The Coupled Effect of Fines Mobilization and Salt Precipitation on CO2 Injectivity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Rocks
2.1.2. Formation Water
2.1.3. Carbonated Water
2.1.4. CO2
2.1.5. Colloid Suspension
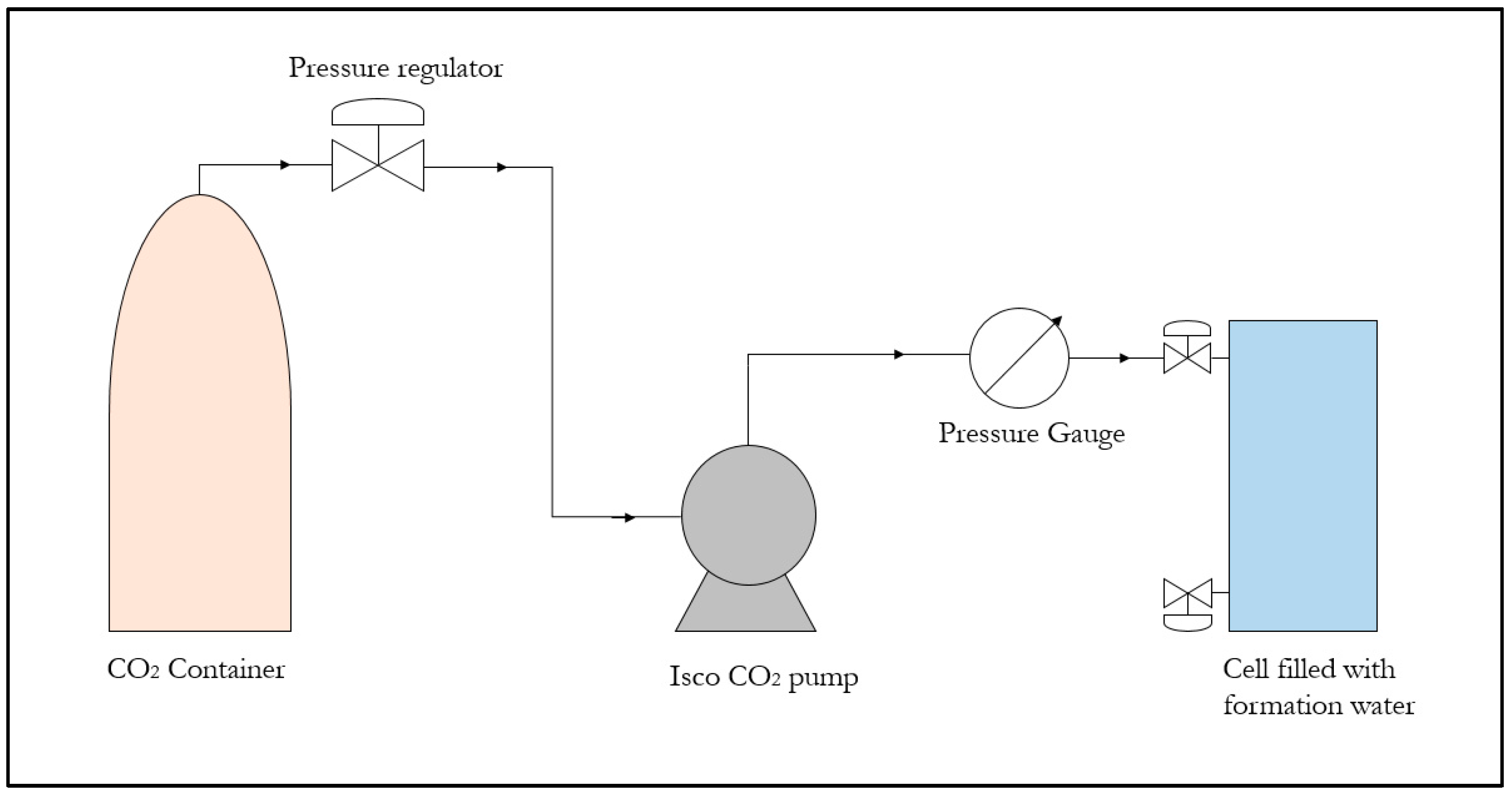
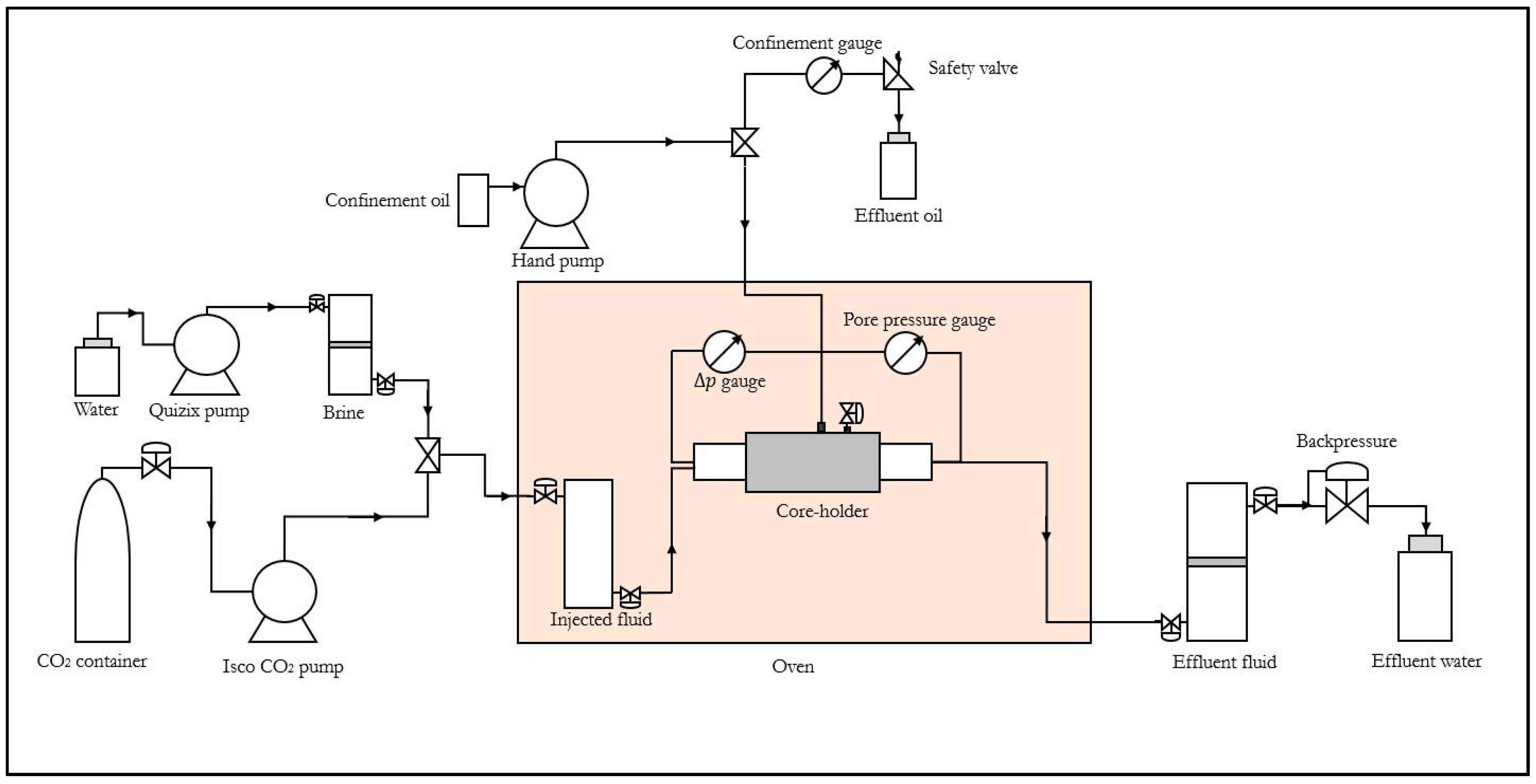
2.2. Experimental Setup
2.3. Methods
2.3.1. Experimental Procedure
- The initial permeability () of the core was measured.
