Alternative Diesel from Waste Plastics
Abstract
:1. Introduction
2. Results and Discussion
2.1. Upgrading of Plastic Waste via Pyrolysis
2.1.1. Pyrolysis Oil Quality
2.1.2. Mid-Distillate Fraction Quality
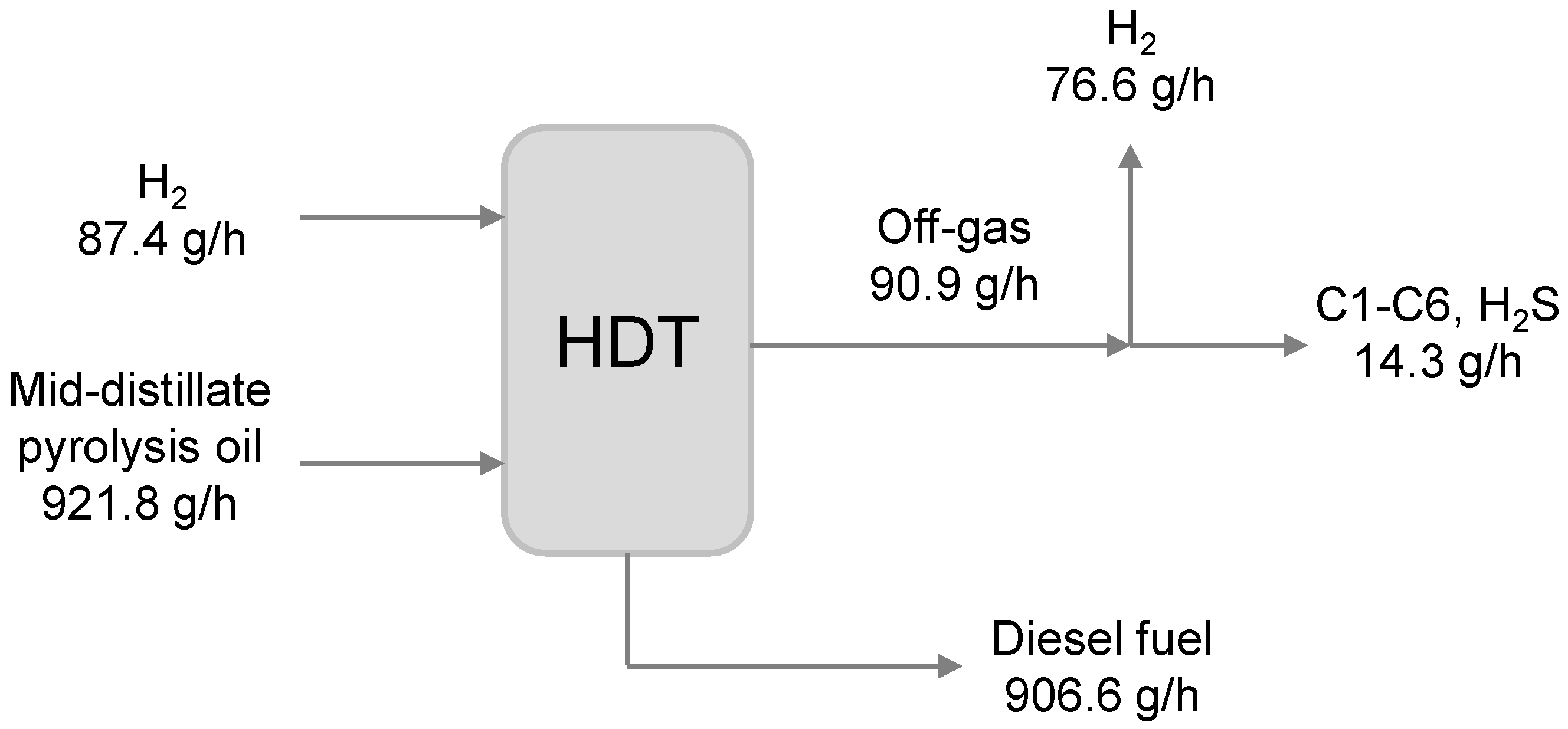
2.2. Pyrolysis Oil Hydrotreatment
2.3. Diesel Fuel Quality
3. Conclusions
4. Materials and Methods
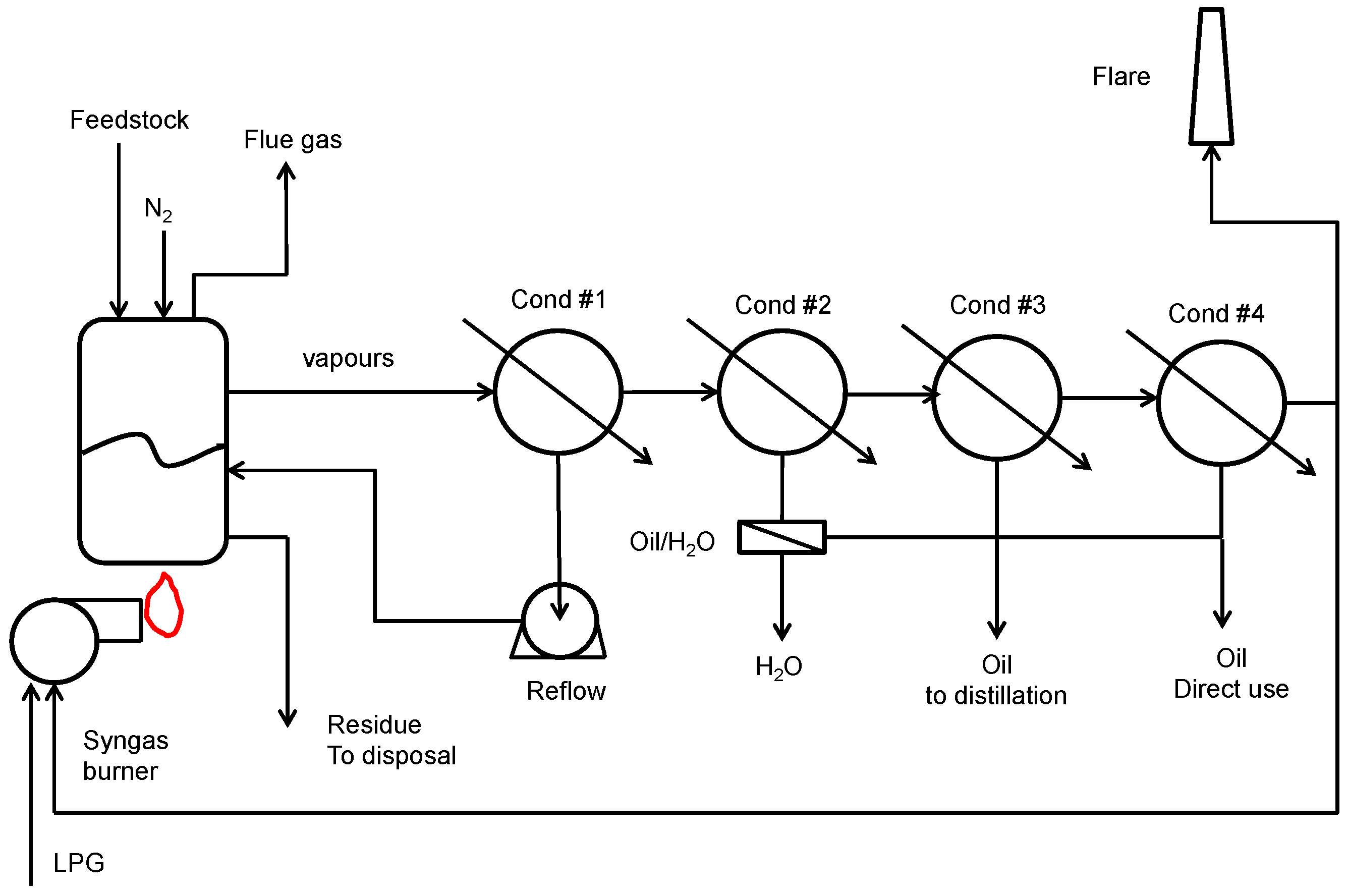
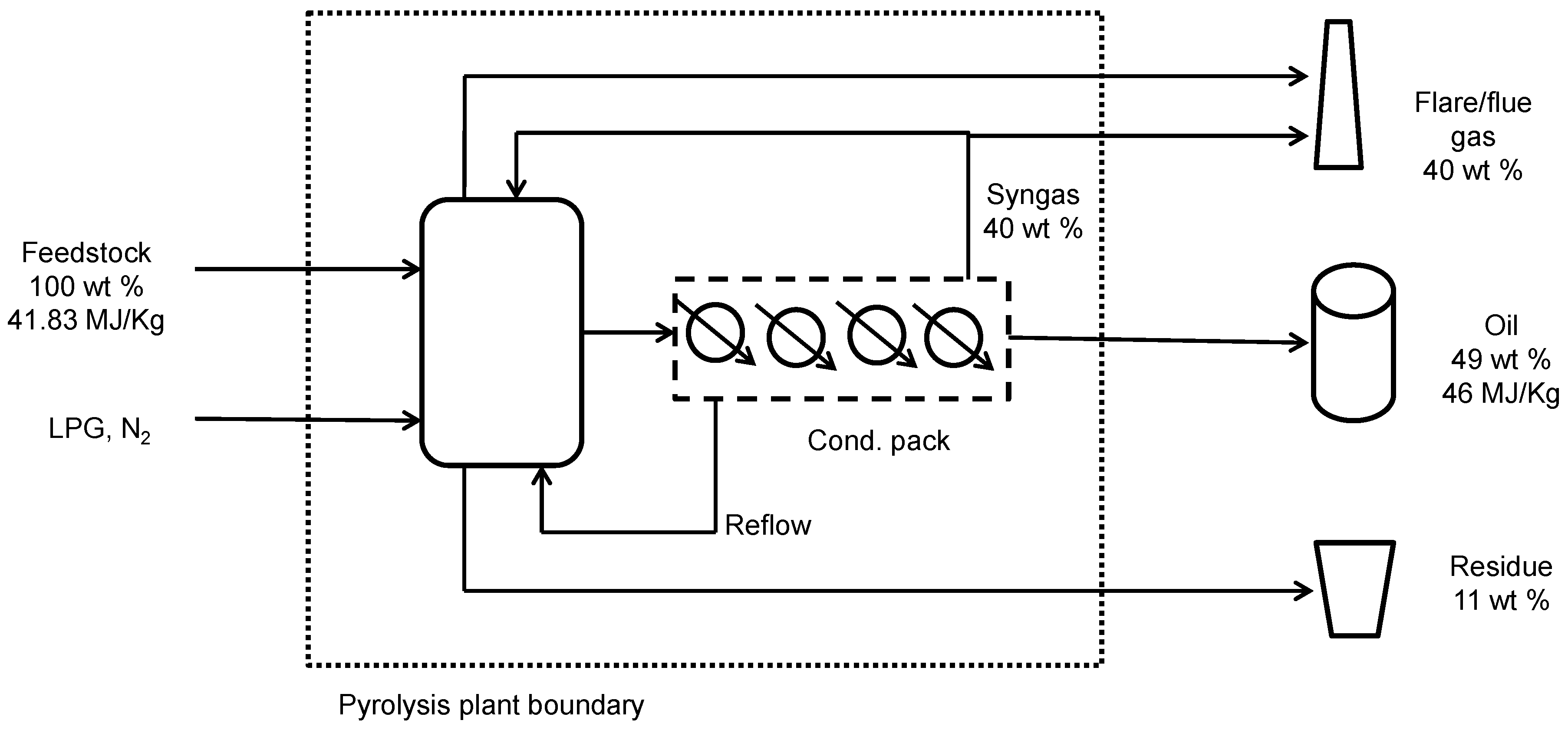
4.1. Plastic Waste Pyrolysis Plant
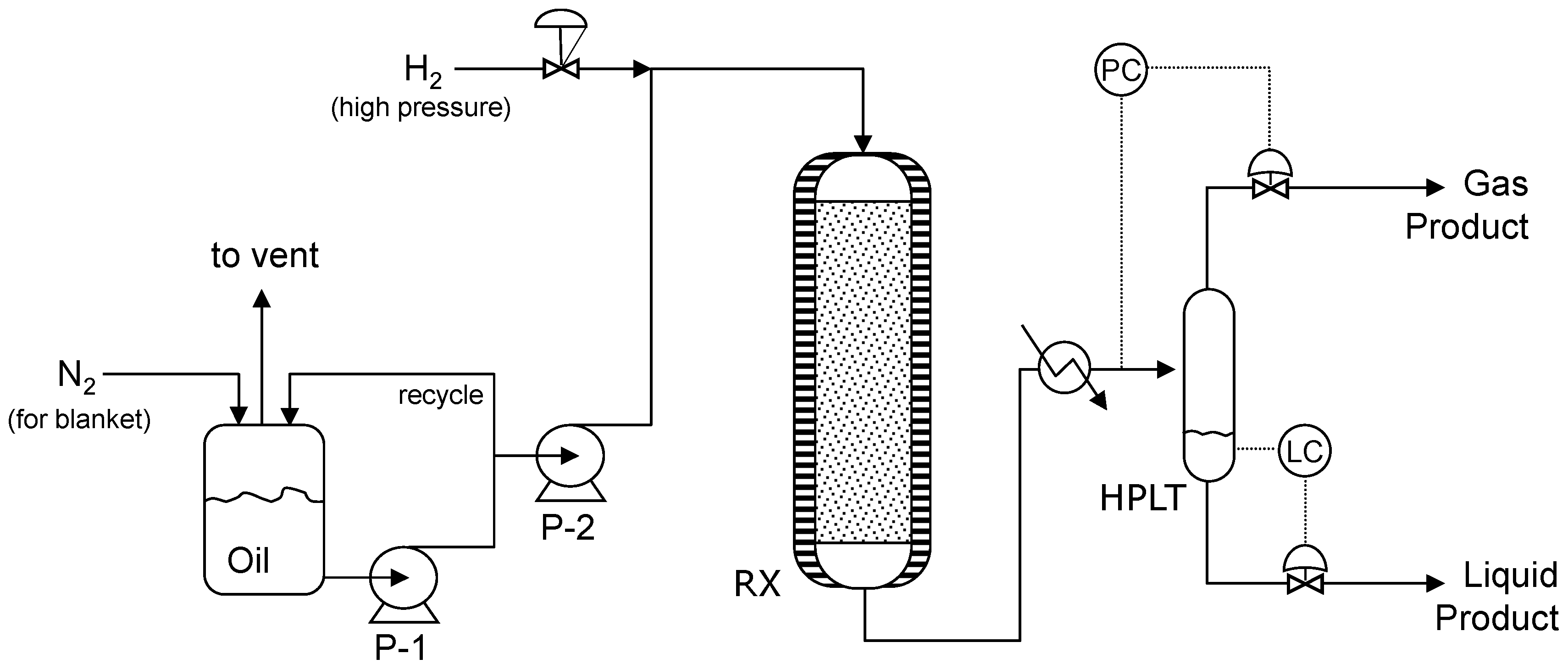
4.2. Catalytic Hydrotreatment Plant
4.3. Final Product Quality
Author Contributions
Conflicts of Interest
References and Notes
- Wong, S.L.; Ngadi, N.; Abdullah, T.A.T.; Inuw, I.M. Current state and future prospects of plastic waste as source of fuel: A review. Renew. Sustain. Energy Rev. 2015, 50, 1167–1180. [Google Scholar] [CrossRef]
- Kalargaris, I.; Tian, G.; Gu, S. The utilisation of oils produced from plastic waste at different pyrolysis temperatures in a DI diesel engine. Energy 2017, 131, 179–185. [Google Scholar] [CrossRef]
