Transport and Deposition of Carbon Nanoparticles in Saturated Porous Media
Abstract
:1. Introduction
2. Material and Methods
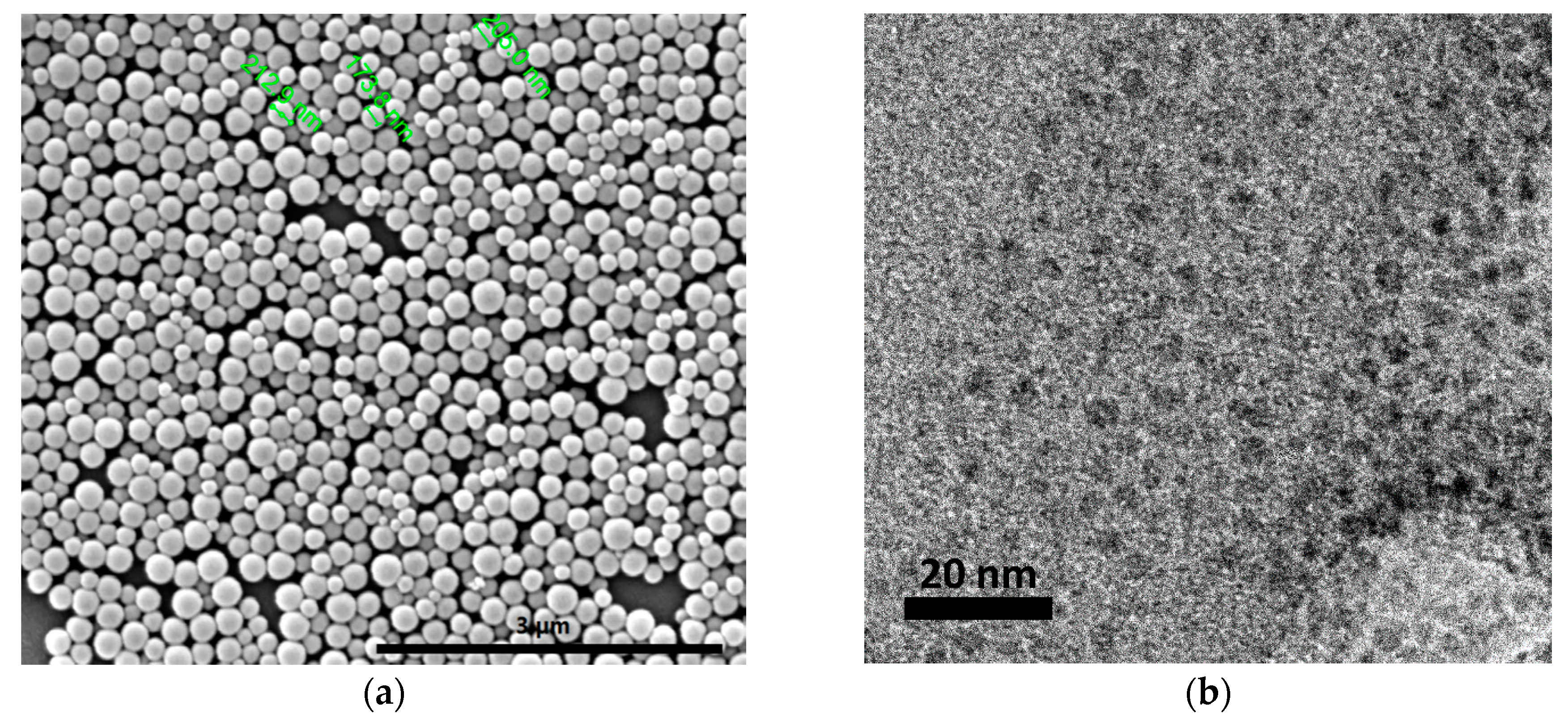
2.1. CNPs Synthesis and Characterization
2.2. Design of Porous Media
2.3. Solution Chemistry
2.4. Column Study
2.5. Mathematical Modelling Method
2.5.1. Nanoparticle Transport Modelling
2.5.2. Derjaguin-Landau-Verwey-Overbeek (DLVO) Interactions Energy Profiles
3. Results and Discussion
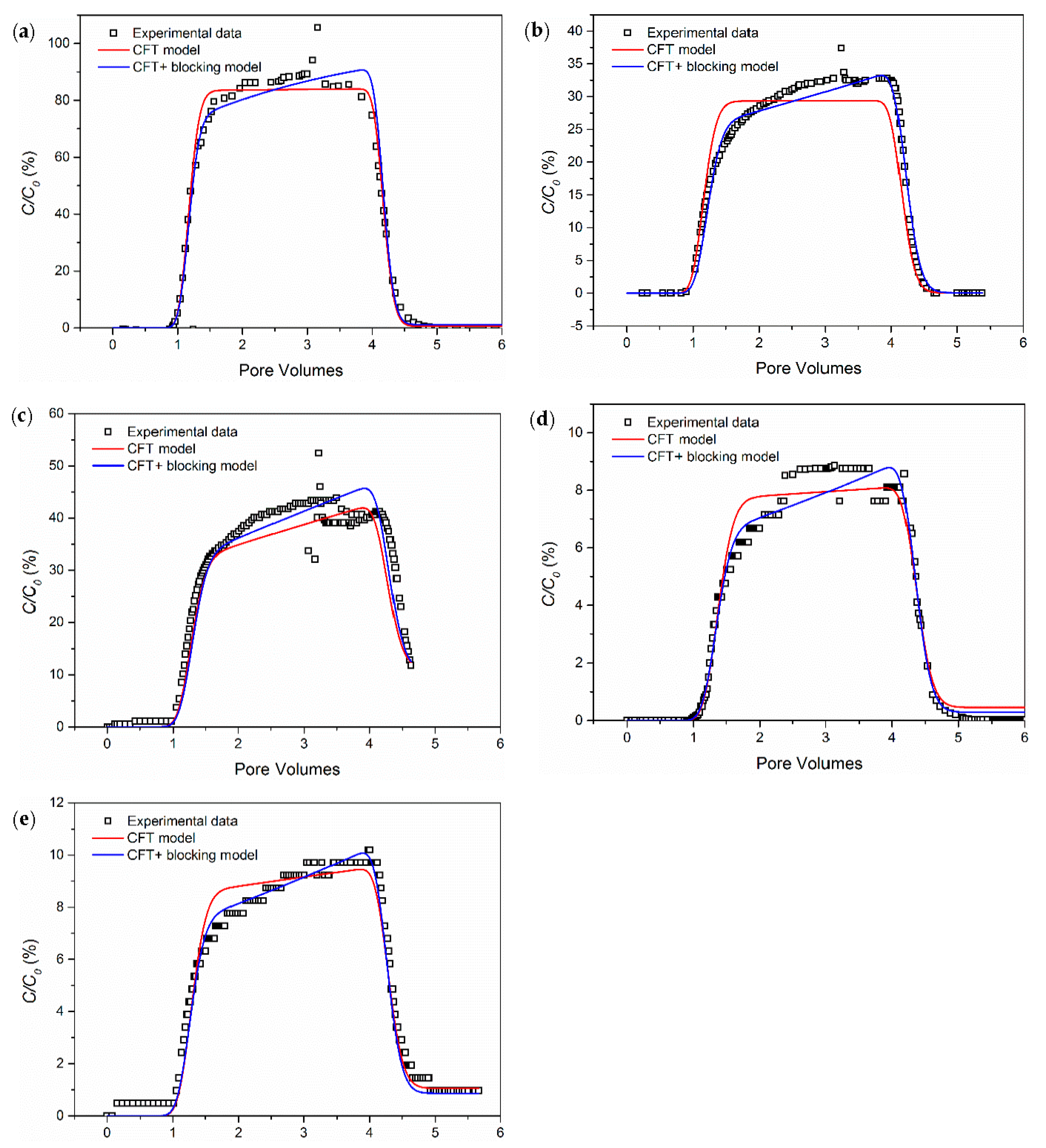
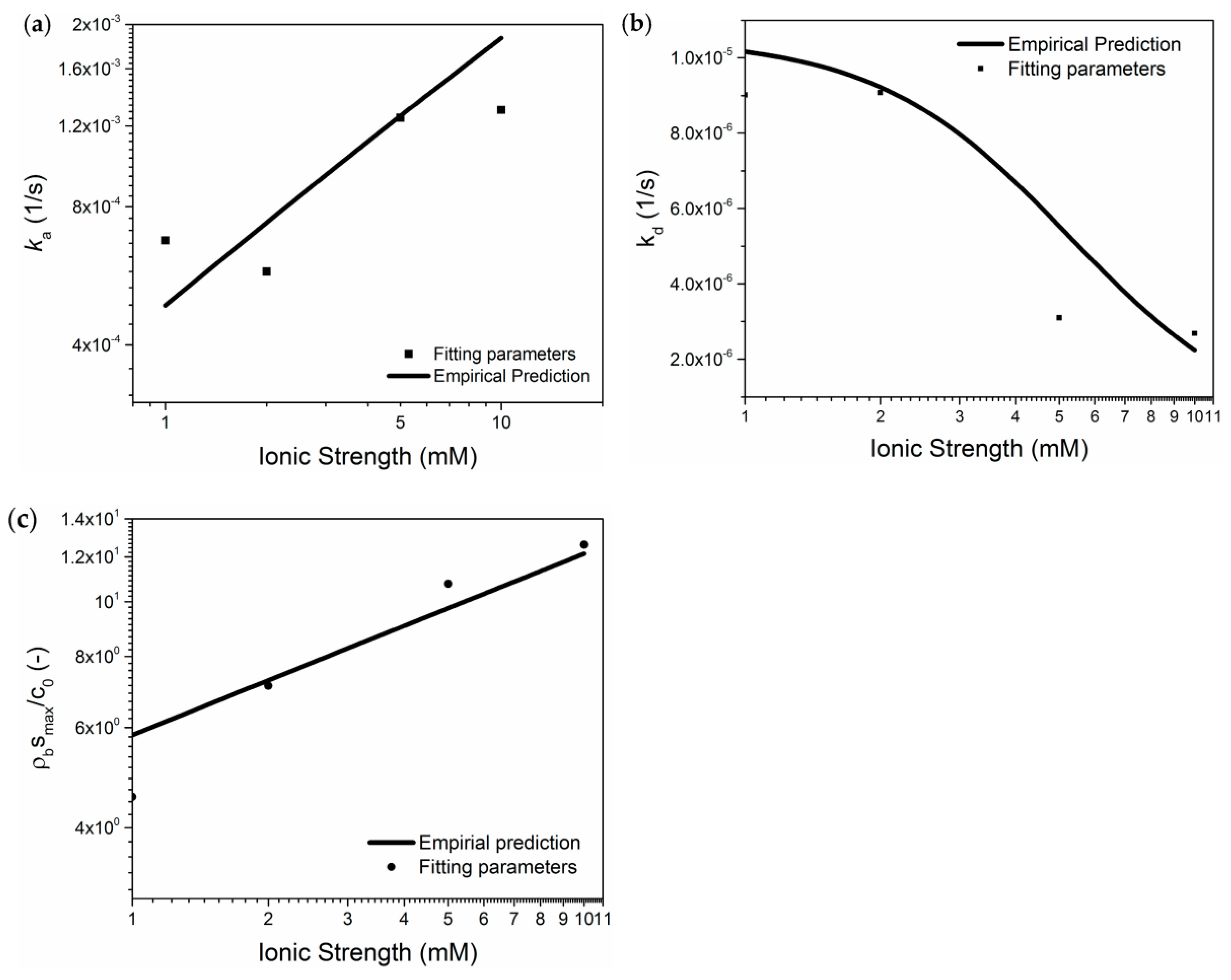
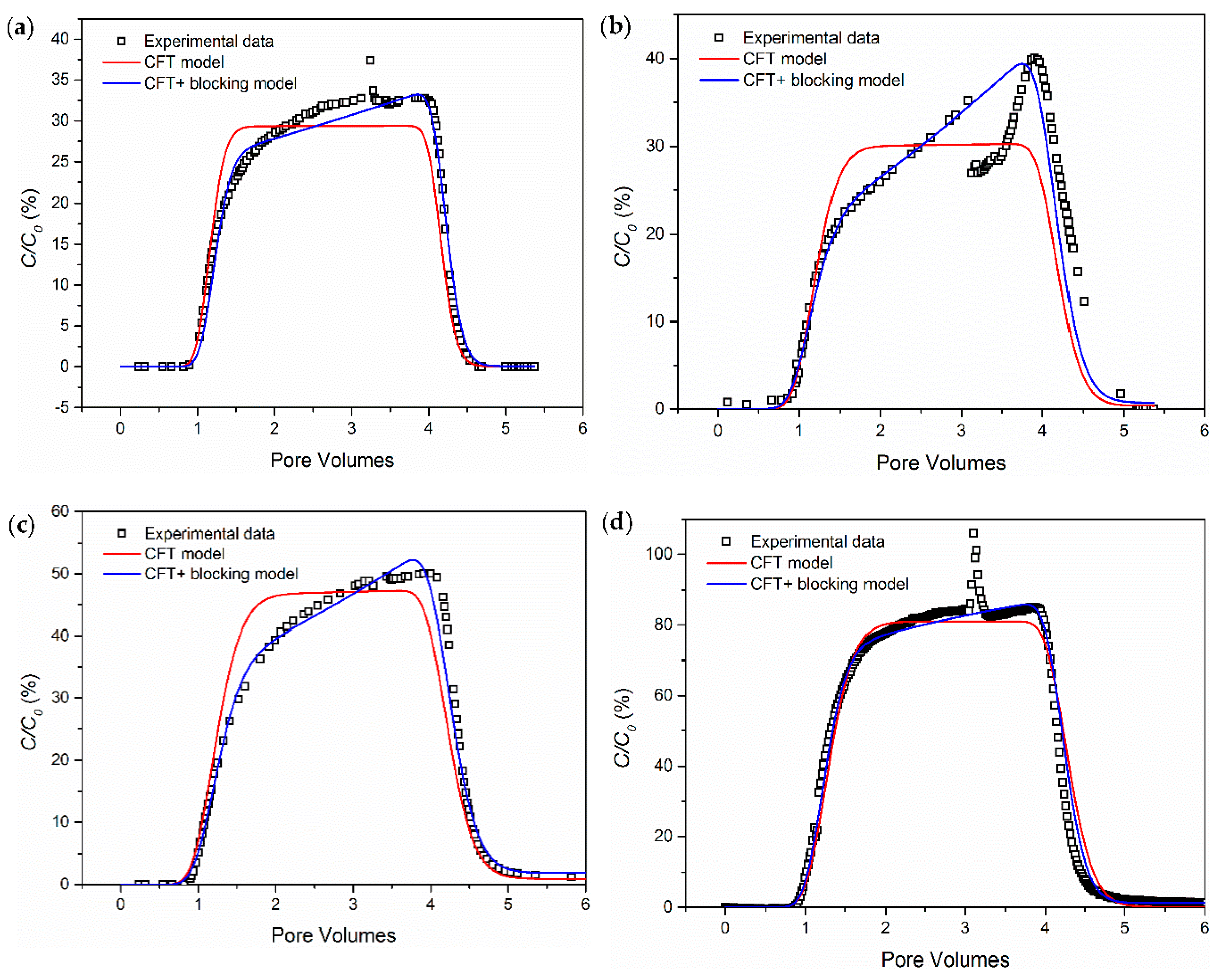
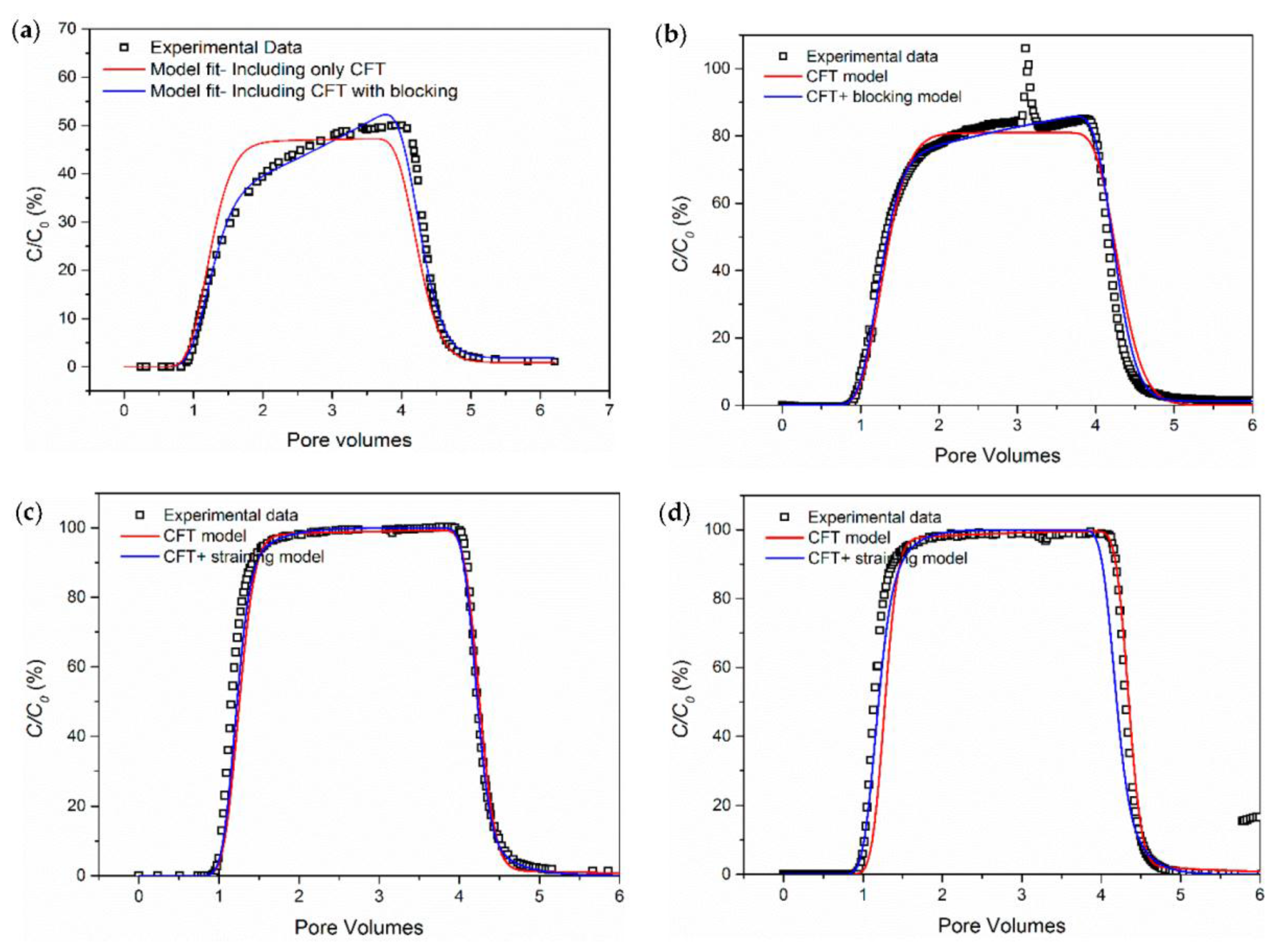
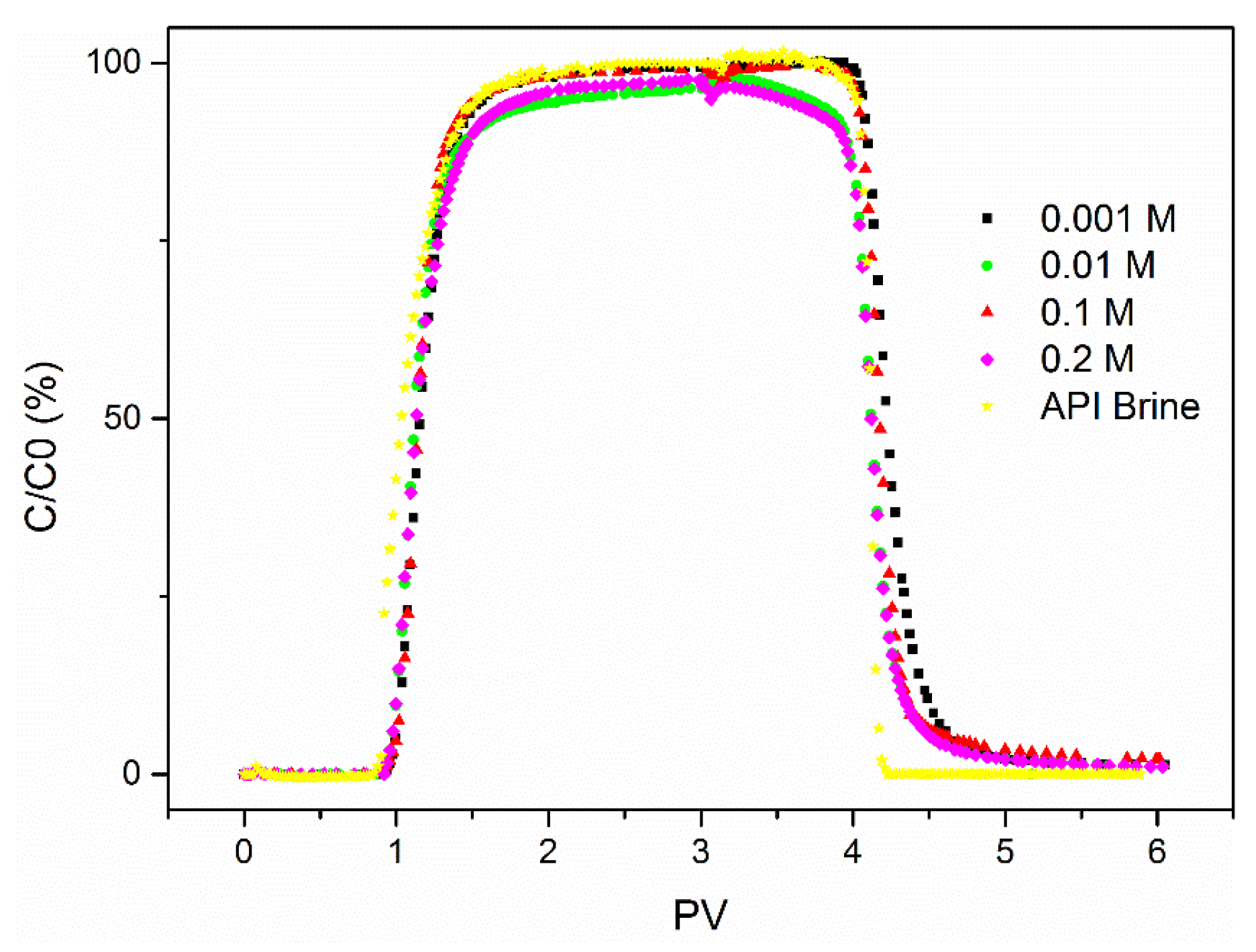
3.1. The Influence of Ionic Strength
3.2. The Influence of Concentration
3.3. The Influence of Particle Size
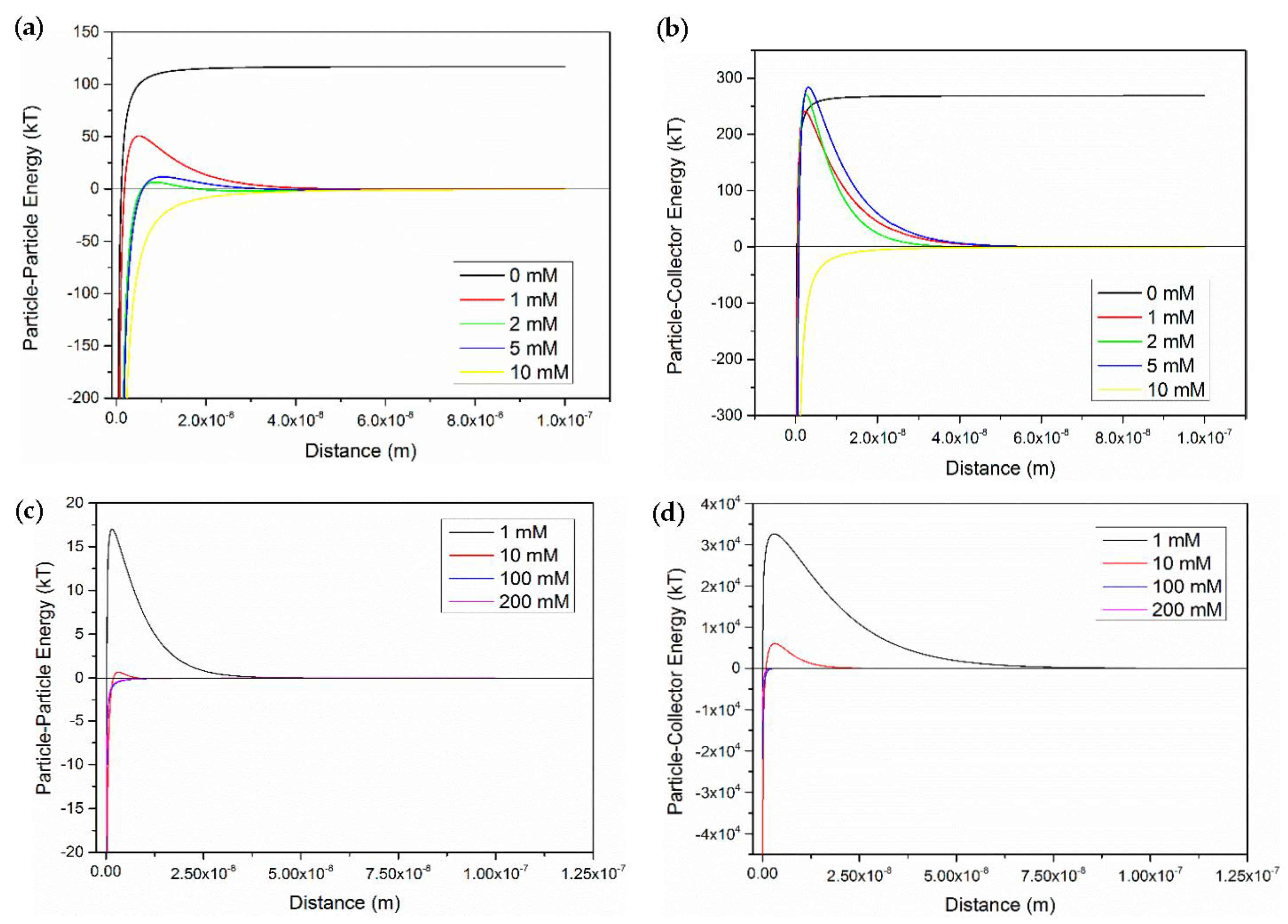
3.4. Applicability of Classic DLVO Theory
4. Conclusions
- Salinity has a strong influence on the breakthrough ability of CNP-200 in a porous medium, which is due to the reduced energy barrier determined by DVLO theory and blocking caused by the formation of large-size aggregation. Hence, the CFT model with blocking effect can fit the BTCs of carbon balls.
- For CNP-200, the retention is very sensitive to the particle concentration, with a higher concentration leading to a higher relative breakthrough (C/Co).
- The increase of ionic strength and concentration are unaffected to the transport behavior of CNP-5, even when the ionic strength increased to the level of API brine. The Kinetic Langmuir effect model shows that the detachment for CNP-5 rate is much higher than its deposition rate.
- The classic DLVO theory without considering other short-range interaction (like Born effect) may be limited to explain the transport behavior of CNP-5.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Alaskar, M. In-Situ Multifunctional Nanosensors for Fractured Reservoir Characterization. Ph.D. Thesis, Stanford University, Stanford, CA, USA, August 2013. [Google Scholar]
- Hwang, C.C.; Wang, L.; Lu, W.; Ruan, G.; Kini, G.C.; Xiang, C.; Samuel, E.L.G.; Shi, W.; Kan, A.T.; Wong, M.S.; et al. Highly stable carbon nanoparticles designed for downhole hydrocarbon detection. Energy Environ. Sci. 2012, 5, 8304. [Google Scholar] [CrossRef]
