Particulate Matter and Gaseous Emission of Hydrous Ethanol Gasoline Blends Fuel in a Port Injection Gasoline Engine
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Engine and Fuels
2.2. Methods and Operating Settings
3. Results and Discussion
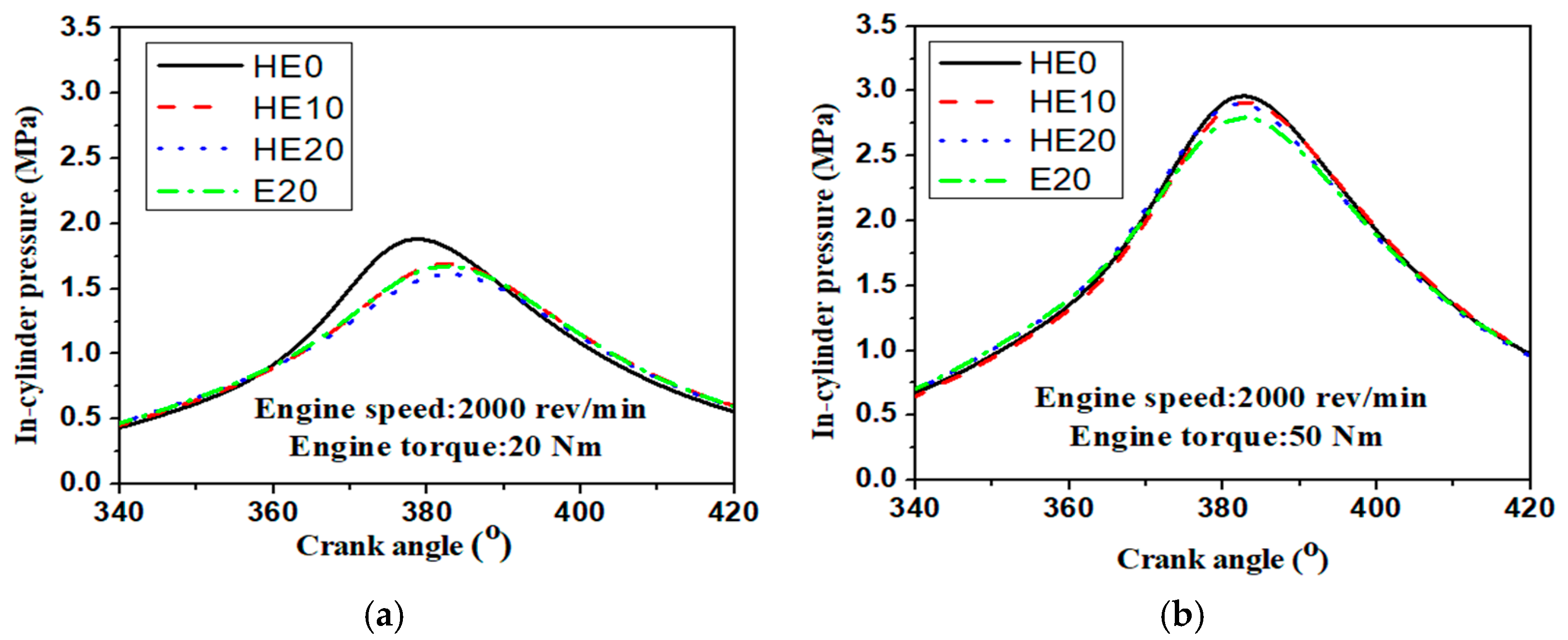
3.1. In-Cylinder Pressure
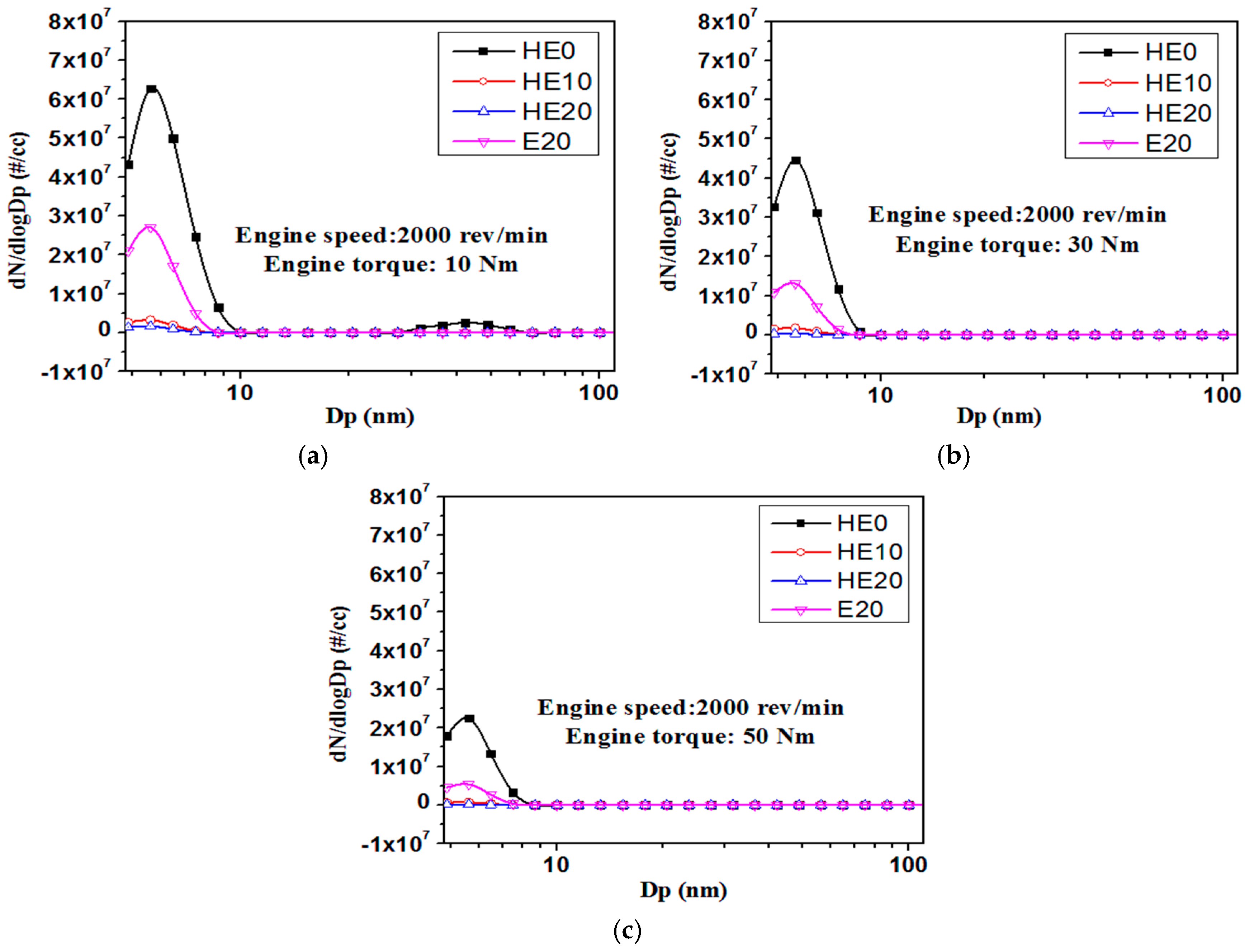
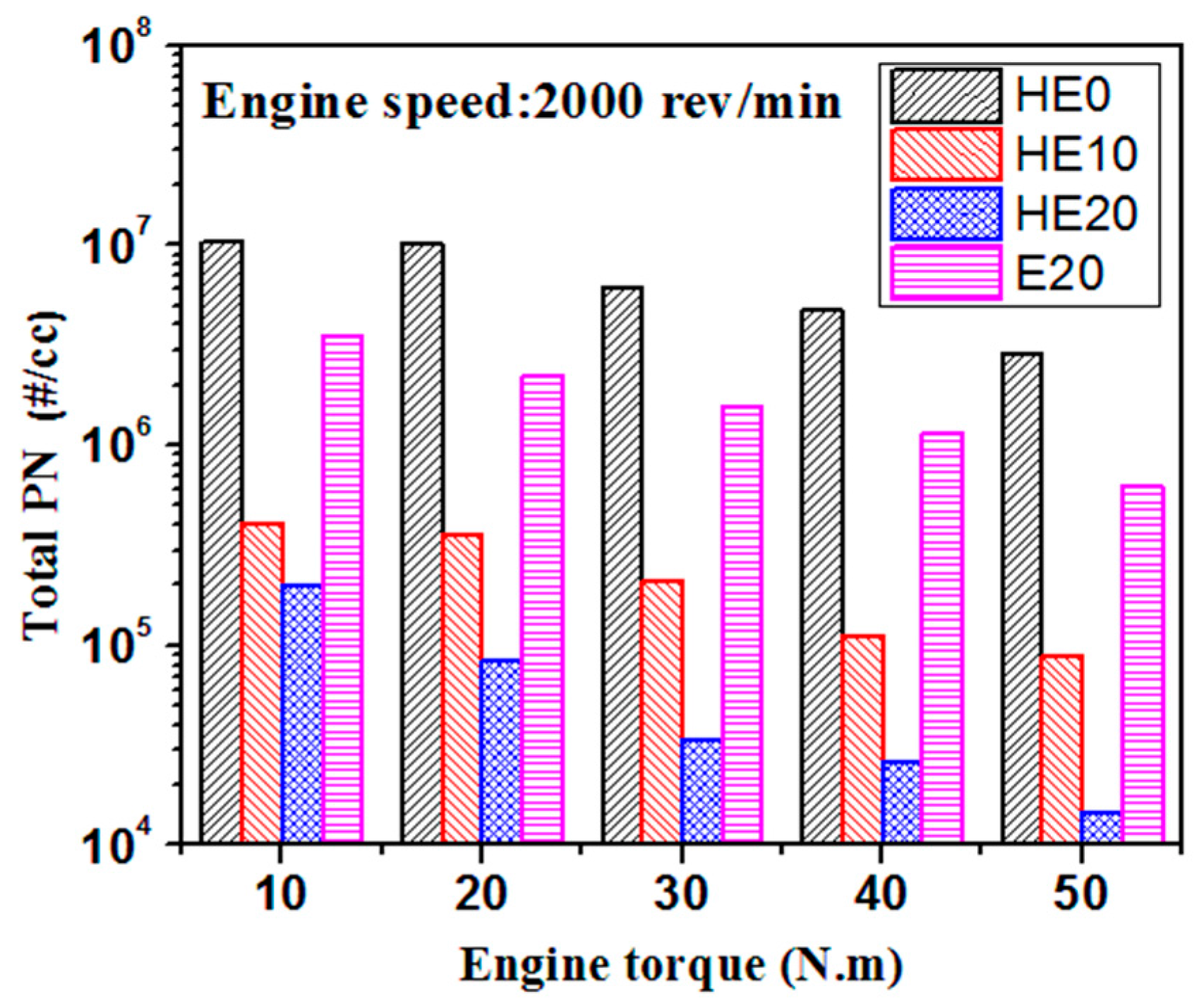
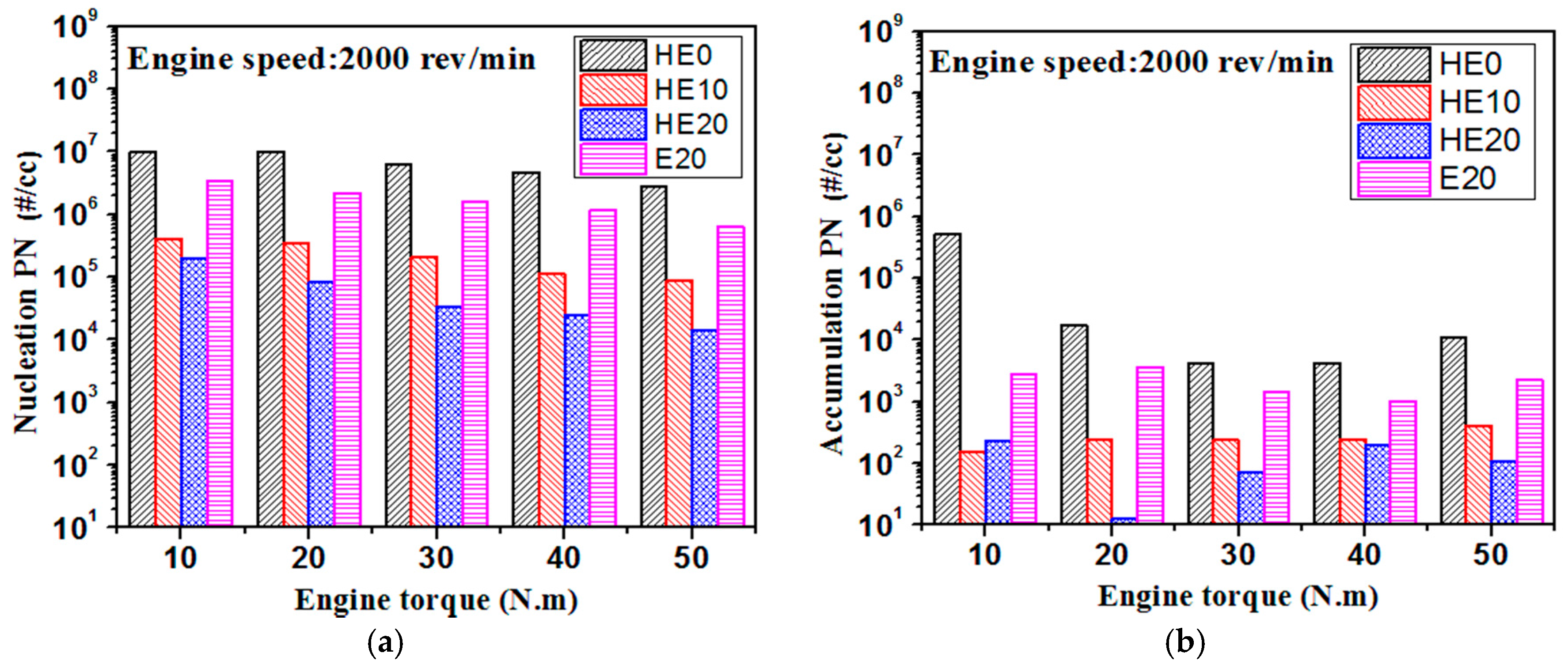
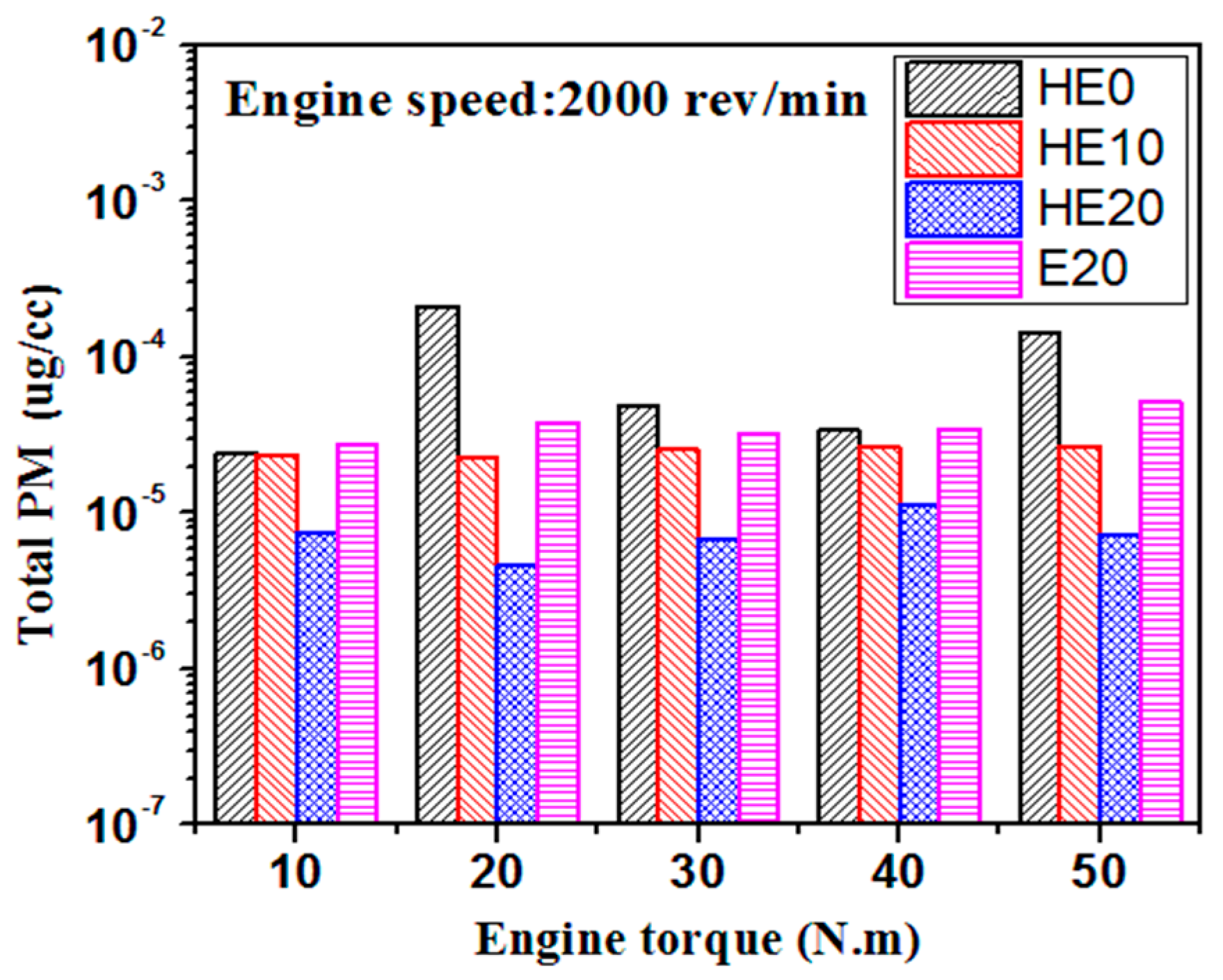
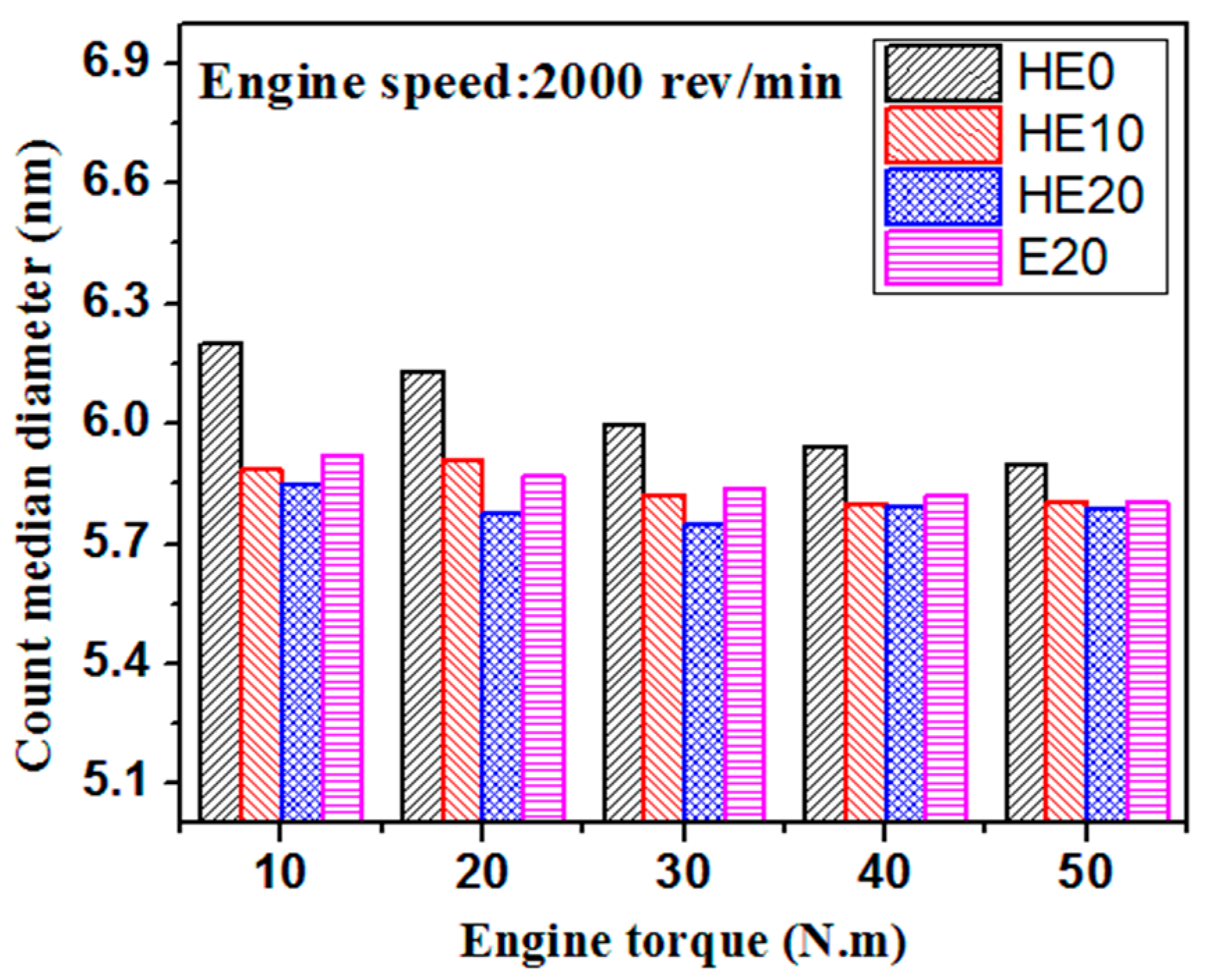
3.2. PM Emission Characteristics
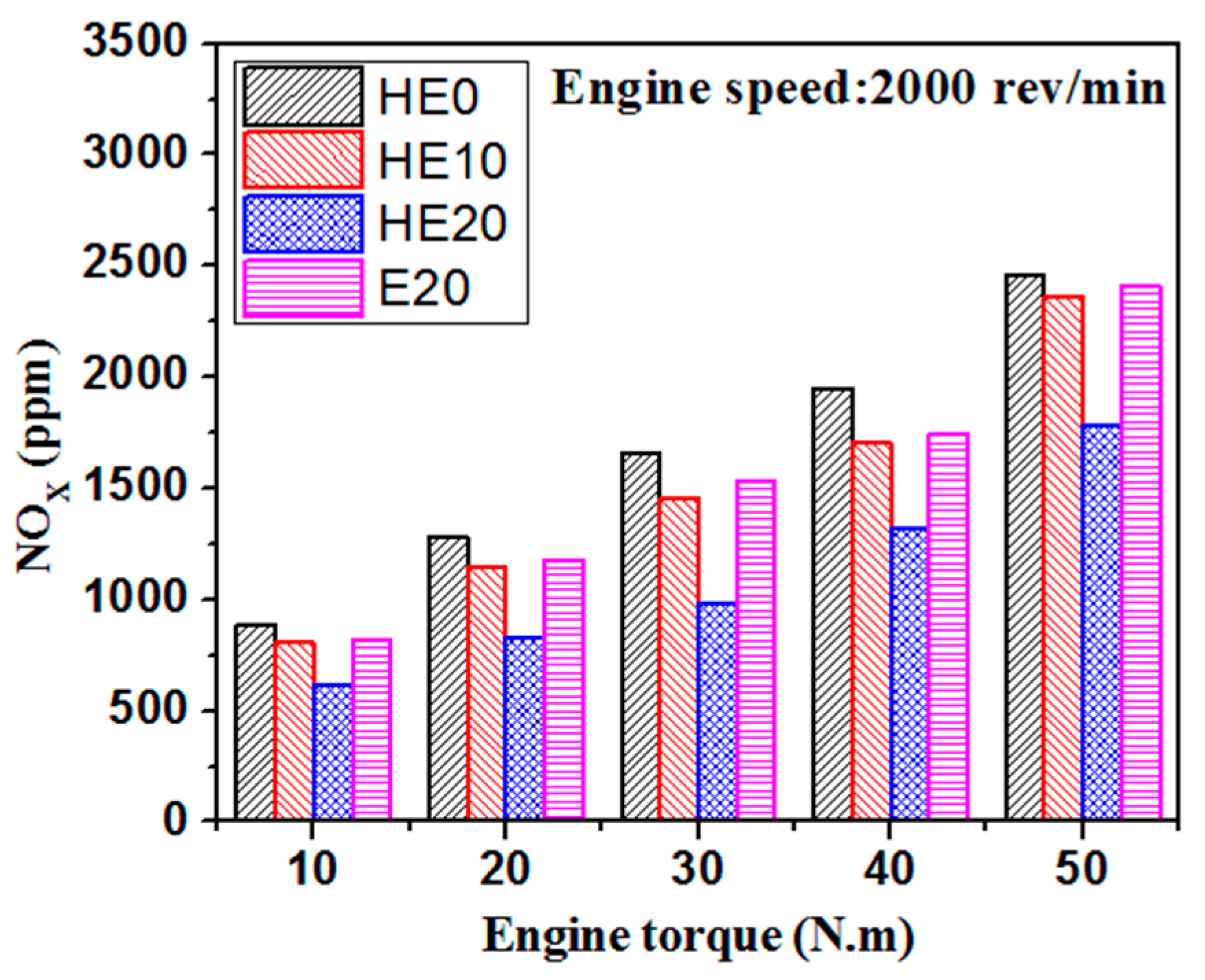
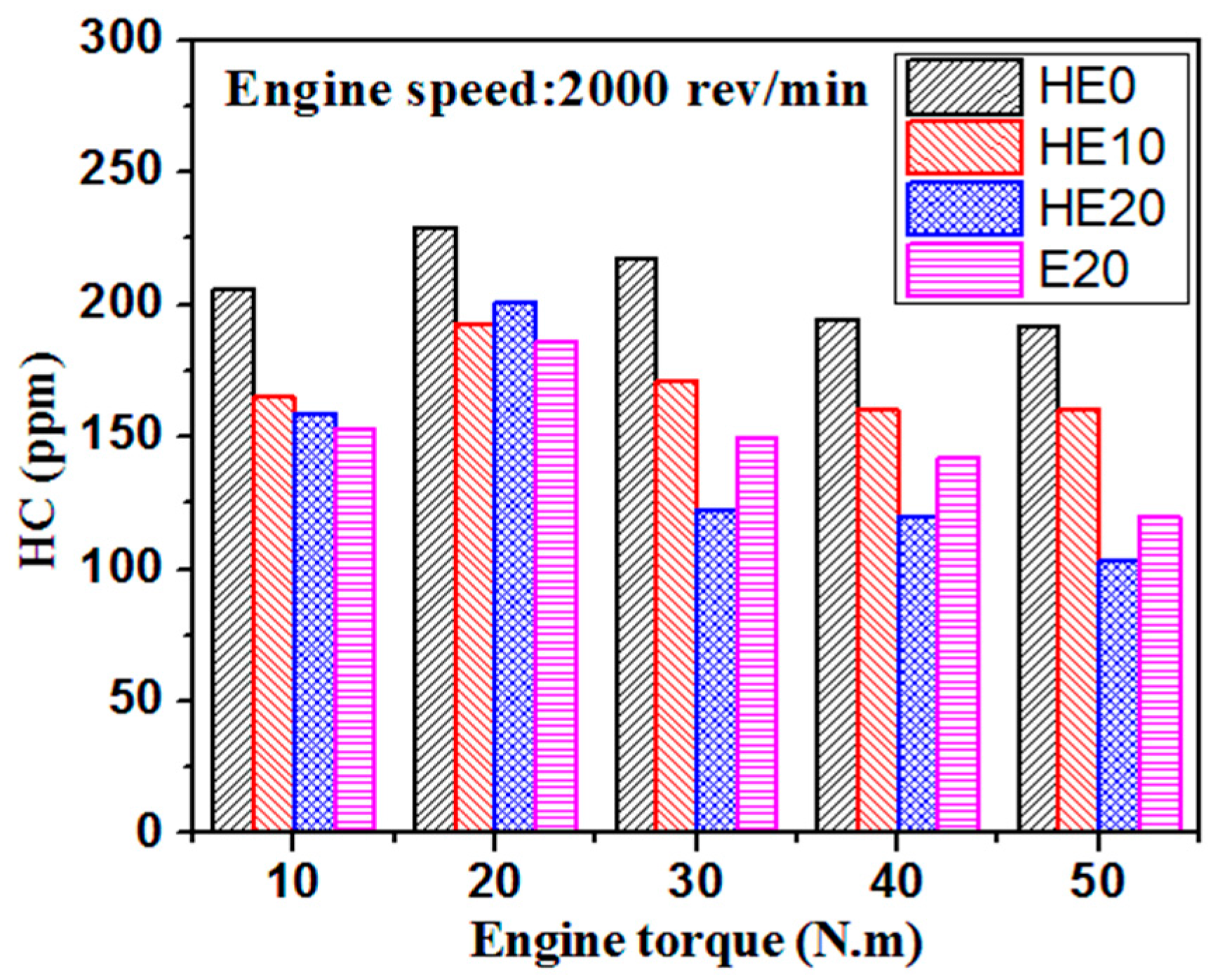
3.3. Gaseous Emission Characteristics
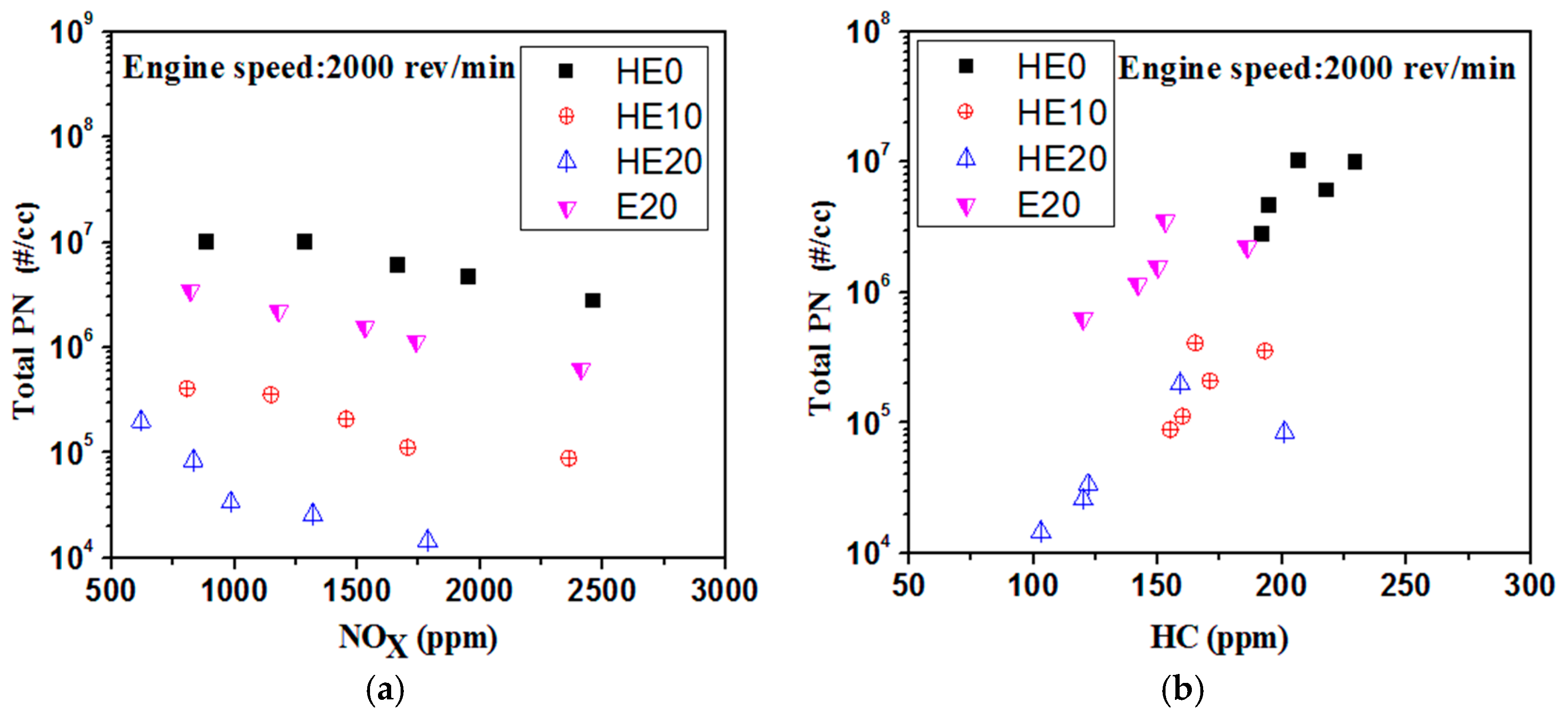
3.4. Correlations between PN-NOx and PN-HC
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chandra, R.; Takeuchi, H.; Hasegawa, T. Methane production from lignocellulosic agricultural crop wastes: A review in context to second generation of biofuel production. Renew. Sustain. Energy Rev. 2012, 16, 1462–1476. [Google Scholar] [CrossRef]
- Ghadikolaei, M.A. Effect of alcohol blend and fumigation on regulated and unregulated emissions of IC engines—A review. Renew. Sustain. Energy Rev. 2016, 57, 1440–1495. [Google Scholar] [CrossRef]
- El-Faroug, M.; Yan, F.; Luo, M.; Fiifi Turkson, R. Spark ignition engine combustion, performance and emission products from hydrous ethanol and its blends with gasoline. Energies 2016, 9, 984. [Google Scholar] [CrossRef]
- Masum, B.; Masjuki, H.; Kalam, M.; Fattah, I.R.; Palash, S.; Abedin, M. Effect of ethanol-gasoline blend on NOx emission in SI engine. Renew. Sustain. Energy Rev. 2013, 24, 209–222. [Google Scholar] [CrossRef]
- Saxena, S.; Schneider, S.; Aceves, S.; Dibble, R. Wet ethanol in HCCI engines with exhaust heat recovery to improve the energy balance of ethanol fuels. Appl. Energy 2012, 98, 448–457. [Google Scholar] [CrossRef]
- Martinez-Frias, J.; Aceves, S.M.; Flowers, D.L. Improving ethanol life cycle energy efficiency by direct utilization of wet ethanol in HCCI engines. J. Energy Resour. Technol. 2007, 129, 332–337. [Google Scholar] [CrossRef]
- Meng, L.; Zeng, C.; Li, Y.; Nithyanandan, K.; Lee, T.H.; Lee, C.-F. An experimental study on the potential usage of acetone as an oxygenate additive in PFI SI engines. Energies 2016, 9, 256. [Google Scholar] [CrossRef]
- Al-lwayzy, S.H.; Yusaf, T. Combustion of microalgae oil and ethanol blended with diesel fuel. Energies 2015, 8, 13985–13995. [Google Scholar] [CrossRef]
- Sharudin, H.; Abdullah, N.R.; Najafi, G.; Mamat, R.; Masjuki, H. Investigation of the effects of iso-butanol additives on spark ignition engine fuelled with methanol-gasoline blends. Appl. Therm. Eng. 2017, 114, 593–600. [Google Scholar] [CrossRef]
