ITO-Free Transparent Organic Solar Cell with Distributed Bragg Reflector for Solar Harvesting Windows
Abstract
:1. Introduction
2. Results
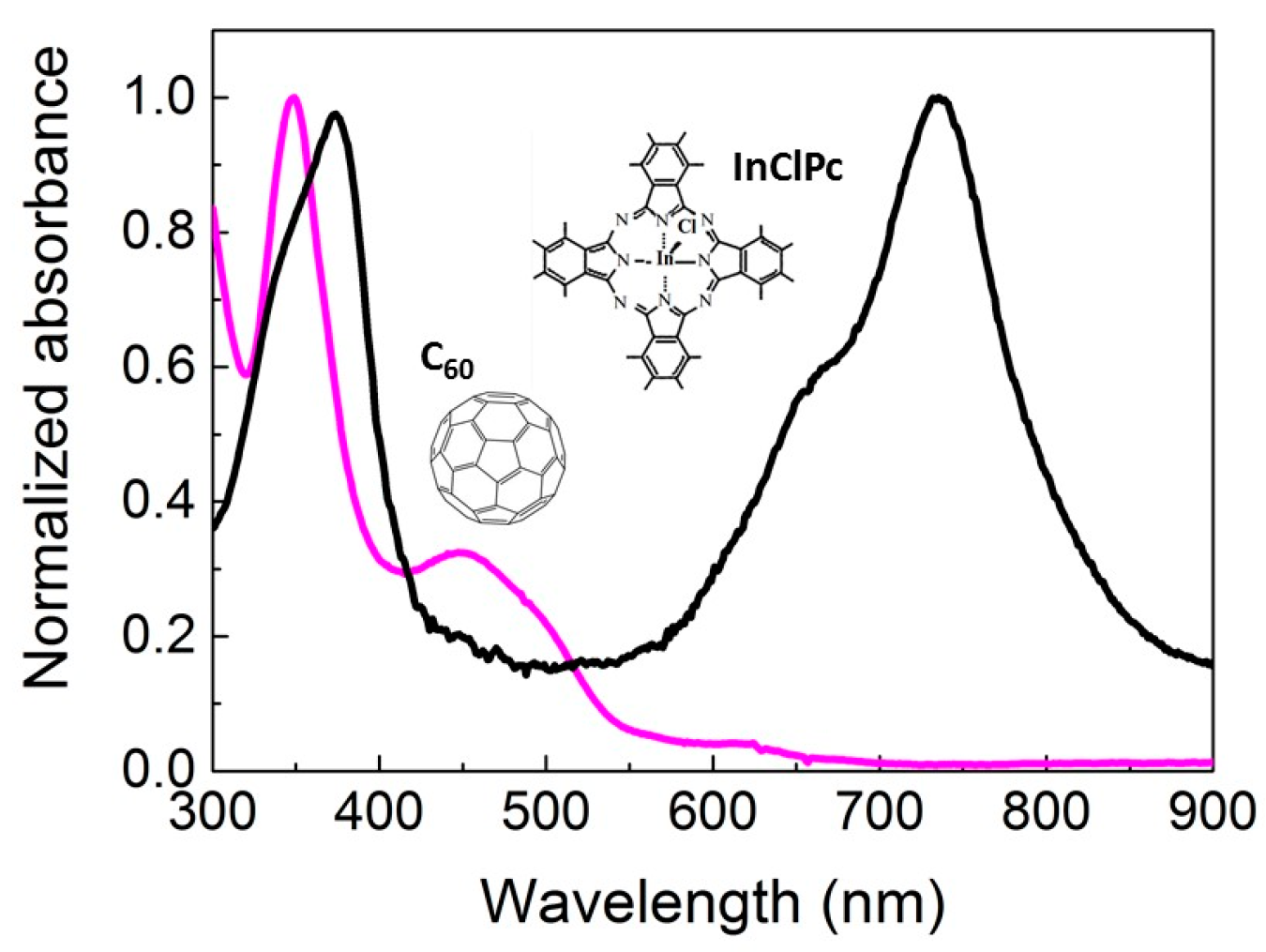
2.1. Donor and Acceptor Layers for the Organic Solar Cell
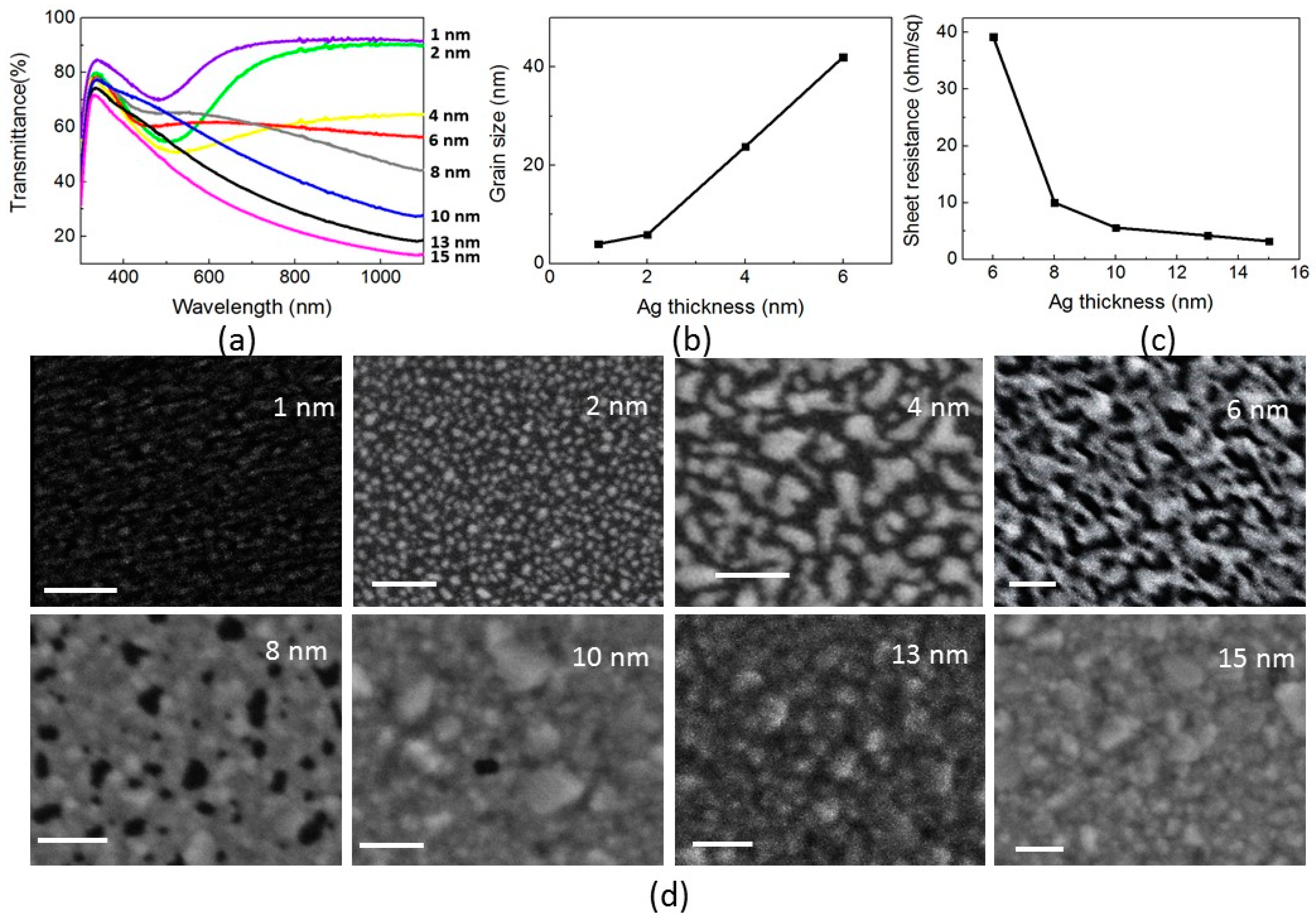
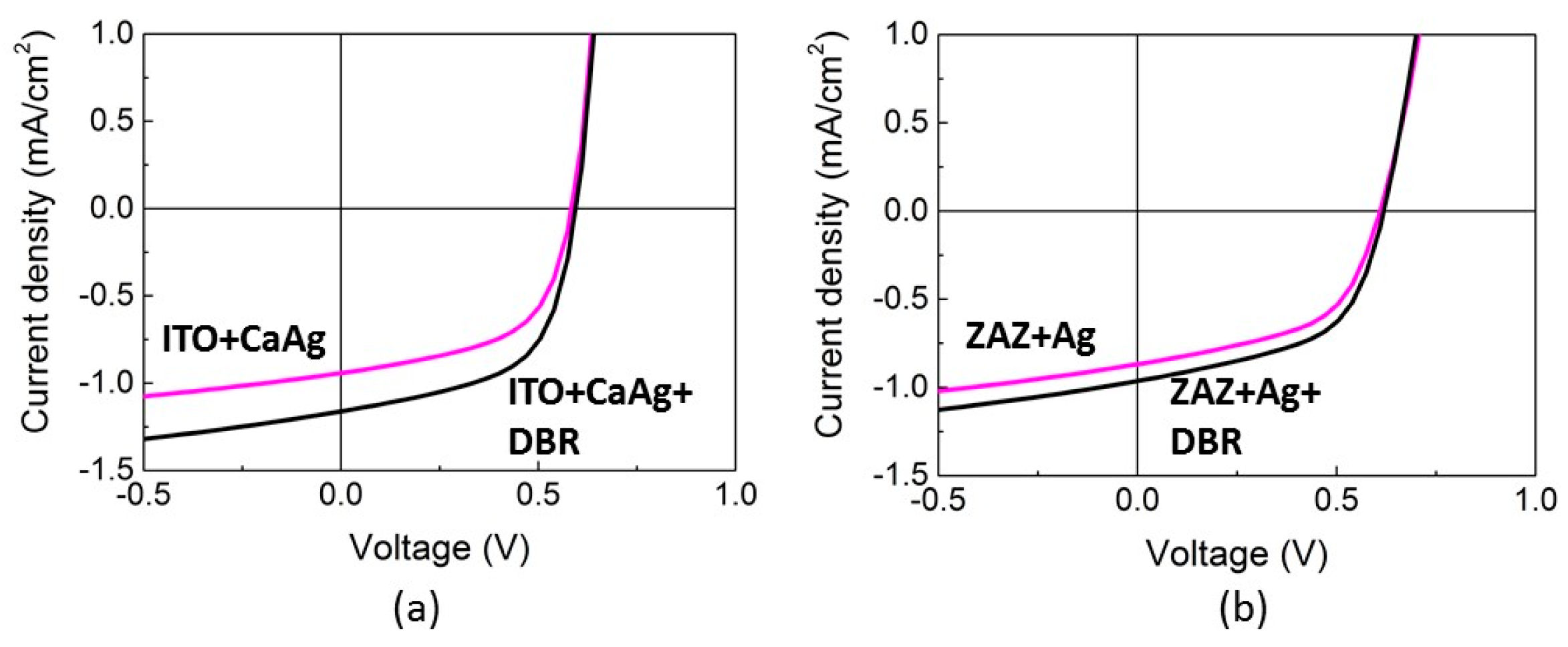
2.2. Transparent Bottom Electrode
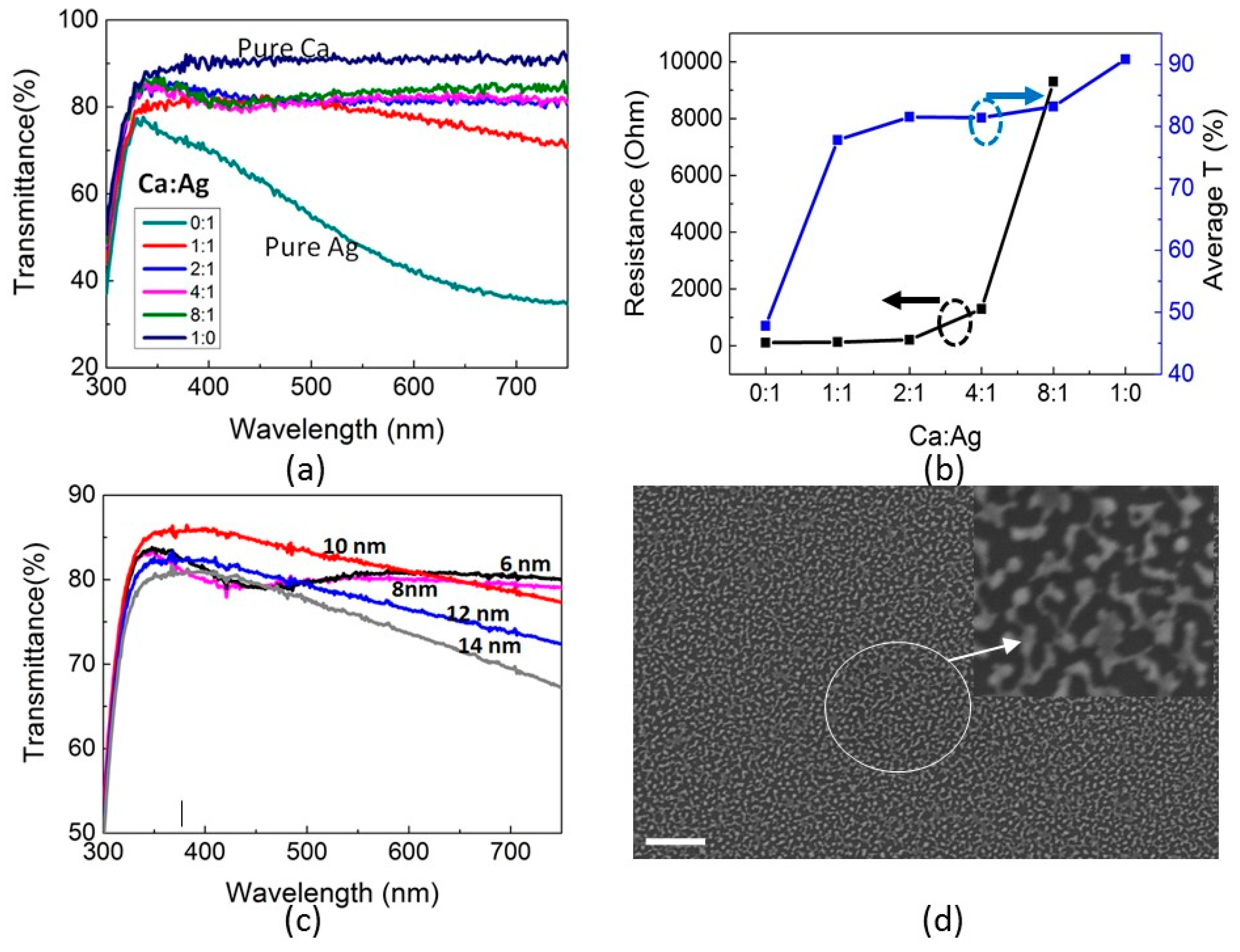
2.3. Transparent Top Electrode
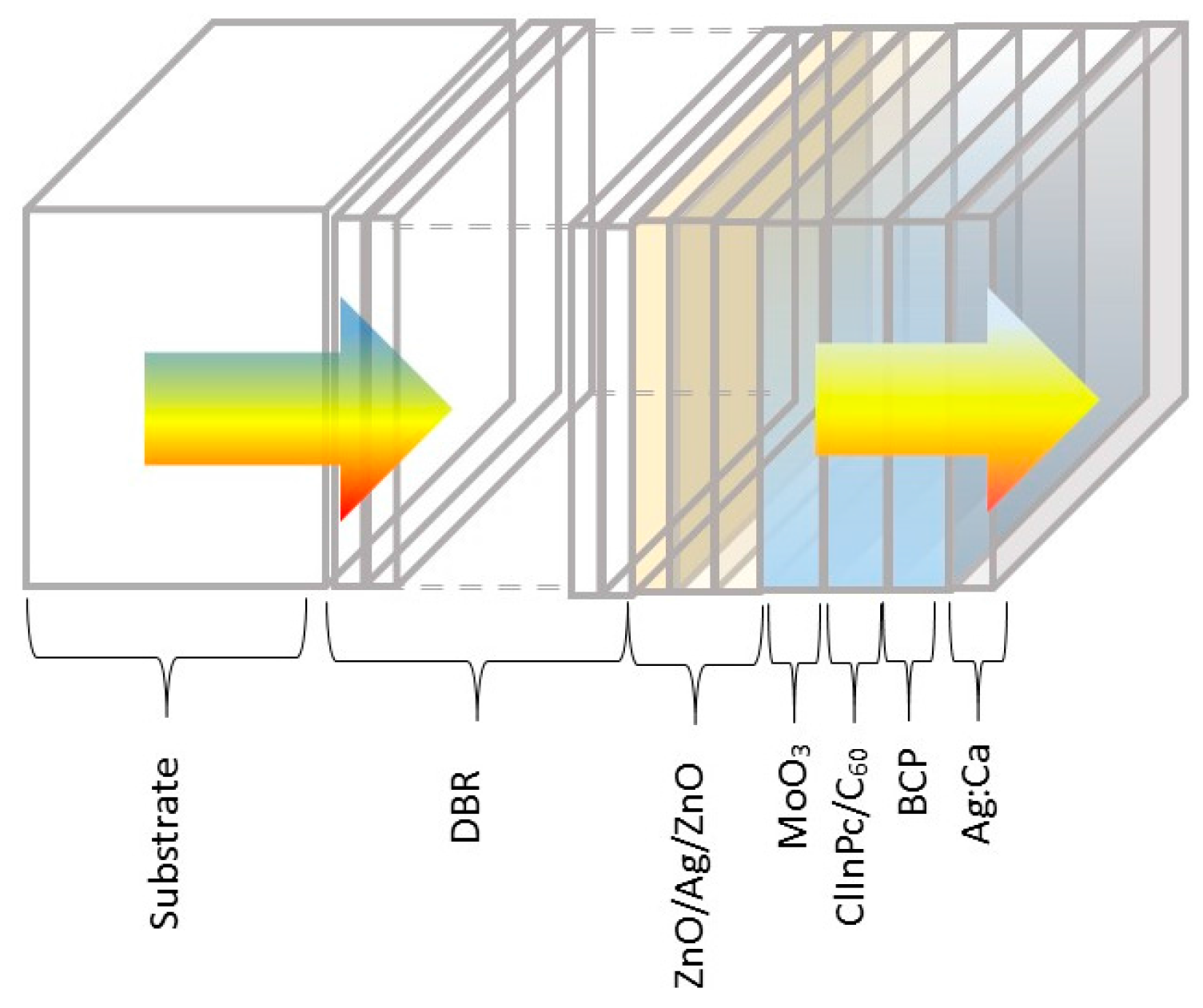
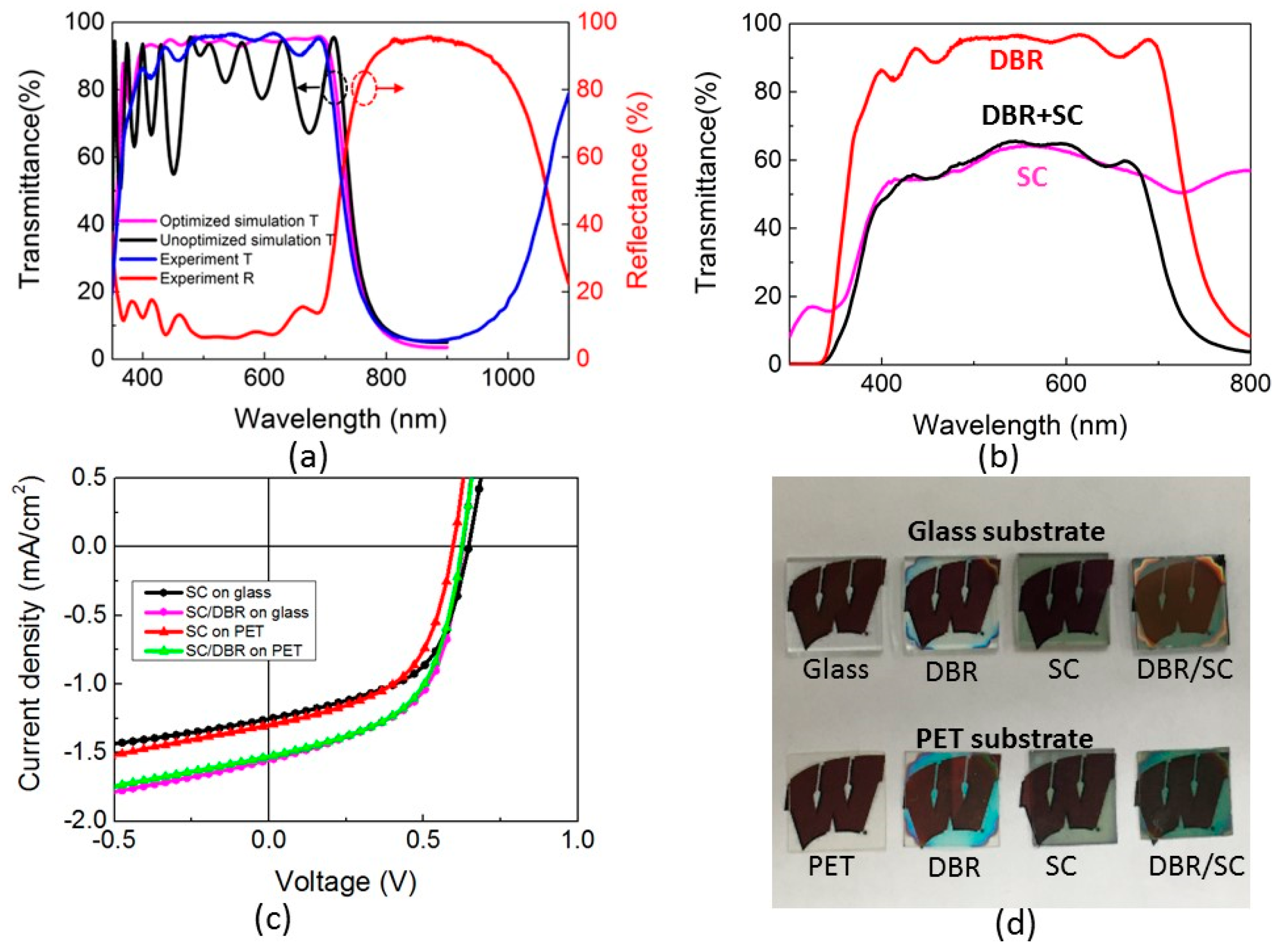
2.4. Transparent Organic Solar Cells
2.5. Adding a Distributed Bragg Reflector to Improve Light Collection and Recycling
3. Discussion
4. Materials and Methods
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tang, C.W. Two-layer organic photovoltaic cell. Appl. Phys. Lett. 1986, 48, 183–185. [Google Scholar] [CrossRef]
- Li, G.; Zhu, R.; Yang, Y. Polymer solar cells. Nat. Photonics 2012, 6, 153–161. [Google Scholar] [CrossRef]
- Service, R. Solar energy. Outlook brightens for plastic solar cells. Science 2011, 332, 293. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Zhang, L.; Andrew, T.L. High open-circuit voltage, high fill factor single-junction organic solar cells. Appl. Phys. Lett. 2014, 105, 083304. [Google Scholar] [CrossRef]
