Thermo-Economic Performance Analysis of a Regenerative Superheating Organic Rankine Cycle for Waste Heat Recovery
Abstract
:1. Introduction
2. Mathematic and Physical Models
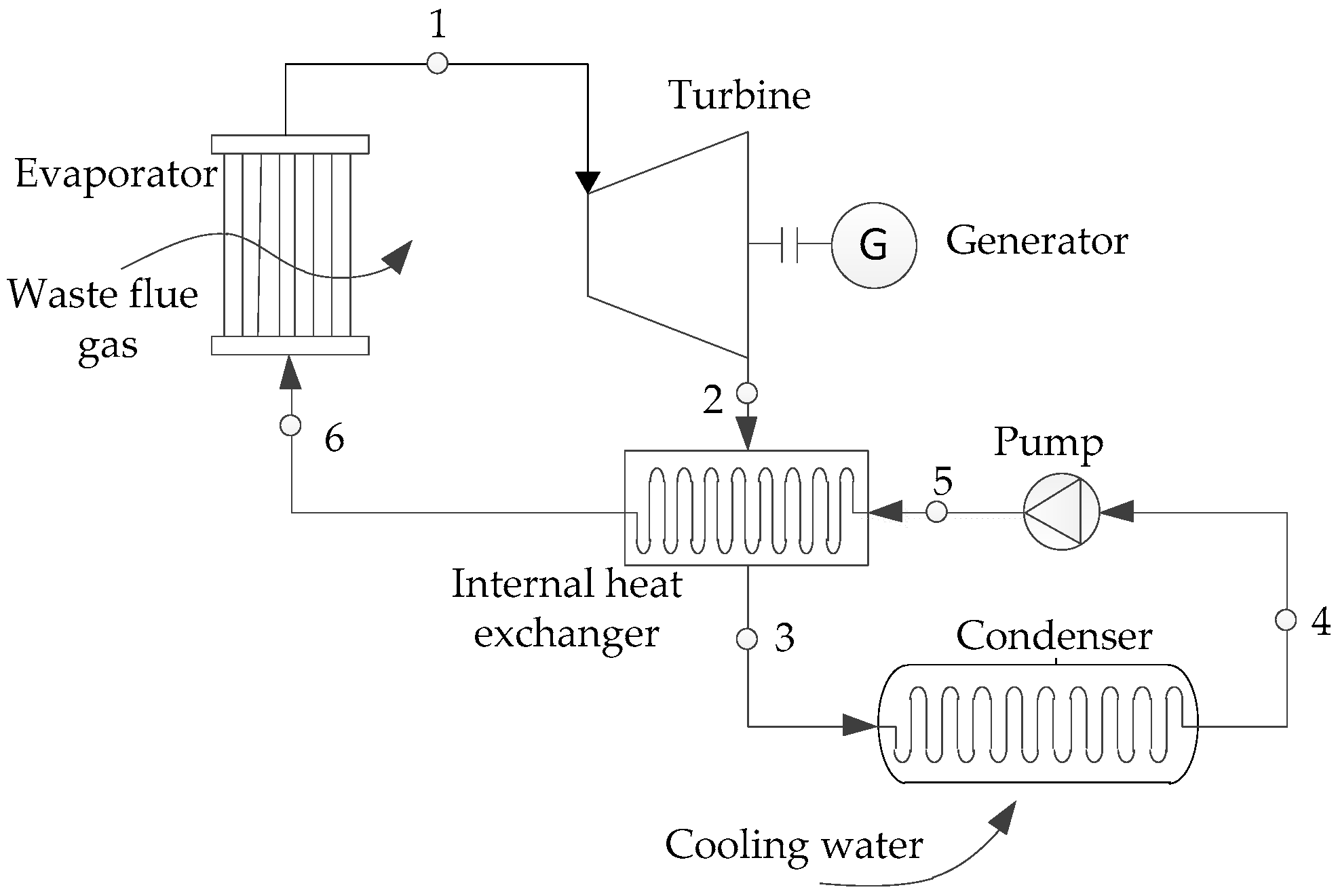
2.1. Structure and Working Principles of the System
2.2. Thermodynamic Model
- (1)