- Hwang, C.C.; Ruan, G.; Wang, L.; Zheng, H.; Samuel, E.L.; Xiang, C.; Lu, W.; Kasper, W.; Huang, K.; Peng, Z.; et al. Carbon-based nanoreporters designed for subsurface hydrogen sulfide detection. ACS Appl. Mater. Interfaces 2014, 6, 7652–7658. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Hendraningrat, L.; Torsater, O. Improved Oil Recovery by Hydrophilic Silica Nanoparticles Suspension: 2-Phase Flow Experimental Studies. In Proceedings of the International Petroleum Technology Conference, Beijing, China, 26–28 March 2013. [Google Scholar]
- Karimi, A.; Fakhroueian, Z.; Bahramian, A.; Pour Khiabani, N.; Darabad, J.B.; Azin, R.; Arya, S. Wettability Alteration in Carbonates using Zirconium Oxide Nanofluids: EOR Implications. Energy Fuels 2012, 26, 1028–1036. [Google Scholar] [CrossRef]
- Roustaei, A.; Saffarzadeh, S.; Mohammadi, M. An evaluation of modified silica nanoparticles’ efficiency in enhancing oil recovery of light and intermediate oil reservoirs. Egypt. J. Pet. 2013, 22, 427–433. [Google Scholar] [CrossRef]
- Ehtesabi, H.; Ahadian, M.M.; Taghikhani, V.; Ghazanfari, M.H. Enhanced Heavy Oil Recovery in Sandstone Cores Using TiO2 Nanofluids. Energy Fuels 2014, 28, 423–430. [Google Scholar] [CrossRef]
- Singh, R.; Mohanty, K.K. Synergy between Nanoparticles and Surfactants in Stabilizing Foams for Oil Recovery. Energy Fuels 2014, 29, 467–479. [Google Scholar] [CrossRef]
- Pei, H.H.; Zhang, G.C.; Ge, J.J.; Zhang, J.; Zhang, Q.; Fu, L.P. Investigation of Nanoparticle and Surfactant Stabilized Emulsion to Enhance Oil Recovery in Waterflooded Heavy Oil Reservoirs. In Proceedings of the SPE Canada Heavy Oil Technical Conference, Calgary, AB, Canada, 9–11 June 2015. [Google Scholar]
- Guo, F.; He, J.; Johnson, P.A.; Aryana, S.A. Stabilization of CO2 foam using by-product fly ash and recyclable iron oxide nanoparticles to improve carbon utilization in EOR processes. Sustain. Energy Fuels 2017, 1, 814–822. [Google Scholar] [CrossRef]
