Electrochemical Hydrogenation of Acetone to Produce Isopropanol Using a Polymer Electrolyte Membrane Reactor
Abstract
1. Introduction
2. Materials and Method
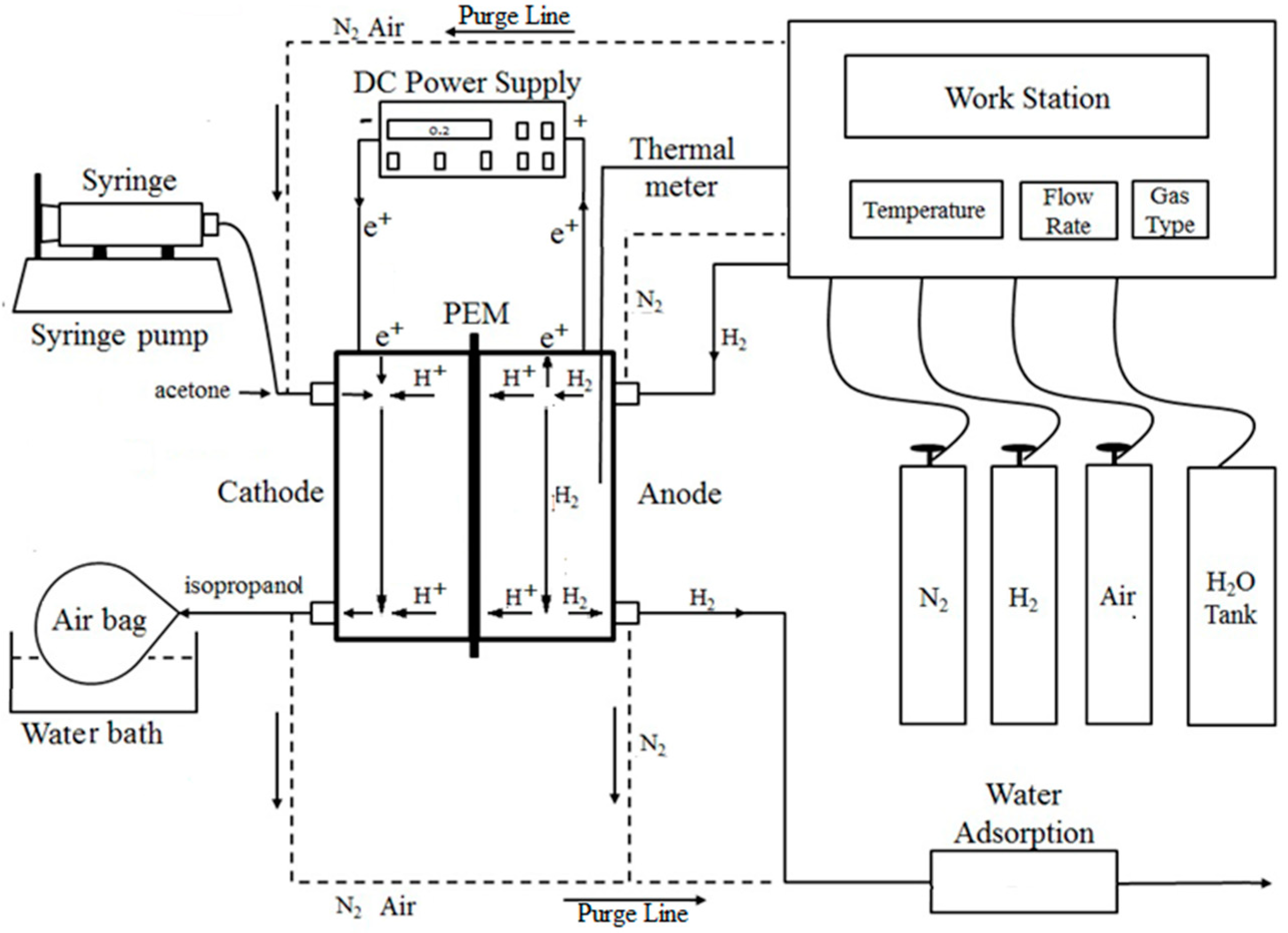
2.1. Material and Experimental Setup
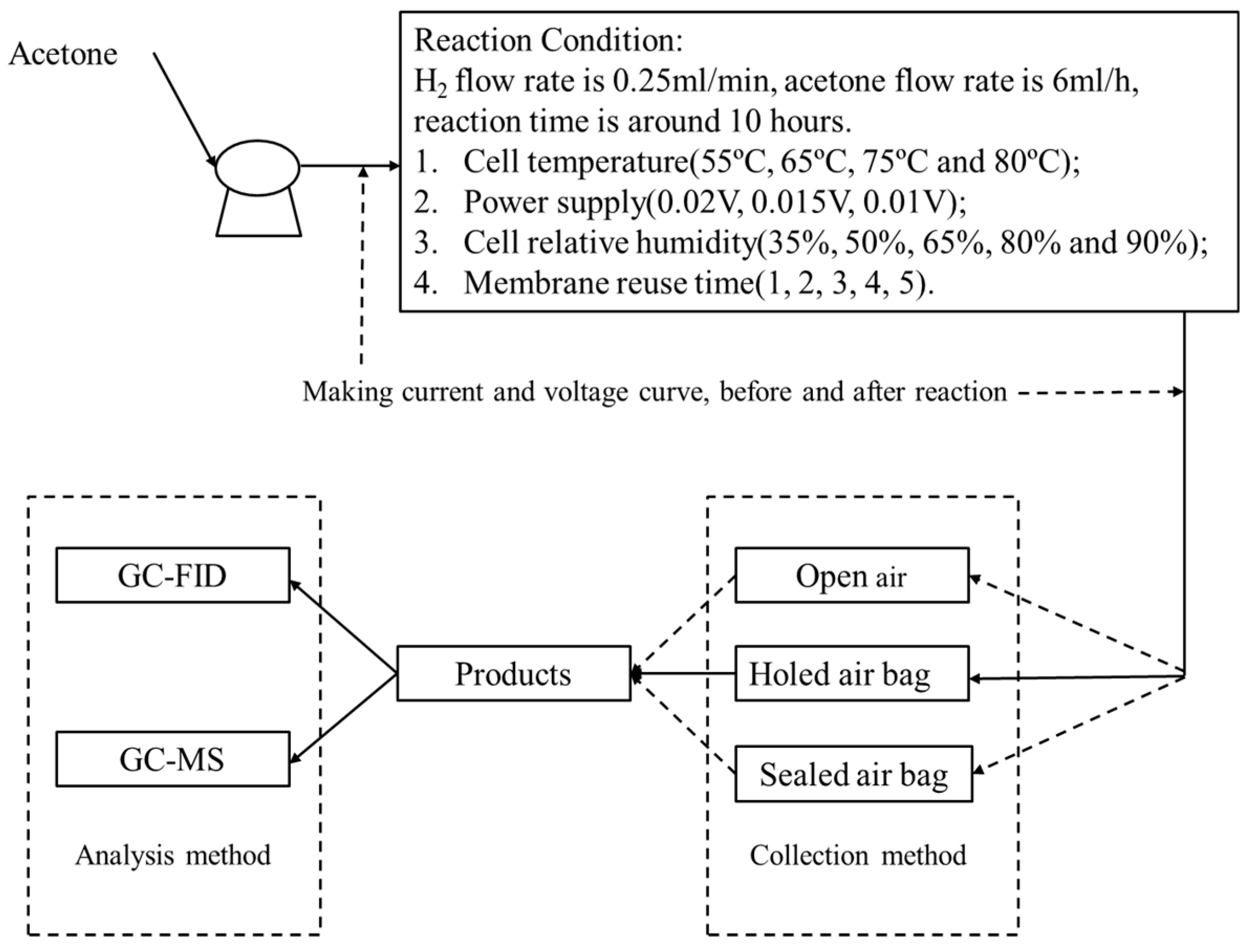
2.2. Electrochemical Hydrogenation and Characterization
3. Results and Discussion
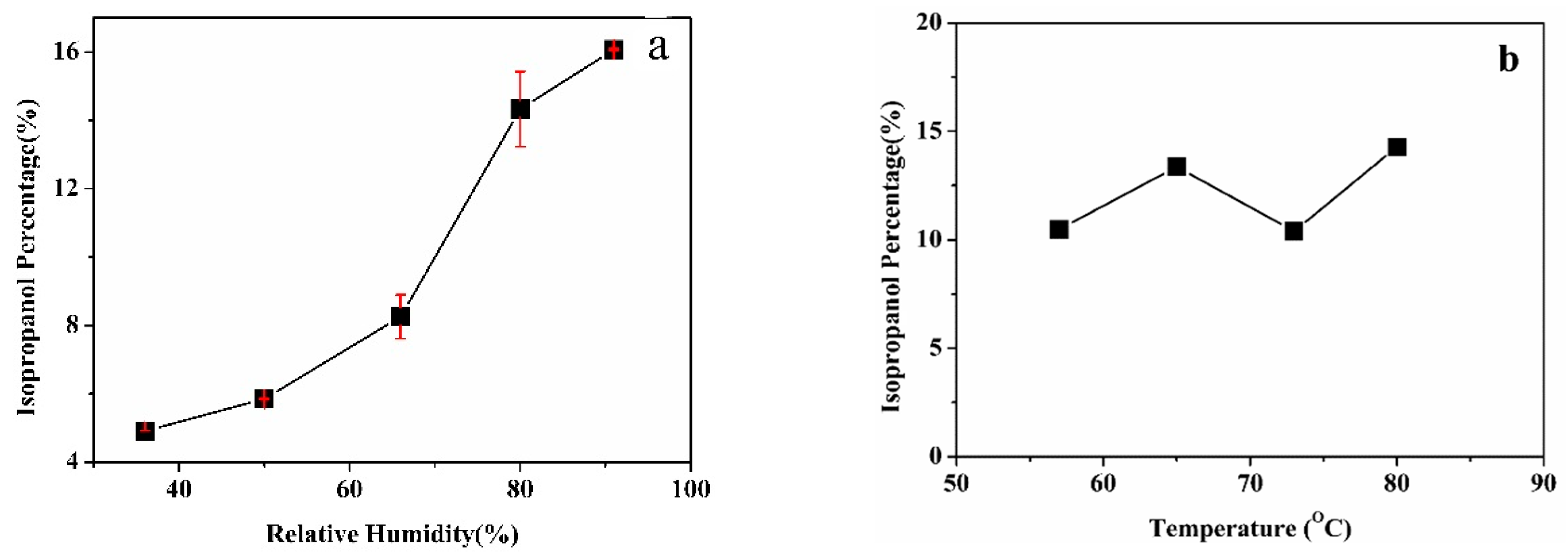
3.1. Product Characterization
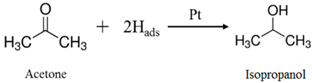
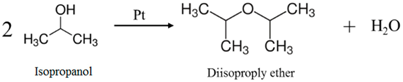
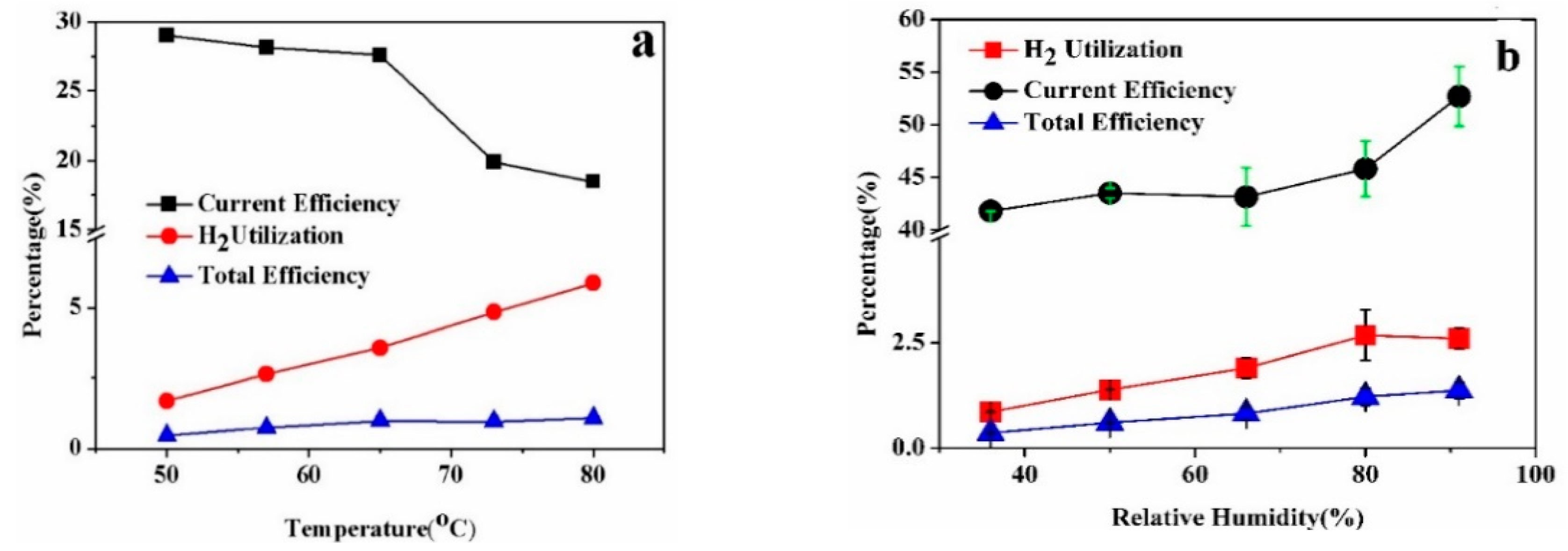
3.2. System Analysis
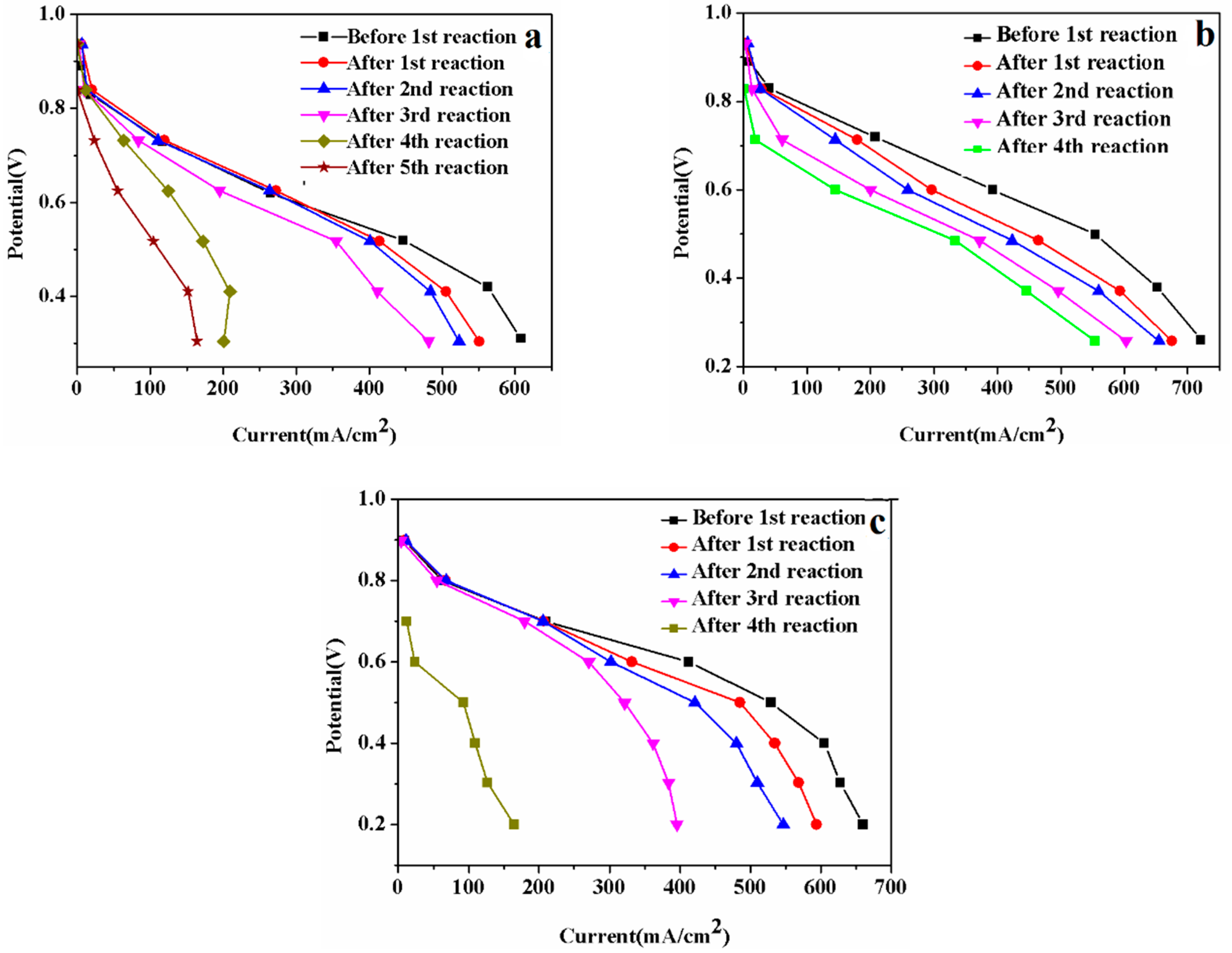
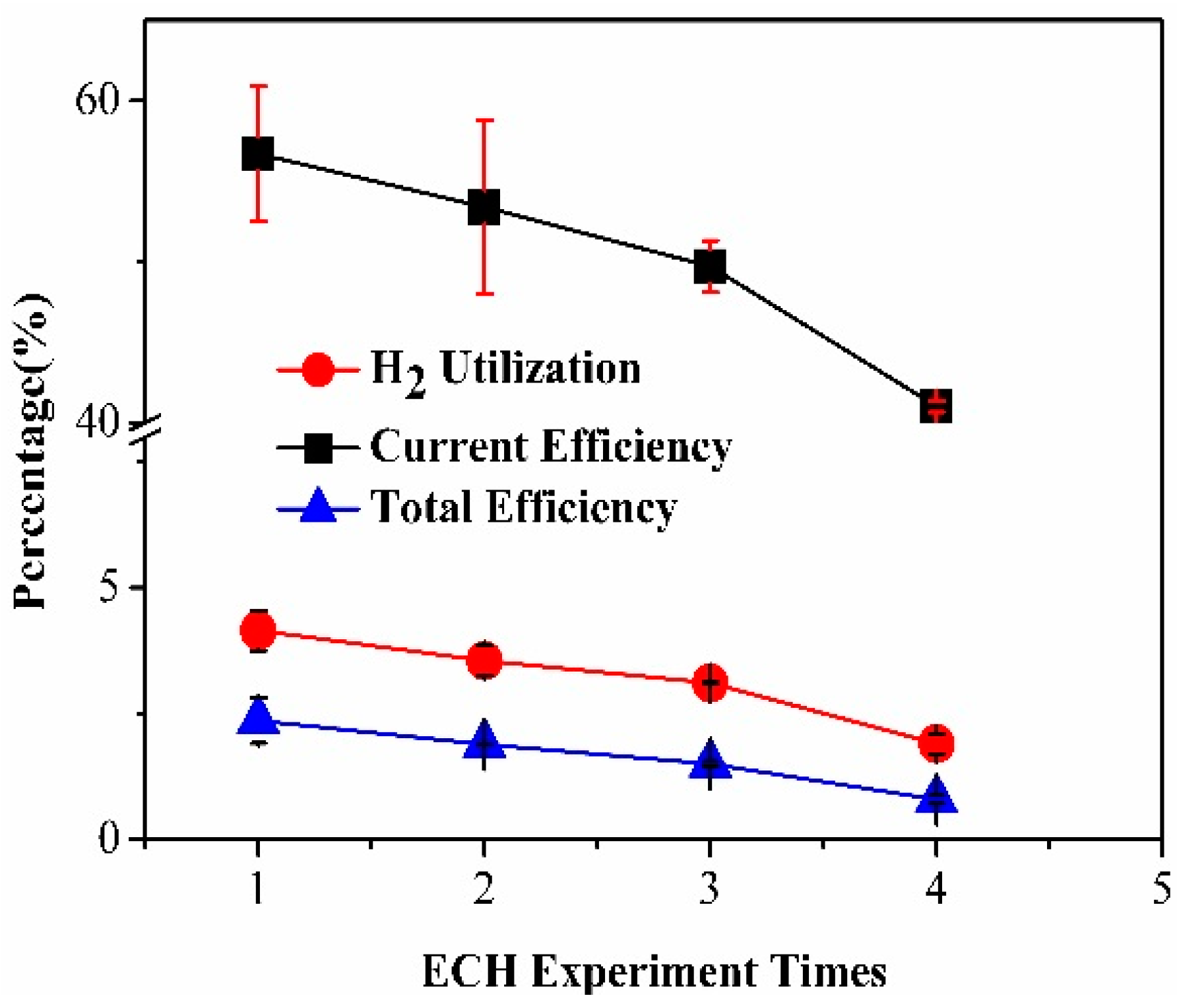
3.3. MEA Degradation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Guide to Local Production: WHO-Recommended Handrub Formulations. 2011, pp. 1123–1156. Available online: http://www.who.int/gpsc/1125may/Guide_to_Local_Production.pdf (accessed on 2 October 2018).
- Ceresana Inc. Market Study Solvents, 4th ed. 2012, p. 10. Available online: https://www.ceresana.com/en/market-studies/chemicals/solvents/ (accessed on 2 October 2018).
- Alt, C. Ullmann’s Encyclopedia of Industrial Chemistry, 1th ed.; Wiley-VCH: New York, NY, USA, 2006; pp. 2345–2351. [Google Scholar]
- Onoue, Y.; Mizutani, Y.; Akiyama, S.; Izumi, Y.; Ihara, H. Isopropyl alcohol by direct hydration of propylene. Bull. Jpn. Pet. Inst. 1973, 15, 50–55. [Google Scholar] [CrossRef]
- John, E.L.; Loke, R.A. Isopropyl Alcohol. In Kirk-Othmer Encyclopedia of Chemical Technology, 5th ed.; John, E., Logsdon, L.R.A., Eds.; Wiley-VCH: New York, NY, USA, 2000; Volume 6, pp. 98–100. ISBN 9780471238966. [Google Scholar]
- Yurieva, T.M.; Plyasova, L.M.; Makarova, O.V.; Krieger, T.A. Mechanisms for hydrogenation of acetone to isopropanol and of carbon oxides to methanol over copper-containing oxide catalysts. J. Mol. Catal. A Chem. 1996, 113, 455–468. [Google Scholar] [CrossRef]
- Cyr, A.; Chiltz, F.; Jeanson, P.; Martel, A.; Brossard, L.; Lessard, J.; Ménard, H. Electrocatalytic hydrogenation of lignin models at Raney nickel and palladium-based electrodes. Can. J. Chem. 2000, 78, 307–315. [Google Scholar] [CrossRef]
