Olive Mill Wastewater: From a Pollutant to Green Fuels, Agricultural Water Source and Bio-Fertilizer—Part 1. The Drying Kinetics
Abstract
1. Introduction
2. Experimental Procedure
2.1. Samples Preparation
2.2. Drying Equipment
3. Theoretical Approach
3.1. Moisture Content
3.2. Drying Kinetic Models
3.3. Diffusion Coefficient
3.4. Activation Energy
4. Results and Discussions
4.1. Experimental Drying Tests
4.2. Drying Kinetics
4.3. Diffusion Coefficient and Activation Energy Determination
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Roig, A.; Cayuela, M.L.; Sanchez-Monedero, M.A. An overview on olive mill wastes and their valorisation methods. Waste Manag. 2006, 26, 960–969. [Google Scholar] [CrossRef] [PubMed]
- Souilem, S.; El-Abbassi, A.; Kiai, H.; Hafidi, A.; Sayadi, S.; Galanakis, C.M. Chapter 1—Olive oil production sector: Environmental effects and sustainability challenges. In Olive Mill Waste: Recent Advances for Sustainable Management; Galanakis, C.M., Ed.; Elsevier-Academic Press: London, United Kingdom, 2017; pp. 1–28. [Google Scholar]
- Zorpas, A.A.; Costa, N.C. Combination of Fenton Oxidation and Composting for the Treatment of the Olive Solid Residue and the Olive Mile Wastewater from the Olive Oil Industry in Cyprus. Biores. Technol. 2010, 101, 7984–7987. [Google Scholar] [CrossRef] [PubMed]
- Zorpas, A.A.; Inglezakis, J.V. Intergraded Applied Methodology for the Treatment of Heavy Polluted Waste Waters from the Olive Oil Industries. Appl. Environ. Soil Sci. 2011, 2011, 537814. [Google Scholar] [CrossRef][Green Version]
- Kraiem, N.; Jeguirim, M.; Limousy, L.; Lajili, M.; Dorge, S.; Michelin, L.; Said, R. Impregnation of olive mill wastewater on dry biomasses: Impact on chemical properties and combustion performances. Energy 2014, 78, 479–489. [Google Scholar] [CrossRef]