- Hoornweg, D.; Bhada-Tata, P. What a Waste—A Global Review of Solid Waste Management. World Bank Urban Development Series Knowledge Papers 2012. Available online: www.worldbank.org/urban (accessed on 12 June 2017).
- United Nations Environmental Program. Converting Waste Plastic into a Resource. 2009. Available online: http://www.unep.or.jp/ (accessed on 12 June 2017).
- Sharuddin, S.D.A.S.; Abnisa, F.; Daud, W.M.A.W.; Aroua, M.K. Energy recovery from pyrolysis of plastic waste: Study on non-recycled plastics (NRP) data as the real measure of plastic waste. Energy Convers. Manag. 2017, 148, 925–934. [Google Scholar] [CrossRef]
- Murugan, S.; Ramaswamy, M.C.; Nagarajan, G. Assessment of pyrolysis oil as an energy source for diesel engines. Fuel Process. Technol. 2009, 90, 67–74. [Google Scholar] [CrossRef]
- Ratnasari, D.K.; Nahil, M.A.; Williams, P.T. Catalytic pyrolysis of waste plastics using staged catalysis for production of gasoline range hydrocarbon oils. J. Anal. Appl. Pyrolysis 2017, 124, 631–637. [Google Scholar] [CrossRef]
- Kaimal, V.K.; Vijayabalan, P. A study on synthesis of energy fuel from waste plastic and assessment of its potential as an alternative fuel for diesel engines. Waste Manag. 2016, 51, 91–96. [Google Scholar] [CrossRef] [PubMed]
- ASTM D86-16a Standard Test Method for Distillation of Petroleum Products and Liquid Fuels at Atmospheric Pressure; ASTM International: West Conshohocken, PA, USA, 2016.
- ASTM D4052-16 Standard Test Method for Density, Relative Density, and API Gravity of Liquids by Digital Density Meter; ASTM International: West Conshohocken, PA, USA, 2016.
- Automotive Fuels-Diesel-Requirements and Test Methods; EN 590:2013; European Committee for Standardization (CEN): Brussel, België, 2013.
- Crude Petroleum and Petroleum Products—Determination of Density—Oscillating U-Tube Method. International Organization for Standardization ISO 12185:1996. Available online: https://www.iso.org/standard/21124.html (accessed on 31 October 2017).
- Petroleum Products—Transparent and Opaque Liquids—Determination of Kinematic Viscosity and Calculation of Dynamic Viscosity. ISO 3104:1994. International Organization for Standardization. Available online: https://www.iso.org/standard/8252.html (accessed on 31 October 2017).
- Petroleum Products—Determination of Sulfur Content of Automotive Fuels—Ultraviolet Fluorescence Method. ISO 20846:2011. International Organization for Standardization. Available online: https://www.iso.org/standard/51774.html (accessed on 31 October 2017).
- Petroleum Products—Determination of Aromatic Hydrocarbon Types in Middle Distillates—High Performance Liquid Chromatography Method with Refractive Index Detection. European Committee for Standardization (CEN) EN 12916:2016. Available online: https://www.sis.se/api/document/preview/8018972/ (accessed on 31 October 2017).
- Petroleum Products—Determination of Distillation Characteristics at Atmospheric Pressure. International Organization for Standardization ISO 3405:2011. Available online: https://www.iso.org/standard/44095.html (accessed on 31 October 2017).