- Li, Y.; Gong, J.; Deng, Y.; Yuan, W.; Fu, J.; Zhang, B. Experimental comparative study on combustion, performance and emissions characteristics of methanol, ethanol and butanol in a spark ignition engine. Appl. Therm. Eng. 2017, 115, 53–63. [Google Scholar] [CrossRef]
- Schifter, I.; Diaz, L.; Gómez, J.; Gonzalez, U. Combustion characterization in a single cylinder engine with mid-level hydrated ethanol-gasoline blended fuels. Fuel 2013, 103, 292–298. [Google Scholar] [CrossRef]
- Venugopal, T.; Sharma, A.; Satapathy, S.; Ramesh, A.; Gajendra Babu, M. Experimental study of hydrous ethanol gasoline blend (E10) in a four stroke port fuel-injected spark ignition engine. Int. J. Energy Res. 2013, 37, 638–644. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Z.; Ni, J.; Liu, S.; Zhou, H. The effects of hydrous ethanol gasoline on combustion and emission characteristics of a port injection gasoline engine. Case Stud. Therm. Eng. 2015, 6, 147–154. [Google Scholar] [CrossRef]
- De Melo, T.C.C.; Machado, G.B.; Belchior, C.R.; Colaço, M.J.; Barros, J.E.; de Oliveira, E.J.; de Oliveira, D.G. Hydrous ethanol-Gasoline blends-Combustion and emission investigations on a flex-fuel engine. Fuel 2012, 97, 796–804. [Google Scholar] [CrossRef]
- Augoye, A.; Aleiferis, P. Characterization of Flame Development with Hydrous and Anhydrous Ethanol Fuels in a Spark-Ignition Engine with Direct Injection and Port Injection Systems; SAE Technical Paper, 2014-01-2623; SAE International: Warrendale, PA, USA, 2014. [Google Scholar]
- Clemente, R.C.; Werninghaus, E.; Coelho, E.P.; Ferraz, L.A.S. Development of an Internal Combustion Alcohol Fueled Engine; SAE Technical Paper, 2001-01-3917; SAE International: Warrendale, PA, USA, 2001. [Google Scholar]
- Costa, R.C.; Sodré, J.R. Hydrous ethanol vs. Gasoline-ethanol blend: Engine performance and emissions. Fuel 2010, 89, 287–293. [Google Scholar] [CrossRef]
- Costa, R.C.; Sodré, J.R. Compression ratio effects on an ethanol/gasoline fuelled engine performance. Appl. Therm. Eng. 2011, 31, 278–283. [Google Scholar] [CrossRef]
- Kyriakides, A.; Dimas, V.; Lymperopoulou, E.; Karonis, D.; Lois, E. Evaluation of gasoline-ethanol-water ternary mixtures used as a fuel for an Otto engine. Fuel 2013, 108, 208–215. [Google Scholar] [CrossRef]
- Amorim, R.J.; Baeta, J.G.C.; Valle, R.M.; Barros, J.; Carvalho, R. Analysis of an Otto cycle engine performance regarding alcohol concentration in gasoline and CNG usage. In Proceedings of the XVIII International Congress of Mechanical Engineering, Ouro Preto, Brazil, 6–11 November 2005. [Google Scholar]