- Del Buono, D.; Said-Pullicino, D.; Proietti, P.; Nasini, L.; Gigliotti, G. Utilization of olive husks as plant growing substrates: Phytotoxicity and plant biochemical responses. Compost Sci. Utili. 2011, 19, 52–60. [Google Scholar] [CrossRef]
- Peri, C.; Proietti, P. Olive mill waste and by-products. Chapter 22. In The Extra-Virgin Olive Oil Handbook; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Vlyssides, A.G.; Loizidou, M.; Gimouhopoulos, K.; Zorpas, A. Olive Oil Processing Wastes Production and Their Characteristics in Relation to Olive Oil Extraction Methods. Fresen. Environ. Bul. 1988, 7, 308–313. [Google Scholar]
- Regni, L.; Gigliotti, G.; Nasini, L.; Proietti, P. Reuse of olive mill waste as soil amendment. In Olive Mill Waste: Recent Advances for Sustainable Management; Galanakis, C.M., Ed.; Elsevier-Academic Press: London, United Kingdom, 2016; Chapter 5; pp. 97–117. [Google Scholar]
- Vlyssides, A.G.; Loizidou, M.; Zorpas, A.A. Characteristics of solid residues from olive oil processing as a bulking material for co-composting with industrial wastewater. J. Environ. Sci. Health Part A 1999, 34, 737–748. [Google Scholar] [CrossRef]
- Gotsi, M.; Kalogerakis, N.; Psillakis, E.; Samaras, P.; Mantzavinos, D. Electrochemical oxidation of olive oil mill wastewaters. Water Res. 2015, 39, 4117–4187. [Google Scholar] [CrossRef] [PubMed]
- El-Gohary, F.A.; Badawy, M.I.; El-Khateeb, M.A.; El-Kalliny, A.S. Integrated treatment of olive mill wastewater (OMW) by the combination of Fenton’s reaction and anaerobic treatment. J. Hazard. Mater. 2009, 162, 1536–1541. [Google Scholar] [CrossRef] [PubMed]
- Christoforou, E.; Fokaides, P.A. A review of olive mill solid wastes to energy utilization techniques. Waste Manag. 2016, 49, 346–363. [Google Scholar] [CrossRef] [PubMed]
- Jeguirim, M.; Chouchène, A.; Réguillon, A.F.; Trouvé, G.; Le Buzit, G. A new valorisation strategy of olive mill wastewater: Impregnation on sawdust and combustion. Resour. Conserv. Recycl. 2012, 59, 4–8. [Google Scholar] [CrossRef]
