Enhanced Thermoelectric Properties of Cu3SbSe4 Compounds via Gallium Doping
Abstract
:1. Introduction
2. Experimental Procedures
3. Results and Discussion
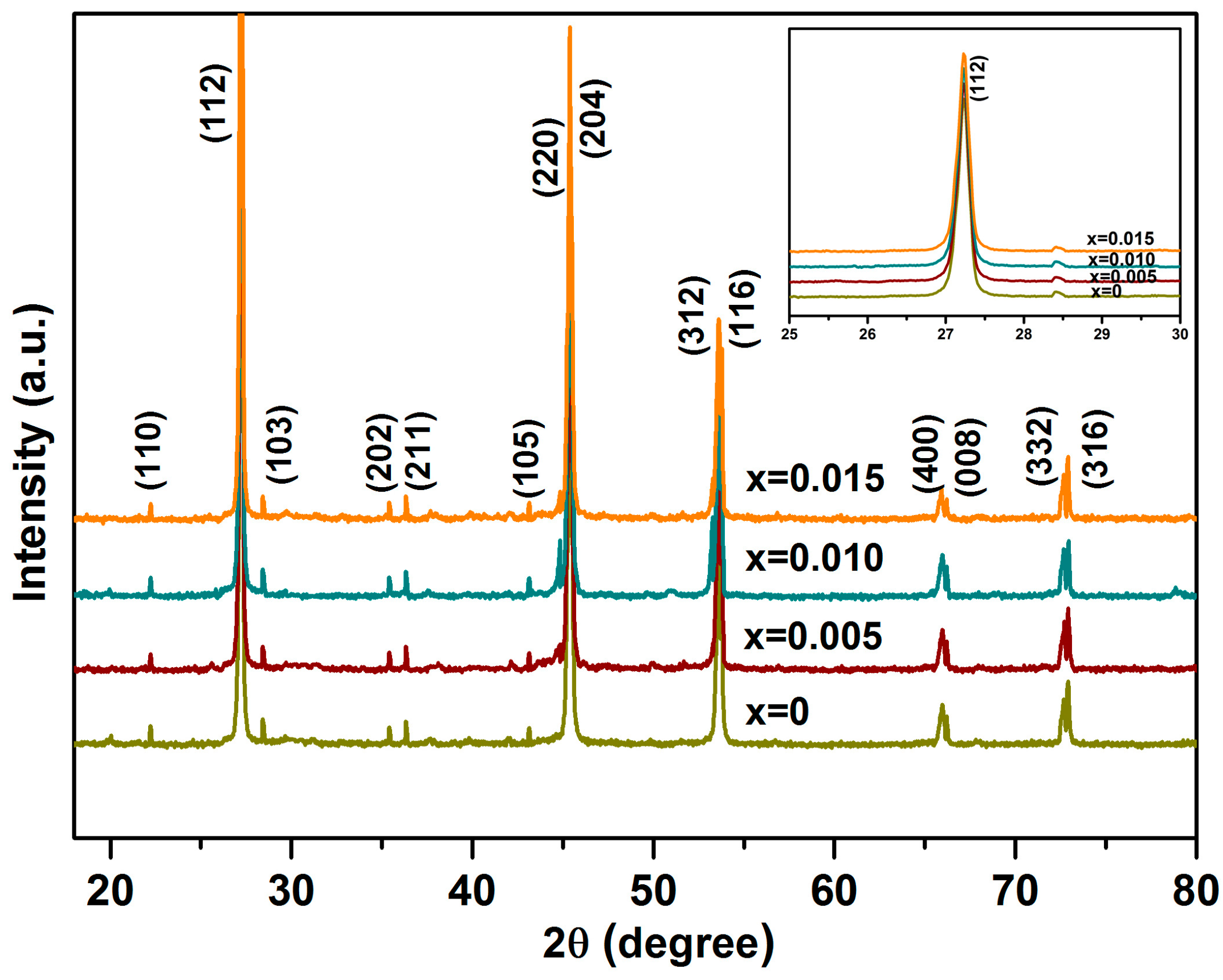
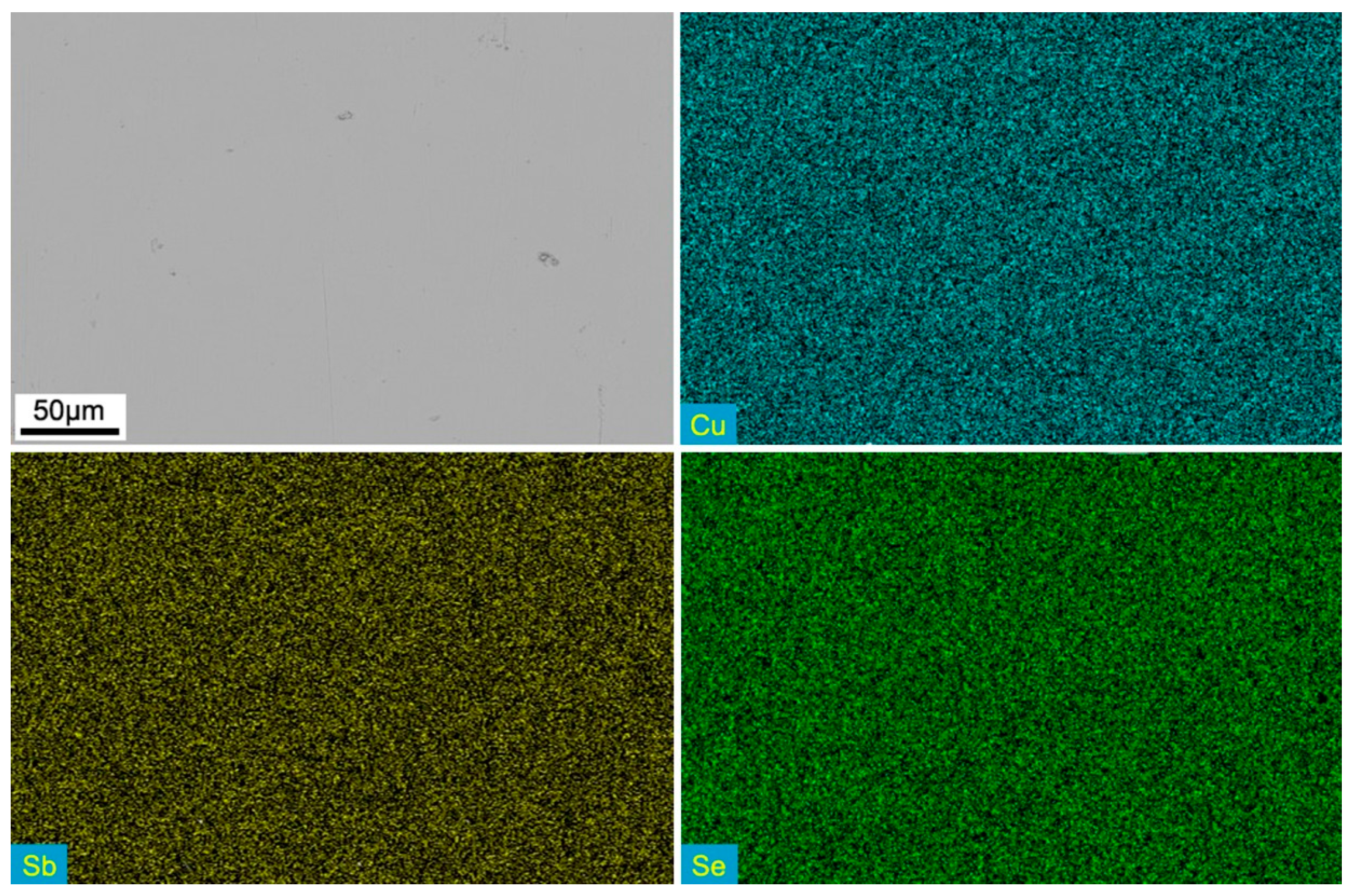
3.1. XRD Analysis and Microstructure
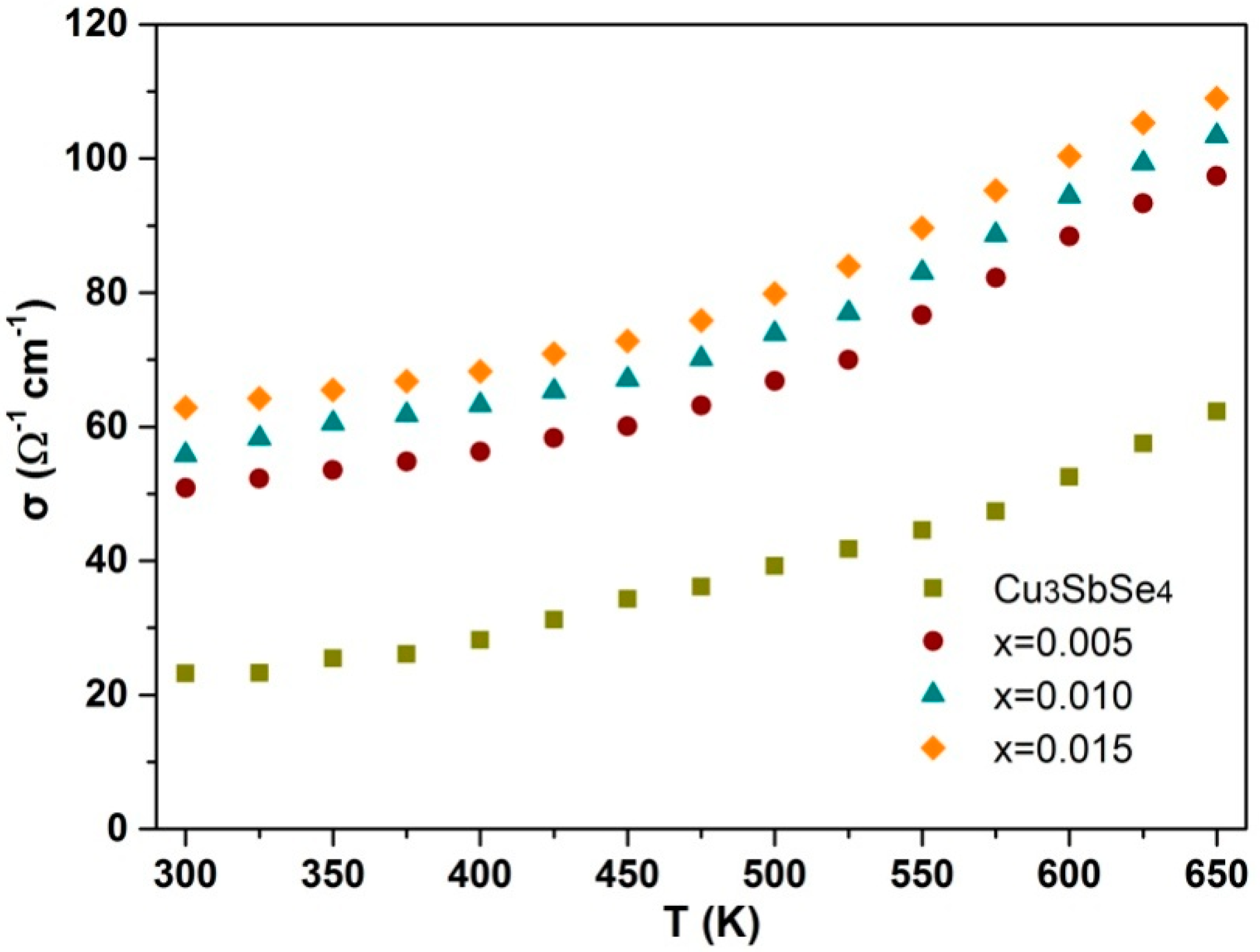
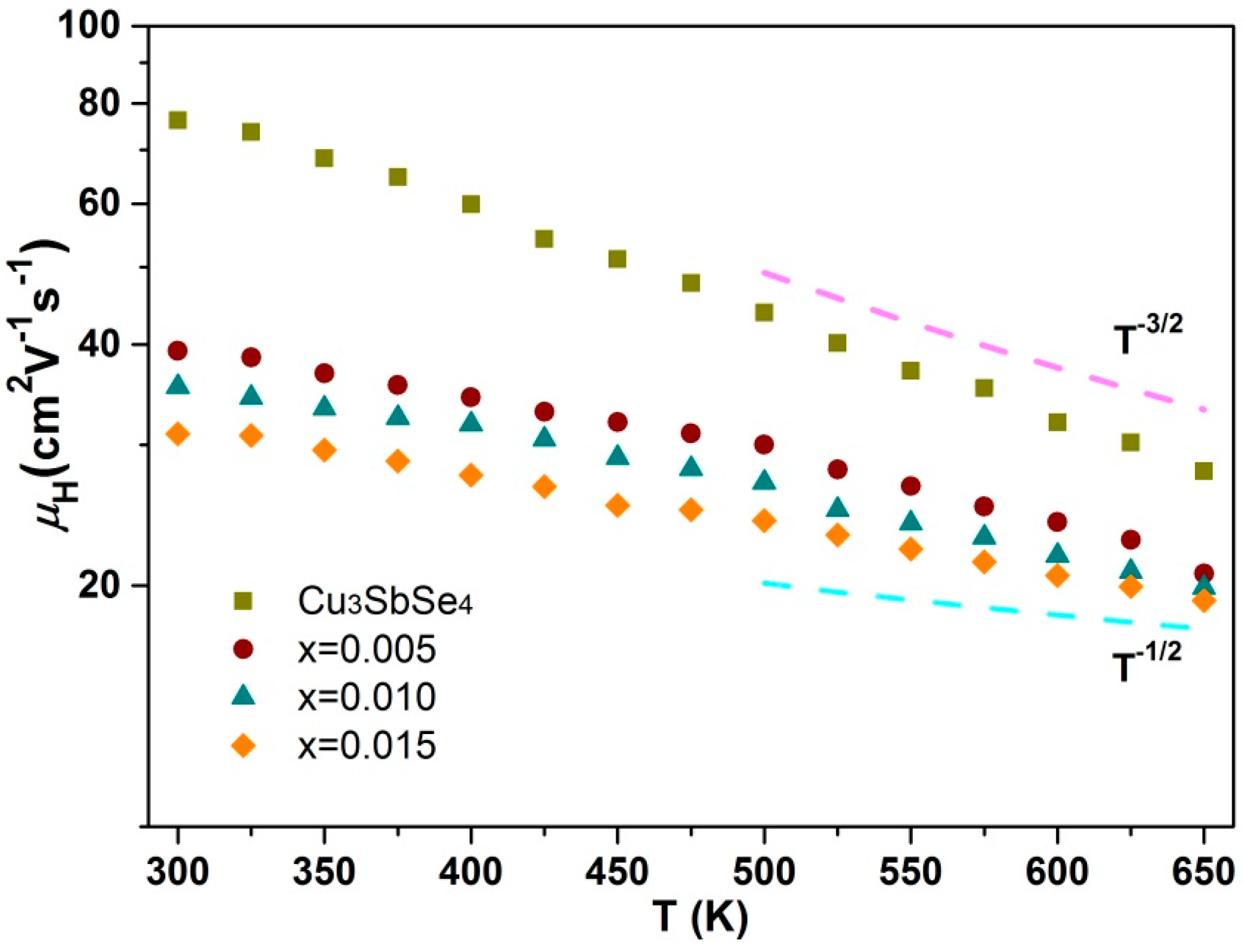
3.2. Electrical Performance
3.3. Thermal Conductivity
3.4. Figure of Merit
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Snyder, G.J.; Toberer, E.S. Complex thermoelectric materials. Nat. Mater. 2008, 7, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.Z.; Shi, X.Y.; LaLonde, A.; Wang, H.; Chen, L.D.; Snyder, G.J. Convergence of electronic bands for high performance bulk thermoelectric. Nature 2011, 473, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.Z.; Wang, H.; Snyder, G.J. Band Engineering of Thermoelectric Materials. Adv. Mater. 2012, 24, 6125–6135. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.; Chen, Z.G.; Pei, Y.Z.; Yang, L.; Zou, J. Limit of ZT enhancement in rocksalt structured chalcogenides by band convergence. Phys. Rev. B 2016, 94, 16120. [Google Scholar] [CrossRef]
- Hong, M.; Chen, Z.G.; Yang, L.; Zou, J. BixSb2−xTe3 nanoplates with enhanced thermoelectric performance due to sufficiently decoupled electronic transport properties and strong wide-frequency phonon scatterings. Nano Energy 2016, 20, 144–155. [Google Scholar]
- Fitriani, R.O.; Long, B.D.; Barma, M.C.; Riaz, M.; Sabri, M.F.; Said, S.M.; Saidur, R. A review on nanostructures of high temperature thermoelectric materials for waste heat recovery. Renew. Sustain. Energy Rev. 2016, 64, 635–659. [Google Scholar] [CrossRef]
- Lu, N.D.; Li, L.; Liu, M. A review of carrier thermoelectric-transport theory in organic semiconductors. Phys. Chem. Chem. Phys. 2016, 18, 19503–19525. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.; Chasapis, T.C.; Chen, Z.G.; Yang, L.; Kanatzidis, M.G.; Snyder, G.J.; Zou, J. N-type Bi2Te3−xSex Nanoplates with enhanced thermoelectric efficiency driven by wide-frequency phonon scatterings and synergistic carrier scatterings. ACS Nano 2016, 10, 4719–4727. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.; Chen, Z.G.; Yang, L.; Zou, J. Enhancing thermoelectric performance of Bi2Te3-based nanostructures through rational structure design. Nanoscale 2016, 8, 8681–8686. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.; Chen, Z.G.; Yang, L.; Chasapis, T.C.; Kang, S.D.; Zou, Y.C.; Auchterlonie, G.J.; Kanatzidis, M.G.; Snyder, G.J.; Zou, J. Enhancing the thermoelectric performance of SnSe1−xTex nanoplates through band engineering. J. Mater. Chem. A 2017, 5, 10713–10721. [Google Scholar]
- Kosuga, A.; Plirdpring, T.; Higashine, R.; Matsuzawa, M.; Kurosaki, K.; Yamanaka, S. High temperature thermoelectric properties of Cu1−xInTe2 with a chalcopyrite structure. Appl. Phys. Lett. 2012, 100, 042108. [Google Scholar] [CrossRef]