- Chen, C.-C.; Dou, L.; Zhu, R.; Chung, C.-H.; Song, T.-B.; Zheng, Y.B.; Hawks, S.; Li, G.; Weiss, P.S.; Yang, Y. Visibly transparent polymer solar cells produced by solution processing. ACS Nano 2012, 6, 7185–7190. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Kumar, A.; Yang, Y. Polarizing organic photovoltaics. Adv. Mater. 2011, 23, 4193–4198. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Meek, G.A.; Levine, B.G.; Lunt, R.R. Near-infrared harvesting transparent luminescent solar concentrators. Adv. Opt. Mater. 2014, 2, 606–611. [Google Scholar] [CrossRef]
- Starr, C.; Evers, C.; Starr, L. Biology: Concepts and Applications without Physiology; Cengage Learning: Boston, MA, USA, 2010; ISBN 0538739258. [Google Scholar]
- Shtein, M. Thin metal films as simple transparent conductors. Int. Soc. Opt. Photonics Newsroom 2009, 10. [Google Scholar] [CrossRef]
- Kim, Y.H.; Sachse, C.; Machala, M.L.; May, C.; Müller-Meskamp, L.; Leo, K. Highly conductive PEDOT: PSS electrode with optimized solvent and thermal post-treatment for ITO-free organic solar cells. Adv. Funct. Mater. 2011, 21, 1076–1081. [Google Scholar] [CrossRef]
- Leem, D.S.; Edwards, A.; Faist, M.; Nelson, J.; Bradley, D.D.; de Mello, J.C. Efficient organic solar cells with solution-processed silver nanowire electrodes. Adv. Mater. 2011, 23, 4371–4375. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhi, L.; Müllen, K. Transparent, conductive graphene electrodes for dye-sensitized solar cells. Nano Lett. 2008, 8, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, H.; Flügge, H.; Winkler, T.; Bülow, T.; Riedl, T.; Kowalsky, W. Efficient semitransparent inverted organic solar cells with indium tin oxide top electrode. Appl. Phys. Lett. 2009, 94, 243302. [Google Scholar] [CrossRef]
- Zakhidov, A.A.; Lee, J.K.; Fong, H.H.; DeFranco, J.A.; Chatzichristidi, M.; Taylor, P.G.; Ober, C.K.; Malliaras, G.G. Hydrofluoroethers as orthogonal solvents for the chemical processing of organic electronic materials. Adv. Mater. 2008, 20, 3481–3484. [Google Scholar] [CrossRef]
- Wu, H.; Kong, D.; Ruan, Z.; Hsu, P.-C.; Wang, S.; Yu, Z.; Carney, T.J.; Hu, L.; Fan, S.; Cui, Y. A transparent electrode based on a metal nanotrough network. Nat. Nanotechnol. 2013, 8, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Sahu, D.; Lin, S.-Y.; Huang, J.-L. ZnO/Ag/ZnO multilayer films for the application of a very low resistance transparent electrode. Appl. Surf. Sci. 2006, 252, 7509–7514. [Google Scholar] [CrossRef]
- Sahu, D.; Huang, J.-L. High quality transparent conductive ZnO/Ag/ZnO multilayer films deposited at room temperature. Thin Solid Films 2006, 515, 876–879. [Google Scholar] [CrossRef]