- Bockris, J. Electrochemical Processing. In Comprehensive Treatise of Electrochemistry, 1th ed.; Bockris, J., Brian, E.C., Yeager, E., White, R.E., Eds.; Springer Science & Business Media: New York, NY, USA, 2013; Volume 2, pp. 537–616. ISBN 1468437852. [Google Scholar]
- Cirtiu, C.M.; Brisach-Wittmeyer, A.; Ménard, H. Electrocatalysis over Pd catalysts: A very efficient alternative to catalytic hydrogenation of cyclohexanone. J. Catal. 2007, 245, 191–197. [Google Scholar] [CrossRef]
- Amouzegar, K.; Savadogo, O. Electrocatalytic hydrogenation of phenol on dispersed Pt: Reaction mechanism and support effect. Electrochim. Acta 1998, 43, 503–508. [Google Scholar] [CrossRef]
- St-Pierre, G.; Chagnes, A.; Bouchard, N.-A.; Harvey, P.D.; Brossard, L.; Ménard, H. Rational design of original materials for the electrocatalytic hydrogenation reactions: Concept, preparation, characterization, and theoretical analysis. Langmuir 2004, 20, 6365–6373. [Google Scholar] [CrossRef] [PubMed]
- Cirtiu, C.M.; Hassani, H.O.; Bouchard, N.-A.; Rowntree, P.A.; Ménard, H. Modification of the surface adsorption properties of alumina-supported Pd catalysts for the electrocatalytic hydrogenation of phenol. Langmuir 2006, 22, 6414–6421. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Chia, S.H.; Sanyal, U.; Gutiérrez, O.Y.; Lercher, J.A. Integrated catalytic and electrocatalytic conversion of substituted phenols and diaryl ethers. J. Catal. 2016, 344, 263–272. [Google Scholar] [CrossRef]
- Li, Z.; Kelkar, S.; Lam, C.H.; Luczek, K.; Jackson, J.E.; Miller, D.J.; Saffron, C.M. Aqueous electrocatalytic hydrogenation of furfural using a sacrificial anode. Electrochim. Acta 2012, 64, 87–93. [Google Scholar] [CrossRef]
- Chu, D.; Hou, Y.; He, J.; Xu, M.; Wang, Y.; Wang, S.; Wang, J.; Zha, L. Nano TiO2 film electrode for electrocatalytic reduction of furfural in ionic liquids. J. Nanopart. Res. 2009, 11, 1805–1809. [Google Scholar] [CrossRef]
- Parpot, P.; Bettencourt, A.; Chamoulaud, G.; Kokoh, K.; Belgsir, E. Electrochemical investigations of the oxidation–reduction of furfural in aqueous medium: Application to electrosynthesis. Electrochim. Acta 2004, 49, 397–403. [Google Scholar] [CrossRef]
- Chamoulaud, G.; Floner, D.; Moinet, C.; Lamy, C.; Belgsir, E. Biomass conversion II: Simultaneous electrosyntheses of furoic acid and furfuryl alcohol on modified graphite felt electrodes. Electrochim. Acta 2001, 46, 2757–2760. [Google Scholar] [CrossRef]