- The regenerative ORC system is under steady state.
- (2)
- Heat losses to or from the environment as well as the kinetic and potential energy changes are neglected.
- (3)
- A saturated liquid state is supposed at the condenser outlet.
- (4)
- Pressure drops in the evaporator, condenser, internal heat exchanger, and related pipelines are ignored.
2.3. Economical Model
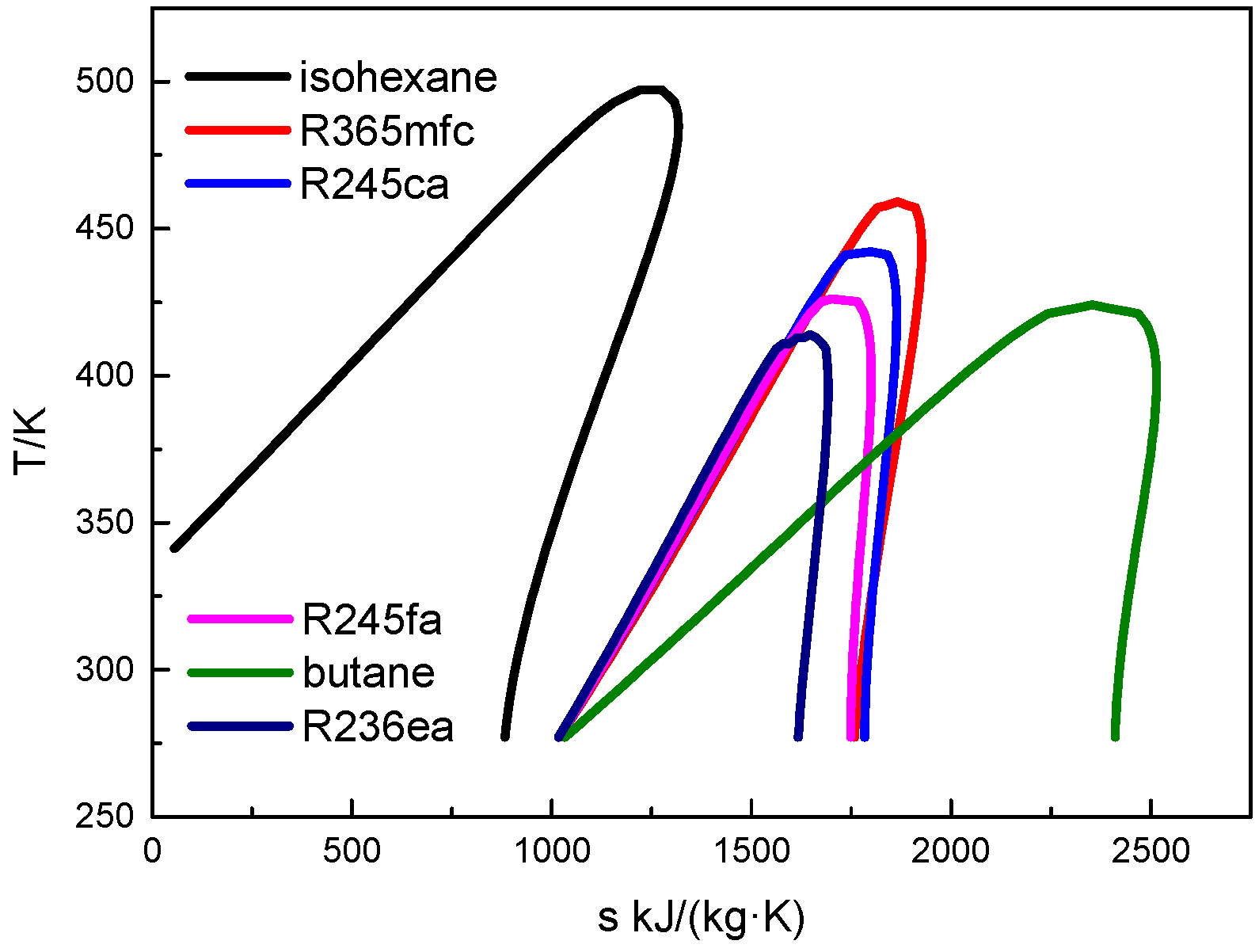
3. Working Fluid Selection and Basic Calculation Parameters
4. Results and Discussion
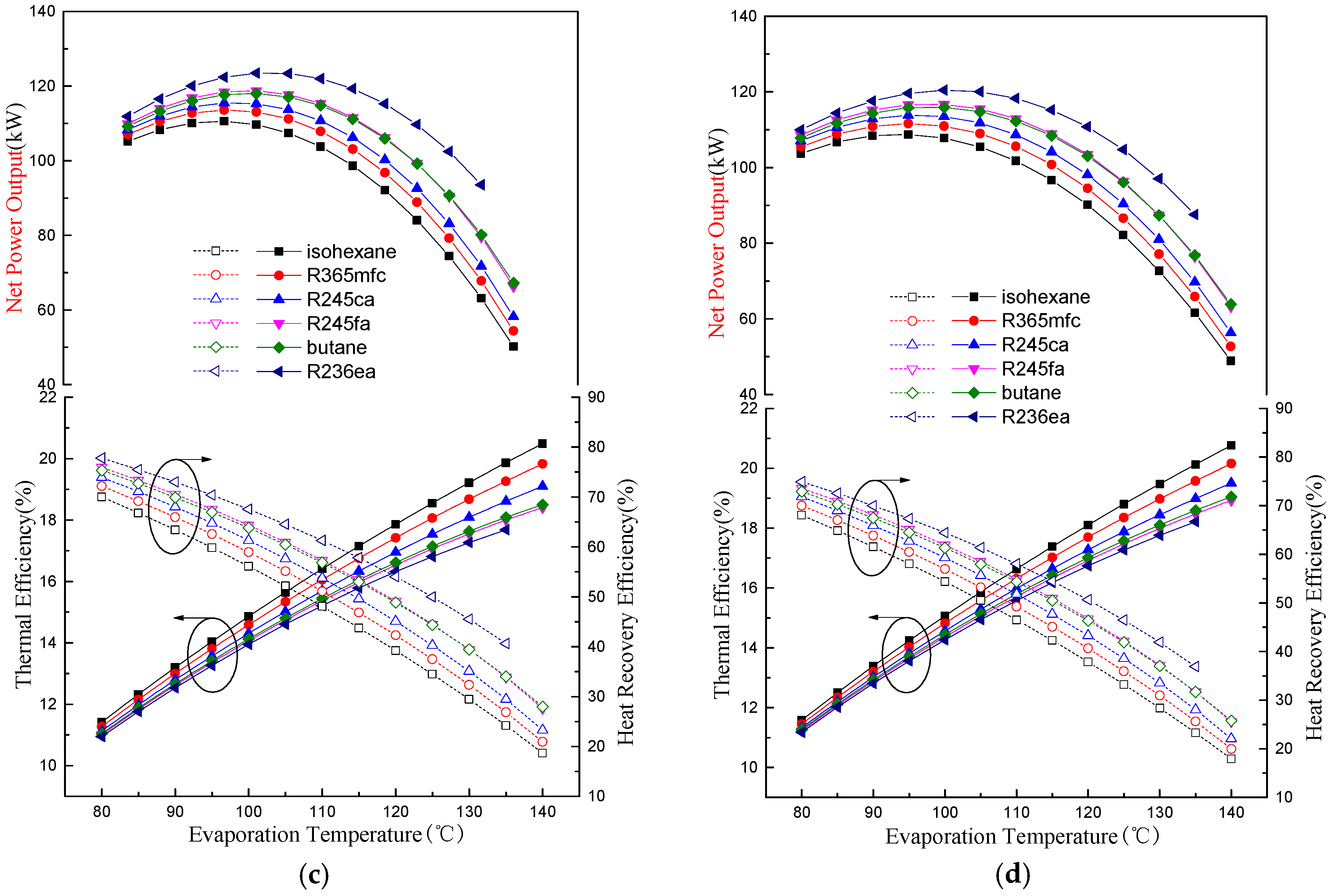
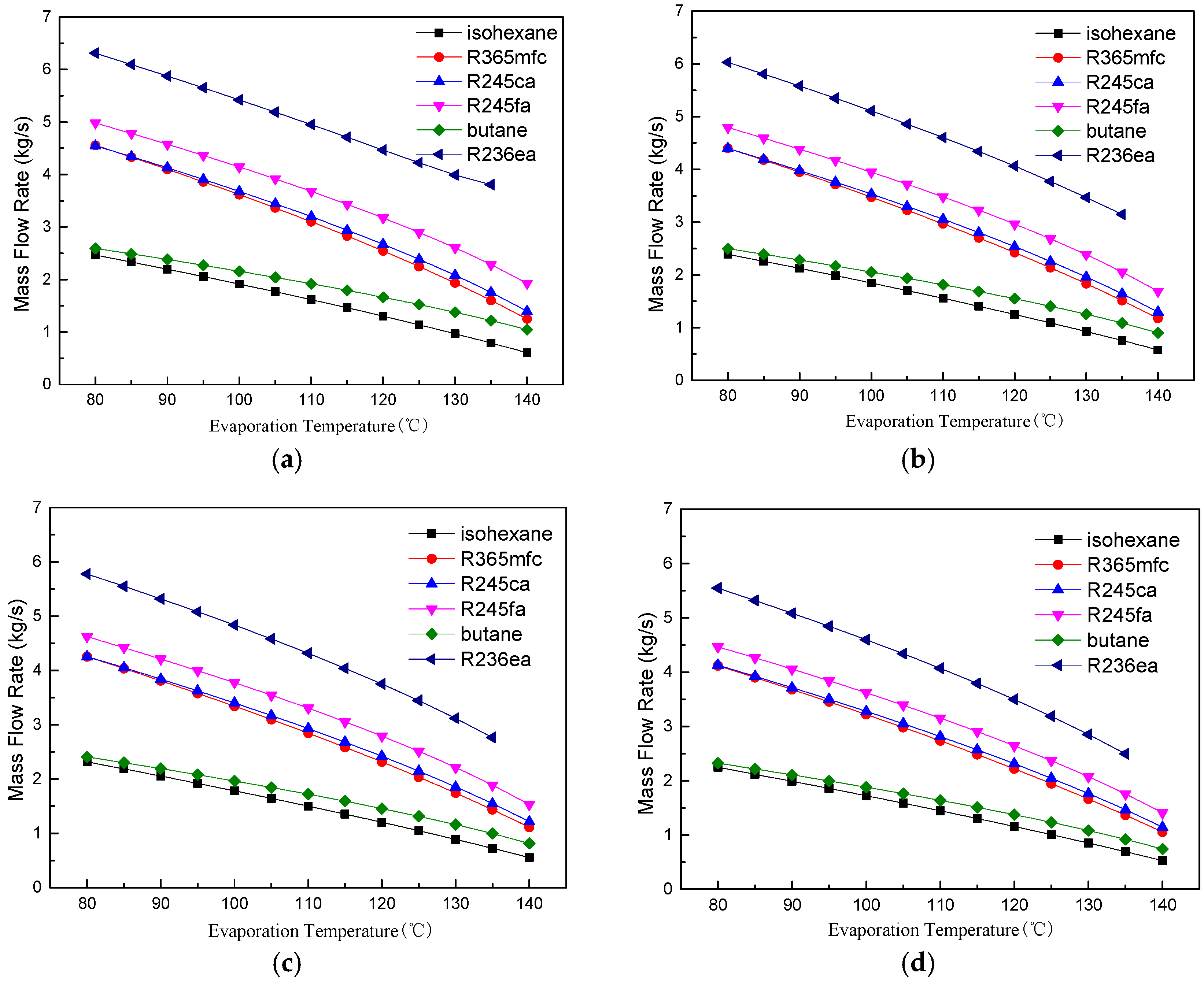
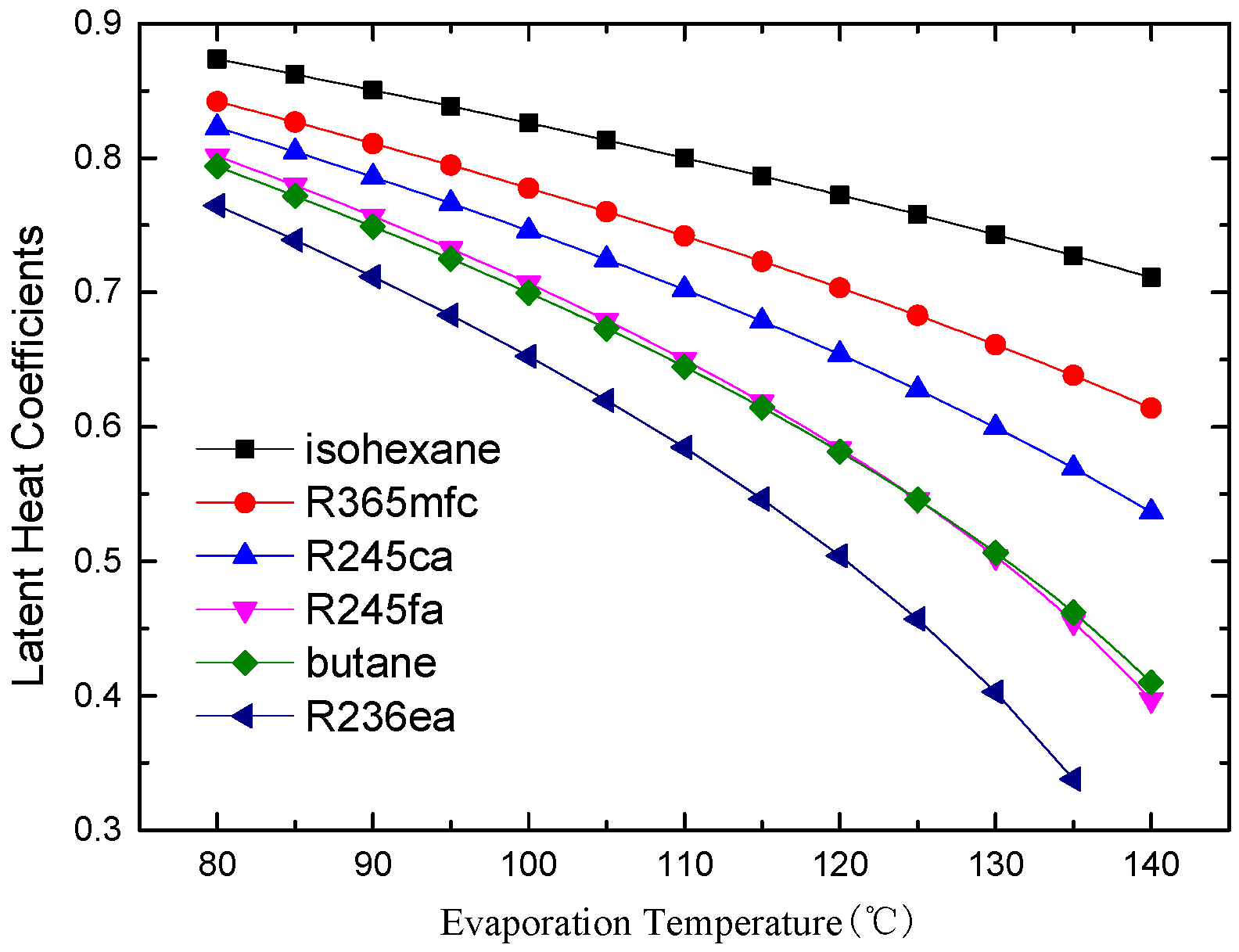