- Liquid Petroleum Products—Determination of Ignition Delay and Derived Cetane Number (DCN) of Middle Distillate Fuels by Combustion in a Constant Volume Chamber. EN 15195:2014. European Committee for Standardization (CEN). Available online: https://infostore.saiglobal.com/preview/is/en/2014/i.s.en15195-2014.pdf?sku=1774892 (accessed on 31 October 2017).
- Petroleum Products—Calculation of Cetane Index of Middle-Distillate Fuels by the Four-Variable Equation. ISO 4264:2007. International Organization for Standardization. Available online: https://www.iso.org/standard/45627.html (accessed on 31 October 2017).
- Determination of Flash Point—Pensky-Martens Closed Cup Method. ISO 2719:2016. International Organization for Standardization. Available online: https://www.iso.org/standard/62263.html (accessed on 31 October 2017).
- Petroleum Products—Corrosiveness to Copper—Copper Strip Test. ISO 2160:1998. International Organization for Standardization. Available online: https://www.iso.org/standard/27552.html (accessed on 31 October 2017).
- Petroleum Products—Determination of Cloud Point. ISO 3015:1992. International Organization for Standardization. Available online: https://www.iso.org/standard/8095.html (accessed on 31 October 2017).
- Petroleum Products—Determination of Pour Point. ISO 3016:1994. International Organization for Standardization. Available online: https://www.iso.org/standard/20721.html (accessed on 31 October 2017).
- CEN. Diesel and Domestic Heating Fuels—Determination of Cold Filter Plugging Point—Stepwise Cooling Bath Method; EN 116:2015; European Committee for Standardization, 2015.
- Diesel Fuel—Assessment of Lubricity Using the High-Frequency Reciprocating Rig (HFRR)—Part 1: Test Method. ISO 12156-1:2016. International Organization for Standardization. Available online: https://www.iso.org/standard/65227.html (accessed on 31 October 2017).
- Petroleum Products—Determination of Carbon Residue—Micro Method. ISO 10370:2014. International Organization for Standardization. Available online: https://www.iso.org/standard/57081.html (accessed on 31 October 2017).
- Petroleum Products—Determination of Ash. ISO 6245:2001. International Organization for Standardization. Available online: https://www.iso.org/standard/31156.html (accessed on 31 October 2017).
- Petroleum Products—Determination of Water—Coulometric Karl Fischer Titration Method. ISO 12937:2000. International Organization for Standardization. Available online: https://www.iso.org/standard/2730.html (accessed on 31 October 2017).
- CEN. Liquid Petroleum Products—Determination of Total Contamination in Middle Distillates, Diesel Fuels and Fatty Acid Methyl Esters; EN 12662:2014; European Committee for Standardization, 2014.
- Petroleum Products—Determination of the Oxidation Stability of Middle-Distillate Fuels. ISO 12205:1995. International Organization for Standardization. Available online: https://www.iso.org/standard/21187.html (accessed on 31 October 2017).
- ASTM D7545-14 Standard Test Method for Oxidation Stability of Middle Distillate Fuels—Rapid Small Scale Oxidation Test (RSSOT); ASTM International: West Conshohocken, PA, USA, 2016.
- CEN. Automotive Fuels—Determination of Manganese and Iron Content in Diesel—Inductively Coupled Plasma Optical Emission Spectrometry (ICP OES) Method; EN 16576:2014; European Committee for Standardization, 2014.
- CEN. Liquid Petroleum Products—Determination of Fatty Acid Methyl Ester (FAME) Content in Middle Distillates—Infrared Spectrometry Method; EN 14078:2014; European Committee for Standardization, 2014.
- ASTM D5291-16 Standard Test Methods for Instrumental Determination of Carbon, Hydrogen, and Nitrogen in Petroleum Products and Lubricants; ASTM International: West Conshohocken, PA, USA, 2016.
- Petroleum Products—Determination of Bromine Number of Distillates and Aliphatic Olefins—Electrometric Method; ISO 3839:1996. International Organization for Standardization. Available online: https://www.iso.org/standard/22154.html (accessed on 31 October 2017).
- ASTM D240-17 Standard Test Method for Heat of Combustion of Liquid Hydrocarbon Fuels by Bomb Calorimeter; ASTM International: West Conshohocken, PA, USA, 2016.