- Olberding, J.; Beyerlein, D.C.S.; Steciak, J.; Cherry, M. Dynamometer Testing of an Ethanol-Water Fueled Transit Van; SAE Technical Paper, 2005-01-3706; SAE International: Warrendale, PA, USA, 2005. [Google Scholar]
- De Melo, T.C.C.; Machado, G.B.; de Oliveira, E.J.; Belchior, C.R.P.; Jos, M.; de Oliveira, D.G. Different Hydrous Ethanol-Gasoline Blends-Ftir Emissions of a Flex-Fuel Engine and Chemical Properties of the Fuels; SAE Technical Paper, 2011-36-0080; SAE International: Warrendale, PA, USA, 2011. [Google Scholar]
- Lanzanova, T.D.; Vielmo, H.A.; Sari, R.L.; Dornelles, H.M.; Tatsch, G.A.; Martins, M.E.; Michels, L. Performance Analysis of a Spark Ignited Engine Running on Different Water-in-Ethanol Mixtures; SAE Technical Paper, 2013-36-0202; SAE International: Warrendale, PA, USA, 2013. [Google Scholar]
- Chen, R.-H.; Chiang, L.-B.; Wu, M.-H.; Lin, T.-H. Gasoline displacement and NOx reduction in an SI engine by aqueous alcohol injection. Fuel 2010, 89, 604–610. [Google Scholar] [CrossRef]
- Liu, S.-H.; Shen, L.-Z.; Ye, N.-Y.; Bi, Y.-H.; Luo, X. Research on effects of E10 hydrous ethanol gasoline blend on performance and emissions of gasoline engine. Chin. Intern. Combust. Engine Eng. 2012, 33, 46–51. (In Chinese) [Google Scholar]
- Suarez-Bertoa, R.; Zardini, A.; Keuken, H.; Astorga, C. Impact of ethanol containing gasoline blends on emissions from a flex-fuel vehicle tested over the worldwide harmonized light duty test cycle (WLTC). Fuel 2015, 143, 173–182. [Google Scholar] [CrossRef]
- Brewster, S.; Railton, D.; Maisey, M.; Frew, R. The Effect of e100 Water Content on High Load Performance of a Spray Guide Direct Injection Boosted Engine; SAE Technical Paper, 2007-01-2648; SAE International: Warrendale, PA, USA, 2007. [Google Scholar]
- El-Faroug, M.O.; Luo, M.-J.; Yan, F.-W.; Liu, Y.-P.; Destech Publicat, I. Experimental investigation on the stability of hydrous ethanol gasoline blends and emission products from SI engine. In Proceedings of the 2016 International Conference on Energy Development and Environmental Protection (Edep 2016), Beijing, China, 11–12 June 2016. [Google Scholar]
- Hu, J.; Yan, W.-S.; Liu, L.-D.; Xu, Y.-F.; Wang, Q.-F.; Liu, X.; Zhang, W.; Liang, X.-Y. Experiment study of E10 hydrous ethanol/gasoline blends on gasoline engine. Intern. Combust. Engine Powerpl. 2007, 1, 001. (In Chinese) [Google Scholar]
- Munsin, R.; Laoonual, Y.; Jugjai, S.; Imai, Y. An experimental study on performance and emissions of a small SI engine generator set fuelled by hydrous ethanol with high water contents up to 40%. Fuel 2013, 106, 586–592. [Google Scholar] [CrossRef]
