1. Introduction
Pyrolysis is an environmentally friendly method to recover hydrocarbon materials due to its good capacity and lower environmental impact. In 2015, the Pollution Control Department of the Ministry of Natural Resources and Environment reported that the amount of municipal solid waste (MSW) in Thailand had continuously increased to approximately 29.09 million tons, of which 13.6 million tons had been disposed. Of this, only 8.4 million tons were disposed of by appropriate methods, i.e., landfilling or incineration. However, about 7.09 million tons of MSW were disposed of through dumping on the ground or in the water, or by combustion in open air [
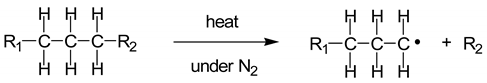
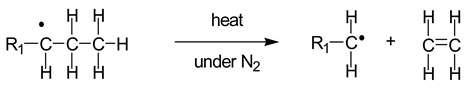
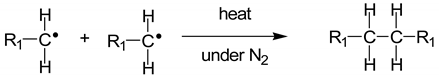
1]. MSW or household waste can be separated into three parts: (1) combustibles such as textiles, paper, wood, and kitchen waste; (2) non-combustibles such as metal, ceramics, and glass; and (3) plastics. The plastic waste collected comprises a mixture of plastics, with major components such as polyethylene (PE), polypropylene (PP), polystyrene (PS), polyvinyl chloride (PVC), and polyethylene terephthalate (PET). In Thailand, hazardous mixed scrap waste comprising used lubricant oil, car batteries, and fluorescent tubes has been found throughout the country. For the pyrolysis of plastic waste, the co-existing hazardous waste must be sorted out. However, used lubricant oil with low hazardous material content can be thermally decomposed to small hydrocarbons. During the pyrolysis reactions of mixed plastic waste, PE and PP are converted into a mixture of paraffins and olefins, while PS is converted into aromatic monomers [
2]. Therefore, mixtures of plastic waste containing PE, PP, and PS and used lubricant oil can undergo pyrolysis to produce oil and small hydrocarbons.
The oil waste used in pyrolysis processes includes automotive engine oil, brake fluid, gear oil, and power steering fluid. These are mixed during collection and storage. The amount of lubricant oil used in automotive engines increases each year due to the increasing number of automobiles. The recycling of used lubricant oil into fuel oil or lubricant oil may be a suitable option for protecting the environment from hazardous waste, as the oil waste typically consists of a mixture of the base oil and additives, which have high concentrations of heavy metals, varnishes, gums, and asphaltic compounds [
3]. Over 80% of waste lubricant oil consists of C
26C
36 compounds, which means it contains a high amount of heavy paraffins [
4]. Bhaskar et al. reported the catalytic thermal treatment of waste lubricant oil with silica-, silica-alumina-, and alumina-supported iron oxide catalysts at 400 °C and atmospheric pressure [
5]. The Fe/SiO
2 catalyst decreased the sulfur content from 1640 to 90 ppm and produced low molecular weight hydrocarbons by cracking the high molecular weight hydrocarbons. Lam et al. treated automotive engine oil using a microwave-induced pyrolysis process [
6]. The results showed that both fresh and waste engine oil were composed mainly of linear and branched paraffins (>85%), and that the long-chain hydrocarbons could be converted into more valuable compounds.
