Dissociation Behaviors of CO2 Hydrate-Bearing Sediment Particle during Settling in Water
Abstract
:1. Introduction
2. Gas Hydrate Dissociation Model
3. Experimental Setup
3.1. Preparation of CHBS Particles
3.1.1. Experimental Apparatus
3.1.2. Preparation Procedure
- The temperature of the cold room was set as 268 K and was kept constant during the formation of CHBS particles.
- CO2 was injected into the vessel through the gas pressurization system. When the gas pressure achieved 5 MPa, the vessel was closed as an isochoric system to form CHBS particles.
- The formation process lasted for 48–72 h until the pressure did not decrease with time.
- The vessel was depressurized to 0.1 MPa after Step 3 by slowly venting gas through the valve.
- The temperature of the cold room was reduced to 253 K for 3–4 h.
- The CHBS particles were obtained as shown in Figure 2a,b. The dissociation experiments were performed at the atmospheric pressure. According to Equation (3), the phase equilibrium temperature at the atmospheric pressure is 257.15 K. The initial sample temperature was set to 253 K, which is about 1.5% lower than the phase equilibrium temperature at the atmospheric pressure. The main purpose was to prevent the hydrate from dissociating immediately when the vessel was depressurized to the atmospheric pressure. If the initial sample temperature was higher than 257.15 K, the hydrate began to dissociate immediately when the prepared CHBS particles were taken out from the vessel, and the released water formed ice to make the particles stick together, as shown in Figure 2c.
3.1.3. Measurement of Hydrate Saturation
- Get a closed container for the CHBS particle. The mass of the container is weighed and recorded as .
