Enhanced Production of Bioethanol by Fermentation of Autohydrolyzed and C4mimOAc-Treated Sugarcane Bagasse Employing Various Yeast Strains
Abstract
:1. Introduction
2. Results
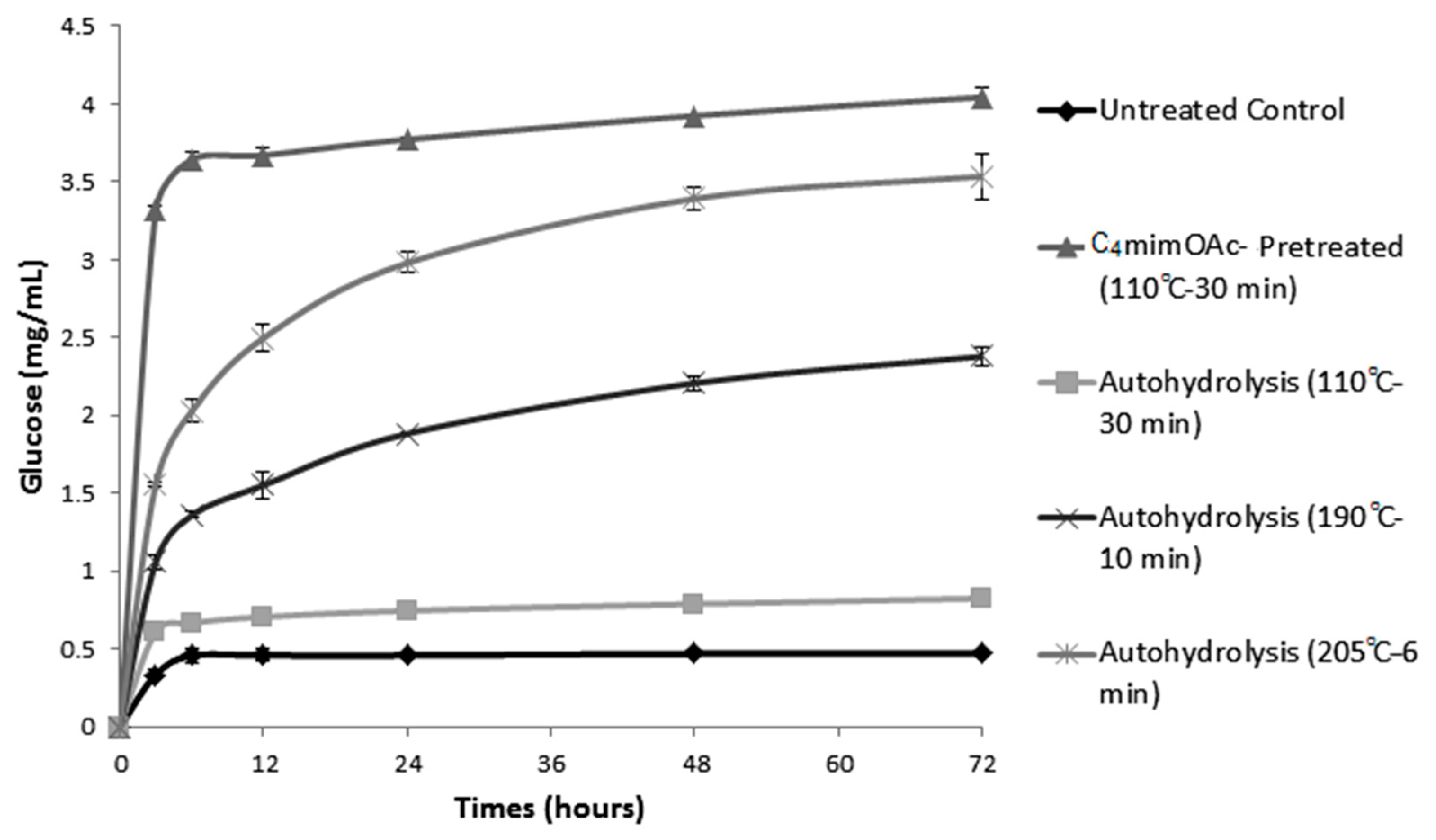
