Crop Characteristics of Aquatic Macrophytes for Use as a Substrate in Anaerobic Digestion Plants—A Study from Germany
Abstract
:1. Introduction
2. Materials and Methods
2.1. Origin of Aquatic Plant Material
2.2. Sample Treatment and Analyses
2.3. Biochemical Methane Potential Test
3. Results and Discussion
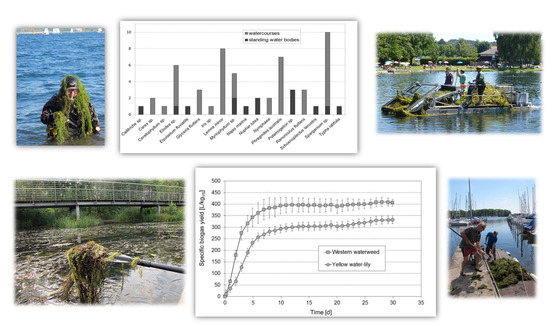
3.1. Aquatic Plant Species
3.2. Sediments and Extraneous Materials
3.3. Properties of the Harvested Material
3.4. Methane Potential of Aquatic Plants
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Guilizzoni, P. The role of heavy metals and toxic materials in the physiological ecology of submersed macrophytes. Aquat. Bot. 1991, 41, 87–109. [Google Scholar] [CrossRef]
- Boyd, C.E. Vascular Aquatic Plants for Mineral Nutrient Removal from Polluted Waters. Econ. Bot. 1970, 24, 95–103. [Google Scholar] [CrossRef]
- Stabenau, N.; Zehnsdorf, A.; Rönicke, H.; Wedwitschka, H.; Moeller, L.; Ibrahim, B.; Stinner, W. A potential phosphorous fertilizer for organic farming: Recovery of phosphorous resources in the course of bioenergy production through anaerobic digestion of aquatic macrophytes. Energy Sustain. Soc. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Verhofstad, M.J.J.M.; Poelen, M.D.M.; van Kempen, M.M.L.; Bakker, E.S.; Smolders, A.J.P. Finding the harvesting frequency to maximize nutrient removal in a constructed wetland dominated by submerged aquatic plants. Ecol. Eng. 2017, 106, 423–430. [Google Scholar] [CrossRef]
- Meier, E.J.; Waliczek, T.M.; Abbott, M.L. Composting Invasive Plants in the Rio Grande River. Invasive Plant Sci. Manag. 2014, 7, 473–482. [Google Scholar] [CrossRef]
- Gollasch, S.; Nehring, S. National checklist for aquatic alien species in Germany. Aquat. Invasions 2006, 1, 245–269. [Google Scholar] [CrossRef]
- Muñoz Escobar, M.; Voyevoda, M.; Fühner, C.; Zehnsdorf, A. Potential uses of Elodea nuttallii-harvested biomass. Energy Sustain. Soc. 2011, 1. [Google Scholar] [CrossRef]
- Zehnsdorf, A.; Korn, U.; Pröter, J.; Naumann, D.; Seirig, M.; Rönicke, H.; Pieper, B. Western waterweed (Elodea nuttallii) as a co-substrate for biogas plants. Agric. Eng. 2011, 66, 136–139. [Google Scholar] [CrossRef]
- Hussner, A.; Nehring, S.; Hilt, S. From first reports to successful control: A plea for improved management of alien aquatic plant species in Germany. Hydrobiologia 2014, 737, 321–331. [Google Scholar] [CrossRef]
- Hussner, A.; Stiers, I.; Verhofstad, M.J.J.M.; Bakker, E.S.; Grutters, B.M.C.; Haury, J.; van Valkenburg, J.L.C.H.; Brundu, G.; Newman, J.; Clayton, J.S.; et al. Management and control methods of invasive alien freshwater aquatic plants: A review. Aquat. Bot. 2017, 136, 112–137. [Google Scholar] [CrossRef]