- Put a single CHBS particle in the closed container. The mass of the container and the particle is weighed by a NewClassic ME analytical balance with an accuracy of g. The mass is recorded as .
- The closed container is kept at room temperature until the hydrate is completely dissociated. After the CO2 produced due to dissociation is released, the mass is weighed and recorded as .
- The particle is dried at 373.15 K for 12 h and then weighed, and the mass is recorded as .
3.2. Dissociation Experiments of Single CHBS Particle
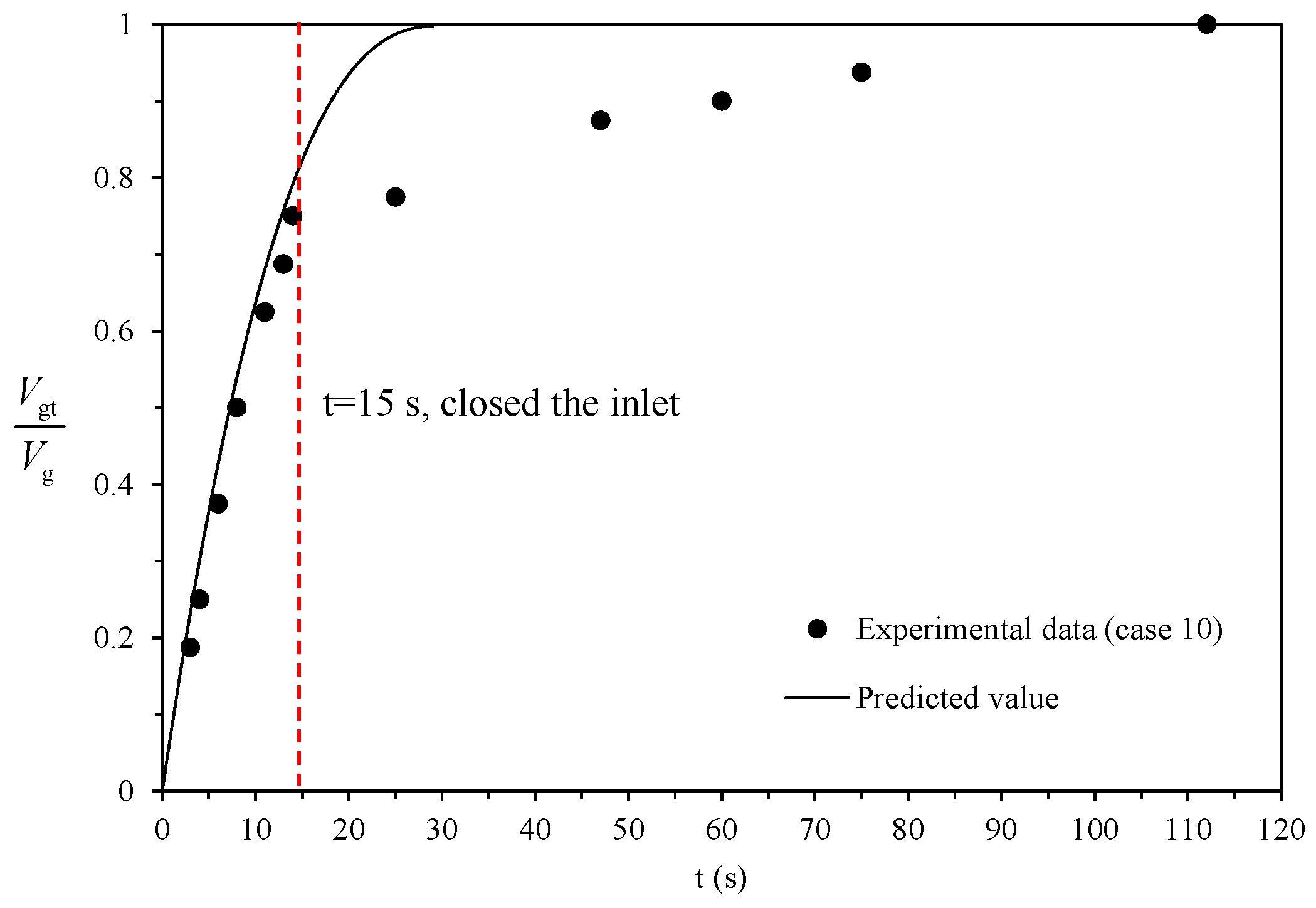
3.3. Dissociation Experiments of Group Particles
4. Results and Discussion
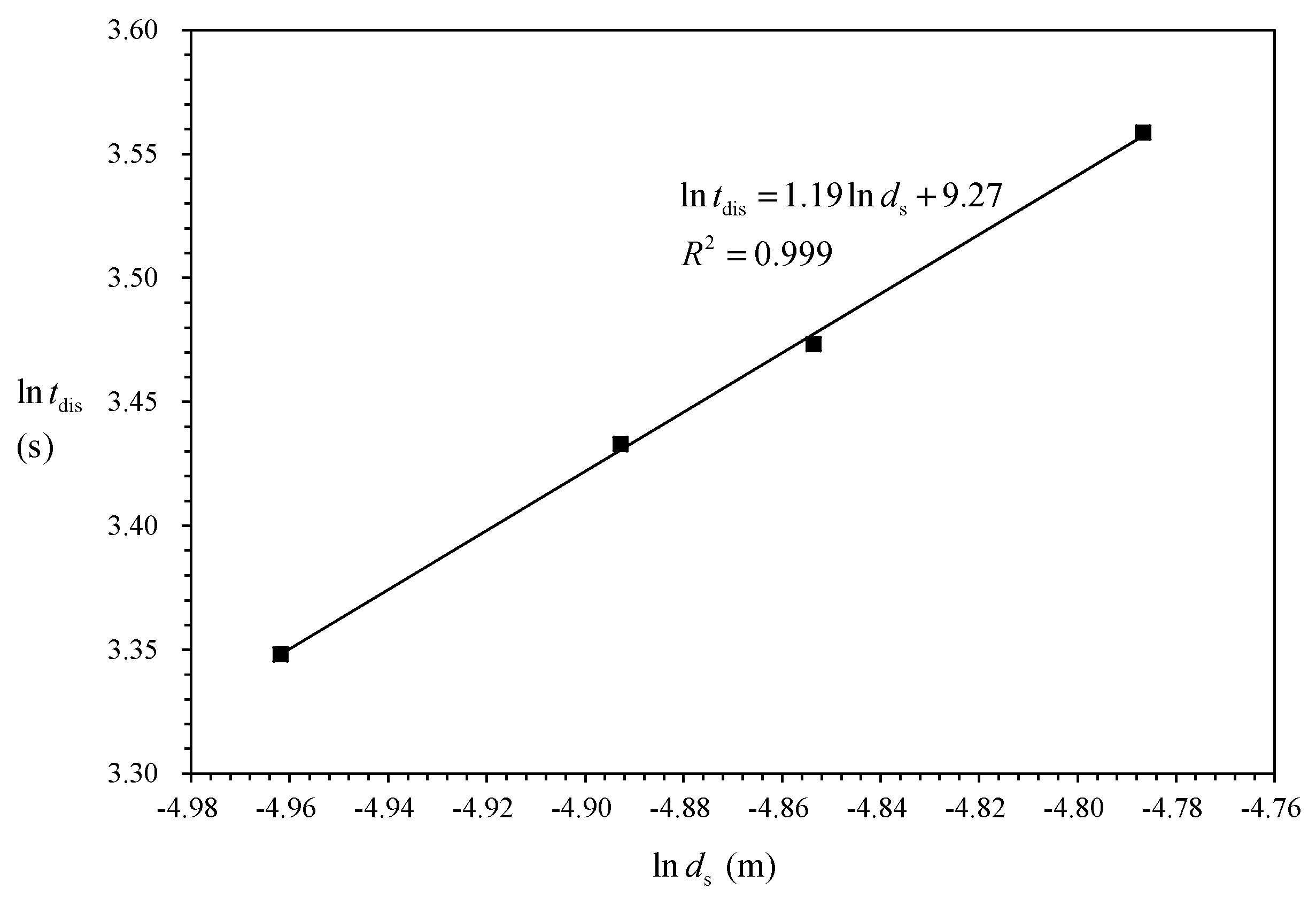
4.1. Determination of the Dissociation Model
4.2. Model Prediction
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kvenvolden, K.A.; Lorenson, T.D. The global occurrence of natural gas hydrate. Geophys. Monogr. 2001, 124, 3–18. [Google Scholar]
- Koh, C.A. Towards a fundamental understanding of natural gas hydrates. Chem. Soc. Rev. 2002, 31, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.C.; Yang, L.; Zhao, J.F.; Liu, W.G.; Yang, M.J.; Li, Y.H.; Liu, Y.; Li, Q.P. The status of natural gas hydrate research in China: A review. Renew. Sustain. Energy Rev. 2014, 31, 778–791. [Google Scholar] [CrossRef]
- Xu, H.L.; Lin, L.C.; Wu, W.R.; Wu, B. Cutter-suction exploitation mode of marine gas hydrate. Acta Sci. Nat. Univ. Sunyatseni Nat. Sci. (China) 2011, 50, 48–52. [Google Scholar]
- Zhang, X.H.; Lu, X.B.; Liu, L.L. Advances in natural gas hydrate recovery methods. Prog. Geophys. (China) 2014, 29, 858–869. [Google Scholar]
- Zhou, S.W.; Chen, W.; Li, Q.P. The green solid fluidization development principle of natural gas hydrate stored in shallow layers of deep water. China Offshore Oil Gas 2014, 26, 1–7. [Google Scholar]