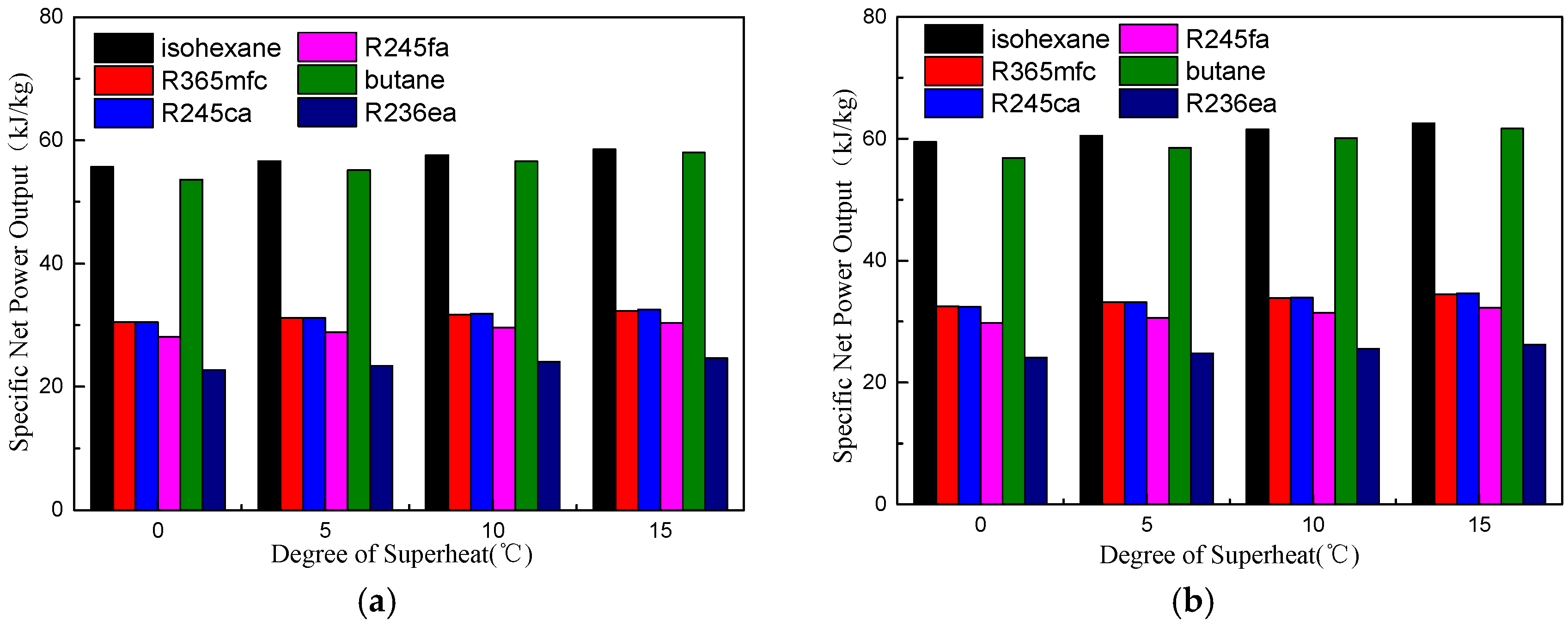
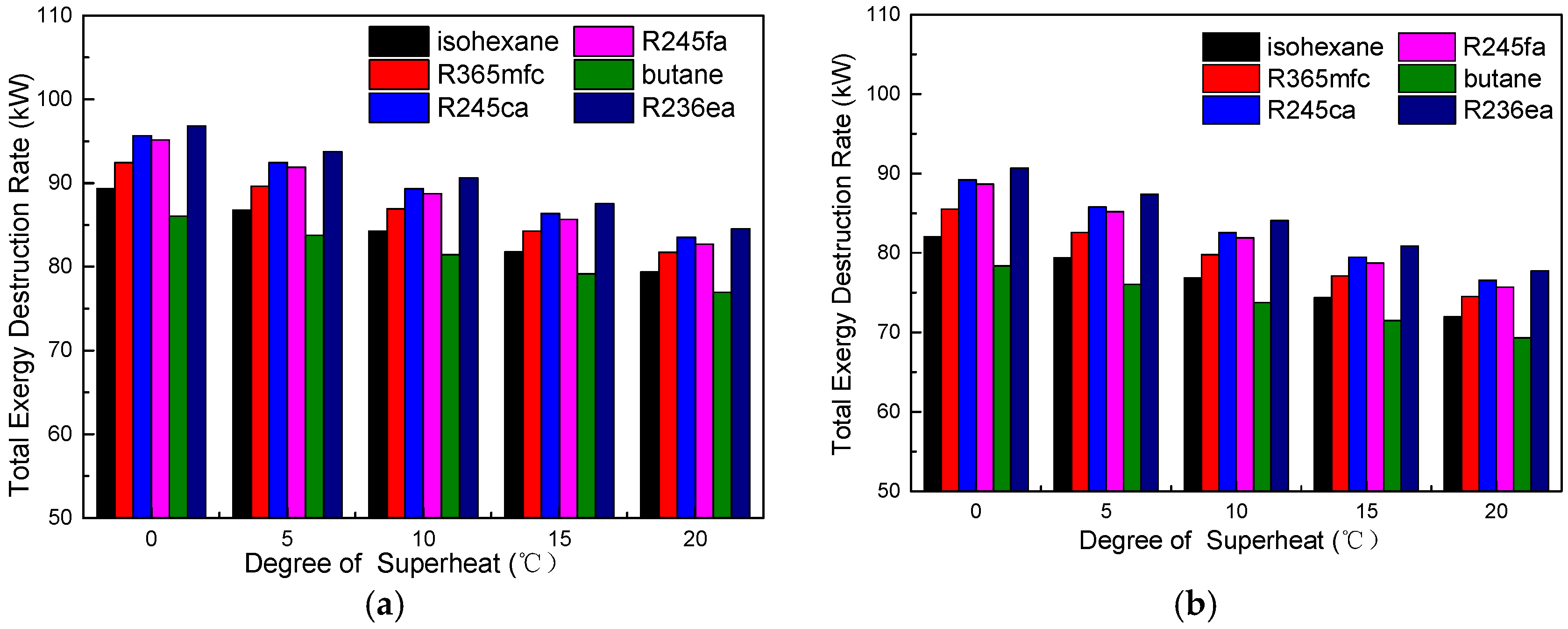
4.1. Thermodynamic Performance Analysis
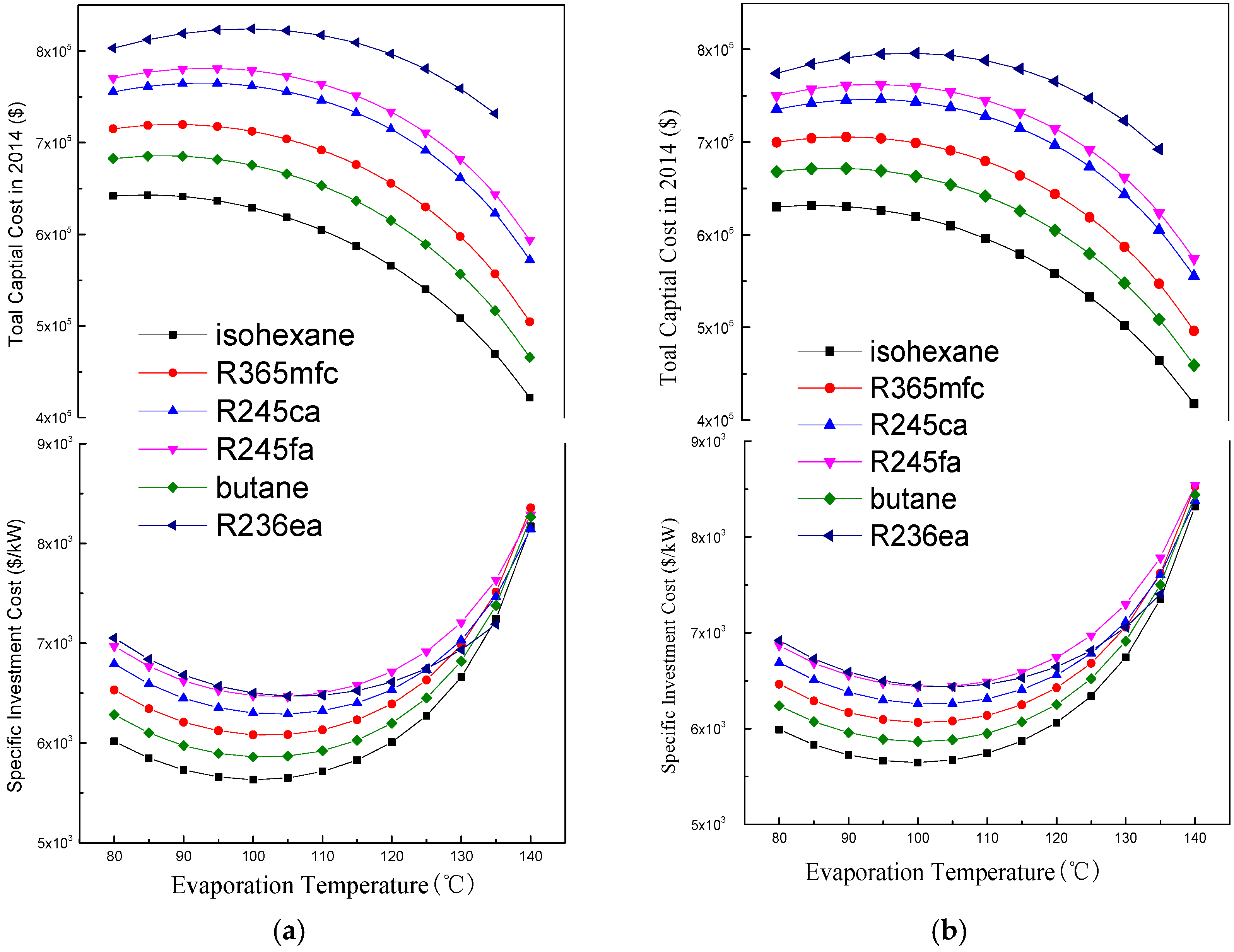
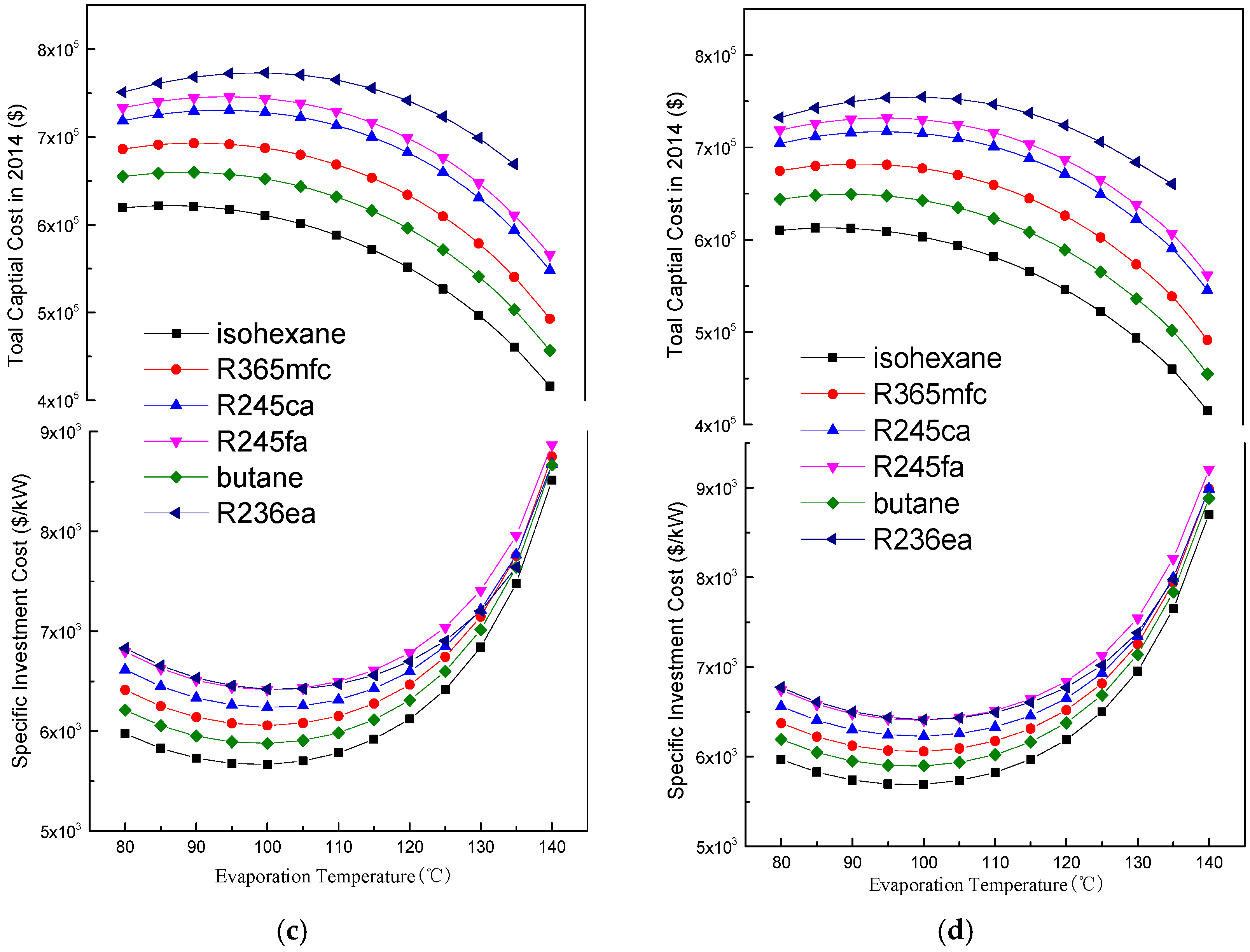
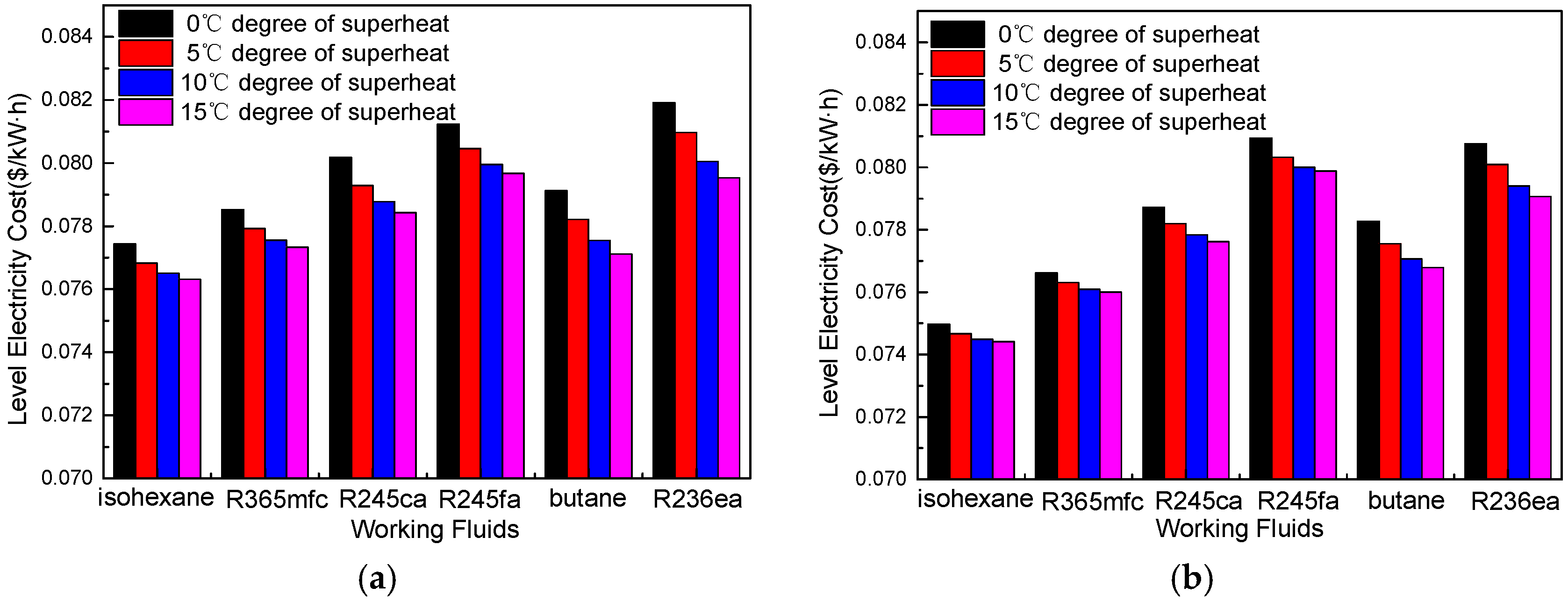
4.2. Economic Performance Analysis
4.3. Suitable Working Fluids and Optimal Parameters
5. Conclusions
- (1)
- There is an optimal evaporation temperature giving a maximum net power output and a minimum SIC. The specific net power output of isohexane and butane is much greater than the other four working fluids due to their greater latent heat.