Co-pyrolysis techniques can provide valuable products through the disposal and conversion of plastic waste and other hydrocarbon sources. Waste lubricant oil is a good source of renewable resources because of its uniform composition. Waste plastic pyrolysis has low heat transfer and high viscosity of melting polymer. When lubricant oil is mixed with plastic waste, it can act as a solvent to decrease viscosity and improve heat transfer in the reactor [
7]. An optimum reactor design must ensure both high heat transfer rates for fast heating of the polymer and reliable temperature control. The operational problems are related to the sticky nature of the fused plastic [
8]. The optimization of conversion parameters such as the choice of catalysts, reactor design, pyrolysis temperature, and plastic-to-catalyst ratio play a very important role in the efficient processing of gasoline and diesel grade fuel [
9]. Serrano et al. studied thermal cracking using a screw kiln reactor in two temperature zones (450 and 500 °C for the first and second zone, respectively) [
10] and Low density polyethylene (LDPE) and lubricant oil base mixtures with compositions of 40:60, 50:50, 60:40, and 70:30 (%wt.). In all cases, near-complete conversion (approximately 90%) with a tendency range of C
1–C
40 hydrocarbons was achieved. The addition of waste motor oil to waste polyolefins not only increased the liquid yield, but also improved the properties of the liquid product, with greater naphtha and paraffinic contents in the products of the co-pyrolysis oil than in the products of the individual waste polyolefins [
11]. However, the proportion of used lubricant oil in the oil/plastic waste blend cannot be more than 50% by raw material weight, because greater oil contents tend to produce oil products that are non-diesel-like [
12,
13]. Bartocci et al. [
14] reported pyrolysis results of glycerol addition in pellet fuels while mixed with sawdust. They showed that the percent yield of gas was increased for the pellets using high glycerol content. The proportion of mixed materials presented a tendency to yield products in proportion to the raw materials mixture [
15].
The co-pyrolysis reactors of used oil blended with plastic wastes with various design for a laboratory scale have been reported. Uҫar et al. [
11] reported their study on co-pyrolysis of individual and blended polyolefin wastes and motor oil waste at 500 °C in a fixed bed reactor. The amount of raw materials studied was 100 g. Serrano et al. [
10] reported thermal and catalytic cracking of a LDPE-lubricant oil base mixture in a continuous screw kiln reactor. A significant enhancement in the product output took place with increasing proportions of the lubricating oil base in the mixture. Miskolczi and Ateş [
16] reported the co-pyrolysis of real municipal plastic waste (MPW) and MPW derived heavy oil (HO) mixtures in the stirred reactor by 750 g of raw materials and 500 °C as a final temperature. Breyer et al. [
15] reported the co-pyrolysis lab scale experiments that were carried out in a 5 L batch reactor with spiral stirrer. Raw materials were the mass of mixture between plastic waste from landfill and used motor oil 412 and 574 g for each experiment. Kim et al. [
17] reported a pyrolysis of mixture of waste of automobile lubricating oil (WALO) and PS. The pyrolysis reaction was carried out in the 1 L of stirred batch reactor, a sample mass of 300 g for all experiment runs, the temperature controlled the pyrolysis temperature in range of 300–500 °C. Most previous works has been focused on a utilization of a stirred batch reactor for co-pyrolysis of used lubricant oil and plastic wastes because the process was simple design and used lubricant oil increasing heat transfer in the reactor.
The aim of this work is to apply a two-stage methodology for a prototype co-pyrolysis process for used lubricant oil blended with mixed waste plastics (HDPE, PP, and PS). The optimum proportion of feedstocks was determined at the laboratory scale, and then studied at the prototype scale in order to optimize the diesel-like oil products.
The novelty and relevance of this work are optimization of proportion of used lubricant oil and mixed plastic wastes. The pyrolysis products are focused on diesel properties and the design melting stage for control proportion of raw materials before feeding to the semi-batch reactor at cracking temperature.
2. Materials and Methods
2.1. Materials and Sample Preparation
The sample of waste lubricant oil was API SN 0W20 from the Thanyaburi Honda Cars Center (viscosity@100 °C: 9.12 cSt; flash point: 194 °C; specific gravity: 0.878). The waste oil was dehydrated by heating at 110 °C for 1 h with stirring at 200 rpm. The HDPE plastic waste samples were collected from drinking water bottles, the PP samples were collected from water cups, and the PS samples were food box packaging. All plastic waste was ground to a particle size of 5–7 mm.