- Zeier, W.G.; Pei, Y.Z.; Pomrehn, G.; Day, T.; Heinz, N.; Heinrich, C.P.; Snyder, G.J.; Tremel, W. Phonon scattering through a local anisotropic structural disorder in the thermoelectric solid solution Cu2Zn1−xFexGeSe4. J. Am. Chem. Soc. 2013, 135, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.J.; Yu, B.; Wang, Y.X.; Zhu, Y.L.; Liu, X.J.; Yu, S.H.; Ren, Z.F. Colloidal synthesis of Cu2CdSnSe4 nanocrystals and hot pressing to enhance the thermoelectric figure of merit. J. Am. Chem. Soc. 2011, 133, 15910–15913. [Google Scholar] [CrossRef] [PubMed]
- Skoug, E.J.; Cain, J.D.; Majsztrik, P.; Kirkham, M.; Morelli, D.T. Doping effects on the thermoelectric properties of Cu3SbSe4. Sci. Adv. Mater. 2011, 3, 602–606. [Google Scholar] [CrossRef]
- Wei, T.R.; Wang, H.; Gibbs, Z.M.; Wu, C.F.; Snyder, G.J.; Li, J.F. Thermoelectric properties of Sn-doped p-type Cu3SbSe4: A compound with large effective mass and small band gap. J. Mater. Chem. A 2014, 2, 13527–13533. [Google Scholar] [CrossRef]