- The core was saturated with brine and flooded with either carbonated water or supercritical CO2 to generate fines or dry the core, respectively.
- The experiment was stopped and the core was cooled for all trapped CO2 to boil out.
- The permeability of the core after mineral impairment was measured.
2.3.2. Injectivity Impairment Quantification
2.3.3 Uncertainty in Experimental Data
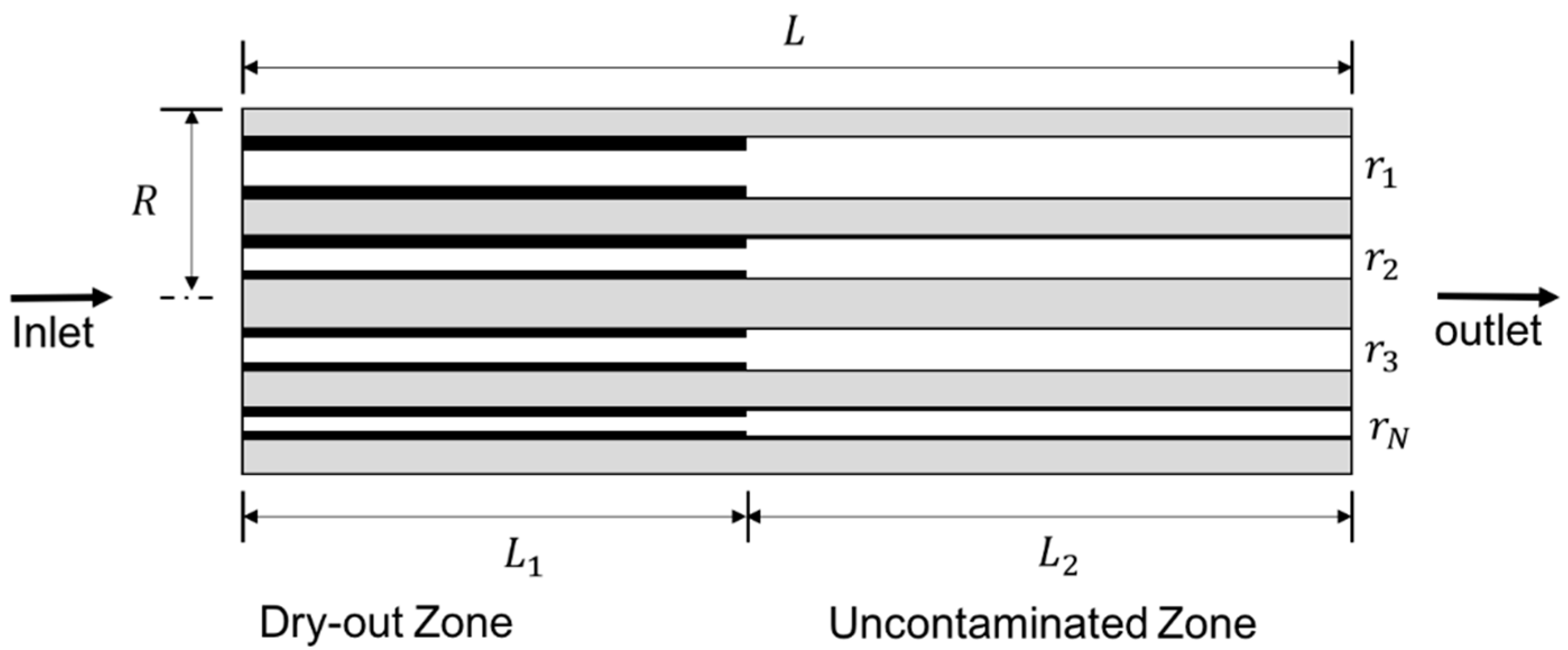
3. Theoretical Modelling
3.1. Background and Assumptions
3.2. Model Formulation
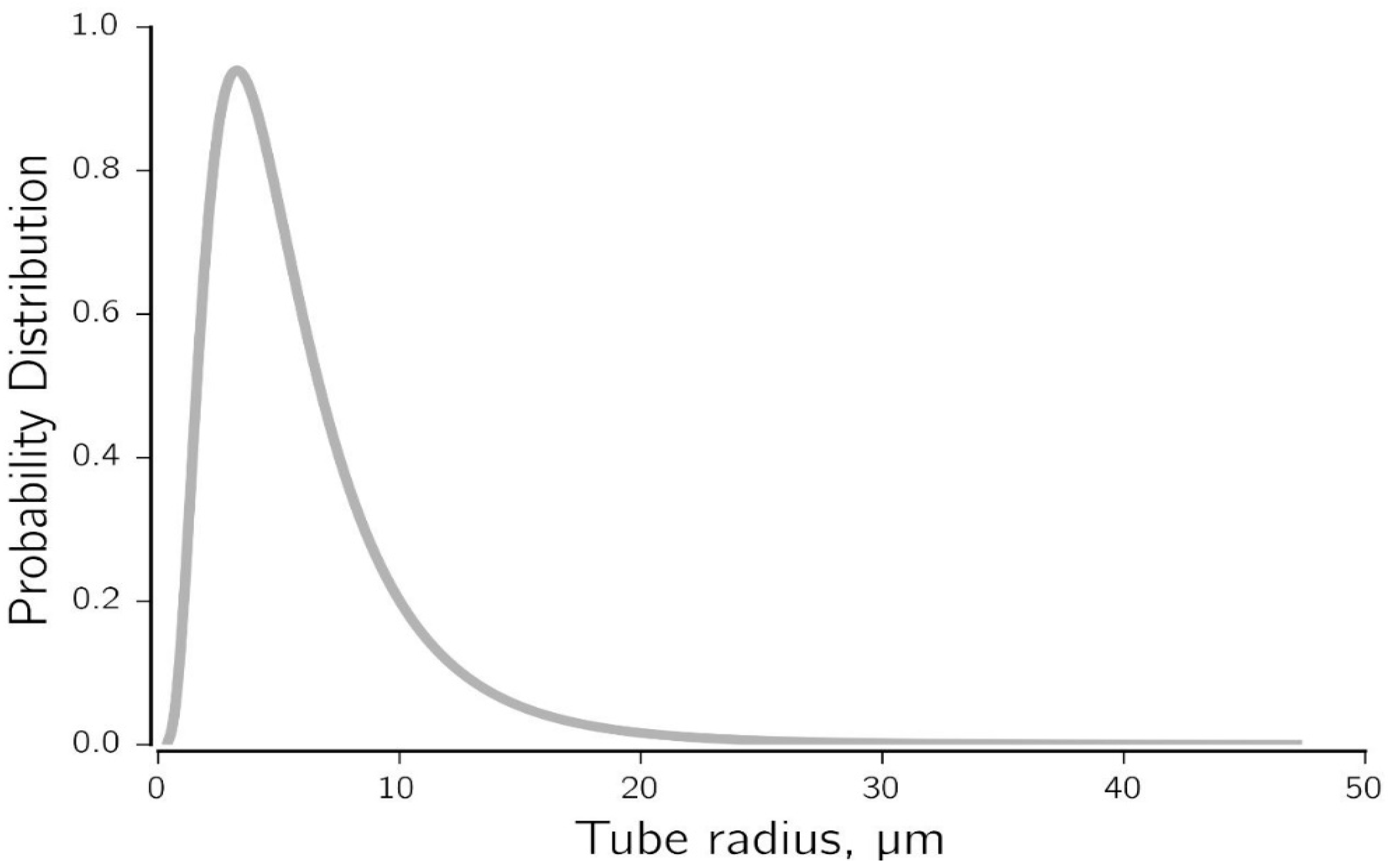
3.3. Number of Capillary Tubes and Distribution of Precipitated Salt