- Green, S.K.; Lee, J.; Kim, H.J.; Tompsett, G.A.; Kim, W.B.; Huber, G.W. The electrocatalytic hydrogenation of furanic compounds in a continuous electrocatalytic membrane reactor. Green Chem. 2013, 15, 1869–1879. [Google Scholar] [CrossRef]
- Nilges, P.; Schroder, U. Electrochemistry for biofuel generation: Production of furans by electrocatalytic hydrogenation of furfurals. Energy Environ. Sci. 2013, 6, 2925–2931. [Google Scholar] [CrossRef]
- Zhao, B.; Chen, M.; Guo, Q.; Fu, Y. Electrocatalytic hydrogenation of furfural to furfuryl alcohol using platinum supported on activated carbon fibers. Electrochim. Acta 2014, 135, 139–146. [Google Scholar] [CrossRef]
- Ilikti, H.; Rekik, N.; Thomalla, M. Electrocatalytic hydrogenation of alkyl-substituted phenols in aqueous solutions at a Raney nickel electrode in the presence of a non-micelle-forming cationic surfactant. J. Appl. Electrochem. 2004, 34, 127–136. [Google Scholar] [CrossRef]
- Laplante, F.; Brossard, L.; Ménard, H. Considerations about phenol electrohydrogenation on electrodes made with reticulated vitreous carbon cathode. Can. J. Chem. 2003, 81, 258–264. [Google Scholar] [CrossRef]
- Song, Y.; Gutiérrez, O.Y.; Herranz, J.; Lercher, J.A. Aqueous phase electrocatalysis and thermal catalysis for the hydrogenation of phenol at mild conditions. Appl. Catal. B Environ. 2016, 182, 236–246. [Google Scholar] [CrossRef]
- Santana, D.S.; Lima, M.V.F.; Daniel, J.R.R.; Navarro, M. Electrocatalytic hydrogenation of organic compounds using current density gradient and sacrificial anode of nickel. Tetrahedron Lett. 2003, 44, 4725–4727. [Google Scholar] [CrossRef]
- Singh, N.; Song, Y.; Gutiérrez, O.Y.; Camaioni, D.M.; Campbell, C.T.; Lercher, J.A. Electrocatalytic Hydrogenation of Phenol over Platinum and Rhodium: Unexpected Temperature Effects Resolved. ACS Catal. 2016, 6, 7466–7470. [Google Scholar] [CrossRef]
- Tountian, D.; Brisach-Wittmeyer, A.; Nkeng, P.; Poillerat, G.; Ménard, H. Effect of support conductivity of catalytic powder on electrocatalytic hydrogenation of phenol. J. Appl. Electrochem. 2009, 39, 411–419. [Google Scholar] [CrossRef]