- Darlington, T.K.; Neigh, A.M.; Spencer, M.T.; Nguyen, O.T.; Oldenburg, S.J. Nanoparticle characteristics affecting environmental fate and transport through soil. Environ. Toxicol. Chem. 2009, 28, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Auffan, M.; Rose, J.; Bottero, J.Y.; Lowry, G.V.; Jolivet, J.P.; Wiesner, M.R. Towards a definition of inorganic nanoparticles from an environmental, health and safety perspective. Nat. Nanotechnol. 2009, 4, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Bhunia, S.K.; Saha, A.; Maity, A.R.; Ray, S.C.; Jana, N.R. Carbon nanoparticle-based fluorescent bioimaging probes. Sci. Rep. 2013, 3, 1473. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Sapelkin, A.V.; Titirici, M.M.; Sukhorukov, G.B. In Situ Synthesis of Fluorescent Carbon Dots/Polyelectrolyte Nanocomposite Microcapsules with Reduced Permeability and Ultrasound Sensitivity. ACS Nano 2016, 10, 9608–9615. [Google Scholar] [CrossRef] [PubMed]
- Lecoanet, H.F.; Wiesner, M.R. Velocity effects on fullerene and oxide nanoparticle deposition in porous media. Environ. Sci. Technol. 2004, 38, 4377–4382. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.L.; Elimelech, M. Aggregation and deposition kinetics of fullerene (C60) nanoparticles. Langmuir 2006, 22, 10994–11001. [Google Scholar] [CrossRef] [PubMed]
- Espinasse, B.; Hotze, E.M.; Wiesner, M.R. Transport and Retention of Colloidal Aggregates of C60 in Porous Media: Effects of Organic Macromolecules, Ionic Composition, and Preparation Method. Environ. Sci. Technol. 2007, 41, 7396–7402. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.L.; Elimelech, M. Interaction of Fullerene (C60) Nanoparticles with Humic Acid and Alginate Coated Silica Surfaces: Measurements, Mechanisms, and Environmental Implications. Environ. Sci. Technol. 2008, 42, 7607–7614. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Y.; Pennell, K.D.; Abriola, L.M. Investigation of the Transport and Deposition of Fullerene (C60) Nanoparticles in Quartz Sands under Varying Flow Conditions. Environ. Sci. Technol. 2008, 42, 7174–7180. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Y.; Fortner, J.D.; Hughes, J.B.; Abriola, L.M.; Pennell, K.D. Transport and retention of nanoscale C60 aggregates in water-saturated porous media. Environ. Sci. Technol. 2008, 42, 3588–3594. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Y.; Pennell, K.D. Influence of Electrolyte Species and Concentration on the Aggregation and Transport of Fullerene Nanoparticles in Quartz Sands. Environ. Toxicol. Chem. 2008, 27, 1860–1867. [Google Scholar] [CrossRef] [PubMed]