- (2)
- The evaporation temperature operation range is affected by the VFR. Butane has the lowest VFR and the largest evaporation temperature operation range among the six working fluids.
- (3)
- A suitable degree of superheat is necessary since it is not only conducive to improving the working capacity of working fluids, but also reduces the total exergy destruction rate, VFR, total capital cost, SIC, and LEC for different working fluids. The system’s thermodynamic and economic performance, as well as the operational stability, are improved.
- (4)
- Compared with other working fluids, butane shows the best comprehensive performance for the regenerative ORC system. Furthermore, the optimal evaporation temperature and degree of superheat are 100 °C and 5 °C, respectively.
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| CEPCI | Chemical engineering plant cost index |
| Com | Operation and maintenance cost |
| CRF | Capital recovery cost |
| GWP | Greenhouse warming potential |
| LEC | Level energy cost |
| NPV | Net present value |
| NSGA-II | Non-dominated sorting genetic algorithm II |
| ODP | Ozone depletion potential |
| ORC | Organic Rankine Cycle |
| PBP | Payback period |
| SIC | Specific investment cost |
| Top | System operation time |
| VFR | Volume flow ratio |
| Nomenclature | |
| a | Thermal diffusivity |
| A | Area (m2) |
| B | Evaluation matrixes |
| C | Investment cost ($) |
| Cb | Basic cost of each equipment ($) |
| CBM | Bare module equipment cost ($) |
| cp | Specific heat (kJ/kg·K) |
| d | Diameter (m) |
| Fbm | Aggregate multiplication |
| Fm | Material factor |
| Fp | Pressure factor |
| h | Specific enthalpy (kJ/kg) |
| i | Interest rate |
| I | Exergy destruction rate (kW) |
| K1, K2, K3, B1, B2, C1, C2, C3 | Constant coefficients for cost evaluation |
| m | Mass flow rate (kg/s) |
| Nu | Nusselt number |
| p | |
| P | Pressure |
| p* | Reduced pressure |
| Pnet | Specific net power output (kJ/kg) |
| Pr | Prandtl number |
| q | Heat flux density (J/m2·s) |
| Q | Heat transfer rate (kW) |
| Re | Reynolds number |
| s | Entropy (kJ/kg·K); Shannon Entropy |
| T | Temperature (°C) |
| Top | System operation time |
| Ts | Saturation temperature (°C) |
| Ty | Plant life time |
| U | Overall heat transfer coefficient (W/m2·K) |
| v | Specific volume (m3/kg) |
| w | Entropy weight |
| W | Power work (kW) |
| x | Vapor mass quality |
| Subscripts | |
| 1-6 | State points corresponding to Figure 1 and Figure 2 |
| 1b, 1d, 2s, 4d, 5s, a-g | State points corresponding to Figure 2 |
| amb | Ambient |
| con | Condenser |
| eva | Evaporator |
| f | Working fluid |
| g | Gaseous |
| gas | Waste flue gas |
| H | Heat source |
| IHE | Internal heat exchanger |
| i | Different regions in heat exchanger |
| in | Inlet; inside |
| l | Liquid |
| L | Cold source |
| max | Maximum |
| min | Minimum |
| net | Net |
| out | Outlet; outside |
| p | Pump |
| s | Isentropic |
| sup | Superheated vapor |
| Total | Total exergy destruction rate |
| T | Turbine |
| the | Thermal |
| Greek Letters | |
| α | Heat transfer coefficient (W/m2·K) |
| γH | Latent heat (kJ/kg) |
| δH | Latent heat coefficient |
| δIHE | Internal heat coefficient |
| δl | Preheating coefficient |
| δsup | Superheating coefficient |
| ε | Effectiveness of internal heat exchanger |
| η | Efficiency |
| λ | Thermal conductivity(W/m·K) |
| μ | Viscosity (m2/s) |
| ρ | Density (kg/m3) |
| ΔTm | Logarithmic mean temperature (°C) |
References
- Feng, Y.Q.; Hung, T.C.; He, Y.L.; Wang, Q.; Wang, S.; Li, B.X.; Lin, J.R.; Zhang, W. Operation Characteristic and Performance Comparison of Organic Rankine Cycle (ORC) for Low-grade Waste Heat Using R245fa, R123 and Their Mixtures. Energy Convers. Manag. 2017, 144, 153–163. [Google Scholar] [CrossRef]
- Javanshir, A.; Sarunac, N.; Razzaghpanah, Z. Thermodynamic Analysis of a Regenerative Organic Rankine Cycle Using Dry Fluids. Appl. Therm. Eng. 2017, 123, 852–864. [Google Scholar] [CrossRef]
- Girgin, I.; Ezgi, C. Design and Thermodynamic and Thermoeconomic Analysis of an Organic Rankine Cycle for Naval Surface Ship Applications. Energy Convers. Manag. 2017, 148, 623–634. [Google Scholar] [CrossRef]
- Dai, Y.; Wang, J.; Gao, L. Parametric Optimization and Comparative Study of Organic Rankine Cycle (ORC) for Low Grade Waste Heat Recovery. Energy Convers. Manag. 2009, 50, 576–582. [Google Scholar] [CrossRef]
- Xu, G.; Song, G.; Zhu, X.; Gao, W.; Li, H.; Quan, Y. Performance Evaluation of a Direct Vapor Generation Supercritical ORC System Driven by Linear Fresnel Reflector Solar Concentrator. Appl. Therm. Eng. 2015, 80, 196–204. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, W.; Qian, C.; Chen, X.; Jin, G. Investigation on the Feasibility and Performance of Ground Source Heat Pump (GSHP) in Three Cities in Cold Climate Zone, China. Renew. Energy 2015, 84, 89–96. [Google Scholar] [CrossRef]
- Ceperley, P.H. A Pistonless Stirling Engine—The Traveling Wave Heat Engine. J. Acoust. Soc. Am. 1979, 66, 1508–1513. [Google Scholar] [CrossRef]
- Markides, C.N.; Smith, T.C.B. A Dynamic Model for the Efficiency Optimization of an Oscillatory Low Grade Heat Engine. Energy 2011, 36, 6967–6980. [Google Scholar] [CrossRef]
- Kirmse, C.J.W.; Oyewunmi, O.A.; Haslam, A.J.; Markides, C.N. Comparison of a Novel Organic-fluid Thermofluidic Heat Converter and an Organic Rankine Cycle Heat Engine. Energies 2016, 9, 47. [Google Scholar] [CrossRef]
- Roy, J.; Mishra, M.; Misra, A. Performance Analysis of an Organic Rankine Cycle with Superheating under Different Heat Source Temperature Conditions. Appl. Energy 2011, 88, 2995–3004. [Google Scholar] [CrossRef]
- Roy, J.; Misra, A. Parametric Optimization and Performance Analysis of a Regenerative Organic Rankine Cycle Using R-123 for Waste Heat Recovery. Energy 2012, 39, 227–235. [Google Scholar] [CrossRef]
