A Theoretical-Experimental Comparison of an Improved Ammonia-Water Bubble Absorber by Means of a Helical Static Mixer
Abstract
1. Introduction
2. Experimental Description
2.1. Test Rig Description
2.2. Absorber Description
2.3. Experimental Results
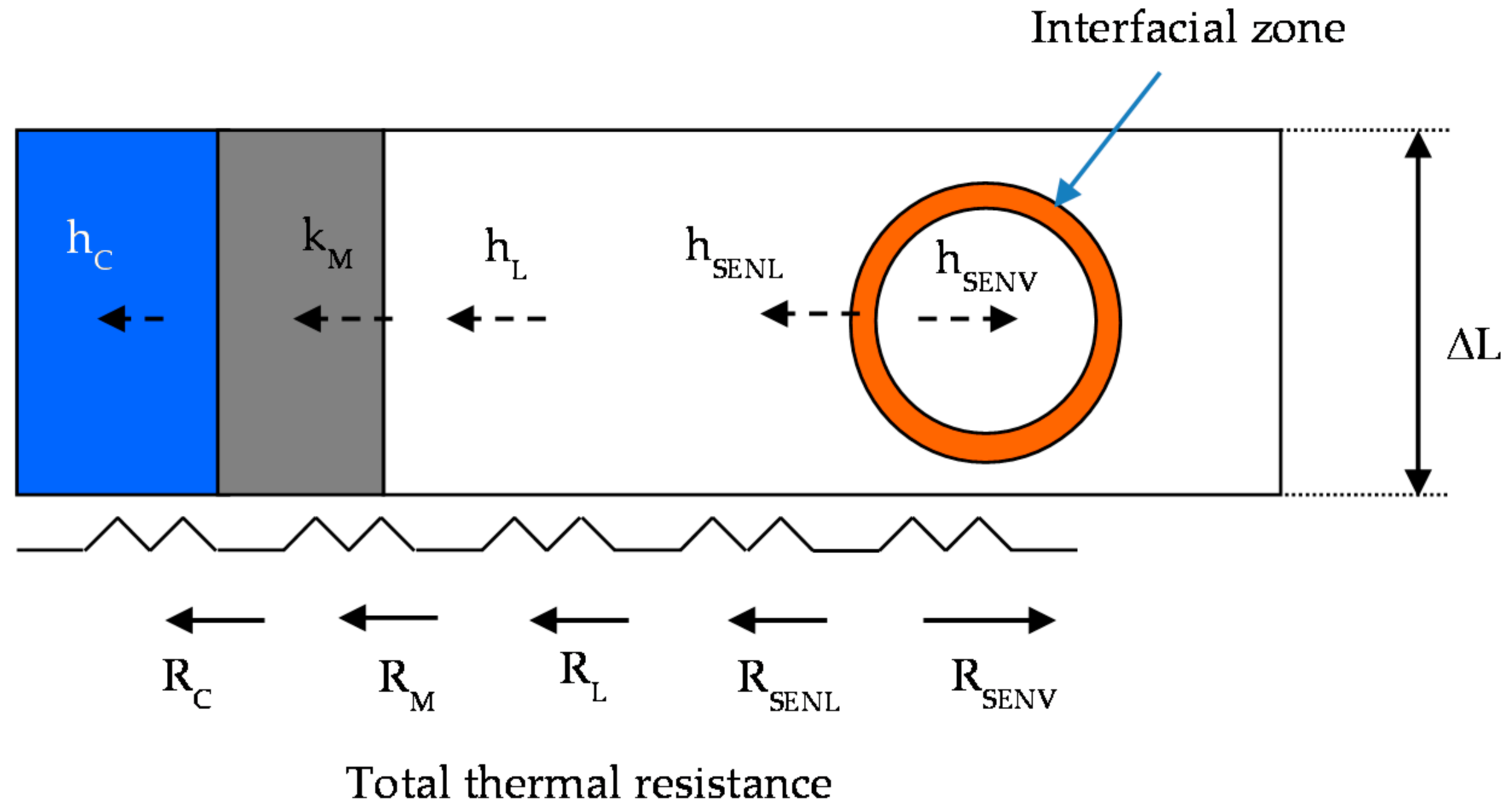
3. Mathematical Model Description
3.1. Assumptions
- The absorption process takes place at steady state conditions.
- The vapor and liquid phases are in equilibrium at the interface zone.
- The bubble velocity is constant.
- The vapor bubble has a spherical shape.
- There is no break-up and no interaction or coalescence amongst bubbles.
- There is no mass transfer in the sensible zone (absorption process is finished).