- Ilikti, H.; Rekik, N.; Thomalla, M. Electrocatalytic hydrogenation of phenol in aqueous solutions at a Raney nickel electrode in the presence of cationic surfactants. J. Appl. Electrochem. 2002, 32, 603–609. [Google Scholar] [CrossRef]
- Tsyganok, A.; Holt, C.M.; Murphy, S.; Mitlin, D.; Gray, M.R. Electrocatalytic hydrogenation of aromatic compounds in ionic liquid solutions over WS2-on-glassy carbon and Raney nickel cathodes. Fuel 2012, 93, 415–422. [Google Scholar] [CrossRef]
- Sáez, A.; García-García, V.; Solla-Gullón, J.; Aldaz, A.; Montiel, V. Electrocatalytic hydrogenation of acetophenone using a Polymer Electrolyte Membrane Electrochemical Reactor. Electrochim. Acta 2013, 91, 69–74. [Google Scholar] [CrossRef]
- Pintauro, P.N.; Gil, M.P.; Warner, K.; List, G.; Neff, W. Electrochemical Hydrogenation of Soybean Oil with Hydrogen Gas. Ind. Eng. Chem. Res. 2005, 44, 6188–6195. [Google Scholar] [CrossRef]
- An, W.; Hong, J.K.; Pintauro, P.N.; Warner, K.; Neff, W. The electrochemical hydrogenation of edible oils in a solid polymer electrolyte reactor. II. Hydrogenation selectivity studies. J. Am. Oil Chem. Soc. 1999, 76, 215–222. [Google Scholar] [CrossRef]
- An, W.; Hong, J.K.; Pintauro, P.N.; Warner, K.; Neff, W. The electrochemical hydrogenation of edible oils in a solid polymer electrolyte reactor. I. Reactor design and operation. J. Am. Oil Chem. Soc. 1998, 75, 917–925. [Google Scholar] [CrossRef]
- Xin, L.; Zhang, Z.; Qi, J.; Chadderdon, D.J.; Qiu, Y.; Warsko, K.M.; Li, W. Electricity Storage in Biofuels: Selective Electrocatalytic Reduction of Levulinic Acid to Valeric Acid or γ-Valerolactone. ChemSusChem 2013, 6, 674–686. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Xin, L.; Chadderdon, D.J.; Qi, J.; Liang, C.; Li, W. Integrated electrocatalytic processing of levulinic acid and formic acid to produce biofuel intermediate valeric acid. Green Chem. 2014, 16, 1305–1315. [Google Scholar] [CrossRef]
- Nilges, P.; dos Santos, T.R.; Harnisch, F.; Schroder, U. Electrochemistry for biofuel generation: Electrochemical conversion of levulinic acid to octane. Energy Environ. Sci. 2012, 5, 5231–5235. [Google Scholar] [CrossRef]
