Desorption Kinetics and Mechanisms of CO2 on Amine-Based Mesoporous Silica Materials
Abstract
:1. Introduction
2. Experimental
2.1. Materials Preparation
2.2. Materials Characterization
2.3. CO2 Desorption Process
3. Results and Discussion
3.1. Material Characterization and Desorption Isotherms
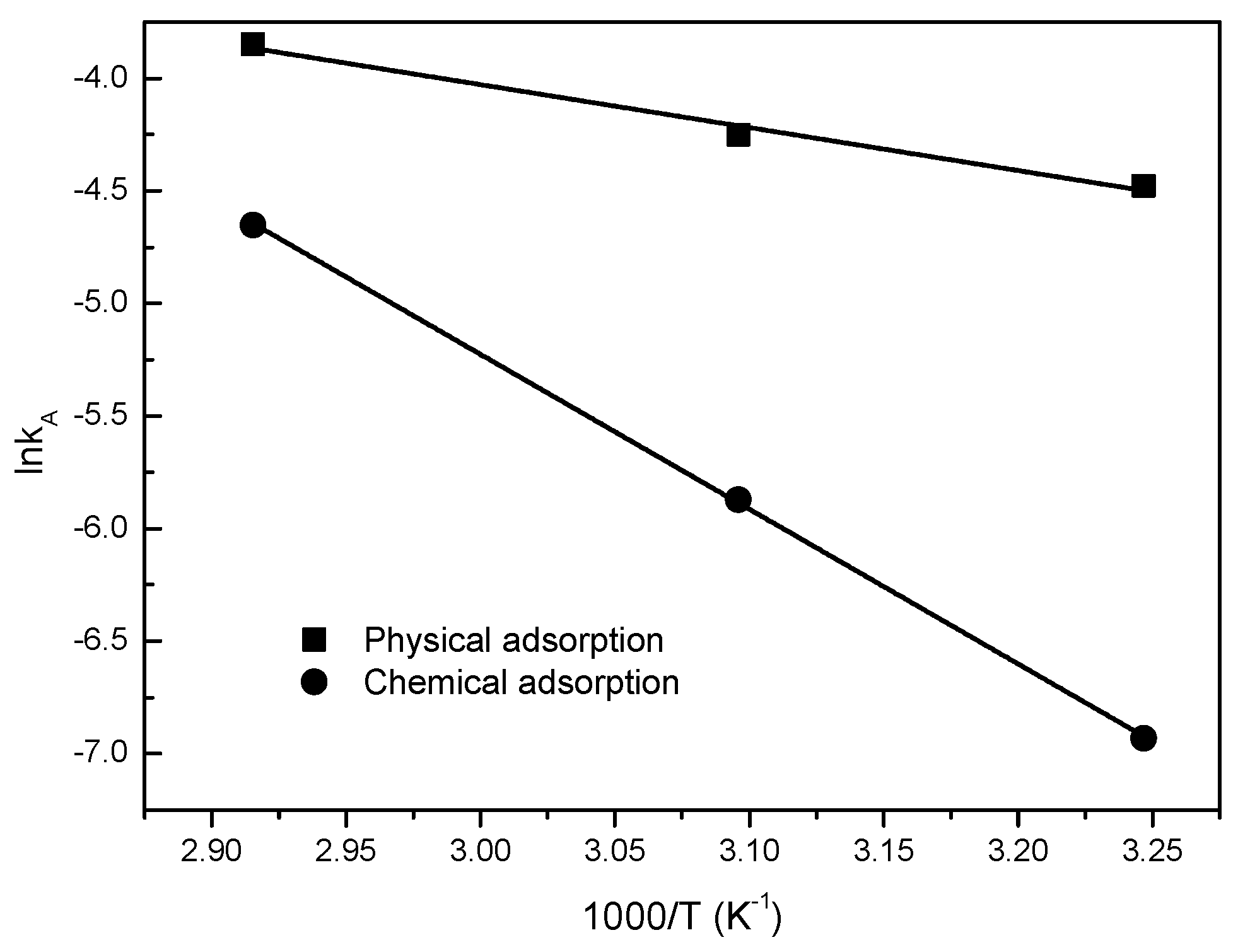
3.2. Desorption Kinetics
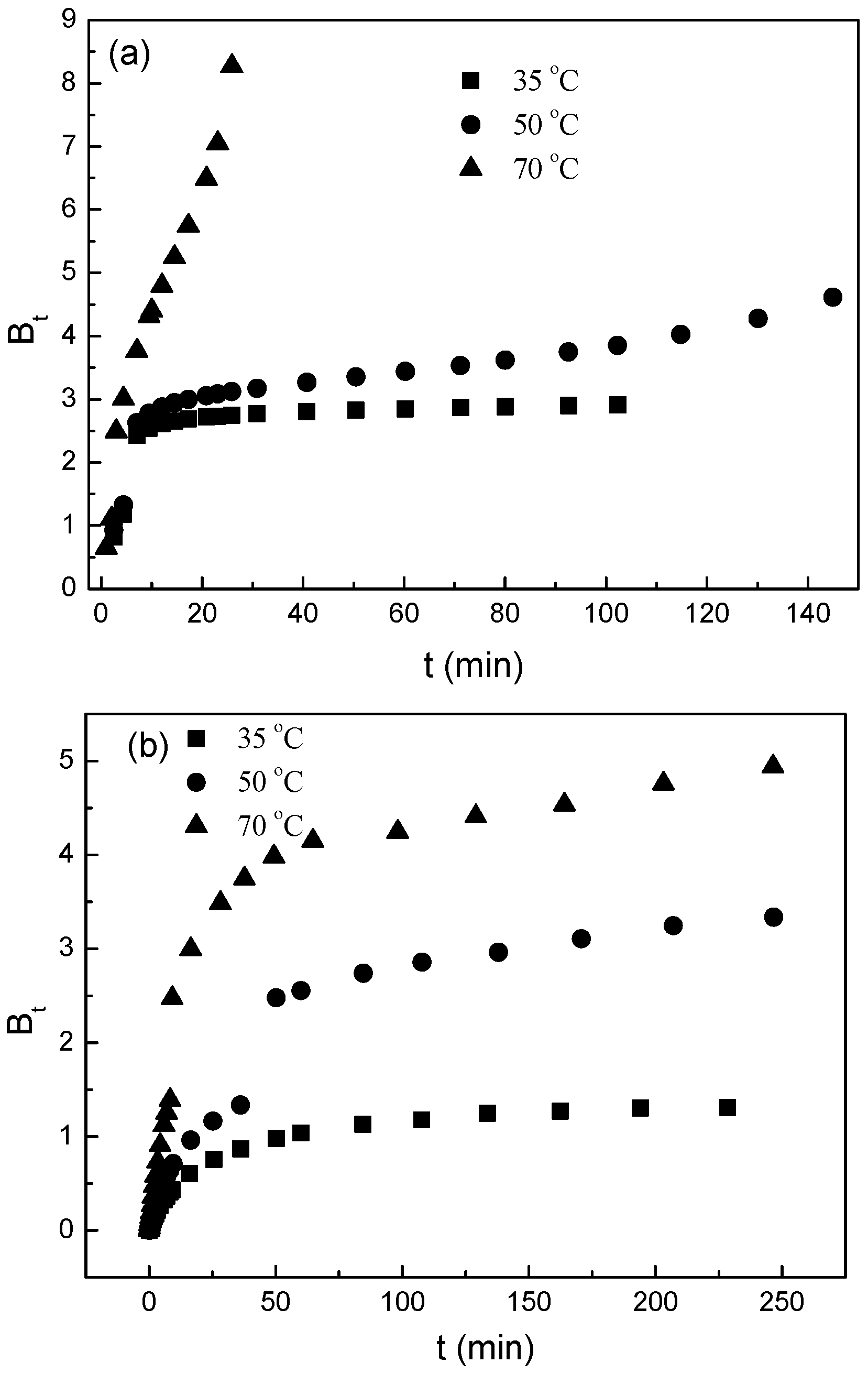
3.3. Desorption Mechnisms
- (a)
- The external resistance of mass transfer is only significant at the beginning of diffusion.
- (b)
- The diffusion direction is radial.
- (c)
- The pore diffusivity is constant.