- Yang, C.Y.; Huang, F.Q.; Wu, L.M.; Xu, K. New stannite-like p-type thermoelectric material Cu3SbSe4. J. Phys. D Appl. Phys. 2011, 44, 295404. [Google Scholar] [CrossRef]
- Suzumura, A.; Watanabe, M.; Nagasako, N.; Asahi, R. Improvement in thermoelectric properties of Se-free Cu3SbSe4 compound. J. Electron. Mater. 2014, 43, 2356–2360. [Google Scholar] [CrossRef]
- Li, X.Y.; Li, D.; Xin, H.X.; Zhang, J.; Song, C.J.; Qin, X.Y. Effects of bismuth doping on the thermoelectric properties of Cu3SbSe4 at moderate temperature. J. Alloys Compd. 2013, 561, 105–108. [Google Scholar] [CrossRef]
- Kumar, A.; Dhama, P.; Saini, D.S.; Banerji, P. Effects of Zn substitution at a Cu site on the transport behavior and thermoelectric properties in Cu3SbSe4. RSC Adv. 2016, 6, 5528–5534. [Google Scholar] [CrossRef]
- Li, D.; Li, R.; Qin, X.Y.; Song, C.J.; Xin, H.X.; Zhang, J.; Guo, G.L.; Zou, T.H.; Zhu, X.G. Co-precipitation synthesis of nanostructured Cu3SbSe4 and its Sn-doped sample with high thermoelectric performance. Dalton Trans. 2014, 43, 1888–1896. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Y.; Qin, X.Y.; Li, D.; Li, X.Y.; Liu, Y.F.; Zhang, J.; Song, C.J.; Xin, H.X. Transport properties and enhanced thermoelectric performance of aluminum doped Cu3SbSe4. RSC Adv. 2015, 5, 31399–31403. [Google Scholar] [CrossRef]
- Chang, C.H.; Chen, C.L.; Chiu, W.T.; Chen, Y.Y. Enhanced thermoelectric properties of Cu3SbSe4 by germanium doping. Mater. Lett. 2017, 186, 227–230. [Google Scholar] [CrossRef]
- Dan, Z.; Yang, J.Y.; Jiang, Q.H.; Fu, L.W.; Xiao, Y.; Zhou, Z.W. Improvement of thermoelectric properties of Cu3SbSe4 compound by In doping. Mater. Des. 2016, 98, 150–154. [Google Scholar]
- Zhang, J.; Qin, X.Y.; Li, D.; Xin, H.X.; Song, C.J.; Li, L.L.; Zhu, X.G.; Wang, Z.M.; Wang, L. Enhanced thermoelectric performance of CuGaTe2 based composites incorporated with nanophase Cu2Se. J. Mater. Chem. A 2014, 2, 2891–2985. [Google Scholar] [CrossRef]