- McKendry, P. Energy production from biomass (part 2): Conversion technologies. Bioresour. Technol. 2002, 83, 47–54. [Google Scholar] [CrossRef]
- Goyal, H.B.; Seal, D.; Saxena, R.C. Biofuels from thermo-chemical conversion of renewable resources. Renew. Sust. Energy Rev. 2008, 12, 504–517. [Google Scholar] [CrossRef]
- Manyà, J.J. Pyrolysis for biochar purposes: A review to establish current knowledge gaps and research needs. Environ. Sci. Technol. 2012, 46, 7939–7954. [Google Scholar] [CrossRef] [PubMed]
- Röser, D.; Mola-Yudego, B.; Sikanen, L.; Prinz, R.; Gritten, D.; Emer, B.; Väätainen, K.; Erkkilä, A. Natural drying treatments during seasonal storage of wood for bioenergy in different European locations. Biomass Bioenergy 2011, 35, 4238–4247. [Google Scholar] [CrossRef]
- Ertekin, C.; Firat, M.Z. A comprehensive review of thin-layer drying models used in agricultural products. Crit. Rev. Food Sci. Nutr. 2017, 57, 701–717. [Google Scholar] [CrossRef] [PubMed]
- Koukouch, A.; Idlimam, A.; Asbik, M.; Sarh, B.; Izrar, B.; Bostyn, S.; Bah, A.; Ansari, O.; Zegaoui, O.E.; Amine, A. Experimental determination of the effective moisture diffusivity and activation energy during convective solar drying of olive pomace waste. Renew. Energy 2017, 101, 565–574. [Google Scholar] [CrossRef]
- Doymaz, I.; Gorel, O.; Akgun, N.A. Drying Characteristics of the Solid By-Product of Olive Oil Extraction. Biosyst. Eng. 2004, 88, 213–219. [Google Scholar] [CrossRef]
- Celma, A.R.; Rojas, S.; López, F.; Montero, I.; Miranda, T. Thin-layer drying behaviour of sludge of olive oil extraction. J. Food Eng. 2007, 80, 1261–1271. [Google Scholar] [CrossRef]
- Montero, I.; Miranda, M.T.; Sepúlveda, F.J.; Arranz, J.I.; Rojas, C.V.; Nogales, S. Solar Dryer Application for Olive Oil Mill Wastes. Energies 2015, 8, 14049–14063. [Google Scholar] [CrossRef]