- Ruiz, F.A.; Cadrazco, M.; López, A.F.; Sanchez-Valdepeñas, J.; Agudelo, J.R. Impact of dual-fuel combustion with n-butanol or hydrous ethanol on the oxidation reactivity and nanostructure of diesel particulate matter. Fuel 2015, 161, 18–25. [Google Scholar] [CrossRef]
- López, A.F.; Cadrazco, M.; Agudelo, A.F.; Corredor, L.A.; Vélez, J.A.; Agudelo, J.R. Impact of n-butanol and hydrous ethanol fumigation on the performance and pollutant emissions of an automotive diesel engine. Fuel 2015, 153, 483–491. [Google Scholar] [CrossRef]
- Hwang, J.T.; Northrop, W.F. Gas and Particle Emissions from a Diesel Engine Operating in a Dual-Fuel Mode Using High Water Content Hydrous Ethanol; ASME Paper No. ICEF2014–5460; ASME: New York, NY, USA, 2014. [Google Scholar]
- Pope III, C.A.; Ezzati, M.; Dockery, D.W. Fine-particulate air pollution and life expectancy in the United States. N. Engl. J. Med. 2009, 2009, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Brook, R.D.; Rajagopalan, S.; Pope, C.A.; Brook, J.R.; Bhatnagar, A.; Diez-Roux, A.V.; Holguin, F.; Hong, Y.; Luepker, R.V.; Mittleman, M.A. Particulate matter air pollution and cardiovascular disease. Circulation 2010, 121, 2331–2378. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, S.V. Cancer Tip: Nanoparticles Can Damage DNA, Increase Cancer Risk. Press Release. 2007. Available online: https://www.sciencedaily.com/releases/2007/04/070417154357.htm (accessed on 24 August 2017).
- United States Environmental Protection Agency. Particulate Matter. Available online: http://cfpub.Epa.Gov/airnow/index.Cfm?Action=aqibasics.Particle (accessed on 10 August 2017).
- Myung, C.-L.; Ko, A.; Lim, Y.; Kim, S.; Lee, J.; Choi, K.; Park, S. Mobile source air toxic emissions from direct injection spark ignition gasoline and LPG passenger car under various in-use vehicle driving modes in Korea. Fuel Process. Technol. 2014, 119, 19–31. [Google Scholar] [CrossRef]
- Fang, S.C.; Cassidy, A.; Christiani, D.C. A systematic review of occupational exposure to particulate matter and cardiovascular disease. Int. J. Environ. Res. Public Health 2010, 7, 1773–1806. [Google Scholar] [CrossRef] [PubMed]
- Valavanidis, A.; Fiotakis, K.; Vlachogianni, T. Airborne particulate matter and human health: Toxicological assessment and importance of size and composition of particles for oxidative damage and carcinogenic mechanisms. J. Environ. Sci. Health Part C 2008, 26, 339–362. [Google Scholar] [CrossRef] [PubMed]
- Samuel, S.; Hassaneen, A.; Morrey, D.; Gonzalez-Oropeza, R. The Effect of Gasoline Additives on Combustion Generated Nano-Scale Particulates; SAE Technical Paper, 2009-01-1823; SAE International: Warrendale, PA, USA, 2009. [Google Scholar]