- Zehnsdorf, A.; Hussner, A.; Eismann, F.; Rönicke, H.; Melzer, A. Management options of invasive Elodea nuttallii and Elodea canadensis. Limnologica 2015, 51, 110–117. [Google Scholar] [CrossRef]
- Herbes, C.; Brummer, V.; Roth, S.; Röhl, M. Using aquatic plant biomass from de-weeding in biogas processes—An economically viable option? Energy Sustain. Soc. 2018, 8, 21. [Google Scholar] [CrossRef]
- Lizasoain, J.; Rincón, M.; Theuretzbacher, F.; Enguídanos, R.; Nielsen, P.J.; Potthast, A.; Zweckmair, T.; Gronauer, A.; Bauer, A. Biogas production from reed biomass: Effect of pretreatment using different steam explosion condition. Biomass Bioenergy 2016, 95, 84–91. [Google Scholar] [CrossRef]
- Yadav, D.; Barbora, L.; Bora, D.; Mitra, S.; Rangan, L.; Mahanta, P. An assessment of duckweed as a potential lignocellulosic feedstock for biogas production. Int. Biodeterior. Biodegrad. 2017, 119, 253–529. [Google Scholar] [CrossRef]
- Gaur, R.Z.; Khan, A.A.; Suthar, S. Effect of thermal pre-treatment on co-digestion of duckweed (Lemna gibba) and waste activated sludge on biogas production. Chemosphere 2017, 174, 754–763. [Google Scholar] [CrossRef] [PubMed]
- Zehnsdorf, A.; Moeller, L.; Stärk, H.-J.; Auge, H.; Röhl, M.; Stinner, W. The study of the variability of biomass from plants of the Elodea genus from a river in Germany over a period of two hydrological years for investigating their suitability for biogas production. Energy Sustain. Soc. 2017, 7, 15. [Google Scholar] [CrossRef]
- Moeller, L.; Zehnsdorf, A. Measures to prevent foam formation in the anaerobic digestion of sugar beet in biogas plants. Agric. Eng. 2017, 72, 13–22. [Google Scholar] [CrossRef]
- Kobayashi, T.; Wu, Y.-P.; Lu, Z.-J.; Xu, K.-Q. Characterization of Anaerobic Degradability and Kinetics of Harvested Submerged Aquatic Weeds Used for Nutrient Phytoremediation. Energies 2015, 8, 304–318. [Google Scholar] [CrossRef]
- Weiland, P. Biogas production: Current state and perspectives. Appl. Microbiol. Biotechnol. 2010, 85, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Salo, T.; Eurola, M.; Rinne, M.; Seppälä, A.; Kaseva, J.; Kousa, T. The Effect of Nitrogen and Phophorus Concentrations on Nutrient Balances of Cereals and Grass Silage. MTT Report No. 147. 2014. Available online: http://jukuri.luke.fi/handle/10024/482918 (accessed on 19 September 2018).
- Bioabfallverordnung BioAbfV 2017 (German Regulation on the Reclamation of Bio Waste of Agricultural, Sivicultural or Horticultural Soils), §4 REQUIREMENTS Regarding Pollutants and Other Parameters. Available online: https://www.gesetze-im-internet.de/bioabfv/ (accessed on 19 September 2018).