- Bezergianni, S.; Kalogianni, A.; Dimitriadis, A. Catalyst evaluation for waste cooking oil hydroprocessing. Fuel 2012, 93, 638–641. [Google Scholar] [CrossRef]
- Bezergianni, S.; Dimitriadis, A.; Chrysikou, L. Quality and Sustainability Comparison of One- vs. Two-step Catalytic Hydroprocessing of Waste Cooking Oil. Fuel 2014, 118, 300–307. [Google Scholar] [CrossRef]
- Bezergianni, S.; Dimitriadis, A.; Kalogianni, A.; Knudsen, K.G. Toward Hydrotreating of Waste Cooking Oil for Biodiesel Production. Effect of Pressure, H2/Oil Ratio, and Liquid Hourly Space Velocity. Ind. Eng. Chem. Res. 2011, 50, 3874–3879. [Google Scholar] [CrossRef]
- Gomes, J.R.; Bezergianni, S.; Luiz Zotin, J.; Falabella Sousa-Aguiar, F. Biofuels Generation via Hydroconversion of Vegetable Oils and Animal Fats. In Catalytic Hydrogenation for Biomass Valorization; RSC Environmental Series; Royal Society of Chemitry: Cambridge, UK, 2014; Chapter 9; pp. 204–222. ISBN 978-1-84973-801-9. [Google Scholar]
- Hita, I.; Gutiérrez, A.; Olazar, M.; Bilbao, J.; Arandes, J.S.; Castaño, P. Upgrading model compounds and Scrap Tires Pyrolysis Oil (STPO) on hydrotreating NiMo catalysts with tailored supports. Fuel 2015, 145, 158–169. [Google Scholar] [CrossRef]
- Dimitriadis, A.; Bezergianni, S. Biomass vs. Plastic Pyrolysis Oil Upgrading via Hydrotreating. In Proceedings of the II Scientific-Technological Symposium Catalytic Hydroprocessing in Oil Refining, Belgrade, Serbia, 17–23 April 2016. [Google Scholar]
- Yang, Z.; Kumar, A.; Huhnke, R.L. Review of Recent Developments to Improve Storage and Transportation Stability of Bio-oil. Renew. Sustain. Energy Rev. 2015, 50, 859–870. [Google Scholar] [CrossRef]
- Elliott, D.C. Biofuel from Fast Pyrolysis and Catalytic Hydrodeoxygenation. Curr. Opin. Chem. Eng. 2015, 9, 59–65. [Google Scholar] [CrossRef]
- Karonis, D.; Chilari, D.; Manou, C. Characterization of Hydroprocessed Used Cooking Oils in Blend with Low Quality Gasoil Samples. SAE Int. J. Fuels Lubr. 2014, 7, 250–262. [Google Scholar] [CrossRef]
- Bezergianni, S.; Dimitriadi, A. Comparison between different types of renewable diesel. Renew. Sustain. Energy Rev. 2013, 21, 110–116. [Google Scholar] [CrossRef]
- Kalitchin, Z.D.; Ivanov, S.K.; Tanielyan, S.K.; Boneva, M.I.; Georgiev, P.T.; Ivanov, A.; Kanariev, K. Chemical stability of diesel fuels and sediment formation therein: 1. Evaluation of the chemical stability of diesel fuels by following the kinetics of sediment. Fuel 1992, 71, 437–442. [Google Scholar] [CrossRef]
- Owczuk, M.; Kołodziejczyk, K. Liquid Fuel Ageing Processes in Long-term Storage. In Storage Stability of Fuels; Biernat, K., Ed.; InTech: Rijeka, Croatia, 2015; pp. 101–129. ISBN 978-953-51-1734-6. [Google Scholar] [CrossRef]
- Pretsch, E.; Bühlmann, P.; Badertscher, M. Structure Determination of Organic Compounds; Springer: Berlin, Germany, 2009; pp. 272–275. ISBN 978-3-540-93809-5. [Google Scholar]
- Coates, J. Interpretation of Infrared Spectra, a Practical Approach. In Encyclopedia of Analytical Chemistry; Meyers, R.A., Ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2000; pp. 10815–10837. [Google Scholar]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J. Spectrometric Identification of Organic Compounds, 7th ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2005; pp. 85–86. ISBN 0-471-39362-2. [Google Scholar]
- Bruno, T.J.; Hsieh, P.Y. A perspective on the origin of lubricity in petroleum distillate motor fuels. Fuel Process. Technol. 2015, 129, 52–60. [Google Scholar] [CrossRef]
- Bacha, J.; Freel, J.; Gibbs, A.; Hemighaus, G.; Hoekman, K.; Horn, J.; Ingham, M.; Jossens, L.; Kohler, D.; Lesnini, D.; et al. Diesel Fuels Technical Review; Chevron: San Ramo, CA, USA, 2007. [Google Scholar]