- El Hajj, A.; Lucas, B.; Chakaroun, M.; Antony, R.; Ratier, B.; Aldissi, M. Optimization of ZnO/Ag/ZnO multilayer electrodes obtained by ion beam sputtering for optoelectronic devices. Thin Solid Films 2012, 520, 4666–4668. [Google Scholar] [CrossRef]
- Sahu, D.; Huang, J.-L. Design of ZnO/Ag/ZnO multilayer transparent conductive films. Mater. Sci. Eng. B 2006, 130, 295–299. [Google Scholar] [CrossRef]
- Xu, W.-F.; Pan, M.-Y.; Fu, P.-H.; Li, S.-W.; Huang, D.-W.; Wei, P.-K. Efficiency enhancement of top-illuminated ITO-free organic solar cells using plasmonic-assisted nanostructured reflective electrodes. J. Mater. Chem. C 2015, 3, 9131–9136. [Google Scholar] [CrossRef]
- Schulze, K.; Uhrich, C.; Schüppel, R.; Leo, K.; Pfeiffer, M.; Brier, E.; Reinold, E.; Baeuerle, P. Efficient vacuum-deposited organic solar cells based on a new low-bandgap oligothiophene and fullerene C60. Adv. Mater. 2006, 18, 2872–2875. [Google Scholar] [CrossRef]
- Zhang, L.; Andrew, T.L. Improved photovoltaic response of a near-infrared sensitive solar cell by a morphology-controlling seed layer. Org. Electron. 2016, 33, 135–141. [Google Scholar] [CrossRef]
- Liu, X.; Cai, X.; Qiao, J.; Mao, J.; Jiang, N. The design of ZnS/Ag/ZnS transparent conductive multilayer films. Thin Solid Films 2003, 441, 200–206. [Google Scholar] [CrossRef]
- Cattin, L.; Bernède, J.; Morsli, M. Toward indium-free optoelectronic devices: Dielectric/metal/dielectric alternative transparent conductive electrode in organic photovoltaic cells. Phys. Status Solidi A 2013, 210, 1047–1061. [Google Scholar] [CrossRef]