- Feriancikova, L.; Xu, S. Deposition and remobilization of graphene oxide within saturated sand packs. J. Hazard. Mater. 2012, 235–236, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Gao, B.; Bradford, S.A.; Wu, L.; Chen, H.; Shi, X.; Wu, J. Transport, retention, and size perturbation of graphene oxide in saturated porous media: Effects of input concentration and grain size. Water Res. 2015, 68, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Jiang, X.; Lu, Y.; Huo, M.; Lin, S.; Geng, Z. Effects of surfactants on graphene oxide nanoparticles transport in saturated porous media. J. Environ. Sci. 2015, 35, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Gao, B.; Ziegler, K.J. High mobility of SDBS-dispersed single-walled carbon nanotubes in saturated and unsaturated porous media. J. Hazard. Mater. 2011, 186, 1766–1772. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; O’Carroll, D.M.; Petersen, E.J.; Huang, Q.; Anderson, C.L. Mobility of multiwalled carbon nanotubes in porous media. Environ. Sci. Technol. 2009, 43, 8153–8158. [Google Scholar] [CrossRef] [PubMed]
- Jaisi, D.P.; Saleh, N.B.; Blake, R.E.; Elimelech, M. Transport of Single-Walled Carbon Nanotubes in Porous Media: Filtration Mechanisms and Reversibility. Environ. Sci. Technol. 2008, 42, 8317–8323. [Google Scholar] [CrossRef] [PubMed]
- Guzman, K.A.; Finnegan, M.P.; Banfield, J.F. Influence of surface potential on aggregation and transport of titania nanoparticles. Environ. Sci. Technol. 2006, 40, 7688–7693. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Tong, M.; Kim, H. Influence of natural organic matter on the transport and deposition of zinc oxide nanoparticles in saturated porous media. J. Colloid Interface Sci. 2012, 386, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Berlin, J.M.; Yu, J.; Lu, W.; Walsh, E.E.; Zhang, L.; Zhang, P.; Chen, W.; Kan, A.T.; Wong, M.S.; Tomson, M.B.; et al. Engineered nanoparticles for hydrocarbon detection in oil-field rocks. Energy Environ. Sci. 2011, 4, 505–509. [Google Scholar] [CrossRef]