- Dalavoy, T.S.; Jackson, J.E.; Swain, G.M.; Miller, D.J.; Li, J.; Lipkowski, J. Mild electrocatalytic hydrogenation of lactic acid to lactaldehyde and propylene glycol. J. Catal. 2007, 246, 15–28. [Google Scholar] [CrossRef]
- Lai, S.C.; Koper, M.T. Electro-oxidation of ethanol and acetaldehyde on platinum single-crystal electrodes. Faraday Discuss. 2009, 140, 399–416. [Google Scholar] [CrossRef]
- Huang, B.; Durante, C.; Isse, A.A.; Gennaro, A. Highly selective electrochemical hydrogenation of acetylene to ethylene at Ag and Cu cathodes. Electrochem. Commun. 2013, 34, 90–93. [Google Scholar] [CrossRef]
- Li, Z.; Kelkar, S.; Raycraft, L.; Garedew, M.; Jackson, J.E.; Miller, D.J.; Saffron, C.M. A mild approach for bio-oil stabilization and upgrading: Electrocatalytic hydrogenation using ruthenium supported on activated carbon cloth. Green Chem. 2014, 16, 844–852. [Google Scholar] [CrossRef]
- Li, Z.; Garedew, M.; Lam, C.H.; Jackson, J.E.; Miller, D.J.; Saffron, C.M. Mild electrocatalytic hydrogenation and hydrodeoxygenation of bio-oil derived phenolic compounds using ruthenium supported on activated carbon cloth. Green Chem. 2012, 14, 2540–2549. [Google Scholar] [CrossRef]
- Kwon, Y.; Koper, M. Electrocatalytic hydrogenation and deoxygenation of glucose on solid metal electrodes. ChemSusChem 2013, 6, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.H.; Lowe, C.B.; Li, Z.; Longe, K.N.; Rayburn, J.T.; Caldwell, M.A.; Houdek, C.E.; Maguire, J.B.; Saffron, C.M.; Miller, D.J. Electrocatalytic upgrading of model lignin monomers with earth abundant metal electrodes. Green Chem. 2015, 17, 601–609. [Google Scholar] [CrossRef]
- Moutet, J.-C. Electrocatalytic hydrogenation on hydrogen-active electrodes. A review. Org. Prep. Proced. Int. 1992, 24, 309–325. [Google Scholar] [CrossRef]
- Mahdavi, B.; Chapuzet, J.M.; Lessard, J. The electrocatalytic hydrogenation of phenanthrene at Raney nickel electrodes: The effect of periodic current control. Electrochim. Acta 1993, 38, 1377–1380. [Google Scholar] [CrossRef]
- Lipkowski, J.; Ross, P.N. Electrocatalysis, 1th ed.; John Wiley&Sons: New York, NY, USA, 1998; pp. 75–290. ISBN 0471246735. [Google Scholar]