3.2. Governing Equations
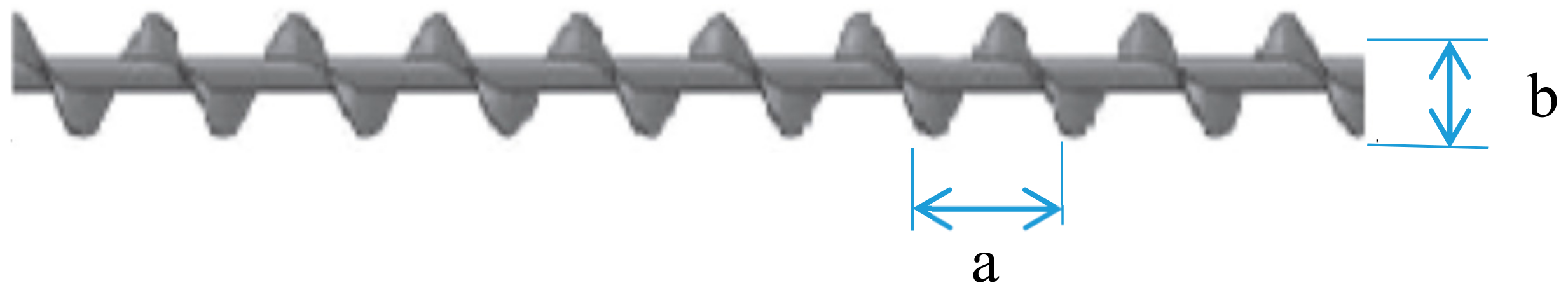
3.3. Empirical Correlations
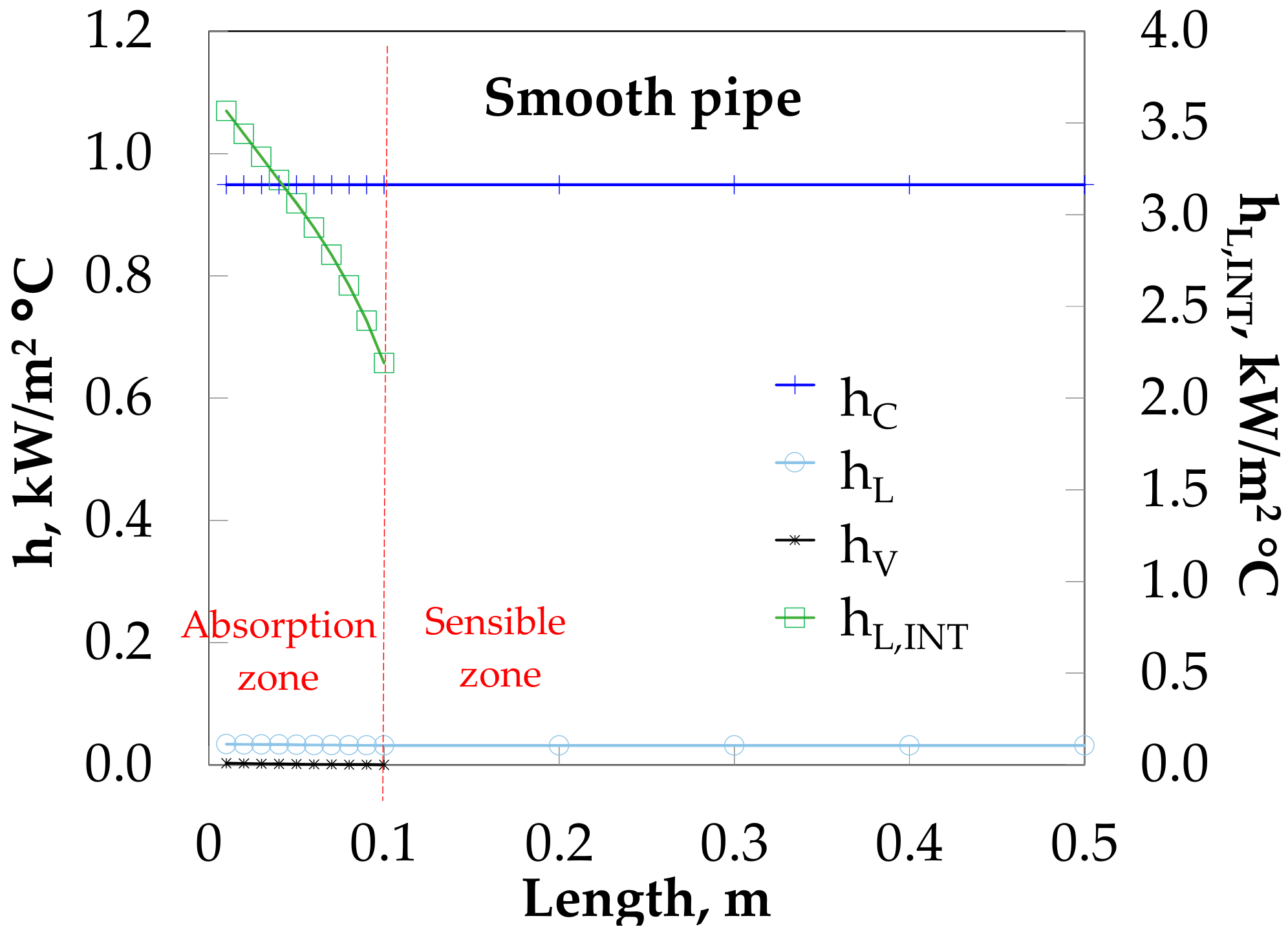
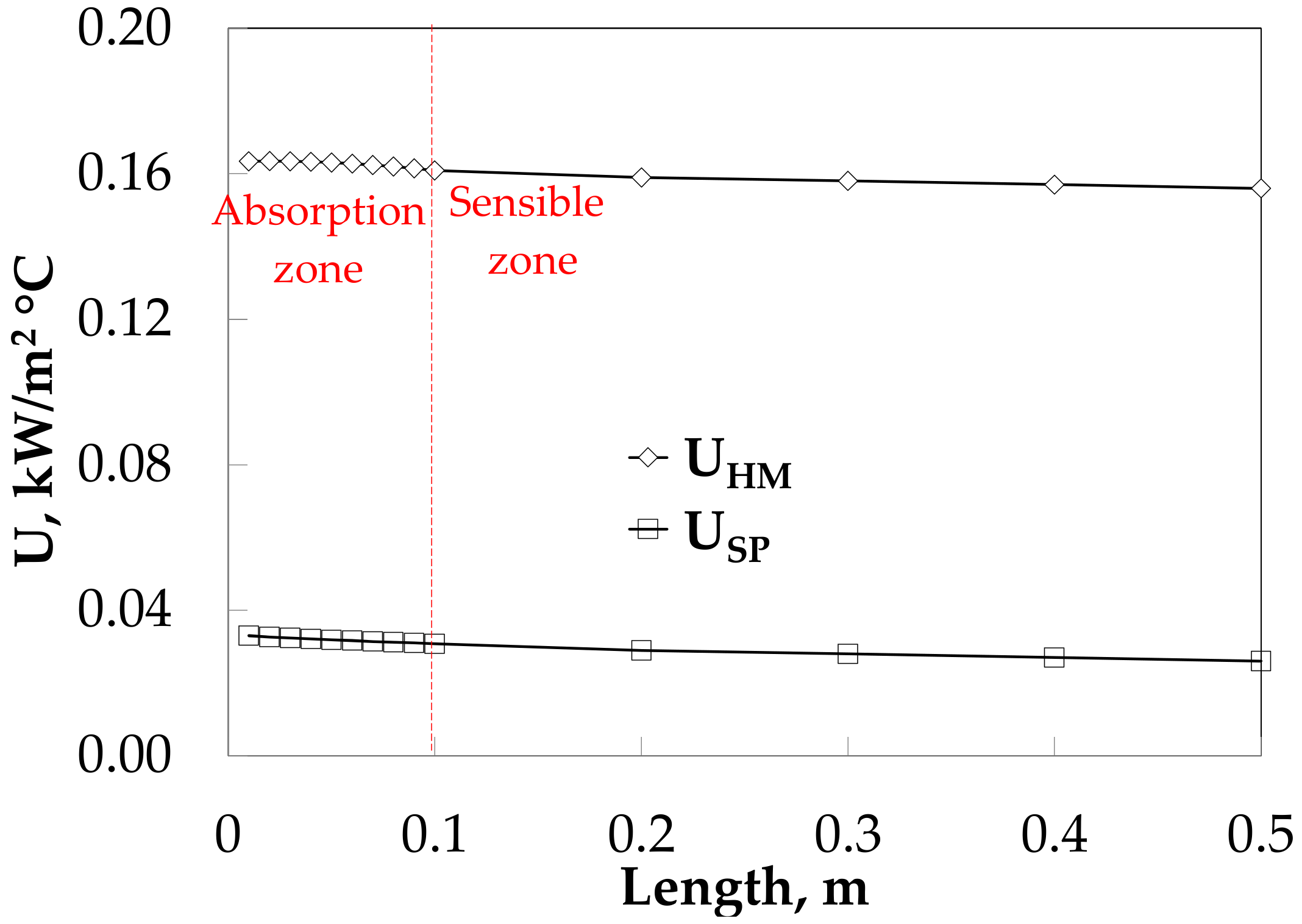
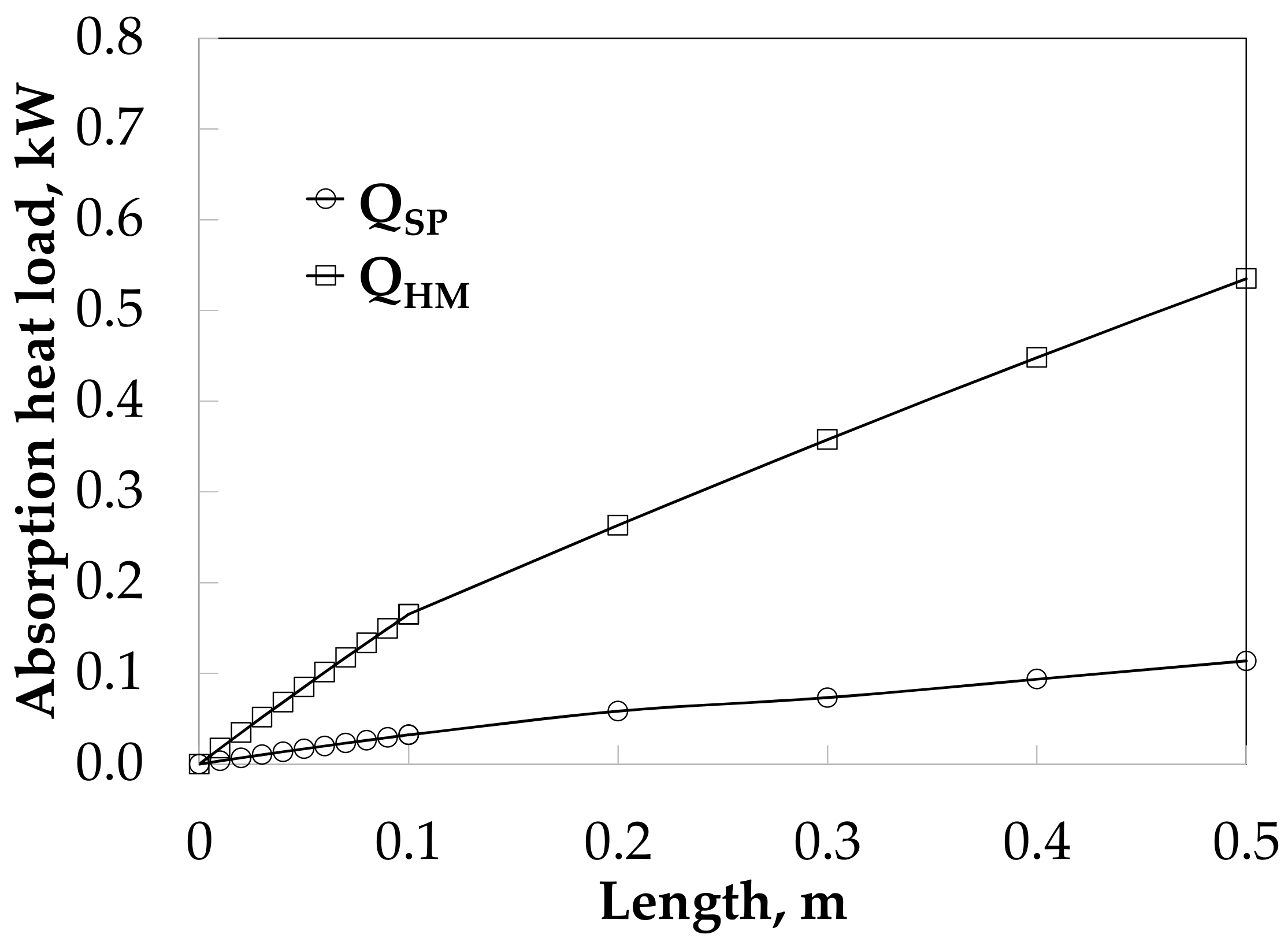
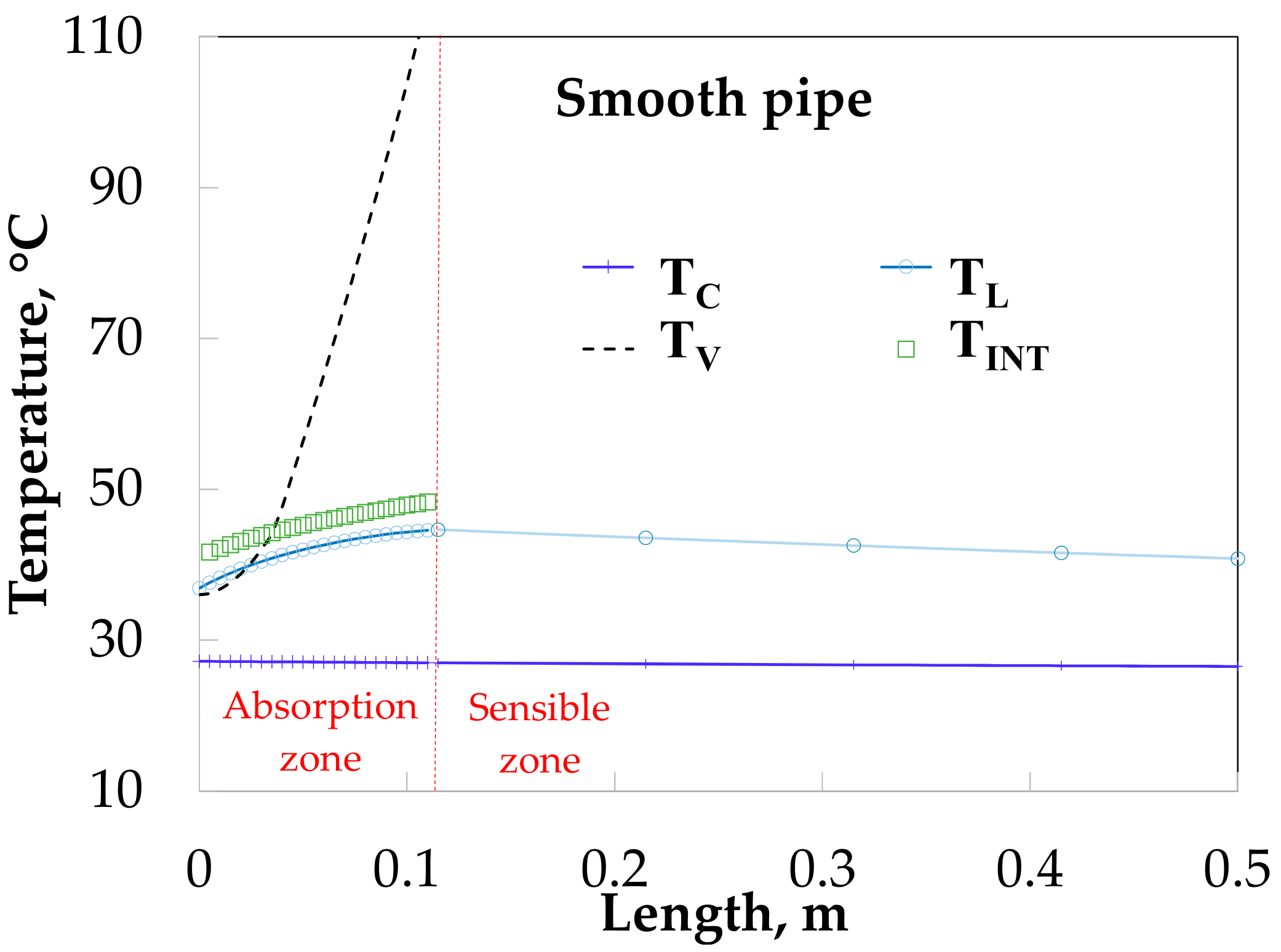
3.4. Absorber Simulation Using Empirical Heat Transfer Coefficient Correlations
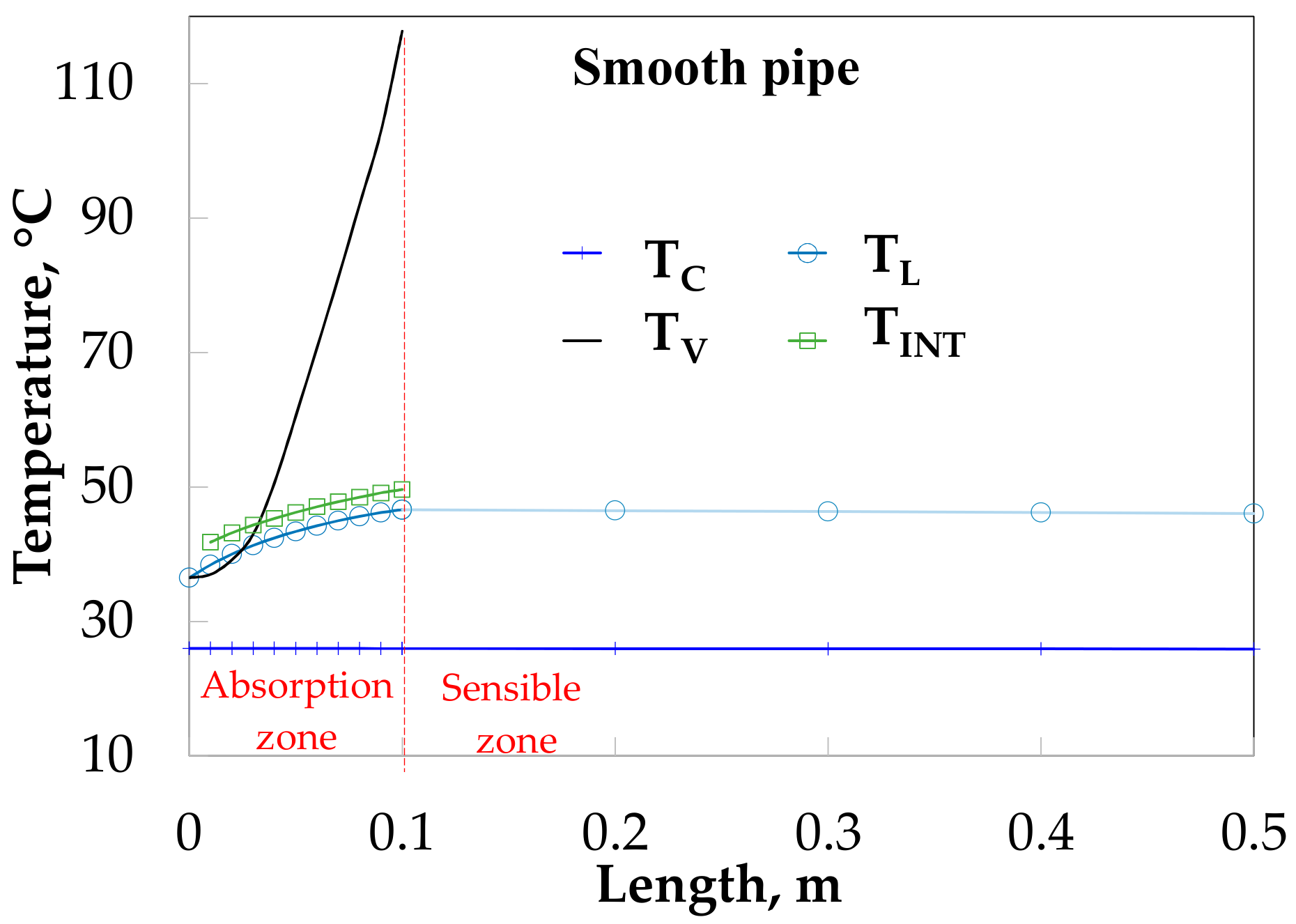
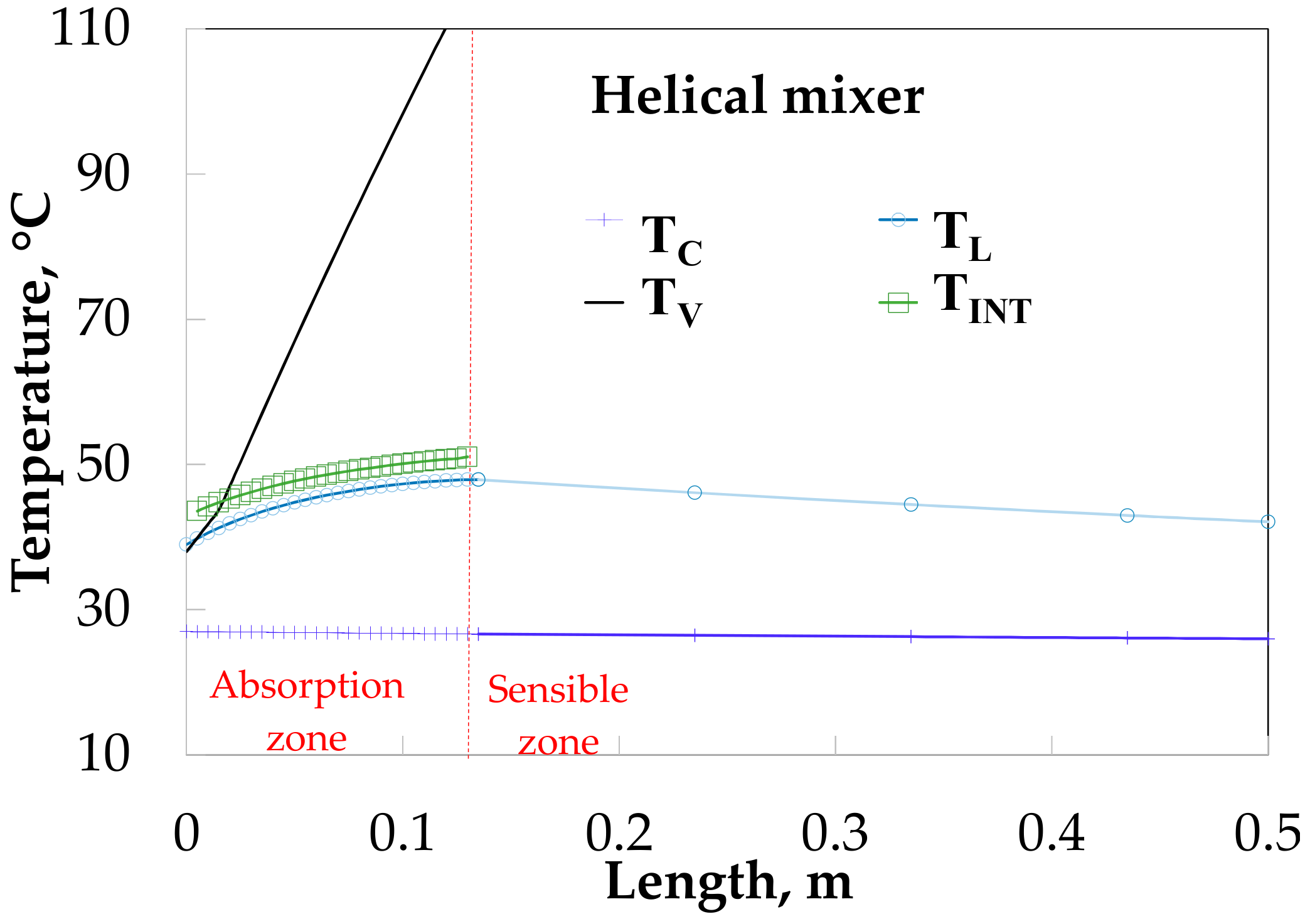
4. Theoretical and Experimental Data Comparison
5. Discussion
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Nomenclature
| Q | absorption heat load, kW |
| T | temperature, °C |
| U | overall heat transfer coefficient, kW/(m2·°C) |
| e | specific enthalpy, kJ/kg |
| h | heat transfer coefficient, kW/(m2·°C) |
| m | mass flow rate, kg/s |
| x | liquid ammonia concentration, % by weight |
| y | vapor ammonia concentration, % by weight |
| R | thermal resistance, °C/kW |
| Km | mass transfer coefficient, kg/m2 s |
| a | length of one twist, m |
| b | diameter of the twist, m |
| s | spacer length, m |
| Greek Symbols | |
| µ | dynamic viscosity, kg/m s |
| σ | surface tension, N/m |
| ρ | density, kg/m3 |
| β | diffusivity, m2/s |
| Subscripts | |
| AB | absorber |
| L | solution, liquid phase |
| V | vapor phase |
| IN | input |
| OUT | output |
| EQ | equilibrium |