- Gouder de Beauregard, A.-C.; Mahy, G. Phytoremediation of heavy metals: The role of macrophytes in a stormwater basin. Ecohydrol. Hydrobiol. 2002, 2, 289–295. [Google Scholar]
- Maleva, M.G.; Nekrasova, G.F.; Bezel, V.S. The response of hydrophytes to environmental pollution with heavy metals. Russ. J. Ecol. 2004, 35, 230–235. [Google Scholar] [CrossRef]
- Demirel, B.; Scherer, P. Trace elements requirements of agricultural biogas digesters during biological conversion of renewable biomass to methane. Biomass Bioenergy 2011, 35, 992–998. [Google Scholar] [CrossRef]
- Pobeheim, H.; Munk, B.; Johansson, J.; Guebitz, G.M. Influence of trace elements on methane formation from a synthetic model substrate for maize silage. Bioresour. Technol. 2010, 101, 836–839. [Google Scholar] [CrossRef] [PubMed]
- Labatut, R.A.; Angenent, L.T.; Scott, N.R. Biochemical methane potential and biodegradability of complex organic substrates. Bioresour. Technol. 2011, 201, 2255–2264. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.; Lizasoain, J.; Theuretzbach, F.; Agger, J.W.; Rincón, M.; Menardo, S.; Saylor, M.K.; Enguídanos, R.; Nielsen, P.J.; Potthast, A.; et al. Steam explosion pretreatment for enhancing biogas production of late harvested hay. Bioresour. Technol. 2014, 166, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Koyama, M.; Yamamoto, S.; Ishikawa, K.; Ban, S.; Toda, T. Anaerobic digestion of submerged macrophytes: Chemical composition and anaerobic digestibility. Ecol. Eng. 2014, 69, 304–309. [Google Scholar] [CrossRef]
- Koyama, M.; Yamamoto, S.; Ishikawa, K.; Ban, S.; Toda, T. Enhancing anaerobic digestibility of lignin-rich submerged macrophyte using thermochemical pre-treatment. Biochem. Eng. J. 2015, 99, 124–130. [Google Scholar] [CrossRef]
- Koyama, M.; Yamamoto, S.; Ishikawa, K.; Ban, S.; Toda, S. Inhibition of anaerobic digestion by dissolved lignin derived from alkaline pre-treatment of an aquatic macrophyte. Chem. Eng. J. 2017, 311, 55–62. [Google Scholar] [CrossRef]
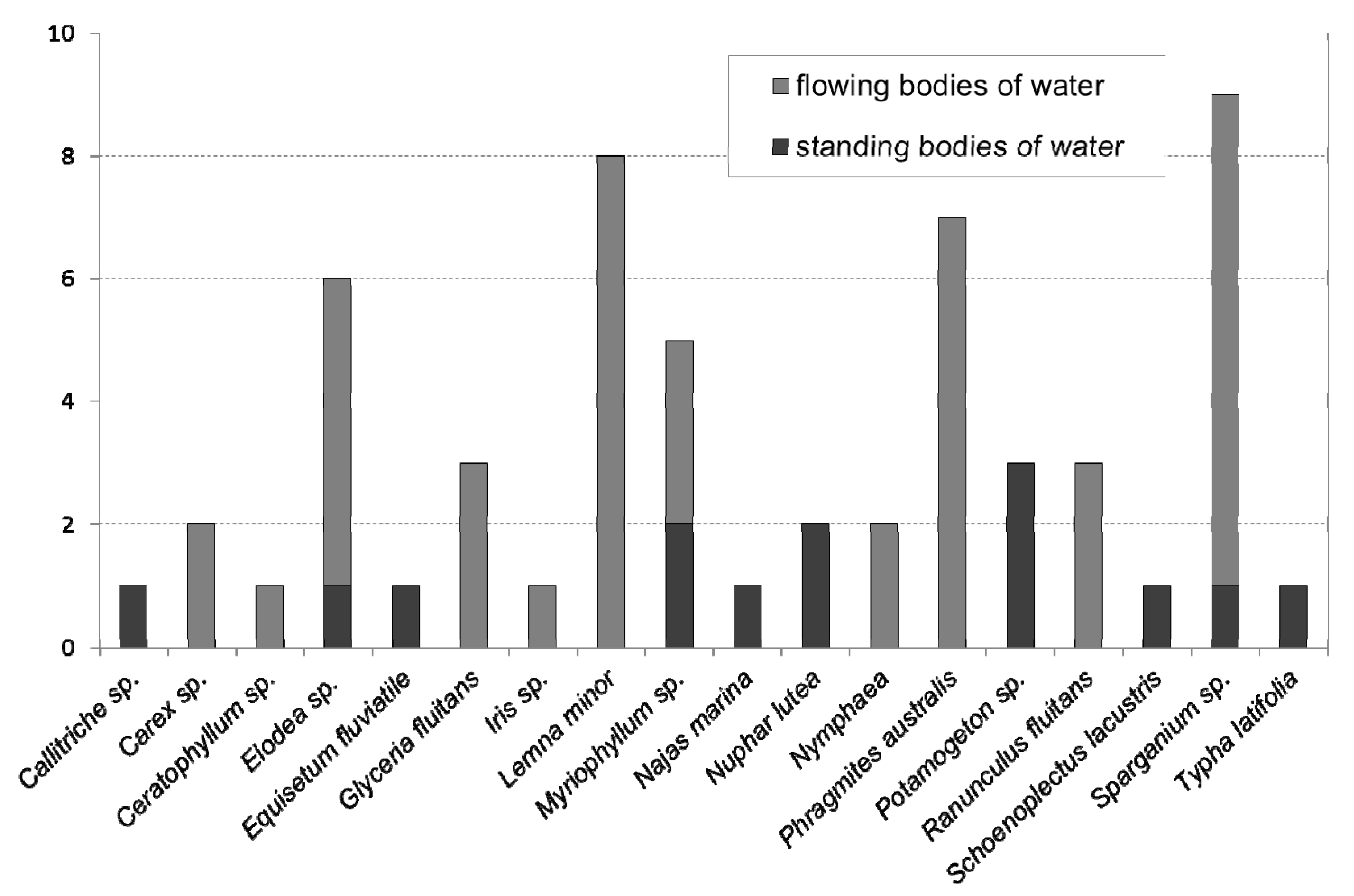
| Sample No. | Federal State | Type of Water Body | Plant Species in the Sample | Sediment Content (% TS) | TSBiomass (%FM) | VSBiomass (% TS) |