- Robin, D.; Comtois, M.; Martel, A.; Lemieux, R.; Cheong, A.K.; Belot, G.; Lessard, J. The electrocatalytic hydrogenation of fused poly cyclic aromatic compounds at Raney nickel electrodes: The influence of catalyst activation and electrolysis conditions. Can. J. Chem. 1990, 68, 1218–1227. [Google Scholar] [CrossRef]
- Garraín, D.; Lechón, Y.; de la Rúa, C. Polymer electrolyte membrane fuel cells (PEMFC) in automotive applications: Environmental relevance of the manufacturing stage. Smart Grid Renew. Energy 2011, 2, 68. [Google Scholar] [CrossRef]
- Montiel, V.; Sáez, A.; Expósito, E.; García-García, V.; Aldaz, A. Use of MEA technology in the synthesis of pharmaceutical compounds: The electrosynthesis of N-acetyl-l-cysteine. Electrochem. Commun. 2010, 12, 118–121. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, K.S.; Mishler, J.; Cho, S.C.; Adroher, X.C. A review of polymer electrolyte membrane fuel cells: Technology, applications, and needs on fundamental research. Appl. Energy 2011, 88, 981–1007. [Google Scholar] [CrossRef]
- Zhang, X.; Pasaogullari, U.; Molter, T. Influence of ammonia on membrane-electrode assemblies in polymer electrolyte fuel cells. Int. J. Hydrogen Energy 2009, 34, 9188–9194. [Google Scholar] [CrossRef]
- Florida Solar Energy Center, Test Protocol for Cell Performance Tests Performed Under DOE. 2009; pp. 19–20. Available online: https://www.energy.gov/sites/prod/files/2014/03/f10/htmwg_may09_pem_single_cell_testing.pdf (accessed on 8 October 2018).
- Green, S.K.; Tompsett, G.A.; Kim, H.J.; Kim, W.B.; Huber, G.W. Electrocatalytic Reduction of Acetone in a Proton-Exchange-Membrane Reactor: A Model Reaction for the Electrocatalytic Reduction of Biomass. ChemSusChem 2012, 5, 2410–2420. [Google Scholar] [CrossRef] [PubMed]
- Dadda, B.; Abboudi, S.; Zarrit, R.; Ghezal, A. Heat and mass transfer influence on potential variation in a PEMFC membrane. Int. J. Hydrogen Energy 2014, 39, 15238–15245. [Google Scholar] [CrossRef]
- Zong, Y.; Zhou, B.; Sobiesiak, A. Water and thermal management in a single PEM fuel cell with non-uniform stack temperature. J. Power Sources 2006, 161, 143–159. [Google Scholar] [CrossRef]
- Berg, L. Separation of Acetone from Isopropanol-Water Mixtures by Extractive Distillation. US 5897750, 1999. Available online: https://patents.justia.com/patent/5897750 (accessed on 8 October 2018).