| C | cooling water |
| SENV | vapor sensible heat |
| SENL | liquid sensible heat |
| NH3 | ammonia |
| H2O | water |
| INT | interfacial zone |
References
- Gugulothu, R.; Reddy, V.; Somanchi, N.S.; Adithya, E.L. A Review on Enhancement of Heat Transfer Techniques. Mater. Today 2017, 4, 1051–1056. [Google Scholar] [CrossRef]
- Lee, K.B.; Chung, B.H.; Lee, J.C.; Lee, C.H.; Kim, S.H. Experimental analysis bubble mode in a plate-type absorber. Chem. Eng. Sci. 2002, 57, 1923–1929. [Google Scholar] [CrossRef]
- Cerezo, J.; Best, R.; Bourouis, M.; Coronas, A. Comparison of numerical and experimental performance criteria of an ammonia-water bubble absorber using plate heat exchangers. Int. J. Heat Mass Transf. 2010, 53, 3379–3386. [Google Scholar] [CrossRef]
- Cardenas, R.; Narayanan, V. A numerical model for ammonia-water absorption into a constrained microscale film. Int. J. Therm. Sci. 2010, 49, 1787–1798. [Google Scholar] [CrossRef]
- Oronel, C.; Amaris, C.; Bourouis, M.; Vallès, M. Heat and mass transfer in a bubble plate absorber with NH3/LiNO3 and NH3/(LiNO3 + H2O) mixtures. Int. J. Therm. Sci. 2013, 63, 105–114. [Google Scholar] [CrossRef]
- Amaris, C.; Bourouis, M.; Vallès, M. Effect of advanced surfaces on the ammonia absorption process with NH3/LiNO3 in a tubular bubble absorber. Int. J. Heat Mass Transf. 2014, 72, 544–552. [Google Scholar] [CrossRef]