2.2. Thermogravimetric Analysis
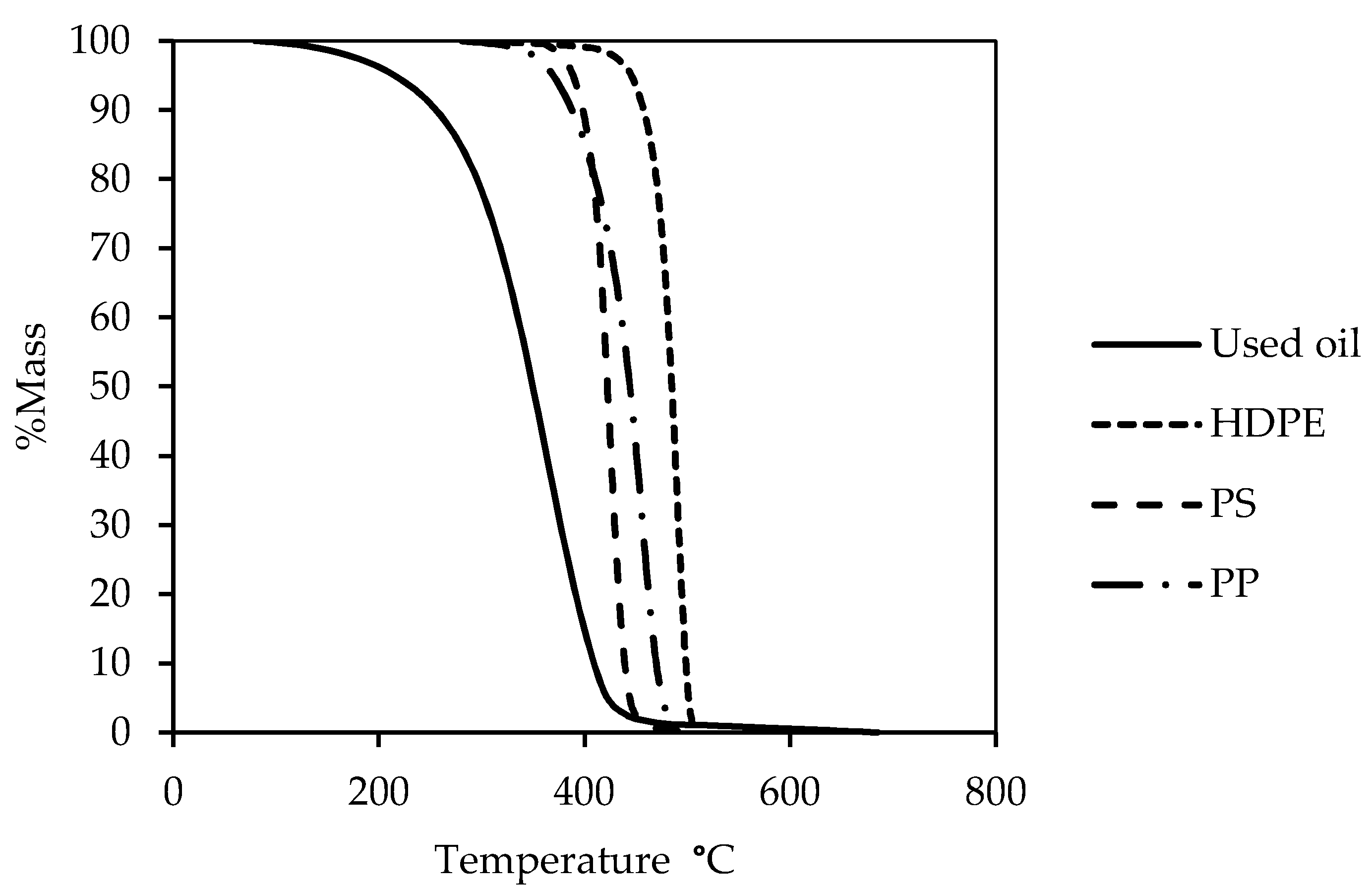
The decomposition of the waste oil, HDPE, PP, and PS samples was monitored by thermogravimetric analysis (TGA) using a TG 209F3 instrument (NETZSCH, Selb, Germany). Samples of approximately 10 mg of PP and HDPE and 5 mg of PS were heated. The samples were maintained at ambient temperature (32 °C) for 10 min and then linearly heated from 32 °C to 700 °C at a heating rate of 20 °C/min for 10 min with a nitrogen gas (N2) flow rate of 20 mL/min.