- Wang, C.; Bobba, A.D.; Attinti, R.; Shen, C.; Lazouskaya, V.; Wang, L.P.; Jin, Y. Retention and transport of silica nanoparticles in saturated porous media: Effect of concentration and particle size. Environ. Sci. Technol. 2012, 46, 7151–7158. [Google Scholar] [CrossRef] [PubMed]
- French, R.A.; Jacobson, A.R.; Kim, B.; Isley, S.L.; Penn, R.L.; Baveye, P.C. Influence of ionic strength, pH, and cation valence on aggregation kinetics of titanium dioxide nanoparticles. Environ. Sci. Technol. 2009, 43, 1354–1359. [Google Scholar] [CrossRef] [PubMed]
- Metin, C.O.; Lake, L.W.; Miranda, C.R.; Nguyen, Q.P. Stability of aqueous silica nanoparticle dispersions. J. Nanopart. Res. 2011, 13, 839–850. [Google Scholar] [CrossRef]
- Lecoanet, H.F.; Bottero, J.Y.; Wiesner, M.R. Laboratory Assessment of the Mobility of Nanomaterials in Porous Media. Environ. Sci. Technol. 2004, 38, 5164–5169. [Google Scholar] [CrossRef] [PubMed]
- Phenrat, T.; Kim, H.J.; Fagerlund, F.; Illangasekare, T.; Tilton, R.D.; Lowry, G.V. Particle Size Distribution, Concentration, and Magnetic Attraction Affect Transport of Polymer-Modified Fe0Nanoparticles in Sand Columns. Environ. Sci. Technol. 2009, 43, 5079–5085. [Google Scholar] [CrossRef] [PubMed]
- Petosa, A.R.; Jaisi, D.P.; Quevedo, I.R.; Elimelech, M.; Tufenkji, N. Aggregation and deposition of engineered nanomaterials in aquatic environments: Role of physicochemical interactions. Environ. Sci. Technol. 2010, 44, 6532–6549. [Google Scholar] [CrossRef] [PubMed]
- Solovitch, N.; Labille, J.; Rose, J.; Chaurand, P.; Borschneck, D.; Wiesner, M.R.; Bottero, J.Y. Concurrent aggregation and deposition of TiO2 nanoparticles in a sandy porous media. Environ. Sci. Technol. 2010, 44, 4897–4902. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Morales, V.L.; Cakmak, M.E.; Salvucci, A.E.; Geohring, L.D.; Hay, A.G.; Parlange, J.Y.; Steenhuis, T.S. Colloid transport and retention in unsaturated porous media: Effect of colloid input concentration. Environ. Sci. Technol. 2010, 44, 4965–4972. [Google Scholar] [CrossRef] [PubMed]
- Kasel, D.; Bradford, S.A.; Simunek, J.; Heggen, M.; Vereecken, H.; Klumpp, E. Transport and retention of multi-walled carbon nanotubes in saturated porous media: Effects of input concentration and grain size. Water Res. 2013, 47, 933–944. [Google Scholar] [CrossRef] [PubMed]