- Zhang, Z.; Pittman, C.U.; Sui, S.; Sun, J.; Wang, Q. Catalytic Upgrading of Bio-Oil by Reacting with Olefins and Alcohols over Solid Acids: Reaction Paths via Model Compound Studies. Energies 2013, 6, 1568–1589. [Google Scholar] [CrossRef]
- Oasmaa, A.; Peacocke, C.; Gust, S.; Meier, D.; McLellan, R. Norms and Standards for Pyrolysis Liquids. End-User Requirements and Specifications. Energy Fuels 2005, 19, 2155–2163. [Google Scholar] [CrossRef]
- Pilusa, T.J.; Shukla, M.; Muzenda, E. Pyrolitic Tyre Derived Fuel: A Review. In Proceedings of the International Conference on Chemical, Mining and Metallurgical Engineering (CMME’2013), Johannesburg, South Africa, 27–28 November 2013. [Google Scholar]
- Oasmaa, A.; Kuoppala, E.; Ardiyanti, A.; Venderbosch, R.H.; Heeres, H.J. Characterization of Hydrotreated Fast Pyrolysis Liquids. Energy Fuels 2010, 24, 5264–5272. [Google Scholar] [CrossRef]
- Okuwaki, A.; Yoshioka, T. The liquefaction of plastic containers and packaging in Japan. In Feedstock Recycling and Pyrolysis of Waste Plastics: Converting Waste Plastics into Diesel and Other Fuels; Wiley: Edithvale, Australia, 2006; pp. 663–708. [Google Scholar]

| Property | Value | Notes |
|---|---|---|
| °API * | 46.3 | - |
| Density | 796.0 kg/m3 | - |
| 0 recovered (IBP **) | 82.0 °C | - |
| 10% recovered | 129.5 °C | - |
| 20% recovered | 157.0 °C | - |
| 30% recovered | 177.5 °C | T 170 at 26.5% |
| 40% recovered | 212.5 °C | - |
| 50% recovered | 244.5 °C | - |
| 60% recovered | 276.0 °C | - |
| 70% recovered | 301.5 °C | - |
| 80% recovered | 325.5 °C | - |
| 90% recovered | 372.5 °C | T 370 at 96% |
| 98% recovered (EP ***) | - | - |
| Light liquid fraction (0–170 °C) | 26.5 wt % | Naphtha |
| Medium liquid fraction (170–370 °C) | 68.5 wt % | Diesel |
| Heavy liquid fraction (>370 °C) | 4 wt % | Wax |
| Property | Value | Unit | Limits | Test Method | |
|---|---|---|---|---|---|
| Min | Max | ||||
| Density at 15 °C | 802.7 | kg/m3 | 820.0 | 845.0 | EN ISO 12185 [12] |
| Viscosity at 40 °C | 2.300 | mm2/s | 2.000 | 4.500 | EN ISO 3104 [13] |
| Sulfur Content | 42.9 | mg/kg | - | 10.0 | EN ISO 20846 [14] |
| Aromatic Hydrocarbons | - | wt % | - | - | EN 12916 [15] |
| Mono | 7.7 | wt % | - | - | - |
| Di | 1.9 | wt % | - | - | - |
| Tri+ | 0.4 | wt % | - | - | - |
| Polyaromatic Hydrocarbons | 2.3 | wt % | - | 8 | EN 12916 [15] |
| Distillation | - | - | - | - | EN ISO 3405 [16] |
| Recovered at 250 °C | 36.3 | % vol | - | 65 | - |
| Recovered at 350 °C | 93.8 | % vol | 85 | - | - |
| 95% (V/V) Recovered at | 354.5 | °C | - | 360 | - |
| Residue | 1.6 | % vol | - | - | - |
| IBP | 133.7 | °C | - | - | - |
| 10% | 204.3 | °C | - | - | - |
| 50% | 291.3 | °C | - | - | - |
| 90% | 346.7 | °C | - | - | - |
| 95% | 354.5 | °C | - | - | - |
| FBP * | 368.7 | °C | - | - | - |
| Cetane Number | 60.7 | - | 51 | - | EN 15195 [17] |
| Cetane Index | 66.7 | - | 46 | - | EN ISO 4264 [18] |
| Flash Point | 48.0 | °C | 55 | - | EN ISO 2719 [19] |
| Copper Corrosion (3 h at 50 °C) | 1A | - | 1A | - | EN ISO 2160 [20] |
| Cloud Point | - | °C | - | - | EN 3015 [21] |
| Pour Point | −2 | °C | - | - | ISO 3016 [22] |
| CFPP ** | −1 | °C | - | - | EN 116 [23] |
| Lubricity Corrected WSD1.4 at 60 °C | 276 | μm | - | 460 | EN ISO 12156-1 [24] |
| Carbon Residue | 0.05 | wt % | - | 0.3 | EN ISO 10370 [25] |
| Ash Content | <0.005 | wt % | - | 0.01 | EN ISO 6245 [26] |
| Water Content | 51 | mg/kg | - | 200 | EN ISO 12937 [27] |
| Total Contamination | <12 | mg/kg | - | 24 | EN 12662 [28] |