- Attar, M.A.; Xu, H. Experimental investigation of impacts of engine hardware, operating parameters and combustion performance on particulate emissions in a DISI engine. Appl. Energy 2016, 177, 703–715. [Google Scholar] [CrossRef]
- Price, P.; Twiney, B.; Stone, R.; Kar, K.; Walmsley, H. Particulate and Hydrocarbon Emissions from a Spray Guided Direct Injection Spark Ignition Engine with Oxygenate Fuel Blends; SAE Technical Paper, 2007-01-0472; SAE International: Warrendale, PA, USA, 2007. [Google Scholar]
- Chen, L.; Stone, R.; Richardson, D. Effect of the valve timing and the coolant temperature on particulate emissions from a gasoline direct-injection engine fuelled with gasoline and with a gasoline-ethanol blend. Proc. Inst. of Mech. Eng. Part D J. Automob. Eng. 2012, 226, 1419–1430. [Google Scholar] [CrossRef]
- Muralidharan, M.; Subramanian, M.; Kanal, P.; Malhotra, R. Characterisation of Particulates with Different Blends of Ethanol-Gasoline in Two Wheelers; SAE Technical Paper, 2009-01-0686; SAE International: Warrendale, PA, USA, 2009. [Google Scholar]
- Catapano, F.; Di Iorio, S.; Lazzaro, M.; Sementa, P.; Vaglieco, B.M. Characterization of Ethanol Blends Combustion Processes and Soot Formation in a Gdi Optical Engine; SAE Technical Paper, 2013-01-1316; SAE International: Warrendale, PA, USA, 2013. [Google Scholar]
- Storey, J.M.; Barone, T.; Norman, K.; Lewis, S. Ethanol blend effects on direct injection spark-ignition gasoline vehicle particulate matter emissions. SAE Int. J. Fuels Lubr. 2010, 3, 650–659. [Google Scholar] [CrossRef]
- Vuk, C.; Vander Griend, S.J. Fuel Property Effects on Particulates in Spark Ignition Engines; SAE Technical Paper, 2013-01-1124; SAE International: Warrendale, PA, USA, 2013. [Google Scholar]
- Maricq, M.M.; Szente, J.J.; Jahr, K. The impact of ethanol fuel blends on PM emissions from a light-duty GDI vehicle. Aerosol Sci. Technol. 2012, 46, 576–583. [Google Scholar] [CrossRef]
- Chen, L.; Stone, R.; Richardson, D. A study of mixture preparation and PM emissions using a direct injection engine fuelled with stoichiometric gasoline/ethanol blends. Fuel 2012, 96, 120–130. [Google Scholar] [CrossRef]
- Bielaczyc, P.; Woodburn, J.; Klimkiewicz, D.; Pajdowski, P.; Szczotka, A. An examination of the effect of ethanol-gasoline blends’ physicochemical properties on emissions from a light-duty spark ignition engine. Fuel Process. Technol. 2013, 107, 50–63. [Google Scholar] [CrossRef]
- Geng, P.; Zhang, H.; Yang, S. Experimental investigation on the combustion and particulate matter (PM) emissions from a port-fuel injection (PFI) gasoline engine fueled with methanol-ultralow sulfur gasoline blends. Fuel 2015, 145, 221–227. [Google Scholar] [CrossRef]