- Chen, Q.; Xu, J.; Chen, H. A New Design Method for Organic Rankine Cycles with Constraint of Inlet and Outlet Heat Carrier Fluid Temperatures Coupling with the Heat Source. Appl. Energy 2012, 98, 562–573. [Google Scholar] [CrossRef]
- Gao, W.; Li, H.; Xu, G.; Quan, Y. Working Fluid Selection and Preliminary Design of a Solar Organic Rankine Cycle System. Environ. Prog. Sustain. Energy 2015, 34, 619–626. [Google Scholar] [CrossRef]
- Liu, B.T.; Chien, K.H.; Wang, C.C. Effect of Working Fluids on Organic Rankine Cycle for Waste Heat Recovery. Energy 2004, 29, 1207–1217. [Google Scholar] [CrossRef]
- Aljundi, I.H. Effect of Dry Hydrocarbons and Critical Point Temperature on the Efficiencies of Organic Rankine Cycle. Renew. Energy 2011, 36, 1196–1202. [Google Scholar] [CrossRef]
- Li, J.; Alvi, J.Z.; Pei, G.; Ji, J.; Li, P.; Fu, H. Effect of Working Fluids on the Performance of a Novel Direct Vapor Generation Solar Organic Rankine Cycle System. Appl. Therm. Eng. 2016, 98, 786–797. [Google Scholar] [CrossRef]
- Invernizzi, C.; Iora, P.; Silva, P. Bottoming Micro-Rankine Cycles for Micro-gas Turbines. Appl. Therm. Eng. 2007, 27, 100–110. [Google Scholar] [CrossRef]
- Larjola, J. Electricity from Industrial Waste Heat Using High-speed Organic Rankine Cycle (ORC). Int. J. Prod. Econ. 1995, 41, 227–235. [Google Scholar] [CrossRef]
- Wu, S.; Li, C.; Xiao, L.; Liu, C.; Li, Y. A Comparative Study on Thermo-economic Performance between Subcritical and Transcritical Organic Rankine Cycles under Different Heat Source Temperatures. Chin. Sci. Bull. 2014, 59, 4379–4387. [Google Scholar] [CrossRef]
- Lecompte, S.; Lemmens, S.; Huisseune, H.; van den Broek, M.; De Paepe, M. Multi-objective Thermo-economic Optimization Strategy for ORCs Applied to Subcritical and Transcritical Cycles for Waste Heat Recovery. Energies 2015, 8, 2714–2741. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Zhao, L. Analysis of Zeotropic Mixtures Used in Low-temperature Solar Rankine Cycles for Power Generation. Sol. Energy 2009, 83, 605–613. [Google Scholar] [CrossRef]
- Feng, Y.; Hung, T.; Greg, K.; Zhang, Y.; Li, B.; Yang, J. Thermoeconomic Comparison between Pure and Mixture Working Fluids of Organic Rankine Cycles (ORCs) for Low Temperature Waste Heat Recovery. Energy Convers. Manag. 2015, 106, 859–872. [Google Scholar] [CrossRef]
- Wu, Y.; Zhu, Y.; Yu, L. Thermal and Economic Performance Analysis of Zeotropic Mixtures for Organic Rankine Cycles. Appl. Therm. Eng. 2016, 96, 57–63. [Google Scholar] [CrossRef]
- Heberle, F.; Brüggemann, D. Thermo-economic Analysis of Zeotropic Mixtures and Pure Working Fluids in Organic Rankine Cycles for Waste Heat Recovery. Energies 2016, 9, 226. [Google Scholar] [CrossRef]
- Desai, N.B.; Bandyopadhyay, S. Process Integration of Organic Rankine Cycle. Energy 2009, 34, 1674–1686. [Google Scholar] [CrossRef]
- Saleh, B.; Koglbauer, G.; Wendland, M.; Fischer, J. Working Fluids for Low-temperature Organic Rankine Cycles. Energy 2007, 32, 1210–1221. [Google Scholar] [CrossRef]
- Li, W.; Feng, X.; Yu, L.; Xu, J. Effects of Evaporating Temperature and Internal Heat Exchanger on Organic Rankine Cycle. Appl. Therm. Eng. 2011, 31, 4014–4023. [Google Scholar] [CrossRef]
- Liu, Q.; Duan, Y.; Yang, Z. Performance Analyses of Geothermal Organic Rankine Cycles with Selected Hydrocarbon Working Fluids. Energy 2013, 63, 123–132. [Google Scholar] [CrossRef]
- Tocci, L.; Pal, T.; Pesmazoglou, I.; Franchetti, B. Small Scale Organic Rankine Cycle (ORC): A Techno-Economic Review. Energies 2017, 10, 413. [Google Scholar] [CrossRef]
- Lemmens, S. Cost Engineering Techniques and Their Applicability for Cost Estimation of Organic Rankine Cycle Systems. Energies 2016, 9, 485. [Google Scholar] [CrossRef]
- Xiao, L.; Wu, S.Y.; Yi, T.T.; Liu, C.; Li, Y.R. Multi-objective Optimization of Evaporation and Condensation Temperatures for Subcritical Organic Rankine Cycle. Energy 2015, 83, 723–733. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, C.; Wang, S.; Xu, X.; Li, Q. Thermo-economic Comparison of Subcritical Organic Rankine Cycle Based on Different Heat Exchanger Configurations. Energy 2017, 123, 728–741. [Google Scholar] [CrossRef]
- Heberle, F.; Brüggemann, D. Thermo-economic Evaluation of Organic Rankine Cycles for Geothermal Power Generation Using Zeotropic Mixtures. Energies 2015, 8, 2097–2124. [Google Scholar] [CrossRef]
- Oyewunmi, O.A.; Markides, C.N. Thermo-economic and Heat Transfer Optimization of Working-fluid Mixtures in a Low-temperature Organic Rankine Cycle system. Energies 2016, 9, 448. [Google Scholar] [CrossRef]
- Wang, J.; Yan, Z.; Wang, M.; Li, M.; Dai, Y. Multi-objective Optimization of an Organic Rankine Cycle (ORC) for Low Grade Waste Heat Recovery Using Evolutionary Algorithm. Energy Convers. Manag. 2013, 71, 146–158. [Google Scholar] [CrossRef]
- Wang, H.; Xu, J.; Yang, X.; Miao, Z.; Yu, C. Organic Rankine Cycle Saves Energy and Reduces Gas Emissions for Cement Production. Energy 2015, 86, 59–73. [Google Scholar] [CrossRef]
- Li, R.; Wang, H.; Yao, E.; Zhang, S. Thermo-Economic Comparison and Parametric Optimizations among Two Compressed Air Energy Storage System Based on Kalina Cycle and ORC. Energies 2016, 10, 15. [Google Scholar] [CrossRef]
- Patel, B.; Desai, N.B.; Kachhwaha, S.S.; Jain, V.; Hadia, N. Thermo-economic Analysis of a Novel Organic Rankine Cycle Integrated Cascaded Vapor Compression–absorption System. J. Clean. Prod. 2017, 154, 26–40. [Google Scholar] [CrossRef]
- Li, Y.; Ren, X.D. Investigation of the Organic Rankine Cycle (ORC) System and the Radial-inflow Turbine Design. Appl. Therm. Eng. 2016, 96, 547–554. [Google Scholar] [CrossRef]
- Costall, A.W.; Hernandez, A.G.; Newton, P.J.; Martinez-Botas, R.F. Design Methodology for Radial Turbo Expanders in Mobile Organic Rankine Cycle Applications. Appl. Energy 2015, 157, 729–743. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, Y.; Li, B.; Yang, J.; Shi, Y. Sensitivity Analysis and Thermoeconomic Comparison of ORCs (Organic Rankine Cycles) for Low Temperature Waste Heat Recovery. Energy 2015, 82, 664–677. [Google Scholar] [CrossRef]