- Liébanes, M.D.; Aragón, J.M.; Palancar, M.C.; Arévalo, G.; Jiménez, D. Fluidized bed drying of 2-phase olive oil mill by-products. Dry. Technol. 2006, 24, 1609–1618. [Google Scholar] [CrossRef]
- Göǧüş, F.; Maskan, M. Air drying characteristics of solid waste (pomace) of olive oil processing. J. Food Eng. 2016, 72, 378–382. [Google Scholar] [CrossRef]
- Celma, A.R.; Rojas, S.; Lopez-Rodriguez, F. Mathematical modelling of thin layer infrared drying of wet olive husk. Chem. Eng. Process. Process Intens. 2009, 47, 1810–1818. [Google Scholar] [CrossRef]
- Gómez-de la Cruz, F.J.; Casanova-Peláez, P.J.; López-García, R.; Cruz-Peragón, F. Review of the drying kinetics of olive oil mill wastes: Biomass recovery. BioResources 2015, 10, 6055–6080. [Google Scholar] [CrossRef]
- Ratti, C. Hot air and freeze-drying of high-value foods: A review. J. Food Eng. 2001, 4, 311–319. [Google Scholar] [CrossRef]
- Nourhène, B.; Mohammed, K.; Nabil, K. Experimental and mathematical investigations of convective solar drying of four varieties of olive leaves. Food Bioprod. Process. 2008, 86, 176–184. [Google Scholar] [CrossRef]
- Celma, A.R.; Cuadros, F.; López-Rodríguez, F. Convective drying characteristics of sludge from treatment plants in tomato processing industries. Food Bioprod. Process. 2012, 90, 224–234. [Google Scholar] [CrossRef]
- Meisami-Asl, E.; Rafiee, S.; Keyhani, A.; Tabatabaee, A. Mathematical Modeling of Moisture Content of Apple Slices (Var. Golab) During Drying. Pak. J. Nutr. 2009, 8, 804–809. [Google Scholar] [CrossRef]
- Jayas, D.S.; Cenkowski, S.; Pabis, S.; Muir, W.E. Review of Thin-Layer Drying and Wetting Equations. Dry Technol. 1991, 9, 551–588. [Google Scholar] [CrossRef]
- Wang, C.; Wang, F.; Yang, Q.; Liang, R. Thermogravimetric studies of the behavior of wheat straw with added coal during combustion. Biomass Bioenergy 2009, 33, 50–56. [Google Scholar] [CrossRef]