- Jia, J.; Zhou, P.; Xie, H.; You, H.; Li, J.; Chen, L. Study of optical and electrical properties of TiO2/Ag/TiO2 multilayers. J. Korean Phys. Soc. 2004, 44, 717–721. [Google Scholar] [CrossRef]
- Fan, J.C.; Bachner, F.J.; Foley, G.H.; Zavracky, P.M. Transparent heat-mirror films of TiO2/Ag/TiO2 for solar energy collection and radiation insulation. Appl. Phys. Lett. 1974, 25, 693–695. [Google Scholar] [CrossRef]
- Choi, K.-H.; Nam, H.-J.; Jeong, J.-A.; Cho, S.-W.; Kim, H.-K.; Kang, J.-W.; Kim, D.-G.; Cho, W.-J. Highly flexible and transparent InZnSnOx∕Ag∕InZnSnOx multilayer electrode for flexible organic light emitting diodes. Appl. Phys. Lett. 2008, 92, 223302. [Google Scholar] [CrossRef]
- Kim, J.H.; Na, J.-Y.; Kim, S.-K.; Yoo, Y.-Z.; Seong, T.-Y. Highly transparent and low-resistance indium-free ZnO/Ag/ZnO multilayer electrodes for organic photovoltaic devices. J. Electron. Mater. 2015, 44, 3967–3972. [Google Scholar] [CrossRef]
- Kim, H.; Horwitz, J.; Kim, W.; Mäkinen, A.; Kafafi, Z.; Chrisey, D. Doped ZnO thin films as anode materials for organic light-emitting diodes. Thin Solid Films 2002, 420, 539–543. [Google Scholar] [CrossRef]