3.4. Implementation of the Model
- The number and distribution of capillary tubes for given properties of the core was calculated from Equation (12) by specifying the average pore radius.
- The initial permeability of the core was calculated from Equation (10) by setting . The calculated permeability was then compared to the measured permeability of the core. If the agreement is not favourable, Step 1 is optimized until the agreement is acceptable.
- base case is established by exposing the tubes to fine particles with average particle size . For simplicity, we assumed that the flowing fluid contains particles of the same size and that each capillary tube has equal chance of being exposed to the particles. Tubes with a jamming ratio greater than 1.0 are isolated and the permeability of the core is recalculated from Equation (10).
- The relative injectivity change, , induced by fines entrapment is calculated from Equation (4).
- For given properties of the saturating formation brine, the thickness of precipitated salt in each capillary tube is computed from Equations (13) and (14).
- Steps 3 and 4 are then repeated to calculate the combined effect of salt precipitation and fines mobilization.
4. Results and Discussion
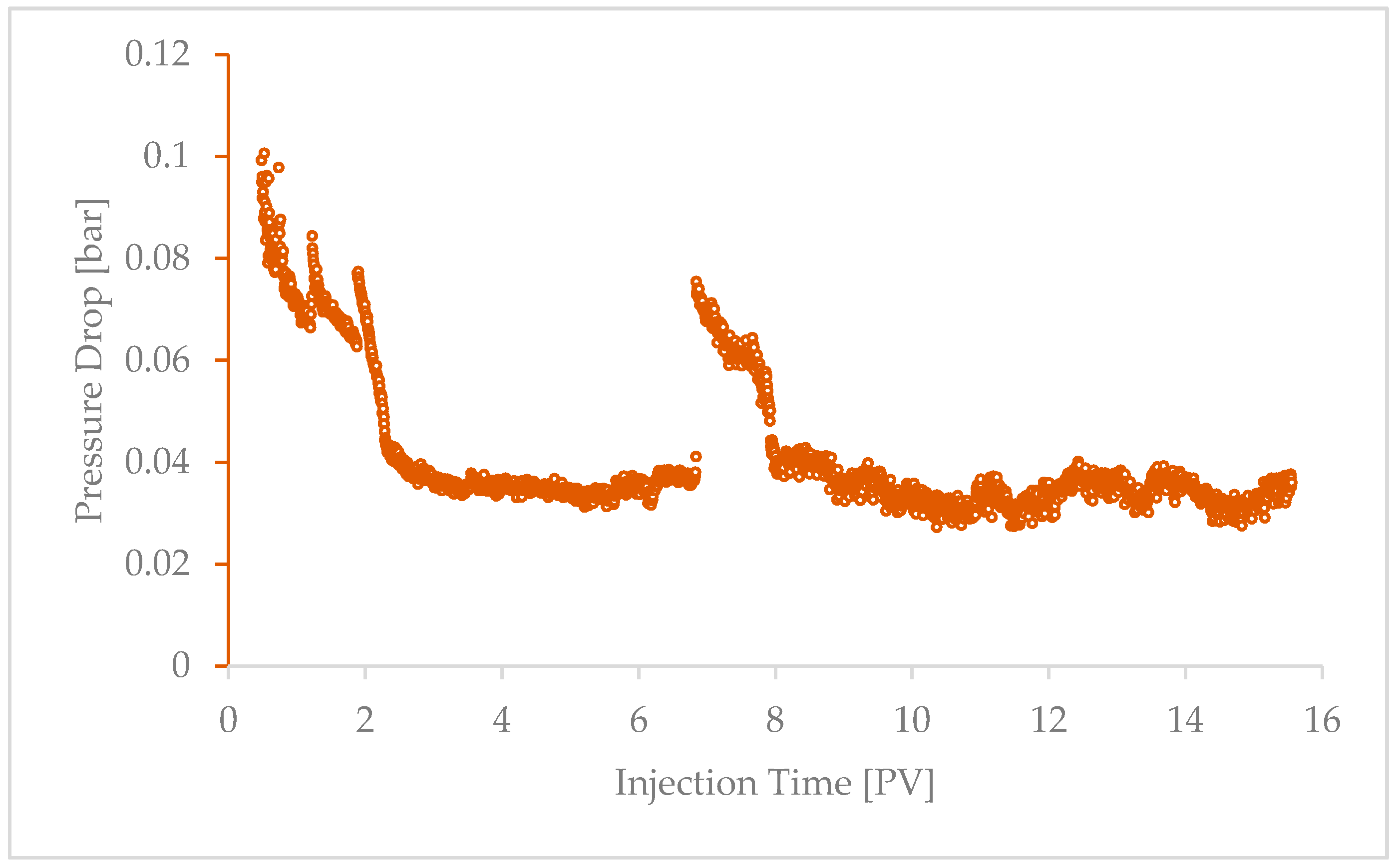
4.1. Injectivity Impairment Induced by Fines Mobilization
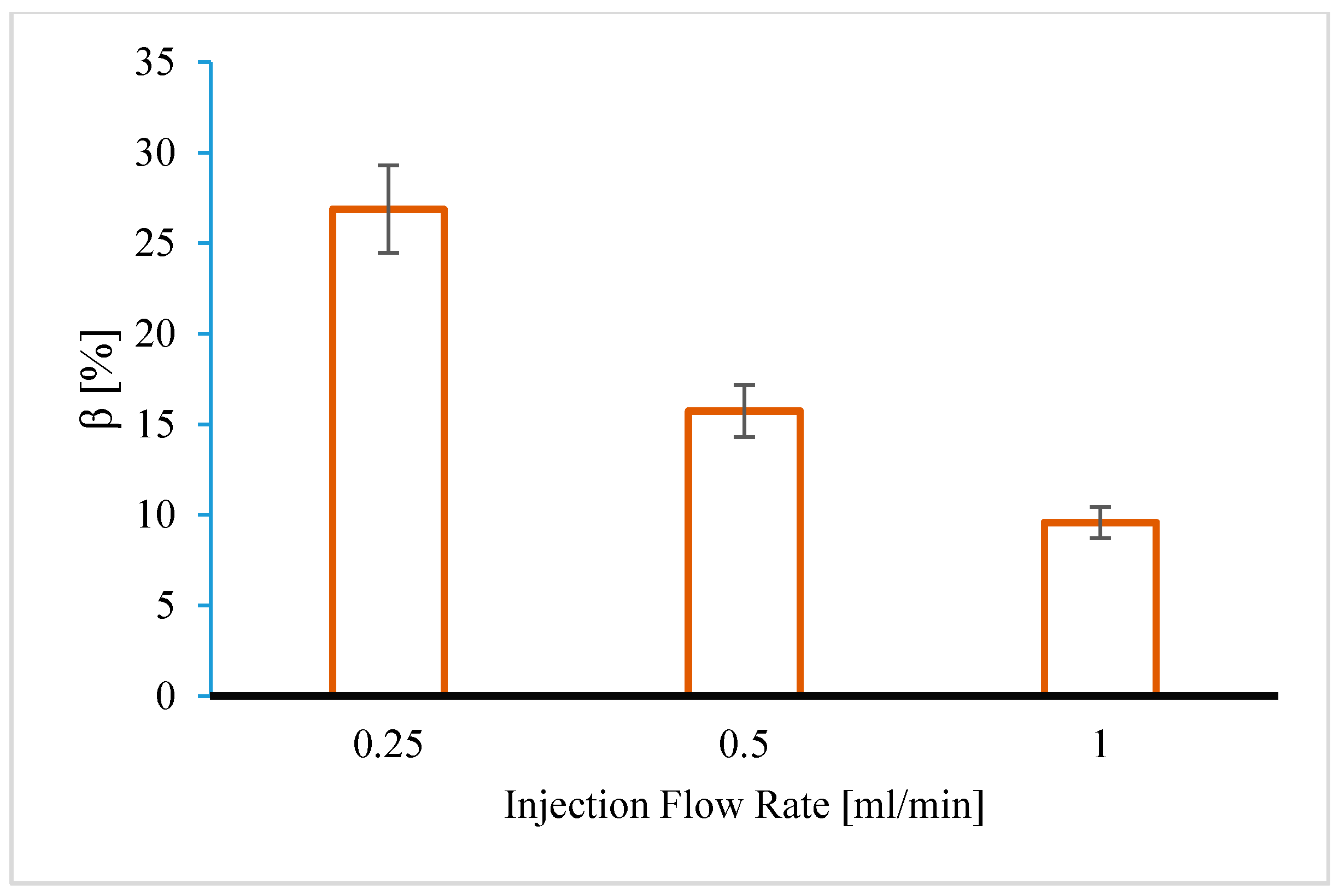
4.1.1. Effect of Injection Flow Rate
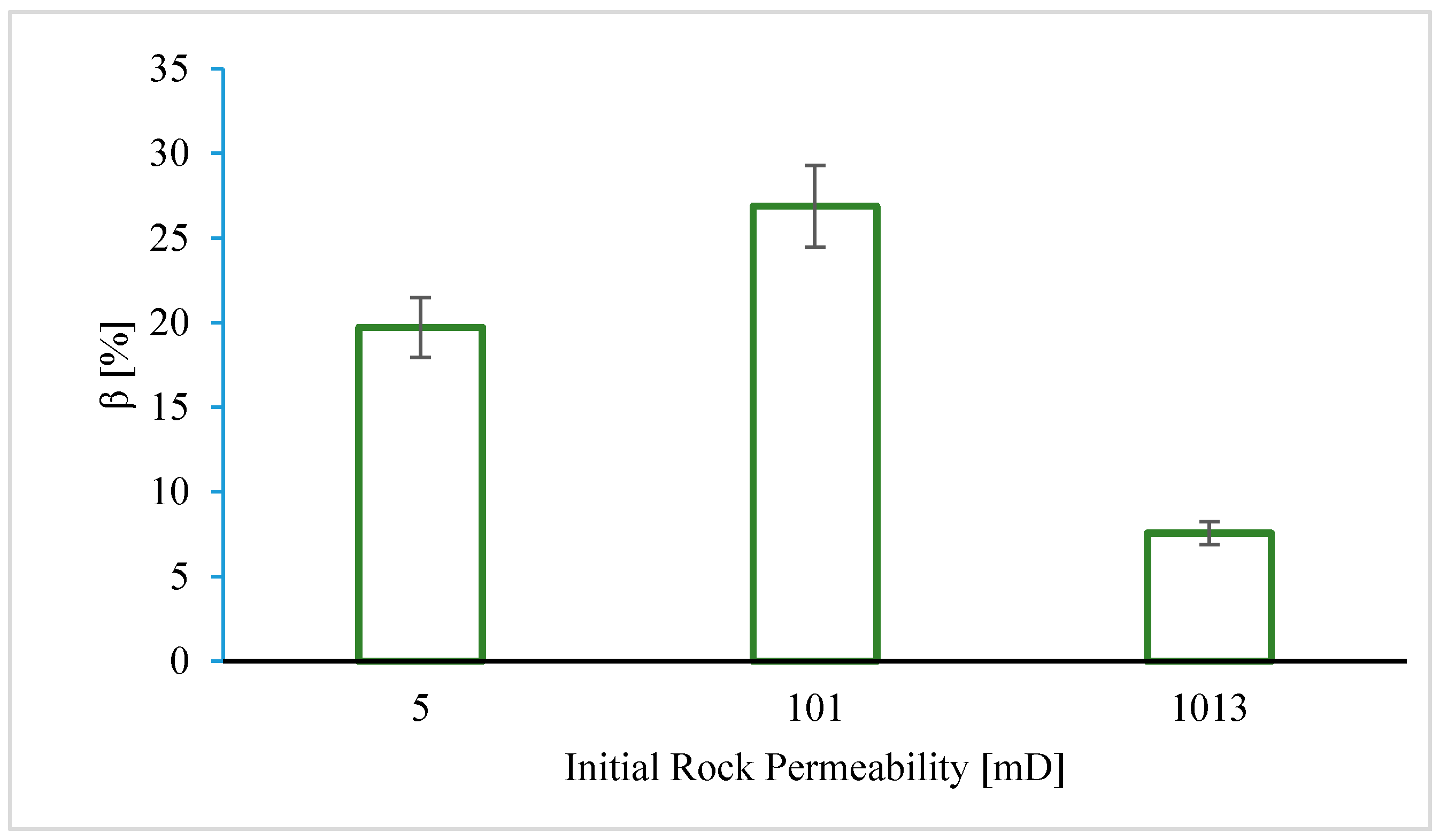
4.1.2. Effect of Initial Core Permeability
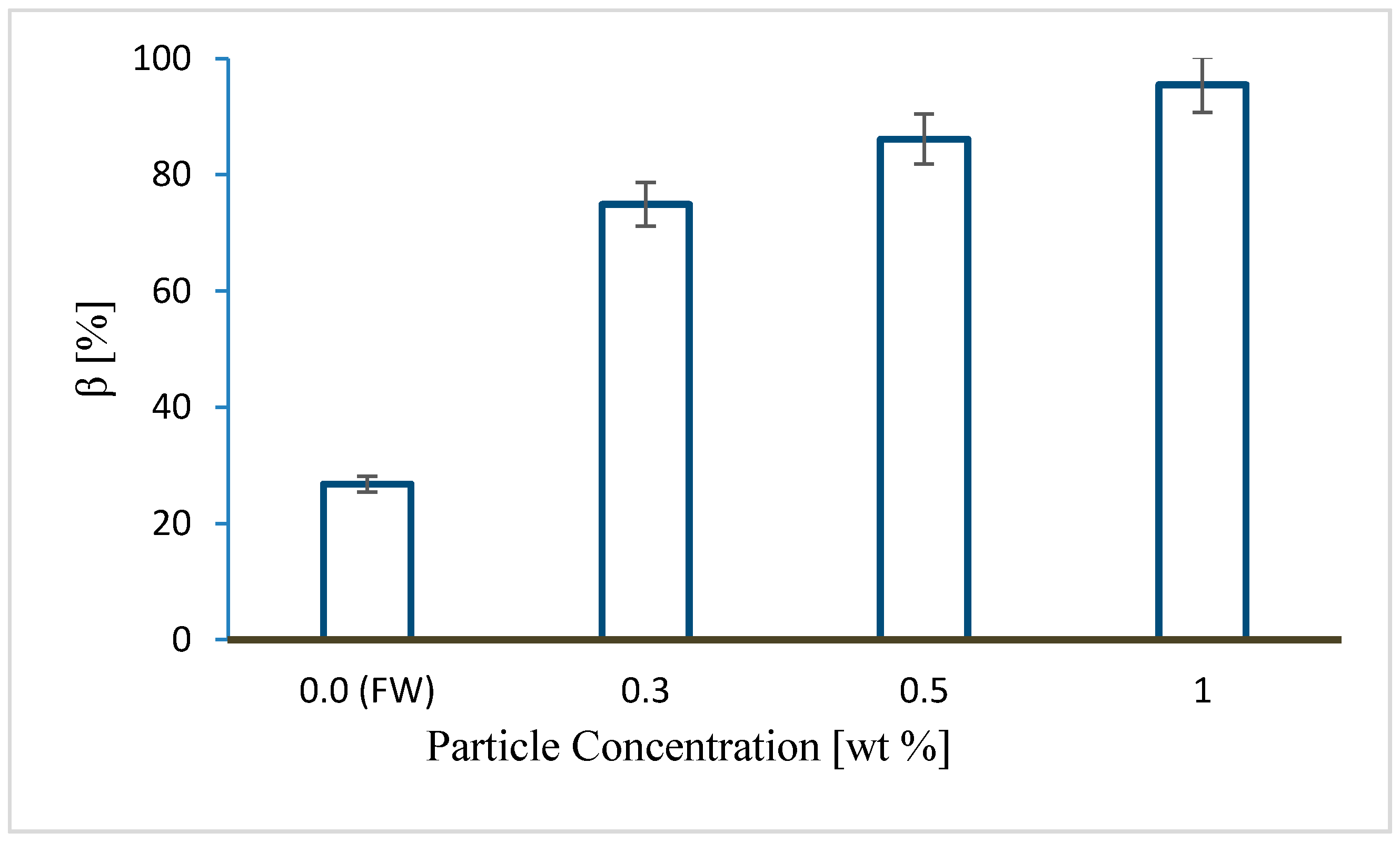
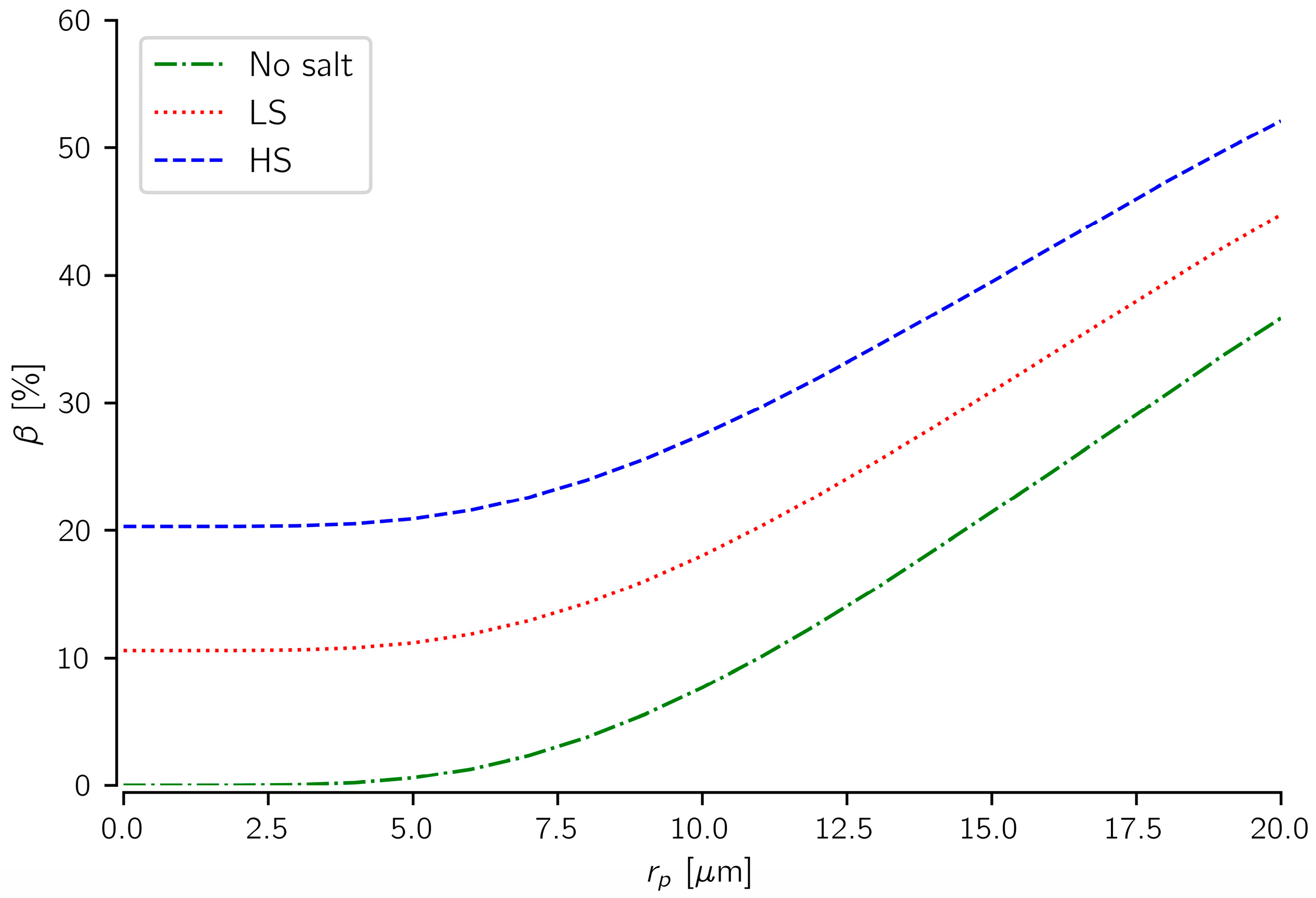
4.2. Comparing the Effects of Salt Precipitation and Fines Mobilization
4.3. Coupled Effect of Fines Mobilization and Salt Precipitation
5. Conclusions
- Fines mobilization could induce severe CO2 injectivity impairment. The experimental results suggest up to about a 26% injectivity impairment could be induced by fines migration.
- Under linear flow conditions, CO2 injectivity impairment induced by fines mobilization could be comparable to injectivity impairment caused by salt precipitation. About a 0.3 wt % particle concentration in the pore fluid induced over twofold injectivity impairment compared to about 10 wt % of total dissolved salt in the formation water.
- The findings also suggest that salt precipitation could compound the impact of fines mobilization on CO2 injectivity. The precipitated salts reduce the pore spaces, making them more susceptible to particle plugging and injectivity reduction.