- Bahreini, R.; Xue, J.; Johnson, K.; Durbin, T.; Quiros, D.; Hu, S.; Huai, T.; Ayala, A.; Jung, H. Characterizing emissions and optical properties of particulate matter from PFI and GDI light-duty gasoline vehicles. J. Aerosol Sci. 2015, 90, 144–153. [Google Scholar] [CrossRef]
- Xing, J.; Shao, L.; Zheng, R.; Peng, J.; Wang, W.; Guo, Q.; Wang, Y.; Qin, Y.; Shuai, S.; Hu, M. Individual particles emitted from gasoline engines: Impact of engine types, engine loads and fuel components. J. Clean. Prod. 2017, 149, 461–471. [Google Scholar] [CrossRef]
- Karavalakis, G.; Short, D.; Chen, V.; Espinoza, C.; Berte, T.; Durbin, T.; Asa-Awuku, A.; Jung, H.; Ntziachristos, L.; Amanatidis, S. Evaluating Particulate Emissions from a Flexible Fuel Vehicle with Direct Injection when Operated on Ethanol and Iso-Butanol Blends; SAE Technical Paper, 2014-01-2768; SAE International: Warrendale, PA, USA, 2014. [Google Scholar]
- Zhu, R.; Hu, J.; Bao, X.; He, L.; Lai, Y.; Zu, L.; Li, Y.; Su, S. Tailpipe emissions from gasoline direct injection (GDI) and port fuel injection (PFI) vehicles at both low and high ambient temperatures. Environ. Pollut. 2016, 216, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Geng, P.; Zhang, H. Combustion and emission characteristics of a direct-injection gasoline engine using the MMT fuel additive gasoline. Fuel 2015, 144, 380–387. [Google Scholar] [CrossRef]
- Dryer, F. Water addition to practical combustion systems—Concepts and applications. Symp. Int. Combust. 1977, 16, 279–295. [Google Scholar] [CrossRef]
- Zhang, W.; Shu, G.; Chen, Z.; Shen, Y.; Weng, J. Chemical kinetics of ignition timing of diesel engine fueled with water emulsion diesel. Trans. Chin. Soc. Agric. Eng. 2012, 28, 59–66. (In Chinese) [Google Scholar]
- Rahman, K.M.; Kawahara, N.; Tsuboi, K.; Tomita, E. Combustion characteristics of wet ethanol ignited using a focused Q-switched Nd: YAG nanosecond laser. Fuel 2016, 165, 331–340. [Google Scholar] [CrossRef]
- Bradley, D.; Lawes, M.; Liao, S.; Saat, A. Laminar mass burning and entrainment velocities and flame instabilities of i-octane, ethanol and hydrous ethanol/air aerosols. Combust. Flame 2014, 161, 1620–1632. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Z.; Long, Y.; Xiang, S.; Wang, J.; Fatouraie, M. Comparative study on alcohol-gasoline and gasoline-alcohol dual-fuel spark ignition (DFSI) combustion for engine particle number (PN) reduction. Fuel 2015, 159, 250–258. [Google Scholar] [CrossRef]
- Roberts, C.E.; Naegeli, D.; Chadwell, C. The Effect of Water on Soot Formation Chemistry; SAE Technical Paper, 2005-01-3850; SAE International: Warrendale, PA, USA, 2005. [Google Scholar]