- Hogg, R.; Healy, T.W.; Fuerstenau, D.W. Mutual coagulation of colloidal dispersions. Trans. Faraday Soc. 1966, 62, 1638–1651. [Google Scholar] [CrossRef]
- Elimelech, M.; Gregory, J.; Jia, X.; Williams, R.A. Particle Deposition & Aggregation: Measurement, Modelling and Simulation, 2nd ed.; Butterworth-Heinemann: Woburn, MA, USA, 1995; ISBN 0-7506-7024-X. [Google Scholar]
- Kallay, N.; Zalac, S. Stability of nanodispersions: A model for kinetics of aggregation of nanoparticles. J. Colloid Interface Sci. 2002, 253, 70–76. [Google Scholar] [CrossRef] [PubMed]
- He, Y.T.; Wan, J.; Tokunaga, T. Kinetic stability of hematite nanoparticles: The effect of particle sizes. J. Nanopart. Res. 2007, 10, 321–332. [Google Scholar] [CrossRef]
- Gao, H.; Wen, D.S.; Sukhorukov, G.B. Composite silica nanoparticle/polyelectrolyte microcapsules with reduced permeability and enhanced ultrasound sensitivity. J. Mater. Chem. B 2015, 3, 1888–1897. [Google Scholar] [CrossRef]
- Shen, C.; Li, B.; Huang, Y.; Jin, Y. Kinetics of Coupled Primary- and Secondary-Minimum Deposition of Colloids under Unfavorable Chemical Conditions. Environ. Sci. Technol. 2007, 41, 6976–6982. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.G.; Li, Y.S.; Costanza, J.; Abriola, L.M.; Pennell, K.D. Enhanced Mobility of Fullerene (C-60) Nanoparticles in the Presence of Stabilizing Agents. Environ. Sci. Technol. 2012, 46, 11761–11769. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.; Qi, J.; Jin, Y. Retention and Transport of Amphiphilic Colloids under Unsaturated Flow Conditions: Effect of Particle Size and Surface Property. Environ. Sci. Technol. 2005, 39, 7853–7859. [Google Scholar] [CrossRef] [PubMed]
- Tufenkji, N.; Elimelech, M. Correlation Equation for Predicting Single-Collector Efficiency in Physicochemical Filtration in Saturated Porous Media. Environ. Sci. Technol. 2004, 38, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Esfandyari Bayat, A.; Junin, R.; Derahman, M.N.; Samad, A.A. TiO2 nanoparticle transport and retention through saturated limestone porous media under various ionic strength conditions. Chemosphere 2015, 134, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Ko, C.H.; Elimelech, M. The “Shadow Effect” in Colloid Transport and Deposition Dynamics in Granular Porous Media: Measurements and Mechanisms. Environ. Sci. Technol. 2000, 34, 3681–3689. [Google Scholar] [CrossRef]