|---|---|---|---|---|---|---|
| 1 | BE | Lake | Water-starwort (Callitriche sp.) | 0.91 | 11.3 | 74.7 |
| 2 | SX | Lake | Watermilfoil (Myriophyllum heterophyllum, M. spicatum) | 2.21 | 6.76 | 81.0 |
| 3 | BW | Lake | Water horsetail (Equisetum fluviatile) | 0.90 | 19.2 | 82.5 |
| 4 | SX | Dam | Spiny water nymph (Najas marina) | 0.44 | 5.00 | 77.6 |
| 5 | BW | Pond | Broadleaf cattail (Typha latifolia), Lakeshore bulrush (Schoenoplectus lacustris) | 0.86 | 9.20 | 93.9 |
| 6 | BW | Pond | Yellow water-lily (Nuphar lutea) | 3.10 | 14.4 | 80.9 |
| 7 | BW | Pond | Yellow water-lily (Nuphar lutea) | 2.70 | 5.90 | 86.3 |
| 8 | NRW | Lido | Watermilfoil (Myriophyllum sp.) | 13.40 | 19.2 | 47.2 |
| 9 | BA | Lake | Bur-reed (Sparganium erectum) | 0.30 | 7.6 | 71.1 |
| 10 | BA | Lake | Sago pondweed (Potamogeton pectinatus) | 0.80 | 24.5 | 75.8 |
| 11 | BA | Lake | Sago pondweed (Potamogeton pectinatus) | 0.52 | 12.1 | 66.3 |
| 12 | BA | Lake | Western waterweed (Elodea nutallii) | 0.04 | 8.31 | 82.4 |
| 13 | BA | Lake | Floating pondweed (Potamogeton natans) | 0.40 | 11.0 | 53.0 |
| Sample No. | Federal State | Type of Water Body | Plant Species in the Sample | Sediment Content (% TS) | TSBiomass (% FM) | VSBiomass (% TS) |
|---|---|---|---|---|---|---|
| 14 | NRW | River | Bur-reed (Sparganium sp.) | 0.57 | 11.9 | 84.2 |
| 15 | NRW | River | Bur-reed (Sparganium sp.) | 0.40 | 8.40 | 89.3 |
| 16 | NRW | Ditch | Reed (Phragmites australis), Hornwort (Ceratophyllum sp.) | 0.68 | 5.62 | 90.5 |
| 17 | MWP | Ditch | Floating sweet-grass (Glyceria fluitans), Duckweed (Lemna minor) | 2.00 | 10.4 | 87.6 |
| 18 | BB | Ditch | Bur-reed (Sparganium sp.) | 0.33 | 19.6 | 84.6 |
| 19 | BB | River | Reed (Phragmites australis), Floating sweet-grass (Glyceria fluitans) | 0.09 | 10.8 | 87.5 |
| 20 | BB | River | Floating sweet-grass (Glyceria fluitans) | 0.68 | 6.07 | 81.6 |
| 21 | BB | River | Reed (Phragmites australis) | 8.70 | 14.0 | 93.8 |
| 22 | BW | Channel | Bur-reed (Sparganium sp.) | 0.73 | 7.71 | 84.4 |
| 23 | BW | Ditch | Western waterweed (Elodea nutallii) | 0.40 | 5.45 | 88.1 |
| 24 | HB | Channel | Watermilfoil (Myriophyllum spicatum) | 1.90 | 10.1 | 77.9 |