- Jian, Q.; Ma, G.; Qiu, X. Influences of gas relative humidity on the temperature of membrane in PEMFC with interdigitated flow field. Renew. Energy 2014, 62, 129–136. [Google Scholar] [CrossRef]
- Anantaraman, A.; Gardner, C. Studies on ion-exchange membranes. Part 1. Effect of humidity on the conductivity of Nafion®. J. Electroanal. Chem. 1996, 414, 115–120. [Google Scholar] [CrossRef]
- Sumner, J.; Creager, S.; Ma, J.; DesMarteau, D. Proton conductivity in Nafion® 117 and in a novel bis [(perfluoroalkyl) sulfonyl] imide ionomer membrane. J. Electrochem. Soc. 1998, 145, 107–110. [Google Scholar] [CrossRef]
- Sone, Y.; Ekdunge, P.; Simonsson, D. Proton conductivity of Nafion 117 as measured by a four-electrode AC impedance method. J. Electrochem. Soc. 1996, 143, 1254–1259. [Google Scholar] [CrossRef]
- Yuan, W.; Tang, Y.; Pan, M.; Li, Z.; Tang, B. Model prediction of effects of operating parameters on proton exchange membrane fuel cell performance. Renew. Energy 2010, 35, 656–666. [Google Scholar] [CrossRef]
- Wong, K.; Loo, K.; Lai, Y.; Tan, S.-C.; Chi, K.T. A theoretical study of inlet relative humidity control in PEM fuel cell. Int. J. Hydrogen Energy 2011, 36, 11871–11885. [Google Scholar] [CrossRef]
- Lee, C.-I.; Chu, H.-S. Effects of cathode humidification on the gas–liquid interface location in a PEM fuel cell. J. Power Sources 2006, 161, 949–956. [Google Scholar] [CrossRef]
- Guvelioglu, G.H.; Stenger, H.G. Flow rate and humidification effects on a PEM fuel cell performance and operation. J. Power Sources 2007, 163, 882–891. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, Y.; Song, C.; Xia, Z.; Li, H.; Wang, H.; Zhang, J. PEM fuel cell relative humidity (RH) and its effect on performance at high temperatures. Electrochim. Acta 2008, 53, 5315–5321. [Google Scholar] [CrossRef]
- Jeon, D.H.; Kim, K.N.; Baek, S.M.; Nam, J.H. The effect of relative humidity of the cathode on the performance and the uniformity of PEM fuel cells. Int. J. Hydrogen Energy 2011, 36, 12499–12511. [Google Scholar] [CrossRef]
- Ikeda, T.; Koido, T.; Tsushima, S.; Hirai, S. MRI investigation of water transport mechanism in a membrane under elevated temperature condition with relative humidity and current density variation. ECS Trans. 2008, 16, 1035–1040. [Google Scholar]
- Misran, E.; Hassan, N.S.M.; Daud, W.R.W.; Majlan, E.H.; Rosli, M.I. Water transport characteristics of a PEM fuel cell at various operating pressures and temperatures. Int. J. Hydrogen Energy 2013, 38, 9401–9408. [Google Scholar] [CrossRef]
- Knights, S.D.; Colbow, K.M.; St-Pierre, J.; Wilkinson, D.P. Aging mechanisms and lifetime of PEFC and DMFC. J. Power Sources 2004, 127, 127–134. [Google Scholar] [CrossRef]
- Abe, T.; Shima, H.; Watanabe, K.; Ito, Y. Study of PEFCs by AC Impedance, Current Interrupt, and Dew Point Measurements I. Effect of Humidity in Oxygen Gas. J. Electrochem. Soc. 2004, 151, A101–A105. [Google Scholar] [CrossRef]
- Saleh, M.M.; Okajima, T.; Hayase, M.; Kitamura, F.; Ohsaka, T. Exploring the effects of symmetrical and asymmetrical relative humidity on the performance of H2/air PEM fuel cell at different temperatures. J. Power Sources 2007, 164, 503–509. [Google Scholar] [CrossRef]
- Xu, H.; Song, Y.; Kunz, H.R.; Fenton, J.M. Effect of elevated temperature and reduced relative humidity on ORR kinetics for PEM fuel cells. J. Electrochem. Soc. 2005, 152, A1828–A1836. [Google Scholar] [CrossRef]
- Uribe, F.A.; Springer, T.E.; Gottesfeld, S. A microelectrode study of oxygen reduction at the platinum/recast—Nafion film interface. J. Electrochem. Soc. 1992, 139, 765–773. [Google Scholar] [CrossRef]
- Jang, J.-H.; Yan, W.-M.; Li, H.-Y.; Chou, Y.-C. Humidity of reactant fuel on the cell performance of PEM fuel cell with baffle-blocked flow field designs. J. Power Sources 2006, 159, 468–477. [Google Scholar] [CrossRef]
- Broka, K.; Ekdunge, P. Oxygen and hydrogen permeation properties and water uptake of Nafion® 117 membrane and recast film for PEM fuel cell. J. Appl. Electrochem. 1997, 27, 117–123. [Google Scholar] [CrossRef]
- Murillo, L.E.; Chen, J.G. Adsorption and reaction of propanal, 2-propenol and 1-propanol on Ni/Pt (111) bimetallic surfaces. Surf. Sci. 2008, 602, 2412–2420. [Google Scholar] [CrossRef]
- Takalloo, P.K.; Nia, E.S.; Ghazikhani, M. Numerical and experimental investigation on effects of inlet humidity and fuel flow rate and oxidant on the performance on polymer fuel cell. Energy Convers. Manag. 2016, 114, 290–302. [Google Scholar] [CrossRef]
- Jiang, R.; Kunz, H.R.; Fenton, J.M. Investigation of membrane property and fuel cell behavior with sulfonated poly(ether ether ketone) electrolyte: Temperature and relative humidity effects. J. Power Sources 2005, 150, 120–128. [Google Scholar] [CrossRef]
- Xu, H.; Kunz, H.R.; Fenton, J.M. Analysis of proton exchange membrane fuel cell polarization losses at elevated temperature 120 °C and reduced relative humidity. Electrochim. Acta 2007, 52, 3525–3533. [Google Scholar] [CrossRef]
- Jiang, R.; Kunz, H.R.; Fenton, J.M. Influence of temperature and relative humidity on performance and CO tolerance of PEM fuel cells with Nafion®–Teflon®–Zr (HPO4) 2 higher temperature composite membranes. Electrochim. Acta 2006, 51, 5596–5605. [Google Scholar] [CrossRef]
