improve oil recovery from fractured North Sea chalk oil reservoirs. Energy Fuels 2009, 23, 2527–2536. [Google Scholar] [CrossRef]
- Kajenthira, A.; Siddiqi, A.; Anadon, L.D. A new case for promoting wastewater reuse in Saudi Arabia: Bringing energy into the water equation. J. Environ. Manag. 2012, 102, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Burnett, D. Desalinating brine from oil and gas operations in Texas. Southwest Hydrol. 2005. Available online: http://www.swhydro.arizona.edu/archive/V4_N6/feature4.pdf (accessed on 27 August 2018).
- Burnett, D.B.; Siddiqui, M. Recovery of Fresh Water Resources from Desalination of Brine Produced during Oil and Gas Production Operations; Texas Engineering Experiment Station: College Station, TX, USA, 2006. [Google Scholar]
- Ayirala, S.C.; Uehara-Nagamine, E.; Matzakos, A.N.; Chin, R.W.; Doe, P.H.; van den Hoek, P.J. A designer water process for offshore low salinity and polymer flooding applications. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 24–28 April 2010. [Google Scholar]
- Collins, I.R. Water Flooding Method. U.S. Patent 7455109B2, 25 November 2008. [Google Scholar]
- Henthorne, L.; Movahed, B. Method and Control Devices for Production of Consistent Water Quality from Membrane-Based Water Treatment for Use in Improved Hydrocarbon Recovery Operations. U.S. Patent 20130213892A1, 22 August 2013. [Google Scholar]
- Curole, M.A.; Greene, E.B. Water Injection Systems and Methods. U.S. Patent 8,789,594, 29 July 2014. [Google Scholar]
- Ligthelm, D.J.; Romanuka, J.; Suijkerbuijk, B.M.J.M. Water Injection Systems and Methods. U.S. Patent 20120090833A1, 19 April 2012. [Google Scholar]
- Ayirala, S.C.B.; Chin, R.W.-Y.; Matzakos, A.N.; Uehara-Nagamine, E. Water Injection Systems and Methods. U.S. Patent 9234413B2, 12 January 2016. [Google Scholar]
- Yousef, A.A.; Ayirala, S.C. A novel water ionic composition optimization technology for SmartWater flooding application in carbonate reservoirs. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 12–16 April 2014. [Google Scholar]
- Ayirala, S.C.; Yousef, A.A. A Critical Review of Alternative Desalination Technologies for Smart Waterflooding. Oil Gas Facil. 2016. [Google Scholar] [CrossRef]
| Ions | Seawater (ppm) | Formation Water (ppm) | ||||
|---|---|---|---|---|---|---|
| Endicott | Ekofisk North Sea | Arabian Gulf | Endicott | Ekofisk North Sea | Arabian Gulf | |
| Na+ | 10,812 | 10,345 | 18,043 | 11,850 | 15,748 | 59,491 |
| K+ | 386 | 391 | 0 | 110 | 0 | 0 |
| Ca2+ | 402 | 521 | 652 | 320 | 9258 | 19,040 |
| Mg2+ | 1265 | 1093 | 2159 | 48 | 607 | 2439 |
| Ba2+ | 0 | 0 | 0 | 7 | 0 | 0 |
| Sr2+ | 7 | 0 | 11 | 24 | 0 | 0 |
| Fe2+ | 0 | 0 | 0 | 10 | 0 | 0 |
| Cl− | 18,964 | 18,719 | 31,808 | 17,275 | 42,437 | 132,060 |
| HCO3− | 147 | 122 | 119 | 2000 | 0 | 350 |
| SO42− | 2645 | 2305 | 4450 | 63 | 0 | 354 |
| CO32− | 0 | 0 | 27 | 0 | 0 | 0 |
| TDS | 34,628 | 33,498 | 57,269 | 31,707 | 68,051 | 213,734 |
| Ionic Strength | 0.688 | 0.659 | 1.146 | 0.541 | 1.453 | 4.317 |
| pH | 7.7 | 6.5 | ||||
| Authors | Field Attributes | Reservoir Temp. (°C) | Formation || Injected Brine (ppm) | Benefits |
|---|---|---|---|---|
| Sandstone reservoirs | ||||
| Webb et al. [210] | Giant Middle Eastern clastic Clay: <5% Oil Viscosity: 0.46–50 cP | 77 | 220,000 || 3000 | 25–50% Sor reduction |
| McGuire et al. [21] | Alaska North Slope Prudhoe Bay field Oil Viscosity: 0.8 cP Distance of Investigation: 3.9–4.2 m, 2.6–2.7 m, and 4.6 m Endicott Field Clay: 7% | 66 103 103 99 | 23,000 || 3000 23,000 || 3000 7000 || 2200 28,000 || 1500 | 8% Sor reduction and 18% incremental recovery 4% Sor reduction and 15% incremental recovery 4% Sor reduction and 8% incremental recovery 9% Sor reduction and 19% incremental recovery |
| Seccombe et al. [173] | Endicott Field | 99 | ||