| 25 | HB | Ditch | Canadian waterweed (Elodea canadiensis), Duckweed (Lemna minor) | 2.85 | 9.28 | 78.6 |
| 26 | SX | River | Western waterweed (Elodea nutalii) | 0.18 | 7.40 | 78.5 |
| 27 | LS | Channel | Sedge (Carex sp.), Bur-reed (Sparganium sp.), Iris (Iris sp.) | 3.90 | 13.2 | 88.7 |
| 28 | LS | Channel | Sedge (Carex sp.), Waterweed (Elodea nutallii, E. canadensis) | 0.17 | 9.30 | 88.9 |
| 29 | BW | Channel | River water-crowfoot (Ranunculus fluitans), Duckweed (Lemna minor) | 0.94 | 8.20 | 85.8 |
| 30 | BB | River | Reed (Phragmites australis) | 0.22 | 22.0 | 91.6 |
| 31 | MWP | River | River water-crowfoot (Ranunculus fluitans) | 0.40 | 8.45 | 81.0 |
| 32 | SA | River | Bur-reed (Sparganium erectum), Duckweed (Lemna minor) | 1.10 | 7.20 | 73.2 |
| 33 | BB | Channel | Watermilfoil (Myriophyllum sp.), Duckweed (Lemna minor), Reed (Phragmites australis) | 0.30 | 10.8 | 59.0 |
| 34 | BB | Channel | Watermilfoil (Myriophyllum sp.), Duckweed (Lemna minor), Water lily (Nymphaea sp.) | 0.60 | 8.1 | 77.7 |
| 35 | LS | Channel | Reed (Phragmites australis), Hedge grasses | 0.80 | 35.8 | 88.5 |
| 36 | BA | River | River water-crowfoot (Ranunculus fluitans) | 0.80 | 5.0 | 61.1 |
| 37 | NRW | River | Bur-reed (Sparganium sp.) | 0.30 | 5.2 | 77.0 |
| 38 | BB | River | Bur-reed (Sparganium erectum), Duckweed (Lemna minor), Water lily (Nymphaea) | 0.22 | 5.2 | 80.4 |
| 39 | SX | River | Duckweed (Lemna minor), Reed (Phragmites australis) | 0.07 | 6.2 | 81.2 |
| Silage | Sediment Content (%TS) |
|---|---|
| Maize #1 | 2.20 |
| Maize #2 | 1.30 |
| Grass #1 | 1.10 |
| Grass #2 | 2.00 |
| Grass #3 | 6.40 |
| Sample No. | Nitrogen (g/kg TS) | Carbon (g/kg TS) | C/N |
|---|---|---|---|
| 1 | 30.1 | 354 | 11.8 |
| 2 | 24.8 | 360 | 14.5 |
| 3 | 24.3 | 385 | 15.8 |
| 4 | 19.9 | 367 | 18.4 |
| 5 | 18.2 | 398 | 21.9 |
| 6 | 16.0 | 397 | 24.8 |
| 7 | 24.3 | 385 | 15.8 |
| 8 | 6.03 | 172 | 28.6 |
| 9 | 36.0 | 396 | 11.0 |
| 10 | 17.3 | 371 | 21.4 |
| 11 | 16.0 | 325 | 20.3 |
| 12 | 20.1 | 283 | 14.1 |
| 13 | 19.9 | 363 | 18.3 |
| Sample No. | Nitrogen (g/kg TS) | Carbon (g/kg TS) | C/N |
|---|---|---|---|
| 14 | 26.6 | 397 | 14.9 |
| 15 | 26.2 | 393 | 15.0 |