- Zhao, D.G.; Ning, J.A.; Wu, D.; Zuo, M. Enhanced thermoelectric performance of Cu2SnSe3 based composites incorporated with nano-fullerene. Materials 2016, 9, 629. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.Y.; Huang, F.Q.; Liu, M.L.; Chen, L.D. Thermoelectric properties of tetrahedrally bonded wide gap stannite compounds Cu2ZnSn1−xInxSe4. J. Am. Chem. Soc. 2009, 94, 122103. [Google Scholar] [CrossRef]
- Kim, H.S.; Gibbs, Z.M.; Tang, Y.L.; Wang, H.; Snyder, G.J. Characterization of Lorenz number with Seebeck coefficient measurement. APL Mater. 2015, 3, 041506. [Google Scholar] [CrossRef]
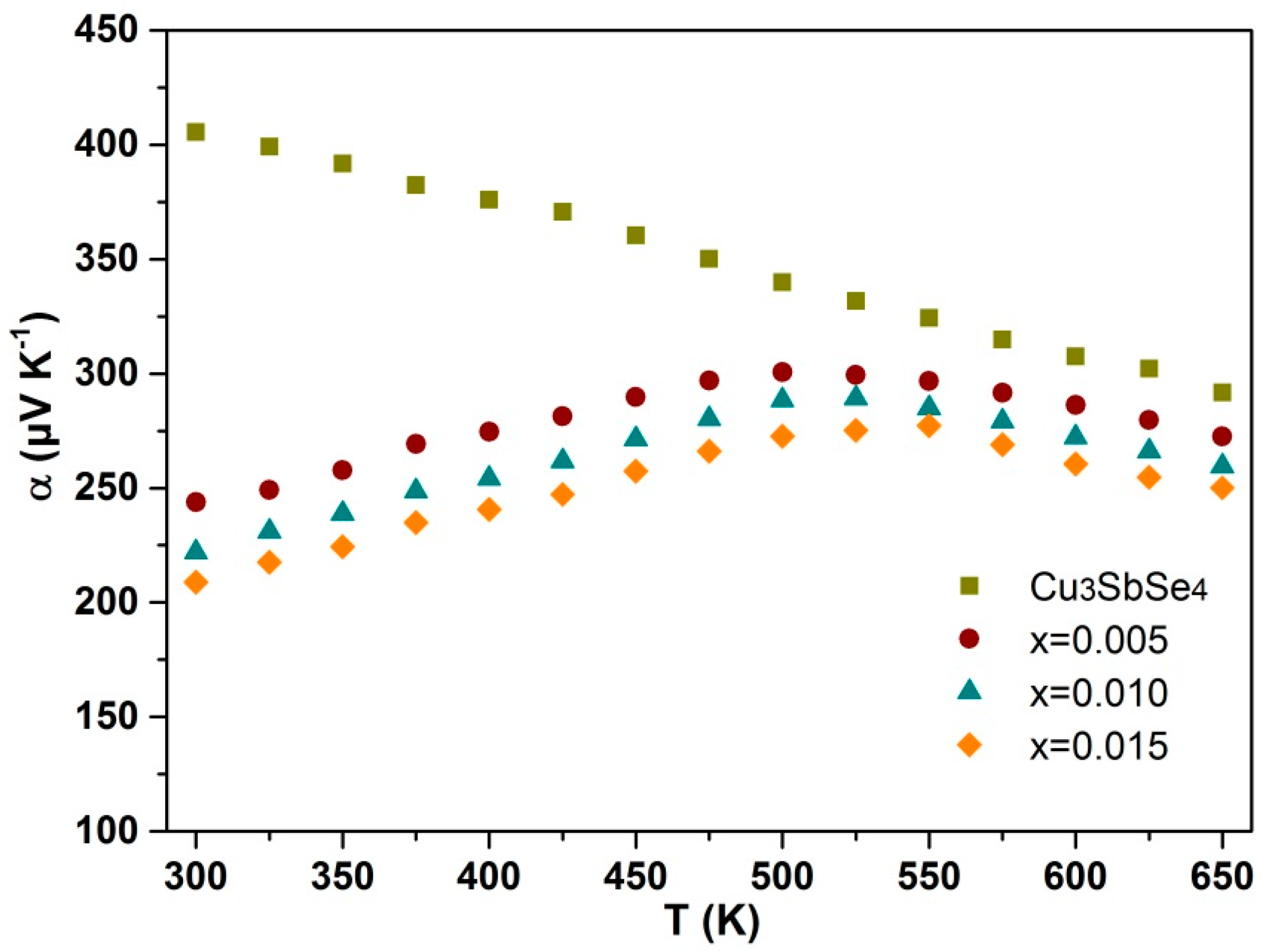
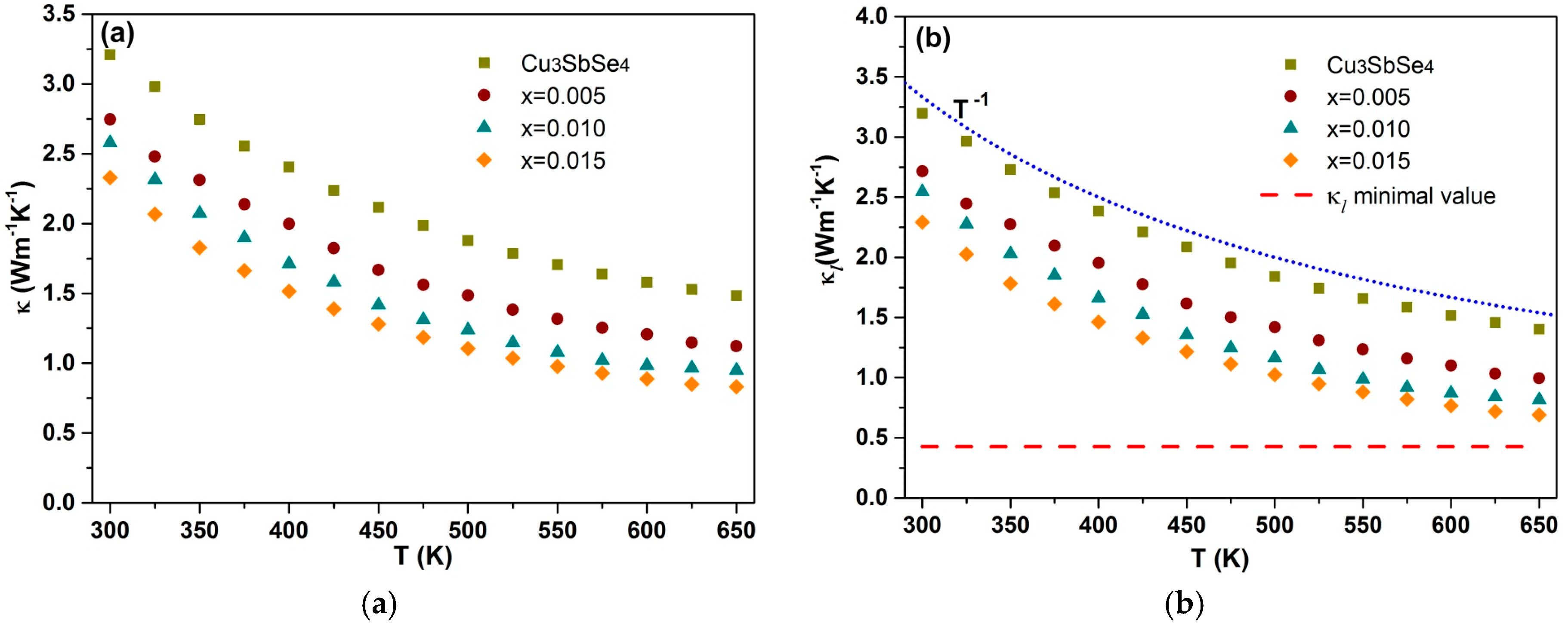
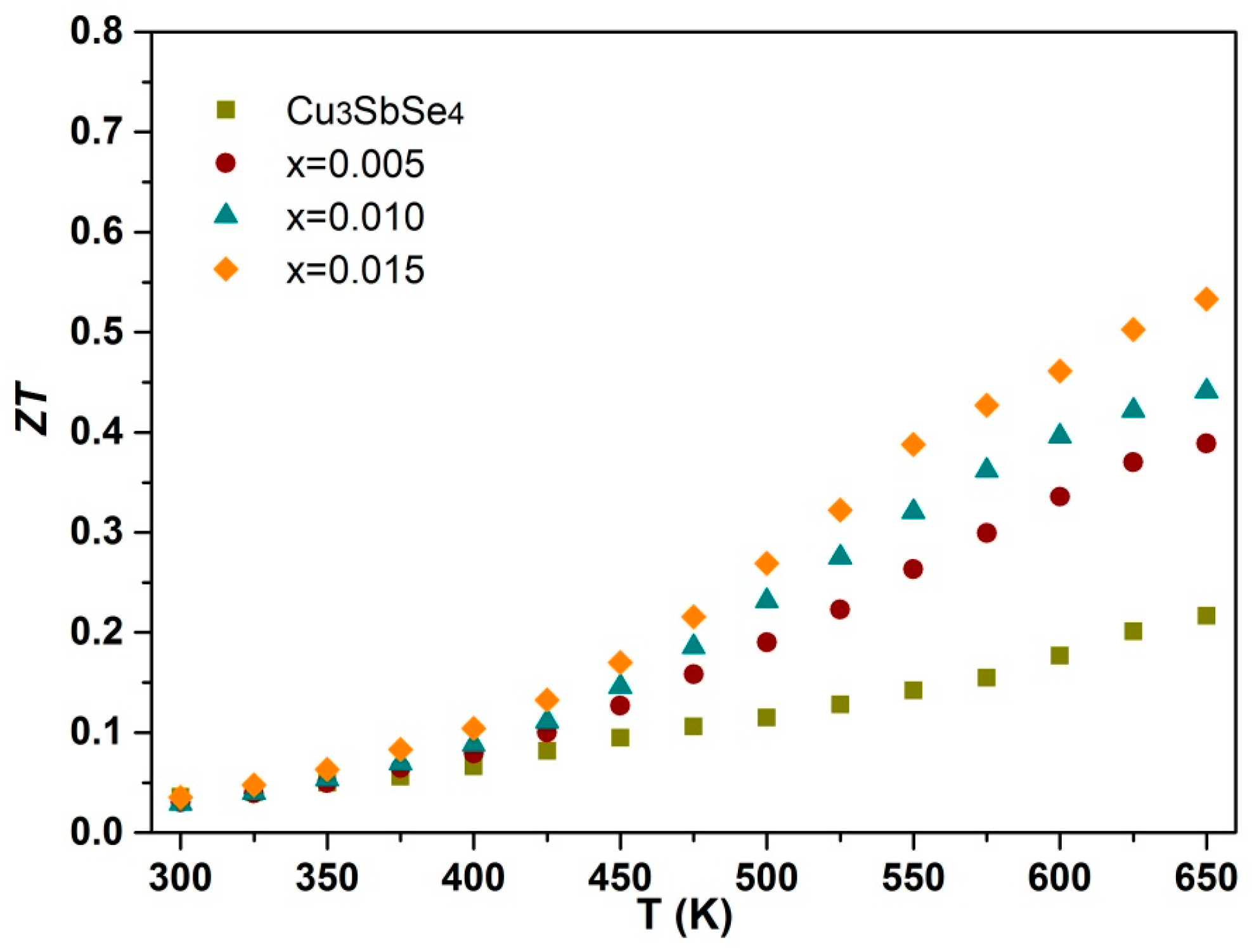
| x | κL (W m−1 K−1) | Relative Density | α (μV/K) | σ (Ω−1 cm−1) | p (1018 cm−3) | μH (cm2/Vs) | m* (m0) |
|---|---|---|---|---|---|---|---|