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| CCUS | CO2 Capture, Utilization and Storage |
| FW | Formation Water |
| I | Injectivity |
| HS | High Salinity brine |
| LS | Low Salinity brine |
References
- Miri, R. Effects of CO2-Brine-Rock Interactions on CO2 Injectivity—Implications for CCS. Ph.D. Thesis, Uniersity of Oslo, Oslo, Norway, July 2015. [Google Scholar]
- Lombard, J.M.; Azaroual, M.; Pironon, J.; Broseta, D.; Egermann, P.; Munier, G.; Mouronval, G. CO2 injectivity in geological storages: An overview of program and results of the GeoCarbone-Injectivity project. Oil Gas Sci. Technol. 2010, 65, 533–539. [Google Scholar] [CrossRef]
- Miri, R.; Hellevang, H. Salt precipitation during CO2 storage—A review. Int. J. Greenh. Gas Control 2016, 51, 136–147. [Google Scholar] [CrossRef]
- Bacci, G.; Durucan, S.; Korre, A. Experimental and Numerical Study of the Effects of Halite Scaling on Injectivity and Seal Performance During CO2 Injection in Saline Aquifers. Energy Procedia 2013, 37, 3275–3282. [Google Scholar] [CrossRef]
- Muller, N.; Qi, R.; Mackie, E.; Pruess, K.; Blunt, M.J. CO2 injection impairment due to halite precipitation. Energy Procedia 2009, 1, 3507–3514. [Google Scholar] [CrossRef]
- Peysson, Y.; André, L.; Azaroual, M. Well injectivity during CO2 storage operations in deep saline aquifers-Part 1: Experimental investigation of drying effects, salt precipitation and capillary forces. Int. J. Greenh. Gas Control 2014, 22, 291–300. [Google Scholar] [CrossRef]
- Zuluaga, E.; Muñoz, N.I.; Obando, G. An Experimental Study to Evaluate Water Vaporisation and Formation Damage Caused by Dry Gas Flow Through Porous Media. In Proceedings of the International Symposium on Oilfield Scale, Aberdeen, UK, 30–31 January 2001. [Google Scholar]
- Kleinitz, W.; Dietzsch, G.; Köhler, M. Halite scale formation in gas-producing wells. Chem. Eng. Res. Des. 2003, 81, 352–358. [Google Scholar] [CrossRef]
- Jasinski, R.; Sablerolle, W.; Amory, M. ETAP: Scale Prediction and Contol for the Heron Cluster. In Proceedings of the SPE Annual Technical Conference and Exhibition, San Antonio, TX, USA, 5–8 October 1997. [Google Scholar]
- Golghanddashti, H.; Saadat, M.; Abbasi, S.; Shahrabadi, A. Experimental investigation of water vaporization and its induced formation damage associated with underground gas storage. J. Porous Media 2013, 16. [Google Scholar] [CrossRef]
- Place, M.C., Jr.; Smith, J.T. An Unusual Case of Salt Plugging in a High-Pressure Sour Gas Well. In Proceedings of the 59th Annual Technical Conference and Exhibition, Houston, TX, USA, 16–19 September 1984; p. 13. [Google Scholar]
- Baumann, G.; Henninges, J.; De Lucia, M. Monitoring of saturation changes and salt precipitation during CO2 injection using pulsed neutron-gamma logging at the Ketzin pilot site. Int. J. Greenh. Gas Control 2014, 28, 134–146. [Google Scholar] [CrossRef]
- Grude, S.; Landrø, M.; Dvorkin, J. Pressure effects caused by CO2 injection in the Tubåen Fm., the Snøhvit field. Int. J. Greenh. Gas Control 2014, 27, 178–187. [Google Scholar] [CrossRef]
- Bacci, G.; Korre, A.; Durucan, S. Experimental investigation into salt precipitation during CO2 injection in saline aquifers. Energy Procedia 2011, 4, 4450–4456. [Google Scholar] [CrossRef]
- Kim, M.; Sell, A.; Sinton, D. Aquifer-on-a-Chip: Understanding pore-scale salt precipitation dynamics during CO2 sequestration. Lab Chip 2013, 13, 2508–2518. [Google Scholar] [CrossRef] [PubMed]
- André, L.; Peysson, Y.; Azaroual, M. Well injectivity during CO2 storage operations in deep saline aquifers—Part 2: Numerical simulations of drying, salt deposit mechanisms and role of capillary forces. Int. J. Greenh. Gas Control 2014, 22, 301–312. [Google Scholar] [CrossRef]
- Tang, Y.; Yang, R.; Du, Z.; Zeng, F. Experimental study of formation damage caused by complete water vaporization and salt precipitation in sandstone reservoirs. Transp. Porous Media 2015, 107, 205–218. [Google Scholar] [CrossRef]
- Sokama-Neuyam, Y.A.; Ursin, J. Experimental and Theoretical Investigations of CO2 injectivity. AGH Drilling Oil Gas 2016, 33, 245–257. [Google Scholar] [CrossRef]
- Giorgis, T.; Carpita, M.; Battistelli, A. 2D modeling of salt precipitation during the injection of dry CO2 in a depleted gas reservoir. Energy Convers. Manag. 2007, 48, 1816–1826. [Google Scholar] [CrossRef]
- Hurter, S.; Berge, J.; Labregere, D. Simulations for CO2 injection projects with Compositional Simulator. In Proceedings of the Offshore Europe, Aberdeen, UK, 4–7 September 2007. [Google Scholar]
- Pruess, K. Formation dry-out from CO2 injection into saline aquifers: 2. analytical model for salt precipitation. Water Resour. Res. 2009, 45, 1–6. [Google Scholar] [CrossRef]
- Zeidouni, M.; Pooladi-Darvish, M.; Keith, D. Analytical solution to evaluate salt precipitation during CO2 injection in saline aquifers. Int. J. Greenh. Gas Control 2009, 3, 600–611. [Google Scholar] [CrossRef]
- Roels, S.M.; Ott, H.; Zitha, P.L.J. μ-CT analysis and numerical simulation of drying effects of CO2 injection into brine-saturated porous media. Int. J. Greenh. Gas Control 2014, 27, 146–154. [Google Scholar] [CrossRef]
- Kleinitz, W.; Koehler, M.; Dietzsch, G.; Gmbh, P.E. The precipitation of salt in gas producing wells. In Proceedings of the SPE European Formation Damage Conference, The Hague, The Netherlands, 21–22 May 2001; pp. 1–7. [Google Scholar]
- Pruess, K.; Muller, N. Formation dry-out from CO2 injection into saline aquifers: 1. Effects of solids precipitation and their mitigation. Water Resour. Res. 2009, 45, 1–11. [Google Scholar] [CrossRef]
- Patton, J.T.; Phelan, P.; Holbrook, S. CO2 Formation Damage Study—First Annual Report; U.S. Department of Energy: Las Cruces, NM, USA, 1981.