- Wang, X.; Baker, P.; Zhang, X.; Garces, H.F.; Bonville, L.J.; Pasaogullari, U.; Molter, T.M. An experimental overview of the effects of hydrogen impurities on polymer electrolyte membrane fuel cell performance. Int. J. Hydrogen Energy 2014, 39, 19701–19713. [Google Scholar] [CrossRef]
- Cheng, X.; Shi, Z.; Glass, N.; Zhang, L.; Zhang, J.; Song, D.; Liu, Z.-S.; Wang, H.; Shen, J. A review of PEM hydrogen fuel cell contamination: Impacts, mechanisms, and mitigation. J. Power Sources 2007, 165, 739–756. [Google Scholar] [CrossRef]
- Benziger, J.; Nehlsen, J. A polymer electrolyte hydrogen pump hydrogenation reactor. Ind. Eng. Chem. Res. 2010, 49, 11052–11060. [Google Scholar] [CrossRef]
- Lundin, M.D.; McCready, M.J. High pressure anode operation of direct methanol fuel cells for carbon dioxide management. J. Power Sources 2011, 196, 5583–5590. [Google Scholar] [CrossRef]
- Scholta, J.; Berg, N.; Wilde, P.; Jörissen, L.; Garche, J. Development and performance of a 10 kW PEMFC stack. J. Power Sources 2004, 127, 206–212. [Google Scholar] [CrossRef]
- Cleghorn, S.; Mayfield, D.; Moore, D.; Moore, J.; Rusch, G.; Sherman, T.; Sisofo, N.; Beuscher, U. A polymer electrolyte fuel cell life test: 3 years of continuous operation. J. Power Sources 2006, 158, 446–454. [Google Scholar] [CrossRef]
- St-Pierre, J.; Wilkinsor, D.; Knights, S.; Bos, M. Relationships between water management, contamination and lifetime degradation in PEFC. J. New Mater. Electrochem. Syst. 2000, 3, 99–106. [Google Scholar]
- Satyapal, S. Hydrogen & Fuel Cells Program Overview. In Proceedings of the Annual Merit Review and Peer Evaluation Meeting, Arlington, VA, USA, 7–11 June 2011. [Google Scholar]
- Wipke, K.; Sprik, S.; Kurtz, J.; Ramsden, T.; Ainscough, C.; Saur, G. VII. 1 Controlled Hydrogen Fleet and Infrastructure Analysis. In Proceedings of the DOE Annual Merit Review and Peer Evaluation Meeting, Washington, DC, USA, 13–17 May 2012. [Google Scholar]
- Wu, J.; Yuan, X.Z.; Martin, J.J.; Wang, H.; Zhang, J.; Shen, J.; Wu, S.; Merida, W. A review of PEM fuel cell durability: Degradation mechanisms and mitigation strategies. J. Power Sources 2008, 184, 104–119. [Google Scholar] [CrossRef]
- Ferreira, P.; Shao-Horn, Y.; Morgan, D.; Makharia, R.; Kocha, S.; Gasteiger, H. Instability of Pt/C electrocatalysts in proton exchange membrane fuel cells a mechanistic investigation. J. Electrochem. Soc. 2005, 152, A2256–A2271. [Google Scholar] [CrossRef]
- Xie, J.; Wood, D.L.; More, K.L.; Atanassov, P.; Borup, R.L. Microstructural changes of membrane electrode assemblies during PEFC durability testing at high humidity conditions. J. Electrochem. Soc. 2005, 152, A1011–A1020. [Google Scholar] [CrossRef]
- Antolini, E. Formation, microstructural characteristics and stability of carbon supported platinum catalysts for low temperature fuel cells. J. Mater. Sci. 2003, 38, 2995–3005. [Google Scholar] [CrossRef]
- Murugan, A.; Brown, A.S. Review of purity analysis methods for performing quality assurance of fuel cell hydrogen. Int. J. Hydrogen Energy 2015, 40, 4219–4233. [Google Scholar] [CrossRef]
- St-Pierre, J.; Angelo, M.; Zhai, Y. Focusing research by developing performance related selection criteria for PEMFC contaminants. ECS Trans. 2011, 41, 279–286. [Google Scholar]
- St-Pierre, J.; Zhai, Y.; Angelo, M.S. Effect of selected airborne contaminants on PEMFC performance. J. Electrochem. Soc. 2014, 161, F280–F290. [Google Scholar] [CrossRef]
- Fukuzumi, S.; Lee, Y.M.; Nam, W. Mechanisms of two-electron versus four-electron reduction of dioxygen catalyzed by earth—Abundant metal complexes. ChemCatChem 2018, 10, 9–28. [Google Scholar] [CrossRef]
- Chen, Y.; Hwang, C.; Liaw, C. One-step synthesis of methyl isobutyl ketone from acetone with calcined Mg/Al hydrotalcite-supported palladium or nickel catalysts. Appl. Catal. A Gen. 1998, 169, 207–214. [Google Scholar] [CrossRef]
- Nikolopoulos, A.; Jang, B.-L.; Spivey, J. Acetone condensation and selective hydrogenation to MIBK on Pd and Pt hydrotalcite-derived MgAl mixed oxide catalysts. Appl. Catal. A Gen. 2005, 296, 128–136. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Sallee, A.M.; Zhang, X.; Kumar, S. Electrochemical Hydrogenation of Acetone to Produce Isopropanol Using a Polymer Electrolyte Membrane Reactor. Energies 2018, 11, 2691. https://doi.org/10.3390/en11102691
Li C, Sallee AM, Zhang X, Kumar S. Electrochemical Hydrogenation of Acetone to Produce Isopropanol Using a Polymer Electrolyte Membrane Reactor. Energies. 2018; 11(10):2691. https://doi.org/10.3390/en11102691
Chicago/Turabian StyleLi, Chen, Ashanti M. Sallee, Xiaoyu Zhang, and Sandeep Kumar. 2018. "Electrochemical Hydrogenation of Acetone to Produce Isopropanol Using a Polymer Electrolyte Membrane Reactor" Energies 11, no. 10: 2691. https://doi.org/10.3390/en11102691
APA StyleLi, C., Sallee, A. M., Zhang, X., & Kumar, S. (2018). Electrochemical Hydrogenation of Acetone to Produce Isopropanol Using a Polymer Electrolyte Membrane Reactor. Energies, 11(10), 2691. https://doi.org/10.3390/en11102691