- Todorov, R.; Lozanova, V.; Knotek, P.; Černošková, E.; Vlček, M. Microstructure and ellipsometric modelling of the optical properties of very thin silver films for application in plasmonics. Thin Solid Films 2017, 628, 22–30. [Google Scholar] [CrossRef]
- Sreenivasan, M.; Malik, S.; Thigulla, S.; Mehta, B.R. Dependence of plasmonic properties of silver island films on nanoparticle size and substrate coverage. J. Nanomater. 2013, 2013, 90. [Google Scholar] [CrossRef]
- Wei, H.; Eilers, H. From silver nanoparticles to thin films: Evolution of microstructure and electrical conduction on glass substrates. J. Phys. Chem. Solids 2009, 70, 459–465. [Google Scholar] [CrossRef]
- Crupi, I.; Boscarino, S.; Strano, V.; Mirabella, S.; Simone, F.; Terrasi, A. Optimization of ZnO: Al/Ag/ZnO: Al structures for ultra-thin high-performance transparent conductive electrodes. Thin Solid Films 2012, 520, 4432–4435. [Google Scholar] [CrossRef]
- Schubert, S.; Müller-Meskamp, L.; Leo, K. Unusually high optical transmission in Ca: Ag blend films: High-performance top electrodes for efficient organic solar cells. Adv. Funct. Mater. 2014, 24, 6668–6676. [Google Scholar] [CrossRef]
- Asanithi, P.; Chaiyakun, S.; Limsuwan, P. Growth of silver nanoparticles by DC magnetron sputtering. J. Nanomater. 2012, 2012, 963609. [Google Scholar] [CrossRef]