- Sean, W.Y.; Sato, T.; Yamasaki, A.; Kiyono, F. CFD and experimental study on methane hydrate dissociation Part I. Dissociation under water flow. AIChE J. 2007, 53, 262–274. [Google Scholar] [CrossRef]
- Kim, H.C.; Bishnoi, P.R.; Heidemann, S.S.; Rizvi, S.S.H. Kinetics of methane hydrate decomposition. Chem. Eng. Sci. 1987, 42, 1645–1653. [Google Scholar] [CrossRef]
- Clarke, M.A.; Bishnoi, P.R. Determination of the intrinsic rate constant and activation energy of CO2 gas hydrate decomposition using in-situ particle size analysis. Chem. Eng. Sci. 2004, 59, 2983–2993. [Google Scholar] [CrossRef]
- Fukumoto, A.; Sean, W.Y.; Sato, T.; Yamasaki, A.; Kiyono, F. Estimation of dissociation rate constant of CO2 hydrate in water flow. Greenh. Gases 2014, 4, 1–11. [Google Scholar] [CrossRef]
- Aaron, D.; Tsouris, C. Separation of CO2 from flue gas: A review. Sep. Sci. Technol. 2005, 40, 321–348. [Google Scholar] [CrossRef]
- Kim, Y.; Wan, J.; Kneafsey, T.J.; Tokunaga, T.K. Dewetting of silica surfaces upon reactions with supercritical CO2 and brine: Pore-scale studies in micromodels. Environ. Sci. Technol. 2012, 46, 4228–4235. [Google Scholar] [CrossRef] [PubMed]
- Mekala, P.; Busch, M.; Mech, D.; Patel, R.S.; Sangwai, J.S. Effect of silica sand size on the formation kinetics of CO2 hydrate in porous media in the presence of pure water and seawater relevant for CO2 sequestration. J. Pet. Sci. Eng. 2014, 122, 1–9. [Google Scholar] [CrossRef]
- Sun, C.Y.; Chen, G.J. Methane hydrate dissociation above 0 °C and below 0 °C. Fluid Phase Equilib. 2006, 242, 123–128. [Google Scholar] [CrossRef]
- Sloan, E.D. Clathrate Hydrate of Natural Gases; Marcel Dekker: New York, NY, USA, 1998. [Google Scholar]
- Zhang, Y.; Li, X.S.; Wang, Y.; Chen, Z.Y.; Li, G. Methane hydrate formation in marine sediment from South China Sea with different water saturations. Energies 2017, 10, 561. [Google Scholar] [CrossRef]