- Cullen, E.; O’Carroll, D.M.; Yanful, E.K.; Sleep, B. Simulation of the subsurface mobility of carbon nanoparticles at the field scale. Adv. Water Resour. 2010, 33, 361–371. [Google Scholar] [CrossRef]
- Rottman, J.J. Fundamentals and Application of Porous Media Filtration for The Removal of Nanoparticles From Industrial Wastewater. Ph.D. Thesis, University of Arizona, Tucson, AZ, USA, July 2012. [Google Scholar]
- Bradford, S.A.; Simunek, J.; Bettahar, M.; Van Genuchten, M.T.; Yates, S.R. Modeling Colloid Attachment, Straining, and Exclusion in Saturated Porous Media. Environ. Sci. Technol. 2003, 37, 2242–2250. [Google Scholar] [CrossRef] [PubMed]
- Tosco, T.; Tiraferri, A.; Sethi, R. Ionic Strength Dependent Transport of Microparticles in Saturated Porous Media: Modeling Mobilization and Immobilization Phenomena under Transient Chemical Conditions. Environ. Sci. Technol. 2009, 43, 4425–4431. [Google Scholar] [CrossRef] [PubMed]
- Torkzaban, S.; Bradford, S.A.; Wan, J.; Tokunaga, T.; Masoudih, A. Release of quantum dot nanoparticles in porous media: Role of cation exchange and aging time. Environ. Sci. Technol. 2013, 47, 11528–11536. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Shapiro, A.A. A mathematical model for non-monotonic deposition profiles in deep bed filtration systems. Chem. Eng. J. 2011, 166, 105–115. [Google Scholar] [CrossRef]
- Johnson, P.R.; Elimelech, M. Dynamics of Colloid Deposition in Porous Media: Blocking Based on Random Sequential Adsorption. Langmuir 1995, 11, 801–812. [Google Scholar] [CrossRef]
- Worthen, A.J.; Tran, V.; Cornell, K.A.; Truskett, T.M.; Johnston, K.P. Steric stabilization of nanoparticles with grafted low molecular weight ligands in highly concentrated brines including divalent ions. Soft Matter 2016, 12, 2025–2039. [Google Scholar] [CrossRef] [PubMed]
| Column | Size | Influent Concentration (ppm) | Ionic Strength (mM CaCl2) | Mass Balance (%) |
|---|---|---|---|---|
| G1 | 200 | 25 | 0 | 5.33 |
| G2 | 200 | 3.1 | 1 | 7.5 |
| G3 | 200 | 3.0 | 2 | 0 |