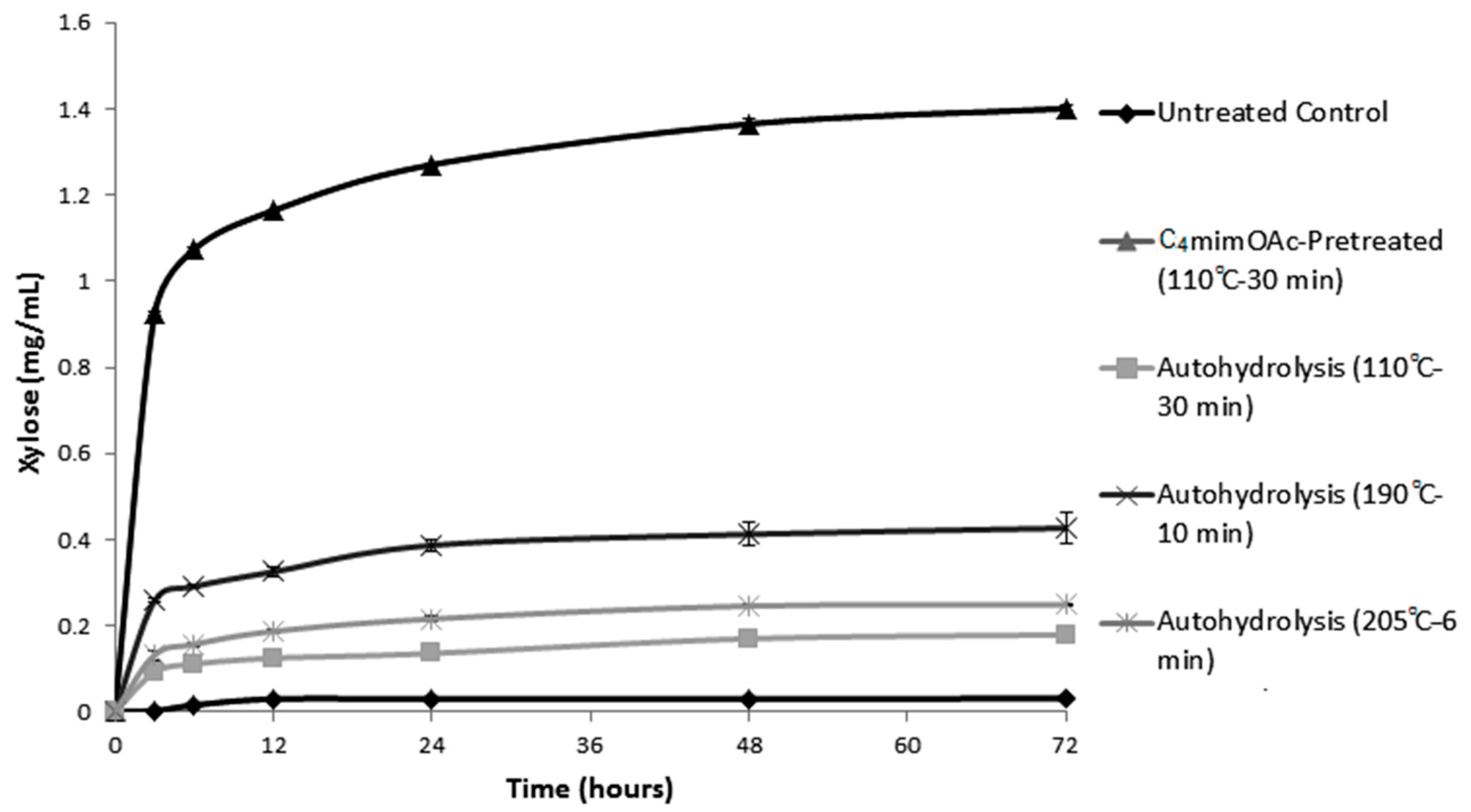
2.1. Enzymatic Hydrolysis
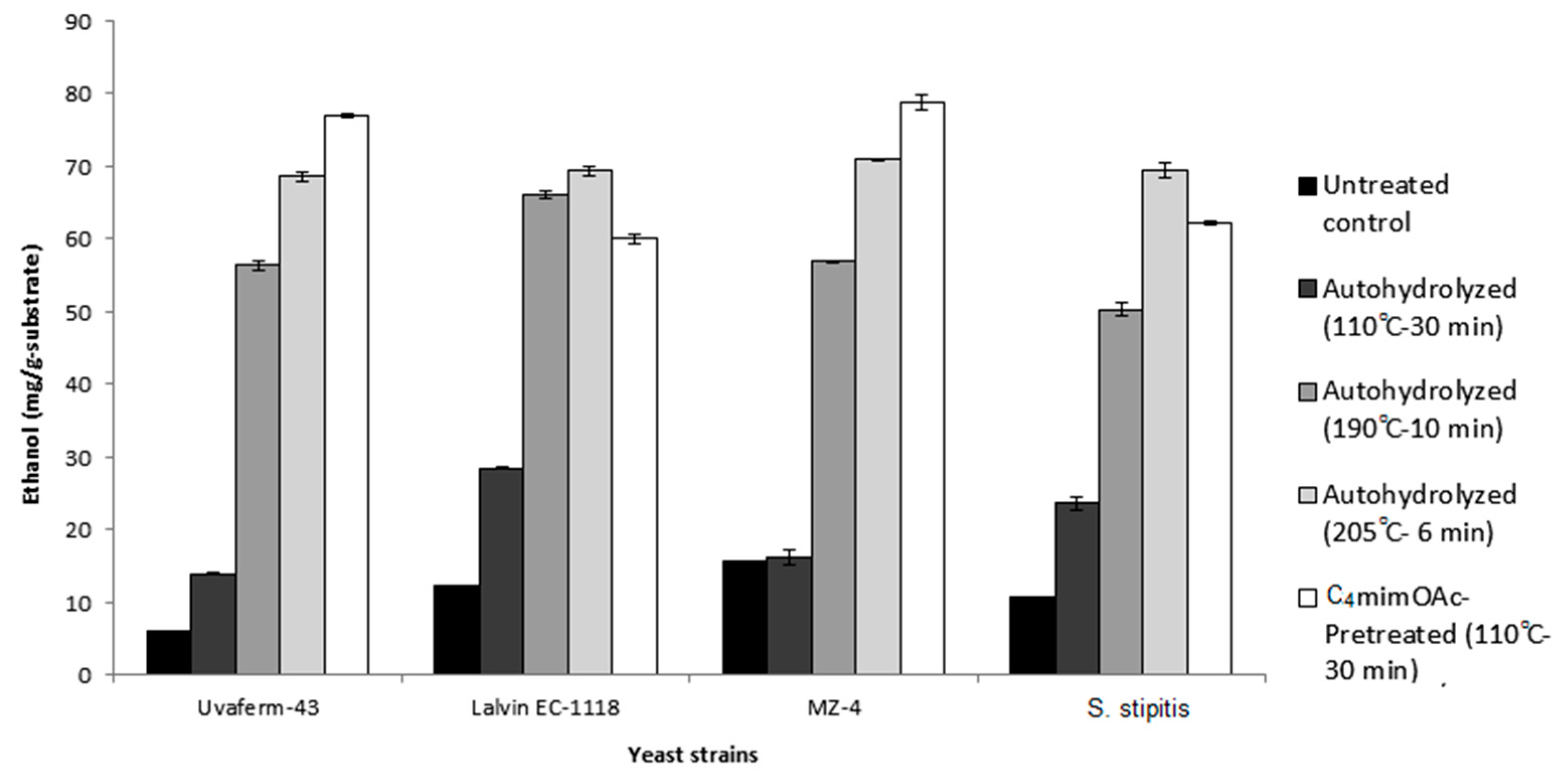
2.2. Fermentation
3. Discussion
4. Materials and Methods
4.1. Yeast Strains
4.2. Fermentation of Pretreated Sugarcane Bagasse
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- U.S. Energy Information Administration, Monthly Energy Review. April 2017. Available online: https://www.eia.gov/totalenergy/data/monthly/ (accessed on 18 May 2017).