- Rao, V.; Bardon, M. The effect of water on gas phase soot formation in laminar diffusion flames. Combust. Flame 1984, 55, 73–78. [Google Scholar] [CrossRef]
- Andrews, G.; Bartle, K.; Pang, S.; Nurein, A.; Williams, P. The Reduction in Diesel Particulate Emissions Using Emulsified Fuels; SAE Technical Paper, 880348; SAE International: Warrendale, PA, USA, 1988. [Google Scholar]
- Huang, J.; Wang, Y.; Li, S.; Roskilly, A.P.; Yu, H.; Li, H. Experimental investigation on the performance and emissions of a diesel engine fuelled with ethanol-diesel blends. Appl. Therm. Eng. 2009, 29, 2484–2490. [Google Scholar] [CrossRef]
- Qi, D.; Bae, C.; Feng, Y.; Jia, C.; Bian, Y. Combustion and emission characteristics of a direct injection compression ignition engine using rapeseed oil based micro-emulsions. Fuel 2013, 107, 570–577. [Google Scholar] [CrossRef]
- Agarwal, A.K. Biofuels (alcohols and biodiesel) applications as fuels for internal combustion engines. Prog. Energy Combust. Sci. 2007, 33, 233–271. [Google Scholar] [CrossRef]
- Rahman, M.M.; Stevanovic, S.; Brown, R.; Ristovski, Z. Influence of different alternative fuels on particle emission from a turbocharged common-rail diesel engine. Procedia Eng. 2013, 56, 381–386. [Google Scholar] [CrossRef]
- Surawski, N.C.; Miljevic, B.; Roberts, B.A.; Modini, R.L.; Situ, R.; Brown, R.J.; Bottle, S.E.; Ristovski, Z.D. Particle emissions, volatility, and toxicity from an ethanol fumigated compression ignition engine. Environ. Sci. Technol. 2009, 44, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Rajan, S. Water-ethanol-gasoline blends—Physical properties, power, and pollution characteristics. J. Eng. Gas Turbines Power 1984, 106, 841–848. [Google Scholar] [CrossRef]
- Li, Y.; Nithyanandan, K.; Lee, T.H.; Donahue, R.M.; Lin, Y.; Lee, C.-F.; Liao, S. Effect of water-containing acetone-butanol-ethanol gasoline blends on combustion, performance, and emissions characteristics of a spark-ignition engine. Energy Convers. Manag. 2016, 117, 21–30. [Google Scholar] [CrossRef]
- Nour, M.; Kosaka, H.; Sato, S.; Bady, M.; Abdel-Rahman, A.K.; Uchida, K. Effect of ethanol/water blends addition on diesel fuel combustion in RCM and DI diesel engine. Energy Convers. Manag. 2017, 149, 228–243. [Google Scholar] [CrossRef]
- Virtanen, A.; Ristimäki, J.; Marjamäki, M.; Vaaraslahti, K.; Keskinen, J.; Lappi, M. Effective Density of Diesel Exhaust Particles as a Function of Size; SAE Technical Paper, 2002-01-0056; SAE International: Warrendale, PA, USA, 2002. [Google Scholar]
- Nabi, M.N.; Zare, A.; Hossain, F.M.; Rahman, M.M.; Bodisco, T.A.; Ristovski, Z.D.; Brown, R.J. Influence of fuel-borne oxygen on european stationary cycle: Diesel engine performance and emissions with a special emphasis on particulate and no emissions. Energy Convers. Manag. 2016, 127, 187–198. [Google Scholar] [CrossRef]