- Sayegh, S.G.; Krause, F.F.; Girard, M.; DeBree, C. Rock/Fluid interactions of carbonated brines in a sandstone reservoir: Pembina Cardium, Alberta, Canada. SPE Form. Eval. 1990, 5, 399–405. [Google Scholar] [CrossRef]
- Tobergte, D.R.; Curtis, S. Experimental perspectives of mineral dissolution and precipitation due to carbon dioxide-water-rock interactions. J. Chem. Inf. Model. 2013, 53, 1689–1699. [Google Scholar]
- Khilar, K.C.; Fogler, H.S. Migrations of Fines in Porous Media; Kluwer Academic Publisher: Dordrecht, The Netherlands, 1998; Volume 12. [Google Scholar]
- Muecke, T.W. Formation fines and factors controlling their movement in porous media. J. Pet. Technol. 1979, 31, 144–150. [Google Scholar] [CrossRef]
- Khilar, K.C.; Fogler, H.S. Water Sensitivity of Sandstone. Soc. Pet. Eng. J. 1983, 23, 55–64. [Google Scholar] [CrossRef]
- Gruesbeck, C.; Collins, R.E. Entrainment and Deposition of Fine Particles in Porous Media. Soc. Pet. Eng. J. 1982, 22, 847–856. [Google Scholar] [CrossRef]
- Sarkar, A.; Sharma, M. Fines Migration in Two-Phase Flow. J. Pet. Technol. 1990, 42, 646–652. [Google Scholar] [CrossRef]
- Sharma, M.M.; Yortsos, Y.C. Fines migration in porous media. AIChE J. 1987, 33, 1654–1662. [Google Scholar] [CrossRef]
- Civan, F. Reservoir Formation Damage: Fundamentals, Modelling, Assessment, and Mitigation; Gulf Professional Publishing: Waltham, MA, USA, 2007. [Google Scholar]
- Aji, K. The Experimental and Theoretical Study of Fines Migration in Porous Media under Particle-rock Repulsion and Attraction. Ph.D. Thesis, University of Adelaide, Adelaide, Australia, October 2014. [Google Scholar]
- Peksa, A.E.; Wolf, K.H.A.A.; Zitha, P.L.J. Bentheimer sandstone revisited for experimental purposes. Mar. Pet. Geol. 2015, 67, 701–719. [Google Scholar] [CrossRef]
- Mohammed, A.S.; Ayoub, A.; Massoud, M. Effects of Low Salinity Water Ion Composition on Wettability Alteration in Sandstone Reservoir Rock: A Laboratory Investigation. J. Nat. Sci. Res. 2014, 4, 34–41. [Google Scholar]
- Dullien, F.A.L.; Dhawan, G.K. Characterization of pore structure by a combination of quantitative photomicrography and mercury porosimetry. J. Colloid Interface Sci. 1974, 47, 337–349. [Google Scholar] [CrossRef]
- Fjelde, I.; Omekeh, A.V.; Sokama-Neuyam, Y.A. Low Salinity Water Flooding: Effect Of Crude Oil Composition. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 12–16 April 2014. [Google Scholar]
- Portier, S.; Rochelle, C. Modelling CO2 solubility in pure water and NaCl-type waters from 0 to 300 C and from 1 to 300 bar Application to the Utsira Formation at Sleipner. Chem. Geol. 2005, 217, 187–199. [Google Scholar] [CrossRef]
- Fogler, H.S. The Existence of a Critical Salt Concentration for Particle Release. J. Colloid Interface Sci. 1984, 101, 214–224. [Google Scholar]
- Shi, J.Q.; Xue, Z.; Durucan, S. Supercritical CO2 core flooding and imbibition in Berea sandstone—CT imaging and numerical simulation. Energy Procedia 2011, 4, 5001–5008. [Google Scholar] [CrossRef]
- Lin, C.; Slattery, J.C. Three-dimensional, randomized, network model for two-phase flow through porous media. AIChE J. 1982, 28, 311–324. [Google Scholar] [CrossRef]
- Yanuka, M.; Dullien, F.A.L.; Elrick, D.E. Serial sectioning and digitization of porous media for two-and three-dimensional analysis and reconstruction. J. Microsc. 1984, 135, 159–168. [Google Scholar] [CrossRef]
- Nelson, P.H. Pore-throat sizes in sandstones, tight sandstones, and shales. Am. Assoc. Pet. Geol. Bull. 2009, 93, 329–340. [Google Scholar] [CrossRef]
- Sen, T.K.; Khilar, K.C. Review on subsurface colloids and colloid-associated contaminant transport in saturated porous media. Adv. Colloid Interface Sci. 2006, 119, 71–96. [Google Scholar]
| Rock | Permeability (mD) | Porosity (%) |
|---|---|---|
| Berea | 90–120 | 17–19 |
| Bentheimer | 1200–2000 | 22–24 |
| Bandera | 4–10 | 19–21 |
| Properties | Data |
|---|---|
| Particle size (µm) | 0.08 |
| Al2O3 content (%) | 39–41 |
| Viscosity (mPa/s) | <90 |
| pH | 6.0–9.0 |
| Density (g/cc) | 1.39 |
| Element | wt % |
|---|---|
| O | 33.56 |
| Fe | 7.78 |
| Ni | 5.02 |
| Na | 17.53 |
| Mg | 0.74 |
| Al | 2.53 |
| Si | 0.35 |
| Cl | 29.79 |
| Ca | 2.52 |
| Co | 0.17 |
| Total | 100.00 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sokama-Neuyam, Y.A.; Forsetløkken, S.L.; Lien, J.-e.; Ursin, J.R. The Coupled Effect of Fines Mobilization and Salt Precipitation on CO2 Injectivity. Energies 2017, 10, 1125. https://doi.org/10.3390/en10081125
Sokama-Neuyam YA, Forsetløkken SL, Lien J-e, Ursin JR. The Coupled Effect of Fines Mobilization and Salt Precipitation on CO2 Injectivity. Energies. 2017; 10(8):1125. https://doi.org/10.3390/en10081125
Chicago/Turabian StyleSokama-Neuyam, Yen Adams, Sindre Langås Forsetløkken, Jhon-eirik Lien, and Jann Rune Ursin. 2017. "The Coupled Effect of Fines Mobilization and Salt Precipitation on CO2 Injectivity" Energies 10, no. 8: 1125. https://doi.org/10.3390/en10081125
APA StyleSokama-Neuyam, Y. A., Forsetløkken, S. L., Lien, J.-e., & Ursin, J. R. (2017). The Coupled Effect of Fines Mobilization and Salt Precipitation on CO2 Injectivity. Energies, 10(8), 1125. https://doi.org/10.3390/en10081125