- Wang, M.; Wu, M.; Huo, H. Life-cycle energy and greenhouse gas emission impacts of different corn ethanol plant types. Environ. Res. Lett. 2007, 2, 024001. [Google Scholar] [CrossRef]
- Verardi, A.; Blasi, A.; De Bari, I.; Calabrò, V. Steam pretreatment of Saccharum officinarum L. bagasse by adding of impregnating agents for advanced bioethanol production. Ecotoxicol. Environ. Saf. 2016, 134, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Verardi, A.; Blasi, A.; Molino, A.; Albo, L.; Calabrò, V. Improving the enzymatic hydrolysis of Saccharum officinarum L. bagasse by optimizing mixing in a stirred tank reactor: Quantitative analysis of biomass conversion. Fuel Process. Technol. 2016, 149, 15–22. [Google Scholar] [CrossRef]
- Xu, Z.; Huang, F. Pretreatment methods for bioethanol production. Appl. Biochem. Biotechnol. 2014, 174, 43–62. [Google Scholar] [CrossRef] [PubMed]
- Boerjan, W.; Ralph, J.; Baucher, M. Lignin biosynthesis. Annu. Rev. Plant Biol. 2003, 54, 519–546. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Block, D.E.; Mills, D.A. Simultaneous consumption of pentose and hexose sugars: An optimal microbial phenotype for efficient fermentation of lignocellulosic biomass. Appl. Microbiol. Biotechnol. 2010, 88, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Ishola, M.M.; Jahandideh, A.; Haidarian, B.; Brandberg, T.; Taherzadeh, M.J. Simultaneous saccharification, filtration and fermentation (SSFF): A novel method for bioethanol production from lignocellulosic biomass. Bioresour. Technol. 2013, 133, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, F.A.; Ruiz, H.A.; dos Santos, E.S.; Teixeira, J.A.; de Macedo, G.R. Bioethanol production by Saccharomyces cerevisiae, Pichia stipitis and Zymomonas mobilis from delignified coconut fibre mature and lignin extraction according to biorefinery concept. Renew. Energy 2016, 94, 353–365. [Google Scholar] [CrossRef] [Green Version]
- Kamzon, M.A.; Abderafi, S.; Bounahmidi, T. Promising bioethanol processes for developing a biorefinery in the Moroccan sugar industry. Int. J. Hydrogen Energy 2016, 41, 20880–20896. [Google Scholar] [CrossRef]
- Yu, Z.; Zhang, H. Ethanol fermentation of acid-hydrolyzed cellulosic pyrolysate with Saccharomyces cerevisiae. Bioresour. Technol. 2004, 93, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Canilha, L.; Kumar Chandel, A.K.; dos Santos Milessi, T.S.; Fernandes Antunes, F.A.; da Costa Freitas, W.L.; das Graças Almeidka Felipe, M.; da Silva, S.S. Bioconversion of sugarcane biomass into ethanol: An overview about composition, pretreatment methods, detoxification of hydrolysates, enzymatic saccharification, and ethanol fermentation. J. Biomed. Biotechnol. 2012, 2012, 989572. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, C.; Wang, L.; Yang, R.; Hou, P.; Liu, J. Direct bioethanol production from wheat straw using xylose/glucose co-fermentation by co-culture of two recombinant yeasts. J. Ind. Microbiol. Biotechnol. 2017, 44, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Kostas, E.T.; White, D.A.; Du, C.; Cook, D.J. Selection of yeast strains for bioethanol production from UK seaweeds. J. Appl. Phycol. 2016, 28, 1427–1441. [Google Scholar] [CrossRef] [PubMed]
- Du Preez, J.C.; Bosch, M.; Prior, B.A. Xylose fermentation by Candida shehatae and Pichia stipitis: Effects of pH, temperature and substrate concentration. Enzym. Microb. Technol. 1986, 8, 360–364. [Google Scholar] [CrossRef]
- Rouhollah, H.; Iraj, N.; Giti, E.; Sorah, A. Mixed sugar fermentation by Pichia stipitis, Sacharomyces cerevisiaea, and an isolated xylosefermenting Kluyveromyces marxianus and their cocultures. Afr. J. Biotechnol. 2007, 6, 1110–1114. [Google Scholar] [CrossRef]
- Silva, J.P.A.; Mussatto, S.I.; Roberto, I.C.; Teixeira, J. Ethanol production from xylose by Pichia stipitis NRRL Y-7124 in a stirred tank bioreactor. Braz. J. Chem. Eng. 2011, 28, 151–156. [Google Scholar] [CrossRef]
- Agbogbo, F.K.; Coward-Kelly, G.; Torry-Smith, M.; Wenger, K.S. Fermentation of glucose/xylose mixtures using Pichia stipitis. Process. Biochem. 2006, 41, 2333–2336. [Google Scholar] [CrossRef]
- Hashmi, M.; Sun, Q.; Tao, J.; Wells, T.; Shah, A.A.; Labbé, N.; Ragauskas, A.J. Comparison of autohydrolysis and ionic liquid 1-butyl-3-methylimidazolium acetate pretreatment to enhance enzymatic hydrolysis of sugarcane bagasse. Bioresour. Technol. 2017, 224, 714–720. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Knierim, B.; Manisseri, C.; Arora, R.; Scheller, H.V.; Auer, M.; Vogel, K.P.; Simmons, B.A.; Singh, S. Comparison of dilute acid and ionic liquid pretreatment of switchgrass: Biomass recalcitrance, delignification and enzymatic saccharification. Bioresour. Technol. 2010, 101, 4900–4906. [Google Scholar] [CrossRef] [PubMed]