- Yaldýz, O.; Ertekýn, C. Thin Layer Solar Drying of Some Vegetables. Dry Technol. 2001, 19, 583–597. [Google Scholar] [CrossRef]
- Diamante, L.M.; Munro, P.A. Mathematical modelling of the thin layer solar drying of sweet potato slices. Sol Energy 1993, 51, 271–276. [Google Scholar] [CrossRef]
- Midilli, A.; Kucuk, H.; Yapar, Z.A. New Model for Single-Layer Drying. Dry Technol. 2002, 20, 1503–1513. [Google Scholar] [CrossRef]
- Zhang, Q.; Litchfield, J.B. An Optimization of Intermittent Corn Drying in a Laboratory Scale Thin Layer Dryer. Dry Technol. 1991, 9, 383–395. [Google Scholar] [CrossRef]
- Overhults, D.G.; White, G.M.; Hamilton, H.E.; Ross, I.J. Drying Soybeans with Heated Air. Trans ASAE 1973, 16, 0112–0113. [Google Scholar] [CrossRef]
- Nadhari, W.N.A.W.; Hashim, R.; Sulaiman, O.; Jumhuri, N. Drying kinetics of oil palm trunk waste in control atmosphere and open air convection drying. Int. J. Heat Mass Transf. 2014, 68, 14–20. [Google Scholar] [CrossRef]
- Montero, I.; Miranda, T.; Arranz, J.I.; Rojas, C.V. Thin layer drying kinetics of by-products from olive oil processing. Int. J. Mol. Sci. 2011, 12, 7885–7897. [Google Scholar] [CrossRef] [PubMed]
- Senadeera, W.; Bhandari, B.R.; Young, G.; Wijesinghe, B. Influence of shapes of selected vegetable materials on drying kinetics during fluidized bed drying. J. Food Eng. 2003, 58, 277–283. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion; Oxford University Press: London, United Kingdom, 1975. [Google Scholar]
- Zogzas, N.P.; Maroulis, Z.B.; Marinos-Kouris, D. Moisture Diffusivity Data Compilation in Foodstuffs. Dry Technol. 1996, 14, 2225–2253. [Google Scholar] [CrossRef]
- Babalis, S.J.; Belessiotis, V.G. Influence of the drying conditions on the drying constants and moisture diffusivity during the thin-layer drying of figs. J Food Eng. 2004, 65, 449–458. [Google Scholar] [CrossRef]
- Doymaz, I. Drying behaviour of green beans. J. Food Eng. 2005, 69, 161–165. [Google Scholar] [CrossRef]