- Sieder, E.N.; Tate, G.E. Heat Eransfer and Pressure Drop of Liquids in Tubes. Ind. Eng. Chem. 1936, 28, 1429–1435. [Google Scholar] [CrossRef]
- Kern, D.Q. Process Heat Transfer; McGraw-Hill: New York, NY, USA, 1950. [Google Scholar]
- Stephan, K.; Abdelsalam, M. Heat-transfer Correlations for Natural Convection Boiling. Int. J. Heat Mass Transf. 1980, 23, 73–87. [Google Scholar] [CrossRef]
- Shah, M.M. A General Correlation for Heat Transfer During Film Condensation Inside Pipes. Int. J. Heat Mass Transf. 1979, 22, 547–556. [Google Scholar] [CrossRef]
- Turton, R.; Bailie, R.C.; Whiting, W.B.; Shaeiwitz, J.A. Analysis, Synthesis and Design of Chemical Processes, 4th ed.; Pearson Education: Ann Arbor, MI, USA, 2013. [Google Scholar]
- Shengjun, Z.; Huaixin, W.; Tao, G. Performance Comparison and Parametric Optimization of Subcritical Organic Rankine Cycle (ORC) and Transcritical Power Cycle System for Low-temperature Geothermal Power Generation. Appl. Energy 2011, 88, 2740–2754. [Google Scholar] [CrossRef]
- Mignard, D. Correlating the Chemical Engineering Plant Cost Index with Macro-economic Indicators. Chem. Eng. Res. Des. 2014, 92, 285–294. [Google Scholar] [CrossRef]
- Nafey, A.; Sharaf, M. Combined Solar Organic Rankine Cycle with Reverse Osmosis Desalination Process: Energy, Exergy, and Cost Evaluations. Renew. Energy 2010, 35, 2571–2580. [Google Scholar] [CrossRef]
- El-Emam, R.S.; Dincer, I. Exergy and Exergoeconomic Analyses and Optimization of Geothermal Organic Rankine Cycle. Appl. Therm. Eng. 2013, 59, 435–444. [Google Scholar] [CrossRef]
- Li, Y.R.; Du, M.T.; Wu, C.M.; Wu, S.Y.; Liu, C. Potential of Organic Rankine Cycle Using Zeotropic Mixtures as Working Fluids for Waste Heat Recovery. Energy 2014, 77, 509–519. [Google Scholar] [CrossRef]
- Cayer, E.; Galanis, N.; Nesreddine, H. Parametric Study and Optimization of a Transcritical Power Cycle Using a Low Temperature Source. Appl. Energy 2010, 87, 1349–1357. [Google Scholar] [CrossRef]
- National Institute of Standards and Technology (NIST). REFPROP: Reference Fluid Thermodynamic and Transport Properties; NIST: Boulder, CO, USA, 2007.
- Li, J.; Li, P.; Pei, G.; Alvi, J.; Li, J. Analysis of a Novel Solar Electricity Generation System Using Cascade Rankine Cycle and Steam Screw Expander. Appl. Energy 2016, 165, 627–638. [Google Scholar] [CrossRef]
- Li, L.; Liu, F.; Li, C. Customer Satisfaction Evaluation Method for Customized Product Development Using Entropy Weight and Analytic Hierarchy Process. Comput. Ind. Eng. 2014, 77, 80–87. [Google Scholar] [CrossRef]
- Chen, T.; Jin, Y.; Qiu, X.; Chen, X. A Hybrid Fuzzy Evaluation Method for Safety Assessment of Food-waste Feed Based on Entropy and the Analytic Hierarchy Process Methods. Expert Syst. Appl. 2014, 41, 7328–7337. [Google Scholar] [CrossRef]
- Feng, Y.; Hung, T.C.; Zhang, Y.; Li, B.; Yang, J.; Shi, Y. Performance Comparison of Low-grade ORCs (Organic Rankine Cycles) Using R245fa, Pentane and Their Mixtures Based on the Thermoeconomic Multi-Objective Optimization and Decision Makings. Energy 2015, 93, 2018–2029. [Google Scholar] [CrossRef]



| Components | K1 | K2 | K3 | C1 | C2 | C3 | B1 | B2 | Fm | Fbm |
|---|---|---|---|---|---|---|---|---|---|---|
| Heat exchanger | 3.2138 | 0.2688 | 0.0796 | −0.0649 | 0.0502 | 0.01474 | 1.80 | 1.50 | 1.25 | - |
| Pump | 3.3892 | 0.0536 | 0.1538 | −0.3935 | 0.3957 | −0.0022 | 1.89 | 1.35 | 1.5 | - |
| Turbine | 3.5140 | 0.5890 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3.50 |
| Item | Unit | Value |
|---|---|---|
| Waste flue gas temperature | °C | 160 |
| Mass flow of waste flue gas temperature | kg·s−1 | 10 |
| Pinch point temperature difference in evaporator | °C | 5 |
| Pinch point temperature difference in IHE | °C | 5 |
| Pinch point temperature difference in condenser | °C | 5 |
| Condensing temperature | °C | 30 |
| Cooling water inlet temperature | °C | 20 |
| Environmental temperature | °C | 20 |
| Turbine isentropic efficiency | % | 85 |
| Pump isentropic efficiency | % | 85 |
| Working Fluids | Molecular Weight (g·mol−1) | Normal Boiling Point (°C) | Critical Temperature (°C) | Critical Pressure (MPa) |
|---|---|---|---|---|
| Isohexane | 86.175 | 60.21 | 224.55 | 3.040 |
| R365mfc | 148.075 | 40.15 | 186.85 | 3.266 |
| R245ca | 134.049 | 25.13 | 174.42 | 3.925 |
| R245fa | 134.048 | 15.14 | 154.01 | 3.651 |
| Butane | 58.122 | −0.49 | 151.98 | 3.923 |
| R236ea | 152.039 | 6.19 | 139.29 | 3.502 |
| Working Fluids | Latent Heat at 95 °C (kJ/kg) | Latent Heat at 100 °C (kJ/kg) |
|---|---|---|
| Isohexane | 295.54 | 291.17 |
| R365mfc | 157.38 | 154.04 |
| R245ca | 155.59 | 151.44 |
| R245fa | 139.32 | 134.48 |
| Butane | 267.66 | 258.26 |
| R236ea | 107.57 | 102.76 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Z.; Li, P.; Han, X.; Mei, Z.; Wang, Z. Thermo-Economic Performance Analysis of a Regenerative Superheating Organic Rankine Cycle for Waste Heat Recovery. Energies 2017, 10, 1593. https://doi.org/10.3390/en10101593
Han Z, Li P, Han X, Mei Z, Wang Z. Thermo-Economic Performance Analysis of a Regenerative Superheating Organic Rankine Cycle for Waste Heat Recovery. Energies. 2017; 10(10):1593. https://doi.org/10.3390/en10101593
Chicago/Turabian StyleHan, Zhonghe, Peng Li, Xu Han, Zhongkai Mei, and Zhi Wang. 2017. "Thermo-Economic Performance Analysis of a Regenerative Superheating Organic Rankine Cycle for Waste Heat Recovery" Energies 10, no. 10: 1593. https://doi.org/10.3390/en10101593
APA StyleHan, Z., Li, P., Han, X., Mei, Z., & Wang, Z. (2017). Thermo-Economic Performance Analysis of a Regenerative Superheating Organic Rankine Cycle for Waste Heat Recovery. Energies, 10(10), 1593. https://doi.org/10.3390/en10101593