- Kim, J.H.; Kang, T.-W.; Na, S.-I.; Yoo, Y.-Z.; Seong, T.-Y. ITO-free inverted organic solar cells fabricated with transparent and low resistance ZnO/Ag/ZnO multilayer electrode. Curr. Appl. Phys. 2015, 15, 829–832. [Google Scholar] [CrossRef]
- Schubert, M.F.; Xi, J.-Q.; Kim, J.K.; Schubert, E.F. Distributed Bragg reflector consisting of high-and low-refractive-index thin film layers made of the same material. Appl. Phys. Lett. 2007, 90, 141115. [Google Scholar] [CrossRef]
- Pettersson, L.A.; Roman, L.S.; Inganäs, O. Modeling photocurrent action spectra of photovoltaic devices based on organic thin films. J. Appl. Phys. 1999, 86, 487. [Google Scholar] [CrossRef]
- Lin, H.-W.; Lin, C.-L.; Chang, H.-H.; Lin, Y.-T.; Wu, C.-C.; Chen, Y.-M.; Chen, R.-T.; Chien, Y.-Y.; Wong, K.-T. Anisotropic optical properties and molecular orientation in vacuum-deposited ter (9, 9-diarylfluorene)s thin films using spectroscopic ellipsometry. J. Appl. Phys. 2004, 95, 881–886. [Google Scholar] [CrossRef]


| Bottom/Top Electrode | Jsc (mA/cm2) | Voc (V) | FF | PCE (%) |
|---|---|---|---|---|
| ITO/Ag | 0.69 ± 0.5 | 0.58 ± 0.02 | 0.48 ± 0.02 | 0.19 ± 0.02 |
| ITO/CaAg | 0.9 ± 0.06 | 0.59 ± 0.02 | 0.56 ± 0.04 | 0.30 ± 0.02 |
| ZAZ/Ag | 0.81 ± 0.04 | 0.59 ± 0.03 | 0.51 ± 0.03 | 0.24 ± 0.01 |
| ZAZ/CaAg (Top Illumination) | 1.25 ± 0.06 | 0.65 ± 0.03 | 0.54 ± 0.02 | 0.44 ± 0.03 |
| ZAZ/CaAg (Bottom Illumination) | 1.28 ± 0.07 | 0.61 ± 0.02 | 0.52 ± 0.03 | 0.41 ± 0.03 |