- Amaris, C.; Bourouis, M.; Vallès, M. Passive intensification of the ammonia absorption process with NH3/LiNO3 using carbon nanotubes and advanced surfaces in a tubular bubble absorber. Energy 2014, 68, 519–528. [Google Scholar] [CrossRef]
- Triché, D.; Bonnot, S.; Perier-Muzet, M.; Boudéhenn, F.; Demasles, H.; Caney, N. Experimental and numerical study of a falling film absorber in an ammonia-water absorption chiller. Int. J. Heat Mass Transf. 2017, 111, 374–385. [Google Scholar] [CrossRef]
- Asfand, F.; Bourouis, M. A review of membrane contactors applied in absorption refrigeration systems. Renew. Sustain. Energy Rev. 2015, 45, 173–191. [Google Scholar] [CrossRef]
- Rader, R.G.; Mutsakis, M.; Grosz-Roell, F.; Maugweiler, W. Better Absorption? Try a Static Mixer. Chem. Eng. 1989, 96, 137–142. [Google Scholar]
- Ghanem, A.; Lemenand, T.; Della Valle, D.; Peerhossaini, H. Static mixers: Mechanisms, applications, and characterization methods—A review. Chem. Eng. Res. Des. 2014, 92, 205–228. [Google Scholar] [CrossRef]
- Rabha, S.; Schubert, M.; Grugel, F.; Banowski, M.; Hampel, U. Visualization and quantitative analysis of dispersive mixing by a helical static mixer in upward co-current gas-liquid flow. Chem. Eng. J. 2015, 262, 527–540. [Google Scholar] [CrossRef]