| Clay: 7% | 28,000 || 1500 | 9% Sor reduction | ||
| Clay: 12% | 28,000 || 10 | 11% Sor reduction | ||
| Clay: 14% | 28,000 || 180 | 17% Sor reduction | ||
| Lager et al. [219] | Alaskan Oil Field | 16,640 || 2600 | 2% Sor reduction | |
| Robertson [220] | Minnelusa field | |||
| Oil Viscosity: 15.2 cP | ||||
| West Semlek | 62 | 60,000 || 10,000 | 27.5% Sor reduction | |
| North Semlek | 60 | 42,000 || 3304 | 32.5% Sor reduction | |
| Moran | 93 | 128,000 || 7948 | 31.5% Sor reduction | |
| Thyne et al. [221] | Wyoming Minnelusa fields | 68–75 | Range (1134–261,982) || Range (300–6000) | no significant benefit |
| Vledder et al. [222] | Omar Oil Field (Isba) Clay: 0.5–4% Oil Viscosity: 0.3 cP | 90,000 || 500 | 10–15% incremental recovery | |
| Skrettingland et al. [36] | Snorre field (Upper Statfjord formation) Clay: 10–20% | 90 | 34,020 || 440 | no significant benefit |
| Abdulla et al. [225] | Burgan Oil field | 140,000 || 5000 | 3% Sor reduction | |
| Al-Qattan et al. [226] | Burgan Oil field (Wara formation) Distance of Investigation: 4.6 m | 54–57 | 148,000 || 692 | 3% Sor reduction |
| Callegaro et al. [20,228] | West African Oil Field Oil Viscosity: 0.6 cP Distance of Investigation: 3.7 m North African Brown Field Oil Viscosity: 6–8 cP Distance of Investigation: 4 m | 88 76 | Range (27,000–87,000) || 200 39,000 || 1000 | no significant benefit 5–11% Sor reduction |
| Zeinijahromi et al. [229,230] | Bastrykskoye Zichebashskoe | 25 | 316,489 || 1986 248,529 || 848 | |
| Akhmetgareev and Khisamov [231] | Pervomaiskoye Oil Viscosity: 5.8 cP | 252,738 || 848 | 5–9% incremental recovery | |
| Carbonate reservoirs | ||||
| Austad [79] | Ekofisk reservoir, North Sea | 130 | 68,050 || 33,498 | Ultimate recovery above 50% of OOIP with two-thirds ascribed to seawater injection |
| Barkved et al. [237], Griffin et al. [238] | Valhall field, North Sea | 90 | Increase in oil production, decrease in GOR and reduced water cut | |
| Yousef et al. [22] | Saudi Arabia Upper Jurassic Oil Viscosity: 0.691 cP | 100 | 57,670 || 5767 57,670 || 28,835 || 5767 | 7% Sor reduction 6% Sor reduction |
| Water Treatment Process | Desalination Methods | Technology Maturity | Selective Ion Removal | Treatment Capability | Comparable Features | |
|---|---|---|---|---|---|---|
| High Saline Water | Produced Water | |||||
| Nanofiltration | Membrane-based | High | Yes | No | No |
|
| Reverse Osmosis | Membrane-based | High | No | No | No |
|
| Chemical Precipitation | Pretreatment | Medium-High | Yes | No | No |
|
| Salt Extraction | Pretreatment | Low | Maybe | Yes | Maybe |
|
| Forward Osmosis | Membrane-based | Low-Medium | No | Yes | Yes |
|
| Membrane Distillation | Combo Membrane & Thermal-based | Medium | No | Yes | No |
|
| Carrier-Gas Extraction | Humidification/Dehumidification | Medium | No | Yes | Yes |
|
| Dynamic Vapor Recompression | Thermal-based | Medium | Maybe | Yes | Yes |
|
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Awolayo, A.N.; Sarma, H.K.; Nghiem, L.X. Brine-Dependent Recovery Processes in Carbonate and Sandstone Petroleum Reservoirs: Review of Laboratory-Field Studies, Interfacial Mechanisms and Modeling Attempts. Energies 2018, 11, 3020. https://doi.org/10.3390/en11113020
Awolayo AN, Sarma HK, Nghiem LX. Brine-Dependent Recovery Processes in Carbonate and Sandstone Petroleum Reservoirs: Review of Laboratory-Field Studies, Interfacial Mechanisms and Modeling Attempts. Energies. 2018; 11(11):3020. https://doi.org/10.3390/en11113020
Chicago/Turabian StyleAwolayo, Adedapo N., Hemanta K. Sarma, and Long X. Nghiem. 2018. "Brine-Dependent Recovery Processes in Carbonate and Sandstone Petroleum Reservoirs: Review of Laboratory-Field Studies, Interfacial Mechanisms and Modeling Attempts" Energies 11, no. 11: 3020. https://doi.org/10.3390/en11113020