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| TEPA | Tetraethylenepentamine |
| CCS | CO2 capture and sequestration |
| ZLC | Zero-Length Column |
| XRD | X-ray diffraction |
| MS | mass spectrometer |
References
- Tan, Y.; Nookuea, W.; Li, H.; Rhorin, E.; Yan, J. Property impacts on carbon capture and storage (CCS) processed: A review. Energy Convers. Manag. 2016, 118, 204–222. [Google Scholar] [CrossRef]
- Serna-Guerrero, R.; Belmabkhout, Y.; Sayari, A. Further investigations of CO2 capture using triamine-grafted pore-expanded mesoporous silica. Chem. Eng. J. 2010, 158, 513–519. [Google Scholar] [CrossRef]
- Bollini, P.; Didas, S.A.; Jones, C.W. Amine-oxide hybrid materials for acid gas separations. J. Mater. Chem. 2011, 21, 15100–15120. [Google Scholar] [CrossRef]
- Loganathan, S.; Ghoshal, A.K. Amine tethered pore-expanded MCM-41: A promising adsorbent for CO2 capture. Chem. Eng. J. 2017, 308, 817–839. [Google Scholar] [CrossRef]
- Chen, C.; Bhattacharjee, S. Trimodal nanoporous silica as a support for amine-based CO2 adsorbents: Improvement in adsorption capacity and kinetics. Appl. Surf. Sci. 2017, 396, 1515–1519. [Google Scholar] [CrossRef]
- Xu, X.; Song, C.; Andersen, J.M.; Mille, B.G.; Scaroni, A.W. Novel polyethylenimine-modified mesoporous molecular sieve of MCM-41 type as high-capacity adsorbent for CO2 capture. Energy Fuels 2002, 16, 1463–1469. [Google Scholar] [CrossRef]
- Bezerra, D.P.; Oliveira, R.S.; Vieira, R.S.; Cavalcante, C.L.; Azevedo, D.C.S. Adsorption of CO2 on nitrogen-enriched activated carbon and zeolite 13X. Adsorpt. J. Int. Adsorpt. Soc. 2011, 17, 235–246. [Google Scholar] [CrossRef]
- Liu, Z.; Teng, Y.; Zhang, K.; Chen, H.; Yang, Y. CO2 adsorption performance of different amine-based siliceous MCM-41 materials. J. Energy Chem. 2015, 24, 322–330. [Google Scholar] [CrossRef]
- Qi, G.; Wang, Y.; Estevez, L.; Duan, X.; Anako, N.; Park, A.-H.A.; Li, W.; Jones, C.W.; Giannelis, E.P. High efficiency nanocomposite sorbents for CO2 capture based on amine-functionalized mesoporous capsules. Energy Environ. Sci. 2011, 4, 444–452. [Google Scholar] [CrossRef]
- Sanz, R.; Calleja, G.; Arencibia, A.; Sanz-Pérez, E.S. CO2 capture with pore-expanded MCM-41 silica modified by double functionalization. Microporous Mesoporous Mater. 2015, 209, 167–171. [Google Scholar] [CrossRef]
- Vaidhyanathan, R.; Iremonger, S.S.; Dawson, K.W.; Shimizu, G.K.H. An amine-functionalized metal organic framework for preferential CO2 adsorption at low pressures. Chem. Commun. 2009, 5230–5232. [Google Scholar] [CrossRef] [PubMed]
- Eic, M.; Ruthven, D.M. A new experimental-technique for measurement of intracrystalline diffusivity. Zeolites 1988, 8, 40–45. [Google Scholar] [CrossRef]
- Cavalcante, CL., Jr.; Ruthven, D.M. Adsorption of Branched and Cyclic Paraffins in Silicalite. 1. Equilibrium. Ind. Eng. Chem. Res. 1995, 34, 177–184. [Google Scholar] [CrossRef]
- Friedrich, D.; Mangano, E.; Brandani, S. Automatic estimation of kinetic and isotherm parameters from ZLC experiments. Chem. Eng. Sci. 2015, 126, 616–624. [Google Scholar] [CrossRef]
- Hu, X.Y.; Mangano, E.; Friedrich, D.; Ahn, H.; Brandani, S. Diffusion mechanism of CO2 in 13X zeolite beads. Adsorption 2014, 20, 121–135. [Google Scholar] [CrossRef]
- Martín-Martínez, J.M.; Torregrosa-Maciá, R.; Mittelmeijer-Hazeleger, M.C. Mechanisms of adsorption of CO2 in the micropores of activated anthracite. Fuel 1995, 74, 111–114. [Google Scholar] [CrossRef]
- Bacsik, Z.; Ahlsten, N.; Ziadi, A.; Zhao, G.; Garcia-Bennett, A.E.; Martín-Matute, B.; Hedin, N. Mechanisms and kinetics for sorption of CO2 on bicontinuous mesoporous silica modified with n-propylamine. Langmuir 2011, 27, 11118–11128. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Teng, Y.; Zhang, K.; Cao, Y.; Pan, W. CO2 adsorption properties and thermal stability of different amine-impregnated MCM-41 materials. J. Fuel Chem. Technol. 2013, 41, 469–475. [Google Scholar] [CrossRef]