| Device | Jsc (mA/cm2) | Voc (V) | FF | PCE (%) |
|---|---|---|---|---|
| Solar cell on glass | 1.25 ± 0.04 | 0.65 ± 0.02 | 0.54 ± 0.03 | 0.44 ± 0.02 |
| Solar cell + DBR on glass | 1.55 ± 0.05 | 0.63 ± 0.03 | 0.54 ± 0.03 | 0.53 ± 0.04 |
| Solar cell on PET | 1.3 ± 0.07 | 0.6 ± 0.02 | 0.53 ± 0.04 | 0.41 ± 0.03 |
| Solar cell + DBR on PET | 1.59 ± 0.08 | 0.62 ± 0.03 | 0.55 ± 0.05 | 0.54 ± 0.03 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, Y.; Zhang, L.; Cheng, N.; Andrew, T.L. ITO-Free Transparent Organic Solar Cell with Distributed Bragg Reflector for Solar Harvesting Windows. Energies 2017, 10, 707. https://doi.org/10.3390/en10050707
Peng Y, Zhang L, Cheng N, Andrew TL. ITO-Free Transparent Organic Solar Cell with Distributed Bragg Reflector for Solar Harvesting Windows. Energies. 2017; 10(5):707. https://doi.org/10.3390/en10050707
Chicago/Turabian StylePeng, Yuelin, Lushuai Zhang, Nongyi Cheng, and Trisha L. Andrew. 2017. "ITO-Free Transparent Organic Solar Cell with Distributed Bragg Reflector for Solar Harvesting Windows" Energies 10, no. 5: 707. https://doi.org/10.3390/en10050707
APA StylePeng, Y., Zhang, L., Cheng, N., & Andrew, T. L. (2017). ITO-Free Transparent Organic Solar Cell with Distributed Bragg Reflector for Solar Harvesting Windows. Energies, 10(5), 707. https://doi.org/10.3390/en10050707