- Zidouni, F.; Krepper, E.; Rzehak, R.; Rabha, S.; Schubert, M.; Hampel, U. Simulation of gas-liquid flow in a helical static mixer. Chem. Eng. Sci. 2015, 137, 476–486. [Google Scholar] [CrossRef]
- Kakaç, S.; Liu, H. Heat Exchangers: Selection, Rating and Thermal Design, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Cerezo, J.; Bourouis, M.; Vallès, M.; Coronas, A.; Best, R. Experimental study of an ammonia-water bubble absorber using a plate heat exchanger for absorption refrigeration machines. Appl. Therm. Eng. 2009, 29, 1005–1011. [Google Scholar] [CrossRef]
- Deckwer, W.D. On the mechanism of heat transfer in bubble column reactor. Chem. Eng. Sci. 1980, 35, 1341–1349. [Google Scholar] [CrossRef]
- Clift, R.; Grace, J.R.; Weber, M.E. Bubbles, Drops and Particles, 1st ed.; Academic Press: New York, NY, USA, 1978. [Google Scholar]
- Incropera, F.P.; De Witt, D.P. Fundamental of Heat and Mass Transfer, 3rd ed.; John Wiley & Sons: New York, NY, USA, 1990. [Google Scholar]
- Sherwood, T.K.; Pigford, R.L.; Wlke, C.R. Mass Transfer; McGraw-Hill: New York, NY, USA, 1975. [Google Scholar]
- Ibrahim, O.M.; Klein, S.A. Thermodynamic properties of ammonia-water mixtures. ASHRAE Trans. Symp. 1993, 99, 1495–1502. [Google Scholar]