- Dinçer, I.; Zanfirescu, C. Drying Phenomena: Theory and Applications; John Wiley & Sons: Chichester, UK, 2015. [Google Scholar]
- Ichikawa, Y.; Selvadura, A.P. Transport Phenomena in Porous Media: Aspects of Micro/Macro Behaviour; Springer Science & Business Media: New York, NY, USA, 2012. [Google Scholar]
- Doymaz, İ. Convective air drying characteristics of thin layer carrots. J. Food Eng. 2004, 61, 359–364. [Google Scholar] [CrossRef]
- Falade, K.O.; Abbo, E.S. Air-drying and rehydration characteristics of date palm (Phoenix dactylifera L.) fruits. J. Food Eng. 2007, 79, 724–730. [Google Scholar] [CrossRef]
- Gupta, P.; Ahmed, J.; Shivhare, U.S.; Raghavan, G.S.V. Drying Characteristics of Red Chilli. Dry Technol. 2002, 20, 1975–1987. [Google Scholar] [CrossRef]
- Bon, J.; Simal, S.; Rosselló, C.; Mulet, A. Drying characteristics of hemispherical solids. J. Food Eng. 1997, 34, 109–122. [Google Scholar] [CrossRef]
- Doymaz, İ. Air-drying characteristics of tomatoes. J. Food Eng. 2007, 78, 1291–1297. [Google Scholar] [CrossRef]
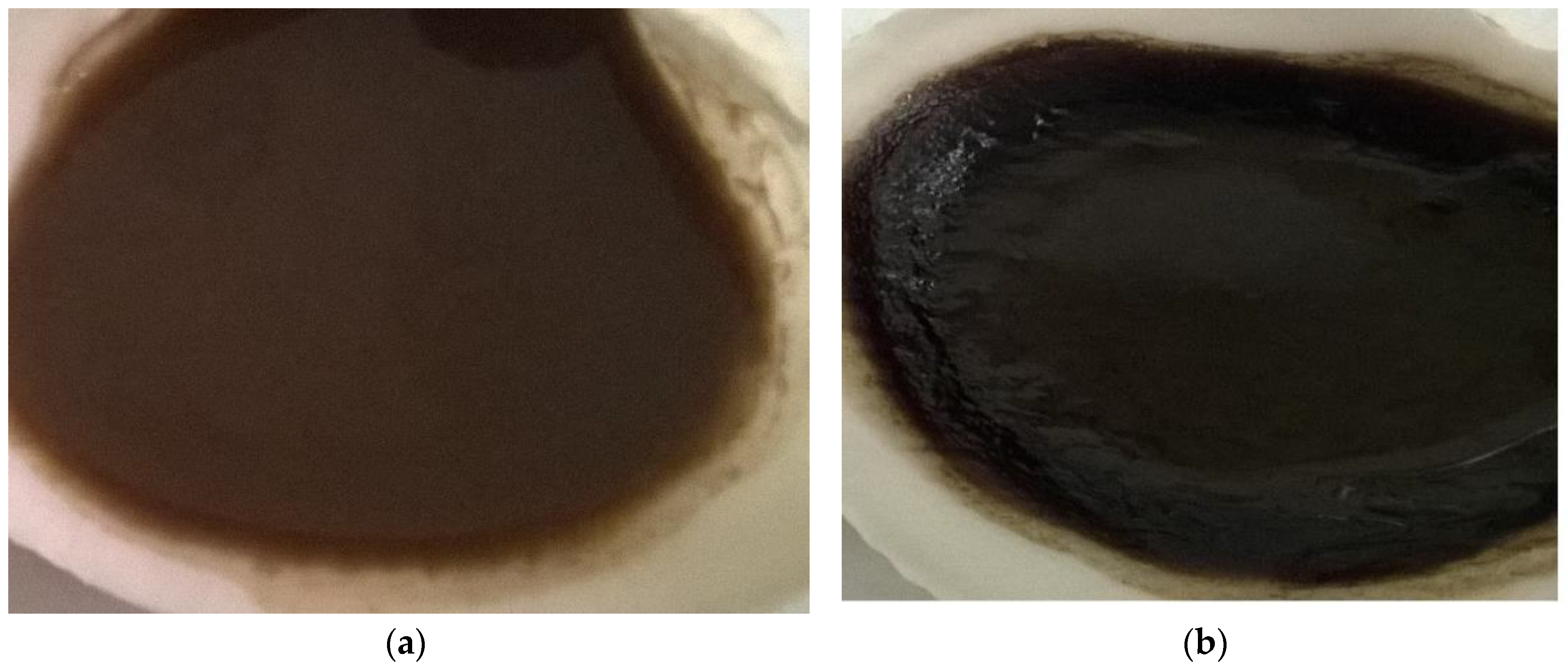

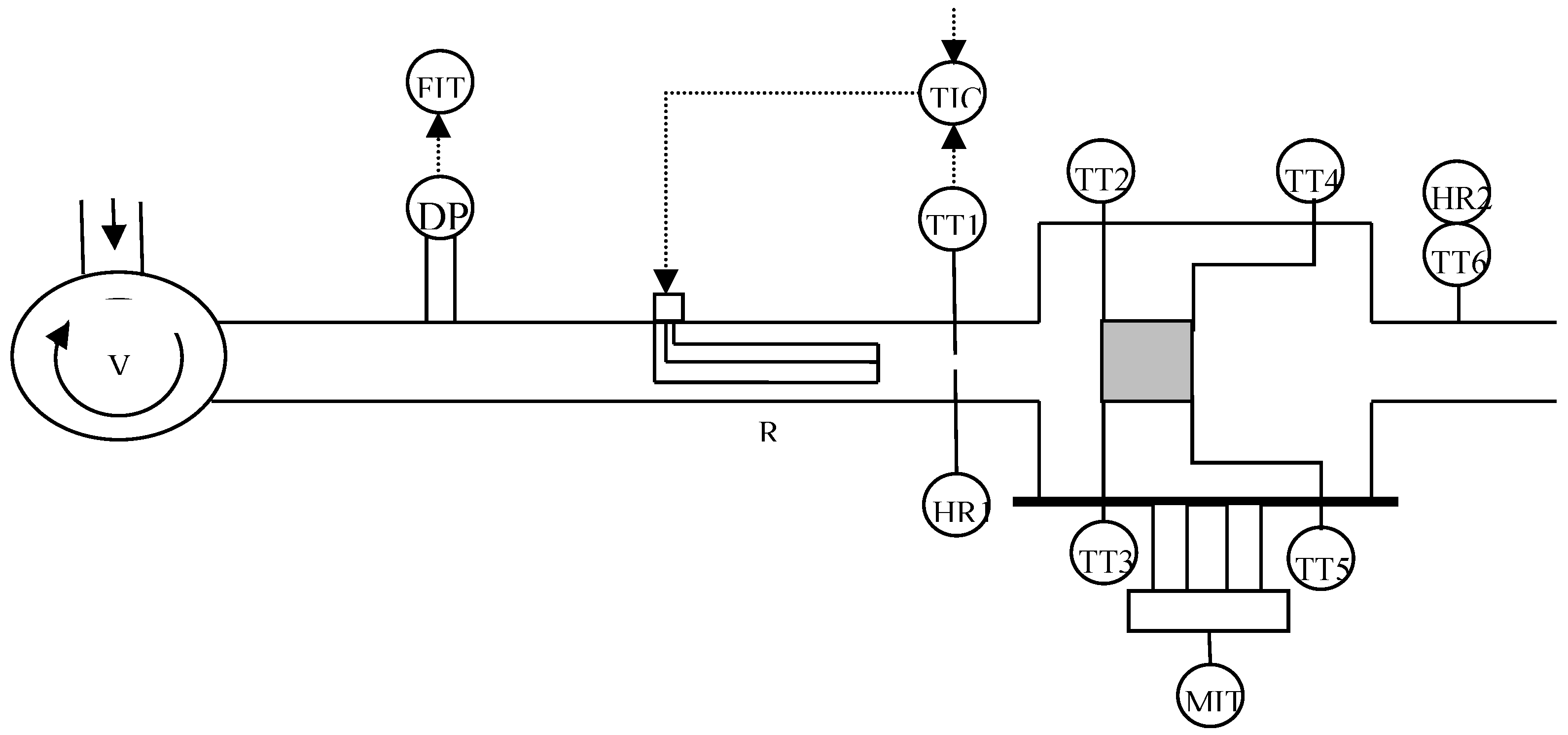


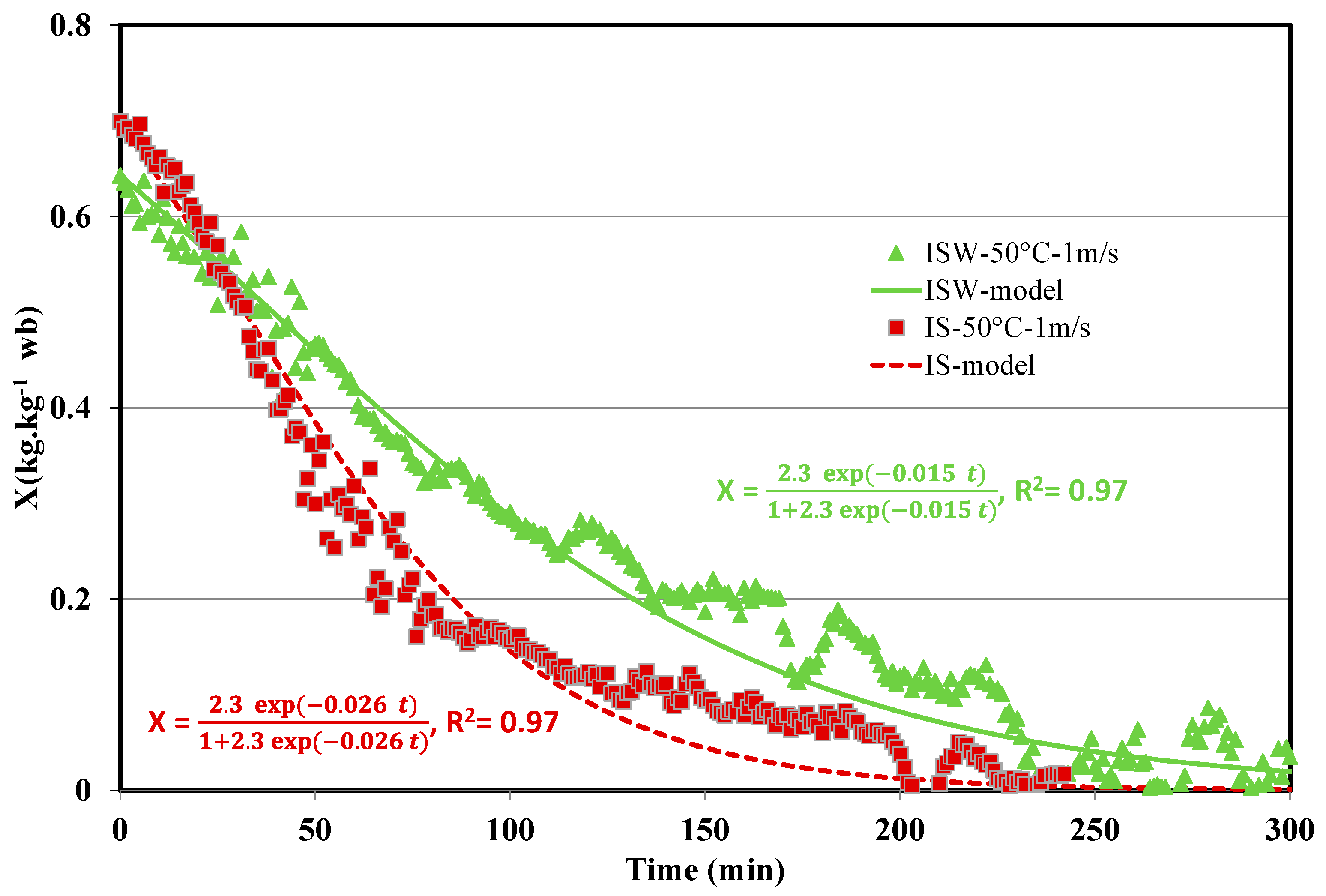
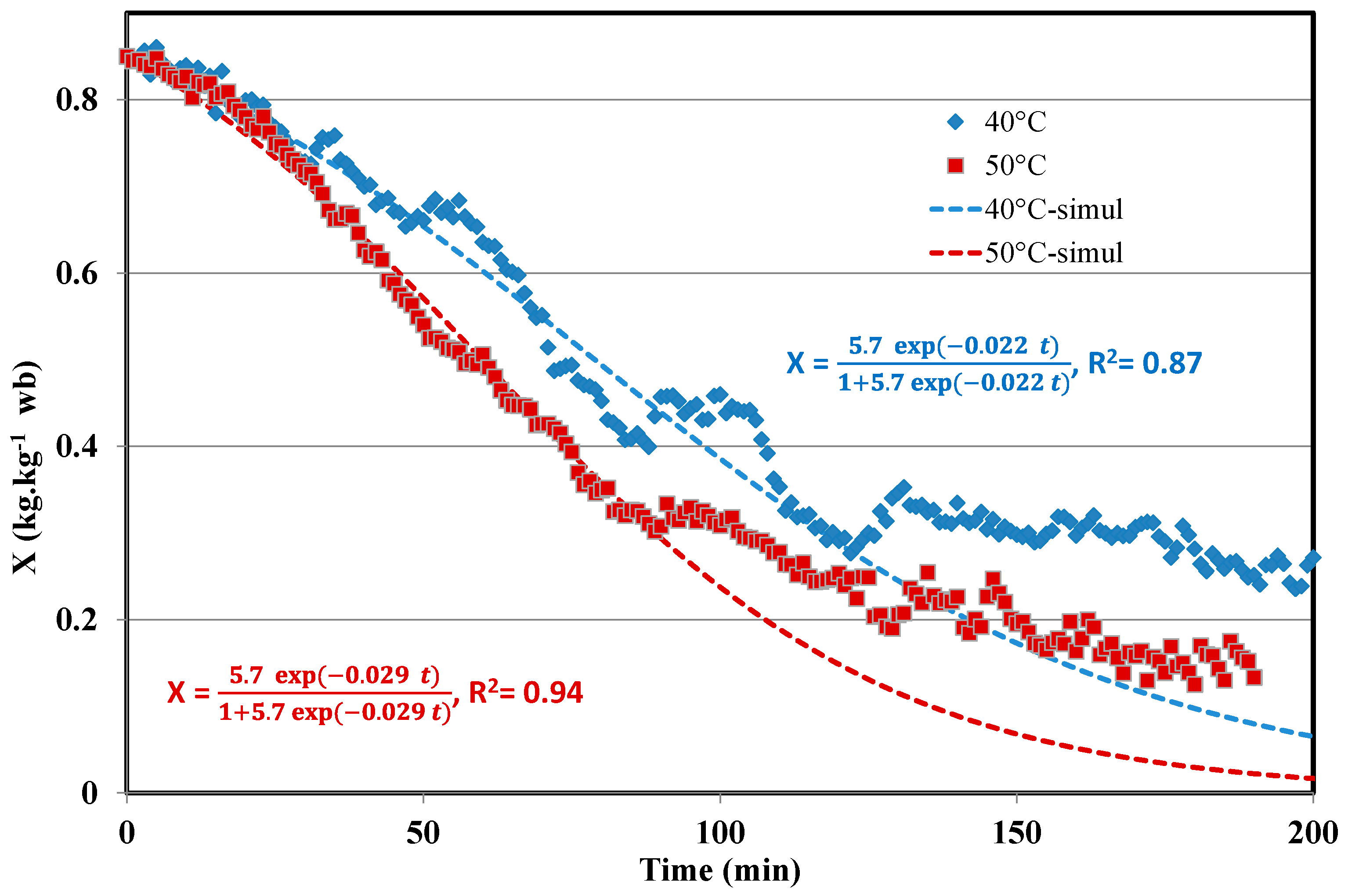


| Parameter | Value |
|---|---|
| pH | 4.80 |
| Electrical conductivity (ms/cm) | 9.20 |
| Water content (%) | 78.99 |
| Total Dissolved salts (g/L) | 16.5 |
| Biological oxygen demand in 5 days (g/L) | 37.5 |
| Chemical oxygen demand (g/L) | 197 |
| Potassium (g/L) | 5.9 |
| Calcium (g/L) | 0.71 |
| Magnesium (g/L) | 2.6 |
| Sodium (g/L) | 1.7 |
| Ammonium: N-NH4+ (g/L) | 1.8 |
| Sample Characteristic | Sawdust | OMSW |
|---|---|---|
| Moisture (%) | 9.7 | 10 |
| Bulk density (kg·m−3) | 103 | 529 |
| Low Heating Value (MJ·kg−1) | 16.4 | 16.9 |
| Fixed Carbon (%) | 14.5 | 25.5 |
| Volatile matter (%) | 75.2 | 61.5 |
| Ashwb (%) | 0.6 | 3 |