- Batalha, L.A.; Han, Q.; Jameel, H.; Chang, H.M.; Colodette, J.L.; Borges Gomes, F.J. Production of fermentable sugars from sugarcane bagasse by enzymatic hydrolysis after autohydrolysis and mechanical refining. Bioresour. Technol. 2015, 180, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Aita, G.M.; Walker, M.S. Effect of ionic liquid pretreatment on the chemical composition, structure and enzymatic hydrolysis of energy cane bagasse. Bioresour. Technol. 2012, 117, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Hongdan, Z.; Shaohua, X.; Shubin, W. Enhancement of enzymatic saccharification of sugarcane bagasse by liquid hot water pretreatment. Bioresour. Technol. 2013, 143, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Krahulec, S.; Kratzer, R.; Longus, K.; Nidetzky, B. Comparison of Scheffersomyces stipitis strains CBS 5773 and CBS 6054 with regard to their xylose metabolism: Implications for xylose fermentation. Microbiol. Open 2012, 1, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Farwick, A.; Bruder, S.; Schadeweg, V.; Oreb, M.; Boles, E. Engineering of yeast hexose transporters to transport D-xylose without inhibition by D-glucose. Proc. Natl. Acad. Sci. USA 2014, 111, 5159–5164. [Google Scholar] [CrossRef] [PubMed]
- Jönsson, L.J.; Carlos, M. Pretreatment of lignocellulose: Formation of inhibitory by-products and strategies for minimizing their effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.A.; Dillon, S.; Kolouchova, R.; Henschke, P.A.; Chambers, P.J. Impacts of variations in elemental nutrient concentration of Chardonnay musts on Saccharomyces cerevisiae fermentation kinetics and wine composition. Appl. Microbiol. Biotechnol. 2011, 91, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Valero, E.; Schuller, D.; Cambon, B.; Casal, M.; Dequin, S. Dissemination and survival of commercial wine yeast in the vineyard: A large-scale, three-years study. FEMS Yeast Res. 2005, 5, 959–969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carreto, L.; Eiriz, M.F.; Gomes, A.C.; Pereira, P.M.; Schuller, D.; Santos, M.A. Comparative genomics of wild type yeast strains unveils important genome diversity. BMC Genom. 2008, 9, 524. [Google Scholar] [CrossRef] [PubMed]
- Yáñez-S, M.; Rojas, J.; Castro, J.; Ragauskas, A.; Baeza, J.; Freer, J. Fuel ethanol production from Eucalyptus globulus wood by autocatalized organosolv pretreatment ethanol—Water and SSF. J. Chem. Technol. Biotechnol. 2013, 88, 39–48. [Google Scholar] [CrossRef]
- Mukhtar, K.; Asgher, M.; Afghan, S.; Hussain, K.; Zia-Ul-Hussnain, S. Comparative study on two commercial strains of Saccharomyces cerevisiae for optimum ethanol production on industrial scale. J. Biomed. Biotechnol. 2010, 2010, 419586. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Ragauskas, A.J. Dilute H2SO4 and SO2 pretreatments of Loblolly pine wood residue for bioethanol production. Ind. Biotechnol. 2012, 8, 22–30. [Google Scholar] [CrossRef]
- Yoo, C.G.; Pu, Y.; Ragauskas, A.J. Ionic liquids: Promising Green Solvents for Lignocellulosic Biomass Utilization. Curr. Opin. Green Sustain. Chem. 2017, 5, 5–11. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hashmi, M.; Shah, A.A.; Hameed, A.; Ragauskas, A.J. Enhanced Production of Bioethanol by Fermentation of Autohydrolyzed and C4mimOAc-Treated Sugarcane Bagasse Employing Various Yeast Strains. Energies 2017, 10, 1207. https://doi.org/10.3390/en10081207
Hashmi M, Shah AA, Hameed A, Ragauskas AJ. Enhanced Production of Bioethanol by Fermentation of Autohydrolyzed and C4mimOAc-Treated Sugarcane Bagasse Employing Various Yeast Strains. Energies. 2017; 10(8):1207. https://doi.org/10.3390/en10081207
Chicago/Turabian StyleHashmi, Muzna, Aamer Ali Shah, Abdul Hameed, and Arthur J. Ragauskas. 2017. "Enhanced Production of Bioethanol by Fermentation of Autohydrolyzed and C4mimOAc-Treated Sugarcane Bagasse Employing Various Yeast Strains" Energies 10, no. 8: 1207. https://doi.org/10.3390/en10081207
APA StyleHashmi, M., Shah, A. A., Hameed, A., & Ragauskas, A. J. (2017). Enhanced Production of Bioethanol by Fermentation of Autohydrolyzed and C4mimOAc-Treated Sugarcane Bagasse Employing Various Yeast Strains. Energies, 10(8), 1207. https://doi.org/10.3390/en10081207