- Cengel, Y.A. Heat and Mass Transfer: A Practical Approach, 2nd ed.; Mc Graw-Hill Professional: New York, NY, USA, 2007. [Google Scholar]
- Ibrahim, E.Z. Augmentation of laminar flow and heat transfer in flat tubes by means of helical screw-tape inserts. Energy Convers. Manag. 2011, 52, 250–257. [Google Scholar] [CrossRef]
- Bourouis, M.; Vallès, M.; Medrano, M.; Coronas, A. Absorption of water vapour in the falling film of water—(LiBr + LiI + LiNO3 + LiCl) in a vertical tube at air-cooling thermal conditions. Int. J. Therm. Sci. 2005, 44, 491–498. [Google Scholar] [CrossRef]

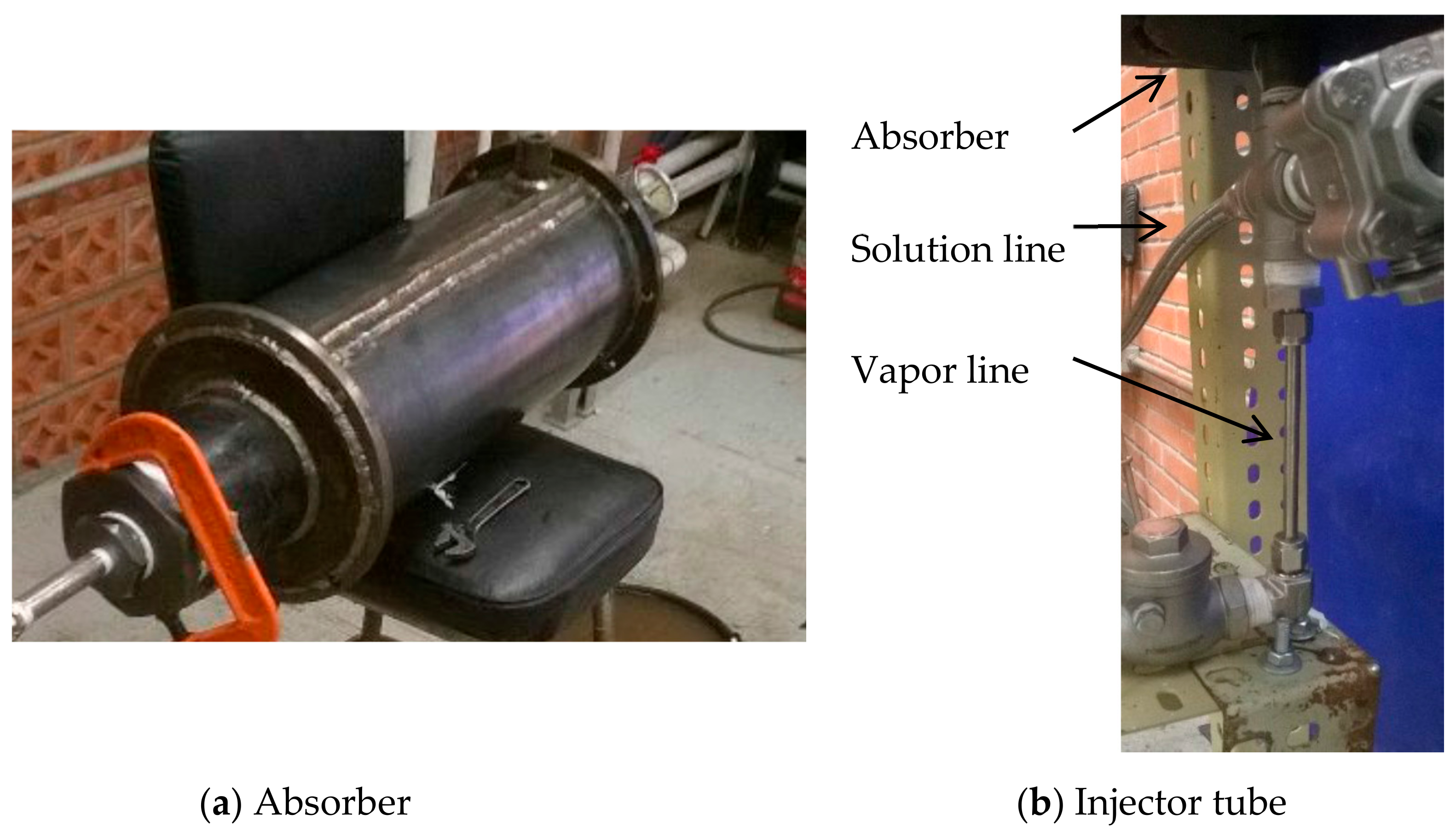



| Sensor | Device | Operating Range | Accuracy |
|---|---|---|---|
| temperature | RTD | −180–520 °C | ±0.20 °C |
| mass flow rate | Coriolis | 0–5 kg/min | ±0.10% |
| density | Coriolis | 700–1200 kg/m3 | ±0.10% |
| pressure | piezoelectric | 0–10 bar | ±0.15% |
| mass flow rate | Turbine | 0–30 kg/min | ±0.20% |
| Geometry | Value |
|---|---|
| Length, m | 0.50 |
| Inside diameter, m | 0.10 |
| Outside diameter, m | 0.20 |
| Tube thickness, mm | 3.0 |
| Number of vapor injection tubes | 1 |
| Orifice diameter of injection, mm | 2 |
| Solution Pressure, bar | 5.7 |
| Solution mass flow rate, kg/s | 0.03 |
| Vapor mass flow rate, kg/s | 0.001 |
| Cooling mass flow rate, kg/s | 0.255 |