| 0 | 3.19 | 98.5% | 405 | 23.2 | 1.90 | 76.2 | 1.2 |
| 0.005 | 2.71 | 98.7% | 244 | 50.8 | 8.01 | 39.2 | 1.4 |
| 0.010 | 2.54 | 98.3% | 222 | 55.9 | 9.84 | 35.5 | 1.6 |
| 0.015 | 2.29 | 98.8% | 208 | 62.7 | 12.7 | 30.8 | 1.5 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, D.; Wu, D.; Bo, L. Enhanced Thermoelectric Properties of Cu3SbSe4 Compounds via Gallium Doping. Energies 2017, 10, 1524. https://doi.org/10.3390/en10101524
Zhao D, Wu D, Bo L. Enhanced Thermoelectric Properties of Cu3SbSe4 Compounds via Gallium Doping. Energies. 2017; 10(10):1524. https://doi.org/10.3390/en10101524
Chicago/Turabian StyleZhao, Degang, Di Wu, and Lin Bo. 2017. "Enhanced Thermoelectric Properties of Cu3SbSe4 Compounds via Gallium Doping" Energies 10, no. 10: 1524. https://doi.org/10.3390/en10101524
APA StyleZhao, D., Wu, D., & Bo, L. (2017). Enhanced Thermoelectric Properties of Cu3SbSe4 Compounds via Gallium Doping. Energies, 10(10), 1524. https://doi.org/10.3390/en10101524