| G4 | 200 | 3.4 | 5 | 0 |
| G5 | 200 | 3.3 | 10 | 0 |
| G6 | 200 | 4.5 | 1 | 3.8 |
| G7 | 200 | 6.4 | 1 | 7.32 |
| G8 | 200 | 8.0 | 1 | 0 |
| G9 | 5 | 24.8 | 1 | 0 |
| G10 | 5 | 10.6 | 1 | 0 |
| CaCl2 (mM) | 0 | 1 | 2 | 5 | 10 | |
|---|---|---|---|---|---|---|
| zeta potential (mV) | CNP-200 | −43.4 | −32.8 | −19.8 | −17 | −0.24 |
| Glass beads | −63.7 | −38 | −20 | −19 | −63.7 | |
| Size (nm) | 176.5 | 304.3 | 691.0 | 743.0 | 750.2 | |
| CaCl2 (mM) | 1 | 10 | 100 | 200 |
|---|---|---|---|---|
| zeta potential (mV) | −40.3 | −10.5 | −0.35 | −0.21 |
| Size (nm) | 25.5 | 26.8 | 16.3 | 13.6 |
| Exp. | Size (nm) | Ionic Strength (mM CaCl2) | Measured Retention after Postflushing (%) | Simulated Retention from Better Fitting (%) | Simulated (µg/g) | (1/h) | (1/h) |
|---|---|---|---|---|---|---|---|
| G1 | 200 | 0 | 10.9 | 10.5 | 2.28 10−5 | 0.616 | 0.062 |
| G2 | 200 | 1 | 27.7 | 27.2 | 3.74 10−5 | 2.434 | 0.036 |
| G3 | 200 | 2 | 44.2 | 43.6 | 5.86 10−5 | 2.081 | 0.033 |
| G4 | 200 | 5 | 62.6 | 61.8 | 8.87 10−5 | 4.572 | 0.011 |
| G5 | 200 | 10 | 62.5 | 62.7 | 1.04 10−4 | 4.644 | 0.010 |
| G6 | 200 | 1 | 46.3 | 45.9 | 2.51 10−5 | 2.970 | 0.014 |
| G7 | 200 | 1 | 35.5 | 36.1 | 9.64 10−5 | 4.104 | 0.015 |
| G8 | 200 | 1 | 18.6 | 18.1 | 1.02 10−5 | 0.587 | 0.057 |
| G9 | 5 | 1 | 0.2 | 0.2 | 1.04 10−4 | 1.447 | 7.452 |
| G10 | 5 | 1 | 1.7 | 1.7 | 3.58 10−4 | 3.460 | 11.952 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Z.; Zhao, J.; Gao, H.; Nourafkan, E.; Wen, D. Transport and Deposition of Carbon Nanoparticles in Saturated Porous Media. Energies 2017, 10, 1151. https://doi.org/10.3390/en10081151
Hu Z, Zhao J, Gao H, Nourafkan E, Wen D. Transport and Deposition of Carbon Nanoparticles in Saturated Porous Media. Energies. 2017; 10(8):1151. https://doi.org/10.3390/en10081151
Chicago/Turabian StyleHu, Zhongliang, Jin Zhao, Hui Gao, Ehsan Nourafkan, and Dongsheng Wen. 2017. "Transport and Deposition of Carbon Nanoparticles in Saturated Porous Media" Energies 10, no. 8: 1151. https://doi.org/10.3390/en10081151
APA StyleHu, Z., Zhao, J., Gao, H., Nourafkan, E., & Wen, D. (2017). Transport and Deposition of Carbon Nanoparticles in Saturated Porous Media. Energies, 10(8), 1151. https://doi.org/10.3390/en10081151