2.3. Pyrolysis Experiments
The lab-scale co-pyrolysis experiments were carried out in an unstirred batch reactor. The reactor consisted of a 1 L borosilicate glass vessel with a proportional–integral–derivative (PID) controller operated under nitrogen at atmospheric pressure. In each experiment, 350 g of raw materials were placed in the reactor, heated from room temperature to 450 °C, and held at the final temperature of 450 °C for 4 h (
Figure 1).
The co-pyrolysis of used lubricant oil mixed with three types of waste plastic was studied using mixtures with proportions (Oil:HDPE:PP:PS) of 50:30:10:10, 50:30:20:0, 50:30:0:20, and 50:0:30:20 percent by weight. The reactor was operated for 4 h to ensure that the reaction was complete. The yield of oil products was determined by weighing the oil collected from vessels using a condenser, and the yield of solid products was determined by measuring the weight of the residue at the end of the reaction. The gaseous product yield was determined by mass balance, assuming that the total weight of all products was equal to the initial weight of the raw materials.
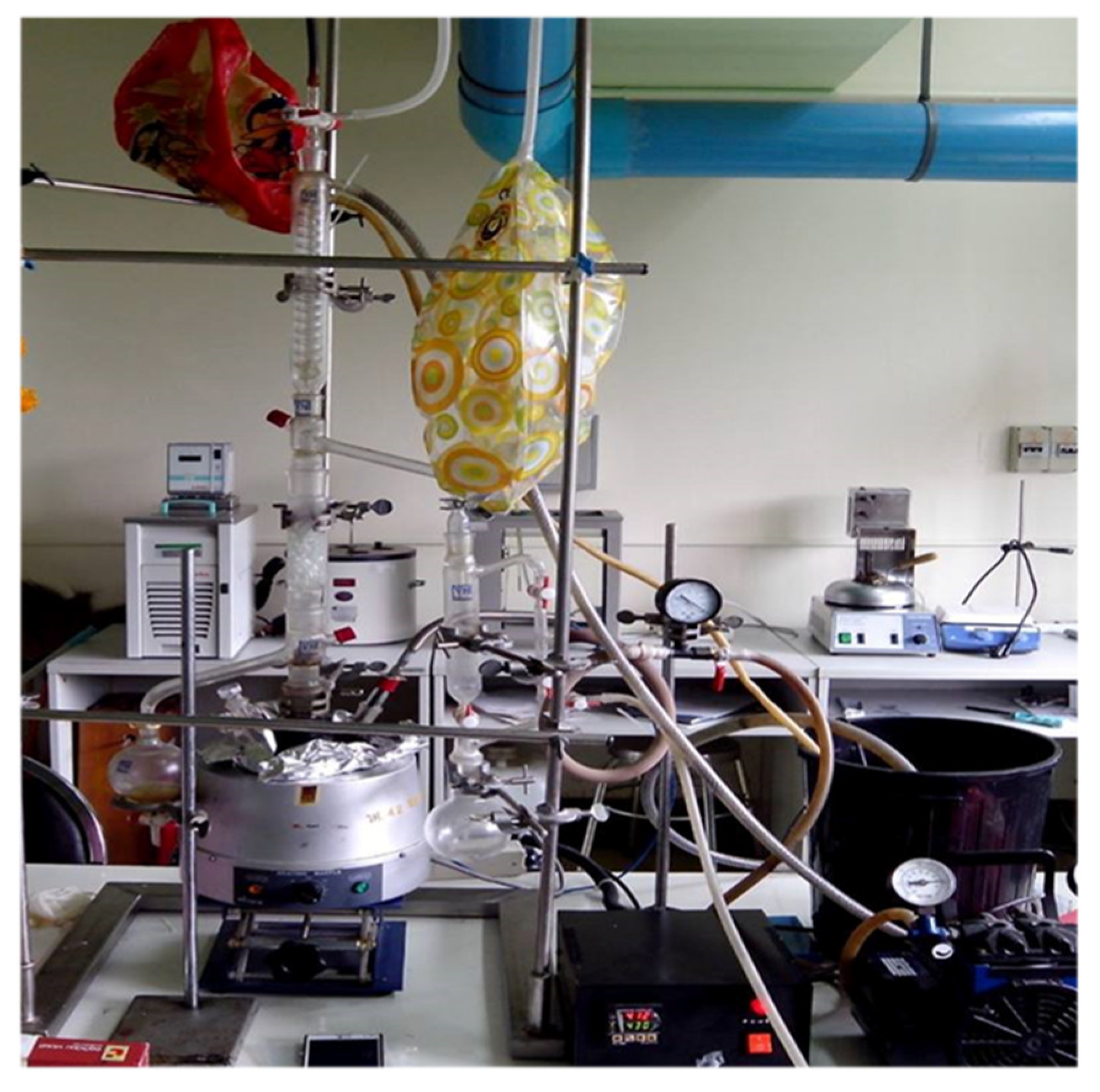
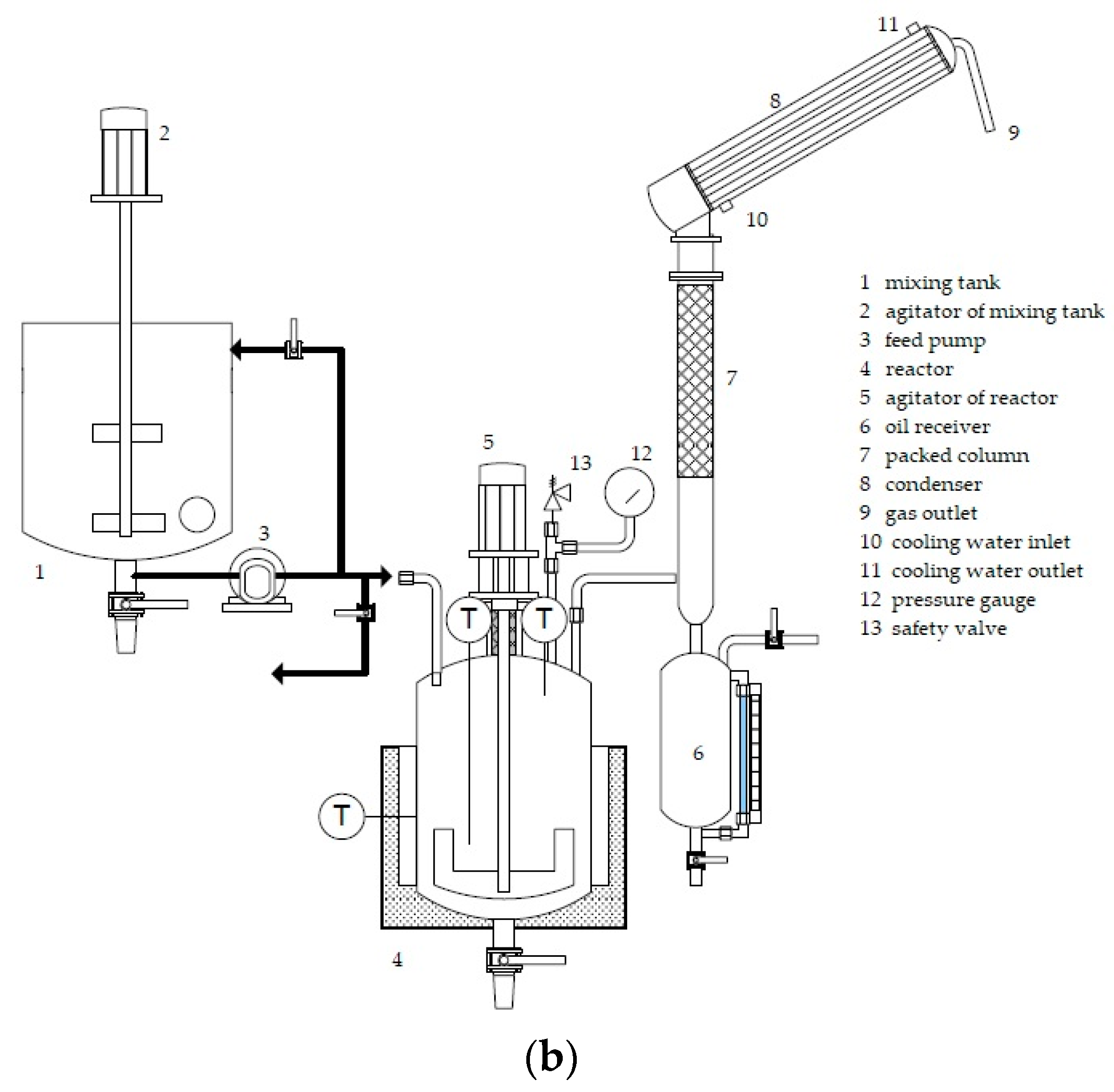
The co-pyrolysis prototype setup is shown in