| Model Name and References | Equation (X (db)) |
|---|---|
| Henderson and Pabis [31] | X = aexp(−kt) |
| Logarithmic [30] | X = aexp(−kt) + c |
| Wang and Singh [32] | X = 1 + at + bt2 |
| Diffusion approach [33] | X = aexp(−kt) + (1 − a)exp(−kbt) |
| Simplified Fick diffusion [34] | X = aexp(−c(t/L2)) |
| Midilli–Kucuk [35] | X = aexp(−ktn) + bt |
| Page [36,37] | X = aexp(−ktn) |
| Conditions | Biomasses Drying Time (min) | |||
|---|---|---|---|---|
| Temperature (°C) | Velocity (m/s) | OMWW | ISW | IS |
| 40 | 0.7 | 353 | 221 | 188 |
| 1.0 | 313 | 167 | 122 | |
| 1.3 | 274 | 121 | 121 | |
| 50 | 0.7 | 238 | 140 | 136 |
| 1.0 | 163 | 92 | 95 | |
| 1.3 | 131 | 105 | 97 | |
| 60 | 0.7 | 124 | 60 | 51 |
| 1.0 | 116 | 52 | 48 | |
| 1.3 | 107 | 51 | 49 | |
| T (°C) | V (m/s) | OMWW | ISW | IS |
|---|---|---|---|---|
| 40 °C | 0.7 | a = 5.67 (r2 = 0.99) k0 = 3.0 × 10−4 s−1 td = 353 min, x = 0.51 | a = 1.80 (r2 = 0.99) k = 1.7 × 10−4 s−1 | a = 2.30 (r2 = 0.96) k = 2.7 × 10−4 s−1 |
| 1.0 | a = 5.67 (r2 = 0.98) k0 = 3.5 × 10−4 s−1 td = 313 min, x = 0.49 | a = 1.80 (r2 = 0.96) k = 2.3 × 10−4 s−1 | a = 2.30 (r2 = 0.95) k = 3.5 × 10−4 s−1 | |
| 1.3 | a = 5.67 (r2 = 0.98) k0 = 3.7 × 10−4 s−1 td = 274 min, x = 0.51 | a = 1.80 (r2 = 0.94) k = 3.0 × 10−4 s−1 | a = 2.30 (r2 = 0.98) k = 3.7 × 10−4 s−1 | |
| 50 °C | 0.7 | a = 5.67 (r2 = 0.98) k0 = 3.2 × 10−4 s−1 td = 238 min, x = 0.80 | a = 1.80 (r2 = 0.98) k = 2.7 × 10−4 s−1 | a = 2.30 (r2 = 0.95) k = 3.0 × 10−4 s−1 |
| 1.0 | a = 5.67 (r2 = 0.99) k0 = 4.7 × 10−4 s−1 td = 238 min, x = 0.72 | a = 1.80 (r2 = 0.97) k = 4.3 × 10−4 s−1 | a = 2.30 (r2 = 0.97) k = 4.5 × 10−4 s−1 | |
| 1.3 | a = 5.67 (r2 = 0.99) k0 = 5.0 × 10−4 s−1 td = 238 min, x = 0.83 | a = 1.80 (r2 = 0.92) k = 4.0 × 10−4 s−1 | a = 2.30 (r2 = 0.97) k = 4.2 × 10−4 s−1 | |
| 60°C | 0.7 | a = 5.67 (r2 = 0.97) k = 5.0 × 10−4 s−1 | a = 1.80 (r2 = 0.97) k = 4.8 × 10−4 s−1 | a = 2.30 (r2 = 0.95) k = 5.8 × 10−4 s−1 |