| 16 | 12.2 | 422 | 34.6 |
| 17 | 14.2 | 419 | 29.5 |
| 18 | 27.3 | 361 | 13.2 |
| 19 | 24.1 | 397 | 16.5 |
| 20 | 29.3 | 424 | 14.5 |
| 21 | 12.8 | 436 | 34.1 |
| 22 | 28.8 | 394 | 13.7 |
| 23 | 25.9 | 389 | 15.0 |
| 24 | 25.1 | 350 | 14.0 |
| 25 | 23.5 | 356 | 15.1 |
| 26 | 32.8 | 354 | 10.8 |
| 27 | 24.2 | 430 | 17.7 |
| 28 | 26.7 | 422 | 15.8 |
| 29 | 30.4 | 456 | 13.7 |
| 30 | 14.0 | 435 | 31.1 |
| 31 | 33.9 | 383 | 11.3 |
| 32 | 26.2 | 345 | 13.2 |
| 33 | 16.2 | 292 | 18.1 |
| 34 | 34.9 | 349 | 10.0 |
| 35 | 11.7 | 400 | 34.1 |
| 36 | 25.9 | 314 | 12.1 |
| 37 | 28.4 | 368 | 13.0 |
| 38 | 27.7 | 403 | 14.6 |
| 39 | 27.2 | 389 | 14.3 |
| Element | Mean Value (mg/kg TS) | Standard Deviation | Minimum (mg/kg TS) | Maximum (mg/kg TS) | Legal Limit 1 (mg/kg TS) |
|---|---|---|---|---|---|
| Al | 827 | 1151 | 13.0 | 3320 | |
| As | 1.47 | 1.32 | 0.17 | 4.35 | |
| B | 19.4 | 10.4 | 6.11 | 42.0 | |
| Ca | 38,860 | 31,906 | 8630 | 119,650 | |
| Cd | 0.17 | 0.21 | 0.02 | 0.47 | 1 |
| Co | 4.44 | 9.71 | 0.07 | 33.0 | |
| Cr | 1.84 | 2.01 | 0.27 | 6.44 | 70 |
| Cu | 16.7 | 21.5 | 1.6 | 62.0 | 70 |
| Fe | 2181 | 2785 | 61 | 7880 | |
| K | 12,662 | 11,780 | 2310 | 46,330 | |
| Mg | 2967 | 1094 | 842 | 4210 | |
| Mn | 868 | 1026 | 28.8 | 2810 | |
| Mo | 0.34 | 0.30 | 0.08 | 0.68 | |
| Ni | 6.46 | 12.1 | 0.43 | 42.0 | 35 |
| P | 1847 | 908 | 474 | 3190 | |
| Pb | 2.40 | 2.81 | 0.11 | 7.80 | 100 |
| S | 3065 | 1441 | 1270 | 6570 | |
| Zn | 57.5 | 90.7 | 8.92 | 305 | 300 |
| Element | Mean Value (mg/kg TS) | Standard Deviation | Minimum (mg/kg TS) | Maximum (mg/kg TS) | Legal Limit 1 (mg/kg TS) |
|---|---|---|---|---|---|
| Al | 844 | 710 | 57.5 | 2690 | |
| As | 3.11 | 4.40 | 0.10 | 22.0 | |
| B | 121 | 209 | 5.01 | 891 | |
| Ca | 24,778 | 32,865 | 4220 | 175,500 | |
| Cd | 0.36 | 0.41 | 0.06 | 1.40 | 1 |
| Co | 4.45 | 6.89 | 0.09 | 27.0 | |
| Cr | 2.23 | 1.82 | 0.19 | 6.60 | 70 |
| Cu | 17.9 | 14.2 | 1.90 | 54.0 | 70 |
| Fe | 6021 | 6125 | 360 | 24,130 | |
| K | 12,247 | 7180 | 1960 | 32,600 | |
| Mg | 1935 | 696 | 662 | 3220 | |
| Mn | 5320 | 6237 | 110 | 26,020 | |
| Mo | 1.01 | 0.86 | 0.14 | 3.80 | |
| Ni | 3.72 | 3.78 | 0.51 | 15.0 | 35 |
| P | 3209 | 1529 | 932 | 8320 | |