| Oxidation Stability | 49.0 | g/m3 | - | 25 | EN ISO 12205 [29] |
| Oxidation Stability | 42.43 | min | - | - | ASTM D7545 [30] |
| Manganese content | <0.5 | mg/L | - | 2 | EN 16576 [31] |
| FAME content *** | <0.1 | % vol | - | 7.0 | EN 14078 [32] |
| Elemental Analysis | - | - | - | - | - |
| Carbon Content | 85.44 | wt % | - | - | ASTM D5291 [33] |
| Hydrogen Content | 14.55 | wt % | - | - | ASTM D5291 [33] |
| Bromine Number | 35.7 | g Br/100 g | - | - | ISO 3839 [34] |
| Calorific Value | - | - | - | - | ASTM D240 [35] |
| Gross | 46.67 | MJ/kg | - | - | - |
| Net | 43.58 | MJ/kg | - | - | - |
| Property | Value | Unit | Limits | Test Method | |
|---|---|---|---|---|---|
| Min | Max | ||||
| Density at 15 °C | 790.6 | kg/m3 | 820.0 | 845.0 | EN ISO 12185 [12] |
| Viscosity at 40 °C | 2.377 | mm2/s | 2.000 | 4.500 | EN ISO 3104 [13] |
| Sulfur Content | 12.1 | mg/kg | - | 10.0 | EN ISO 20846 [14] |
| Aromatic Hydrocarbons | - | wt % | - | - | EN 12916 [15] |
| Mono | 1.8 | wt % | - | - | - |
| Di | - | wt % | - | - | - |
| Tri+ | - | wt % | - | - | - |
| Polyaromatic Hydrocarbons | - | wt % | - | 8 | EN 12916 [15] |
| Distillation | - | - | - | - | EN ISO 3405 [16] |
| Recovered at 250 °C | 41.0 | vol % | - | 65 | - |
| Recovered at 350 °C | 95.2 | % vol | 85 | - | - |
| 95% (V/V) Recovered at | 349.5 | °C | - | 360 | - |
| Residue | 1.4 | vol % | - | - | - |
| IBP | 146.6 | °C | - | - | - |
| 10% | 194.7 | °C | - | - | - |
| 50% | 263.6 | °C | - | - | - |
| 90% | 331.7 | °C | - | - | - |
| 95% | 349.5 | °C | - | - | - |
| FBP | 360.7 | °C | - | - | - |
| Derived Cetane Number | 74.7 | - | 51 | - | EN 16144 [53] |
| Cetane Index | 71.5 | - | 46 | - | EN ISO 4264 [18] |
| Flash Point | 52.5 | °C | 55 | - | EN ISO 2719 [19] |
| Copper Corrosion (3 h at 50 °C) | 1A | - | 1A | - | EN ISO 2160 [20] |
| Cloud Point | 8 | °C | - | - | EN 23015 [21] |
| Pour Point | 2 | °C | - | - | ISO 3016 [22] |
| CFPP | 2 | °C | - | - | EN 116 [23] |
| Lubricity Corrected WSD1.4 at 60 °C | 552 | μm | - | 460 | EN ISO 12156-1 [24] |
| Carbon Residue | 0.01 | wt % | - | 0.3 | EN ISO 10370 [25] |
| Ash Content | - | wt % | - | 0.01 | EN ISO 6245 [26] |
| Water Content | 40 | mg/kg | - | 200 | EN ISO 12937 [27] |
| Total Contamination | 1.2 | mg/kg | - | 24 | EN 12662 [28] |
| Oxidation Stability | 2.1 | g/m3 | - | 25 | EN ISO 12205 [29] |
| Oxidation Stability | 148 | min | - | - | ASTM D7545 [30] |
| Manganese content | - | mg/L | - | 2 | EN 16576 [31] |
| FAME content | - | % vol | - | 7.0 | EN 14078 [32] |
| Elemental Analysis | - | - | - | - | - |
| Carbon Content | 85.14 | wt % | - | - | ASTM D5291 [33] |
| Hydrogen Content | 14.85 | wt % | - | - | ASTM D5291 [33] |
| Bromine Number | <0.1 | g Br/100 g | - | - | ISO 3839 [34] |
| Calorific Value | - | - | - | - | ASTM D240 [35] |
| Gross | 46.98 | MJ/kg | - | - | - |
| Net | 43.83 | MJ/kg | - | - | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bezergianni, S.; Dimitriadis, A.; Faussone, G.-C.; Karonis, D. Alternative Diesel from Waste Plastics. Energies 2017, 10, 1750. https://doi.org/10.3390/en10111750
Bezergianni S, Dimitriadis A, Faussone G-C, Karonis D. Alternative Diesel from Waste Plastics. Energies. 2017; 10(11):1750. https://doi.org/10.3390/en10111750
Chicago/Turabian StyleBezergianni, Stella, Athanasios Dimitriadis, Gian-Claudio Faussone, and Dimitrios Karonis. 2017. "Alternative Diesel from Waste Plastics" Energies 10, no. 11: 1750. https://doi.org/10.3390/en10111750
APA StyleBezergianni, S., Dimitriadis, A., Faussone, G.-C., & Karonis, D. (2017). Alternative Diesel from Waste Plastics. Energies, 10(11), 1750. https://doi.org/10.3390/en10111750