- Tse, H.; Leung, C.; Cheung, C. Investigation on the combustion characteristics and particulate emissions from a diesel engine fueled with diesel-biodiesel-ethanol blends. Energy 2015, 83, 343–350. [Google Scholar] [CrossRef]
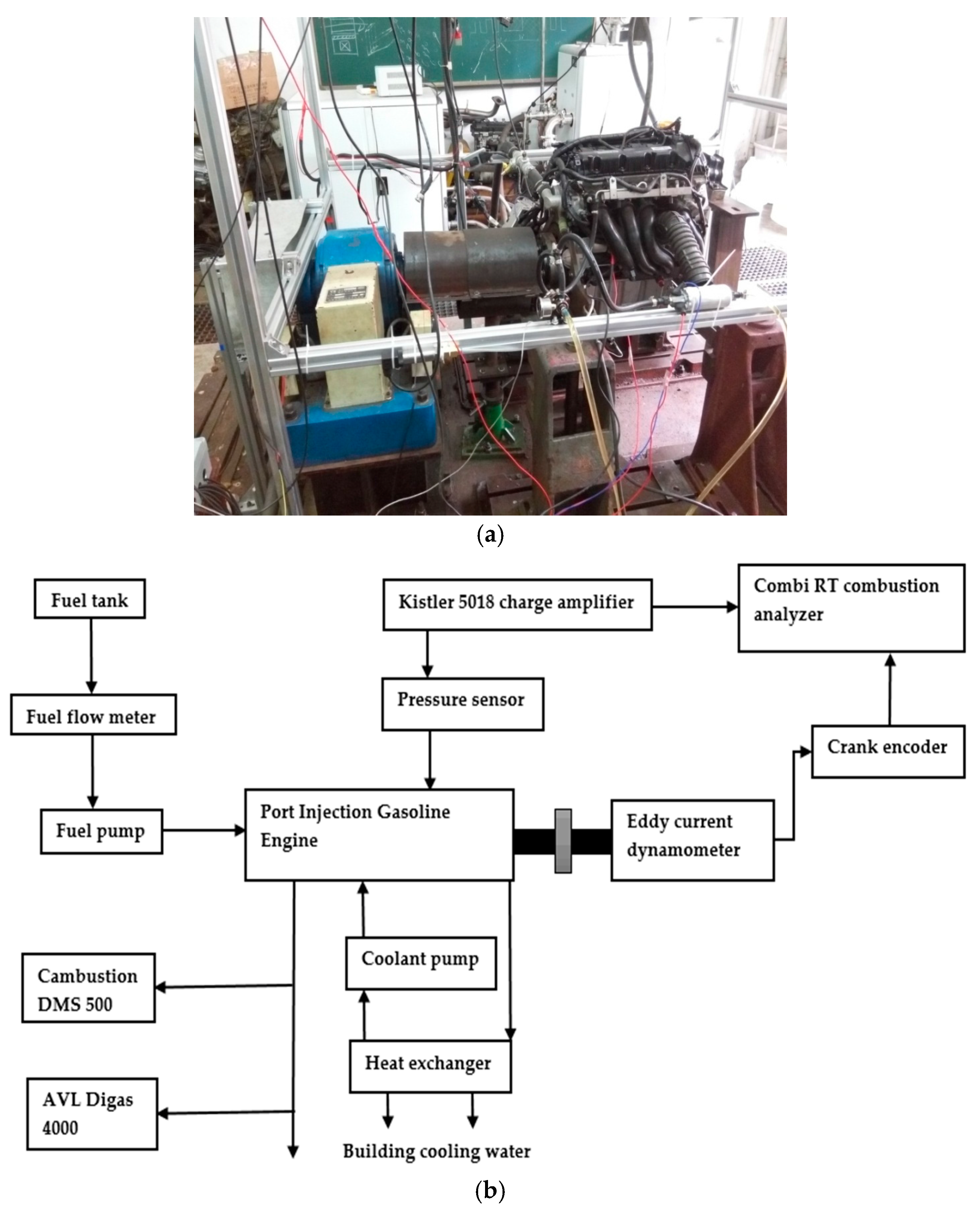

| Cylinders | Four in a Row |
|---|---|
| Injection type | Aspirate port fuel injection |
| Bore × stroke | 75.0 × 84.8 (mm) |
| Connecting road length | 143.70 (mm) |
| Compression ratio | 10.50:1 |
| Work order | 1-3-4-2 |
| Rated power | 78/600(kW/(rev min−1)) |
| Rated torque | 135/4500 (Nm/(rev min−1)) |
| Intake valve opening | 353 (°CA ATDC) |
| Intake valve closing | −141 (°CA ATDC) |
| Exhaust valve opening | 135 (°CA ATDC) |
| Exhaust valve closing | −349 (°CA ATDC) |
| Ambient temperature | 25 ± 3 (°C) |
| Lambda | 1 |
| Spark timing @ 2000 rev/min | 31.3 (°CA BTDC) |
| Intake fuel pressure @ 2000 rev/min | 3.09 (bar) |
| Intake air pressure @ 2000 rev/min | 0.38 (bar) |
| Property | Gasoline | Anhydrous Ethanol | Hydrous Ethanol |
|---|---|---|---|
| Water content (vol/vol %) | 0 | 0 | 4.0–5.0 |
| Latent heat of vaporization (kJ/kg) | 380–500 | 900–920 | 948 |
| LHV (MJ/kg) | 42.7–43.4 | 26.8 | 24.76–25.235 |
| Stoichiometric–A/F ratio (w/w) | 14.7 | 9 | 8.7–8.8 |
| RON | 88–100 | 108.6 | 111.1 |
| MON | 80–90 | 89.7 | 91.8–103.3 |
| Carbon content (mass %) | 87.4 | 52.2 | 50.59–50.7 |
| Hydrogen content (mass %) | 12.6 | 13 | 12.89–13 |
| Oxygen content (mass %) | 0 | 34.7 | 36.3–36.42 |
| H/C (atom ratio) | 1.795 | 3 | 3 |
| O/C (atom ratio) | 0 | 0.5 | 0.53 |
| Sulphur content (ppm) | 9 | 0 | 0 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, M.; O. El-Faroug, M.; Yan, F.; Wang, Y. Particulate Matter and Gaseous Emission of Hydrous Ethanol Gasoline Blends Fuel in a Port Injection Gasoline Engine. Energies 2017, 10, 1263. https://doi.org/10.3390/en10091263
Luo M, O. El-Faroug M, Yan F, Wang Y. Particulate Matter and Gaseous Emission of Hydrous Ethanol Gasoline Blends Fuel in a Port Injection Gasoline Engine. Energies. 2017; 10(9):1263. https://doi.org/10.3390/en10091263
Chicago/Turabian StyleLuo, Maji, Musaab O. El-Faroug, Fuwu Yan, and Yinan Wang. 2017. "Particulate Matter and Gaseous Emission of Hydrous Ethanol Gasoline Blends Fuel in a Port Injection Gasoline Engine" Energies 10, no. 9: 1263. https://doi.org/10.3390/en10091263
APA StyleLuo, M., O. El-Faroug, M., Yan, F., & Wang, Y. (2017). Particulate Matter and Gaseous Emission of Hydrous Ethanol Gasoline Blends Fuel in a Port Injection Gasoline Engine. Energies, 10(9), 1263. https://doi.org/10.3390/en10091263