| Case # | Initial Particle Temperature (K) | Hydrate Saturation (%) | Water Temperature (K) | Particle Diameter (m) | Dissociation Time (s) |
|---|---|---|---|---|---|
| 1 | 253.15 | 3.22 | 283.15 | 7.00 × 10−3 | 28.450 |
| 2 | 253.15 | 3.22 | 283.15 | 7.50 × 10−3 | 30.970 |
| 3 | 253.15 | 3.22 | 283.15 | 7.80 × 10−3 | 32.240 |
| 4 | 253.15 | 3.22 | 283.15 | 8.34 × 10−3 | 35.120 |
| 5 | 253.15 | 3.22 | 288.15 | 7.50 × 10−3 | 26.180 |
| 6 | 253.15 | 3.22 | 294.15 | 7.50 × 10−3 | 22.538 |
| 7 | 253.15 | 3.22 | 298.15 | 7.50 × 10−3 | 20.513 |
| Case # | Initial Particle Temperature (K) | Hydrate Saturation (%) | Water Temperature (K) | Initial Height of Particles (m) | Initial Particle Volume Fraction | Particle Diameter (m) | Gas Production (mL) |
|---|---|---|---|---|---|---|---|
| 8 | 253.15 | 8.10 | 293.15 | 0.12 | 0.557 | 7.50 × 10−3 | 570 |
| 9 | 253.15 | 5.44 | 298.15 | 0.15 | 0.500 | 8.00 × 10−3 | 429 |
| 10 | 253.15 | 5.18 | 293.15 | 0.15 | 0.500 | 8.00 × 10−3 | 409 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.; Zhang, X.; Lu, X. Dissociation Behaviors of CO2 Hydrate-Bearing Sediment Particle during Settling in Water. Energies 2018, 11, 2896. https://doi.org/10.3390/en11112896
Li P, Zhang X, Lu X. Dissociation Behaviors of CO2 Hydrate-Bearing Sediment Particle during Settling in Water. Energies. 2018; 11(11):2896. https://doi.org/10.3390/en11112896
Chicago/Turabian StyleLi, Peng, Xuhui Zhang, and Xiaobing Lu. 2018. "Dissociation Behaviors of CO2 Hydrate-Bearing Sediment Particle during Settling in Water" Energies 11, no. 11: 2896. https://doi.org/10.3390/en11112896
APA StyleLi, P., Zhang, X., & Lu, X. (2018). Dissociation Behaviors of CO2 Hydrate-Bearing Sediment Particle during Settling in Water. Energies, 11(11), 2896. https://doi.org/10.3390/en11112896