| Solution concentration, % weight | 40.50 |
| Vapor concentration, % weight | 99.00 |
| Parameters | SP | HM |
|---|---|---|
| TL,IN (°C) | 36.5 | 36.7 |
| xIN (% weight) | 40.91 | 42.32 |
| mL,IN (kg/s) | 0.030 | 0.028 |
| PL,IN (bar) | 5.72 | 5.64 |
| TL,OUT (°C) | 33.2 | 32.5 |
| TC,IN (°C) | 26.4 | 26.4 |
| TC,OUT (°C) | 26.7 | 26.8 |
| mC (kg/s) | 0.255 | 0.256 |
| QL (kW) | 0.384 | 0.498 |
| QC (kW) | 0.352 | 0.385 |
| QAVE (kW/m2) | 1.17 | 1.40 |
| ΔΤML | 8.25 | 7.88 |
| hC (kW/(m2·°C)) | 0.95 | 0.95 |
| hL | 0.37 | 0.59 |
| Parameters | SP | HM |
|---|---|---|
| TL,IN (°C) | 36.9 | 37.0 |
| xIN (% weight) | 40.89 | 40.76 |
| mIN (kg/s) | 0.028 | 0.028 |
| PL,IN (bar) | 5.49 | 5.77 |
| TL,OUT (°C) | 38.9 | 38.4 |
| XOUT (% weight) | 42.83 | 42.77 |
| mOUT (kg/s) | 0.029 | 0.030 |
| PL,OUT (bar) | 5.45 | 5.74 |
| TC,IN (°C) | 26.6 | 26.0 |
| TC,OUT (°C) | 27.5 | 27.4 |
| mC (kg/s) | 0.253 | 0.255 |
| QAB (kW) | 1.026 | 1.406 |
| QC (kW) | 0.973 | 1.515 |
| QAB,AVE (kW/m2) | 3.18 | 4.65 |
| QINC (%) | - | 31.61 |
| ΔP (bar) | 0.04 | 0.03 |
| mABS (kg/s) | 0.0008 | 0.0010 |
| hC (kW/(m2·°C)) | 0.95 | 0.95 |
| hL (kW/(m2·°C)) | 0.27 | 0.45 |
| ΔTML,EQ | 29.4 | 31.7 |
| Parameters | Smooth | Helical | ||
|---|---|---|---|---|
| EXP | TEO | EXP | TEO | |
| TL,IN (°C) | 36.9 | 37.0 | ||