Figure 2. Waste motor oil blended with HDPE, PP, and PS in a proportion of 50:30:20:0 percent by weight was used for co-pyrolysis with a final temperature of 450 °C for 80 min and without the addition of any catalyst. The waste oil (5 kg) was placed in a mixing tank and heated to 180 °C. 5 kg of plastic waste consisting of HDPE (3 kg) and PP (2 kg) was melted into a homogenous phase, and then fed into the stirred reactor and heated to 300 °C (10 °C/min) under 0.5 bar of nitrogen gas. The mixing tank and the reactor were made of 304 stainless steel, and both had a capacity of 20 L. The internal temperature at the top and bottom of the reactor vessel was measured using a thermocouple, and the jacket temperature was controlled by a PID controller. Gases from the reactor were driven through a packed column, and the pyrolysis oil was condensed by cooling water from a chiller (cooling water temperature at inlet = 10 °C). The pyrolysis oil was collected using an oil receiver at three reactor temperature ranges: 300–400 °C, 400–425 °C, and 425–450 °C. Uncondensed gases were exhausted outside. The comparison of process specification between lab-scale and prototype are shown in
Table 1.
2.4. Product Analysis
The properties of the oil samples were tested as follows: the flash point (ASTM D 93) was determined using a Pensky Martens model HFP 380 (Walter Herzog GmbH, Lauda-Königshofen, Germany); the viscosity @ 40 °C (ASTM D 445) using a viscometer bath model TV2500B (PM Tamson Instruments, Bleiswijk, Netherlands); the colour measurement (ASTM D 1500-96) using a model Comparator 3000 series (Lovibond, Dortmund, Germany);the specific gravity (ASTM D1298) using a glass hydrometer; and the distillation temperature (ASTM D86) and cetane index (ASTM D 976-06) using an Automated Distillation Tester model AD-6 (TANAKA Scientific Limited, Tokyo, Japan). The pyrolysis oil flash point, specific gravity, distillation temperature at 90% recovery, cetane index (calculated from the density and temperature of distillation at 50% recovery), viscosity, and colour were compared with standards for diesel oil specified by the Department of Energy Business, Ministry of Energy of Thailand. The chemical composition of the hydrocarbon compounds of the pyrolytic oils was analysed using a gas chromatography and mass spectrometry analyser (GC-MS, QP2010, Shimadzu, Kyoto, Japan) with an HP-5 column 30 m in length and 0.25 mm in diameter, a 10:1 split, a Helium gas flowrate of 0.9 mL/min, and an oven temperature of 170 °C to 320 °C (10 °C/min).