| Pb | 4.80 | 5.04 | 0.37 | 17.0 | 100 |
| S | 3535 | 1593 | 1520 | 6760 | |
| Zn | 103 | 162 | 15.0 | 815 | 300 |
| Origin/Sample No. | Aquatic Plant Species | Test System | Methane Yield (L/kg FM) | Specific Methane Yield (SMY) (L/kg VS) |
|---|---|---|---|---|
| 3 | Water horsetail (Equisetum fluviatile) | AMPTS | 20.2 ± 1.1 | 190 ± 10 |
| 7 | Yellow water-lily (Nuphar lutea) | FBTS | 19.9 ± 2.7 | 202 ± 22 |
| 12 | Western waterweed (Elodea nuttallii) | AMPTS | 13.9 ± 0.4 | 204 ± 6.0 |
| 20 | Floating sweet-grass (Glyceria fluitans) | AMPTS | 18.1 ± 0.9 | 372 ± 19 |
| 21 | Reed (Phragmites australis) | AMPTS | 22.2 ± 1.5 | 169 ± 12 |
| 37 | Bur-reed (Sparganium sp.) | AMPTS | 16.6 ± 0.2 | 223 ± 2.6 |
| Fresh material | ||||
| Parthe | Western waterweed (Elodea nuttallii) | FBTS | 12.3 ± 0.5 | 233 ± 11 |
| Karl Heine Canal | Watermilfoil (Myriophyllum heterophyllum) | FBTS | 8.8 ± 2.2 | 160 ± 26 |
| Parthe | River water-crowfoot (Ranunculus fluitans) | FBTS | 15.7 ± 2.4 | 222 ± 6.0 |
| Parthe | Water-starwort (Callitriche sp.) | FBTS | 12.0 ± 0.5 | 292 ± 34 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moeller, L.; Bauer, A.; Wedwitschka, H.; Stinner, W.; Zehnsdorf, A. Crop Characteristics of Aquatic Macrophytes for Use as a Substrate in Anaerobic Digestion Plants—A Study from Germany. Energies 2018, 11, 3016. https://doi.org/10.3390/en11113016
Moeller L, Bauer A, Wedwitschka H, Stinner W, Zehnsdorf A. Crop Characteristics of Aquatic Macrophytes for Use as a Substrate in Anaerobic Digestion Plants—A Study from Germany. Energies. 2018; 11(11):3016. https://doi.org/10.3390/en11113016
Chicago/Turabian StyleMoeller, Lucie, Aline Bauer, Harald Wedwitschka, Walter Stinner, and Andreas Zehnsdorf. 2018. "Crop Characteristics of Aquatic Macrophytes for Use as a Substrate in Anaerobic Digestion Plants—A Study from Germany" Energies 11, no. 11: 3016. https://doi.org/10.3390/en11113016
APA StyleMoeller, L., Bauer, A., Wedwitschka, H., Stinner, W., & Zehnsdorf, A. (2018). Crop Characteristics of Aquatic Macrophytes for Use as a Substrate in Anaerobic Digestion Plants—A Study from Germany. Energies, 11(11), 3016. https://doi.org/10.3390/en11113016