| TL,OUT (°C) | 38.9 | 40.8 | 34.4 | 42.1 |
| TC,IN (°C) | 26.6 | 26.0 | ||
| TC,OUT (°C) | 27.47 | 27.20 | 27.4 | 27.0 |
| QAB (kW) | 1.00 | 0.72 | 1.46 | 1.14 |
| QDIF (%) | 28.0 | 21.9 | ||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cerezo, J.; Best, R.; Chan, J.J.; Romero, R.J.; Hernandez, J.I.; Lara, F. A Theoretical-Experimental Comparison of an Improved Ammonia-Water Bubble Absorber by Means of a Helical Static Mixer. Energies 2018, 11, 56. https://doi.org/10.3390/en11010056
Cerezo J, Best R, Chan JJ, Romero RJ, Hernandez JI, Lara F. A Theoretical-Experimental Comparison of an Improved Ammonia-Water Bubble Absorber by Means of a Helical Static Mixer. Energies. 2018; 11(1):56. https://doi.org/10.3390/en11010056
Chicago/Turabian StyleCerezo, Jesús, Roberto Best, Jorge Jesús Chan, Rosenberg J. Romero, Jorge I. Hernandez, and Fernando Lara. 2018. "A Theoretical-Experimental Comparison of an Improved Ammonia-Water Bubble Absorber by Means of a Helical Static Mixer" Energies 11, no. 1: 56. https://doi.org/10.3390/en11010056
APA StyleCerezo, J., Best, R., Chan, J. J., Romero, R. J., Hernandez, J. I., & Lara, F. (2018). A Theoretical-Experimental Comparison of an Improved Ammonia-Water Bubble Absorber by Means of a Helical Static Mixer. Energies, 11(1), 56. https://doi.org/10.3390/en11010056