| 1.0 | a = 5.67 (r2 = 0.95) k = 5.2 × 10−4 s−1 | a = 1.80 (r2 = 0.91) k = 5.8 × 10−4 s−1 | a = 2.30 (r2 = 0.99) k = 7.2 × 10−4 s−1 | |
| 1.3 | a = 5.67 (r2 = 0.92) k = 5.3 × 10−4 s−1 | a = 1.80 (r2 = 0.93) k = 6.7 × 10−4 s−1 | a = 2.30 (r2 = 0.97) k = 7.2 × 10−4 s−1 |
| T (°C) | V (m/s) | OMWW | ISW | IS |
|---|---|---|---|---|
| 40 °C | 0.7 | 4.8 | 2.8 | 4.4 |
| 1.0 | 5.7 | 3.7 | 5.7 | |
| 1.3 | 6.0 | 4.9 | 6.0 | |
| 50 °C | 0.7 | 5.2 | 4.4 | 4.9 |
| 1.0 | 7.6 | 7.0 | 7.3 | |
| 1.3 | 8.1 | 6.5 | 6.8 | |
| 60 °C | 0.7 | 8.1 | 7.8 | 9.4 |
| 1.0 | 8.4 | 9.4 | 11.7 | |
| 1.3 | 8.6 | 10.9 | 11.7 |
| ISW | IS | |||||
|---|---|---|---|---|---|---|
| V (m/s) | 0.7 | 1.0 | 1.3 | 0.7 | 1.0 | 1.3 |
| Ea (KJ/mol) | 44.9 | 40.2 | 34.7 | 32.9 | 31.2 | 28.7 |
| 104D0 (m2/s) | 84.8 | 20.2 | 2.9 | 1.2 | 0.9 | 0.3 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeguirim, M.; Dutournié, P.; Zorpas, A.A.; Limousy, L. Olive Mill Wastewater: From a Pollutant to Green Fuels, Agricultural Water Source and Bio-Fertilizer—Part 1. The Drying Kinetics. Energies 2017, 10, 1423. https://doi.org/10.3390/en10091423
Jeguirim M, Dutournié P, Zorpas AA, Limousy L. Olive Mill Wastewater: From a Pollutant to Green Fuels, Agricultural Water Source and Bio-Fertilizer—Part 1. The Drying Kinetics. Energies. 2017; 10(9):1423. https://doi.org/10.3390/en10091423
Chicago/Turabian StyleJeguirim, Mejdi, Patrick Dutournié, Antonis A. Zorpas, and Lionel Limousy. 2017. "Olive Mill Wastewater: From a Pollutant to Green Fuels, Agricultural Water Source and Bio-Fertilizer—Part 1. The Drying Kinetics" Energies 10, no. 9: 1423. https://doi.org/10.3390/en10091423
APA StyleJeguirim, M., Dutournié, P., Zorpas, A. A., & Limousy, L. (2017). Olive Mill Wastewater: From a Pollutant to Green Fuels, Agricultural Water Source and Bio-Fertilizer—Part 1. The Drying Kinetics. Energies, 10(9), 1423. https://doi.org/10.3390/en10091423