- Brandani, S.; Ruthven, D.M. Moments analysis of the Zero Length Column method. Ind. Eng. Chem. Res. 1996, 35, 315–319. [Google Scholar] [CrossRef]
- Yousef, R.I.; El-Eswed, B.; Al-Muhtaseb, A.H. Adsorption characteristics of natural zeolites as solid adsorbents for phenol removal from aqueous solutions: Kinetics, mechanism, and thermodynamics studies. Chem. Eng. J. 2011, 171, 1143–1149. [Google Scholar] [CrossRef]
- Serna-Guerrero, R.; Sayari, A. Modeling adsorption of CO2 on amine-functionalized mesoporous silica. 2: Kinetics and breakthrough curves. Chem. Eng. J. 2010, 161, 182–190. [Google Scholar] [CrossRef]
- Liu, F.Q.; Wang, L.; Huang, Z.G.; Li, C.Q.; Li, W.; Li, R.X.; Li, W.H. Amine-retheredadsorbents based on three-dimensional macroporoussilica for CO2 capture from simulated flue gas and air. ACS Appl. Mater. Interfaces 2014, 6, 4371–4381. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Yan, Q.; Kong, C.; Chen, L. Polyethyleneimine incorporated metal-organic frameworks adsorbent for highly selective CO2 capture. Sci. Rep. 2013, 3, 1859. [Google Scholar] [CrossRef] [PubMed]
- Dzubak, A.L.; Lin, L.-C.; Kim, J.; Swisher, J.A.; Poloni, R.; Maximoff, S.N.; Smit, B.; Gagliardi, L. Ab initio carbon capture in open-site metal–organic frameworks. Nat. Chem. 2012, 4, 810–816. [Google Scholar] [CrossRef] [PubMed]
- Wurzbacher, J.A.; Gebald, C.; Steinfeld, A. Separation of CO2 from air by temperature-vacuum swing adsorption using diamine-functionalized silica gel. Energy Environ. Sci. 2011, 4, 3584–3592. [Google Scholar] [CrossRef]
- Jeppu, G.P.; Clement, T.P. A modified Langmuir-Freundlich isotherm model for simulating pH-dependent adsorption effects. J. Contam. Hydrol. 2012, 129–130, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Gargiulo, N.; Peluso, A.; Aprea, P.; Pepe, F.; Caputo, D. CO2 Adsorption on polyethylenimine-functionalized SBA-15 mesoporous silica: Isotherms and modeling. J. Chem. Eng. Data 2014, 59, 896–902. [Google Scholar] [CrossRef]
- Haerifar, M.; Azizian, S. Mixed surface reaction and diffusion-controlled kinetic model for adsorption at the solid/solution interface. J. Phys. Chem. C 2013, 117, 8310–8317. [Google Scholar] [CrossRef]
- Wang, Y.X.; Liu, B.S.; Zheng, C. Preparation and adsorption properties of corncob-derived activated carbon with high surface area. J. Chem. Eng. Data 2010, 55, 4669–4676. [Google Scholar] [CrossRef]
- Kinefuchi, I.; Yamaguchi, H.; Sakiyama, Y.; Takagi, S.; Matsumoto, Y. Inhomogeneous decomposition of ultrathin oxide films on Si(100): Application of Avrami kinetics to thermal desorption spectra. J. Chem. Phys. 2008, 128, 164712. [Google Scholar] [CrossRef] [PubMed]
- Moura, K.O.; Pastore, H.O. Comparative adsorption of CO2 by mono-, di-, and triamino-organofunctionalized magnesium phyllosilicates. Environ. Sci. Technol. 2013, 47, 12201–12210. [Google Scholar] [CrossRef] [PubMed]
- Lopes, E.C.N.; dos Anjos, F.S.C.; Vieira, E.F.S.; Cestari, A.R. An alternative Avrami equation to evaluate kinetic parameters of the interaction of Hg(II) with thin chitosan membranes. J. Colloid Interface Sci. 2003, 263, 542–547. [Google Scholar] [CrossRef]
- Liu, Q.; Shi, J.; Zheng, S.; Tao, M.; He, Y.; Shi, Y. Kinetics studies of CO2 adsorption/desorption on amine-functionalized multiwalled carbon nanotubes. Ind. Eng. Chem. Res. 2014, 53, 11677–11683. [Google Scholar] [CrossRef]
- Sun, Z.; Fan, M.; Argyle, M. Desorption kinetics of the monoethanolamine/macroporous TiO2-based CO2 separation process. Energy Fuels 2011, 25, 2988–2996. [Google Scholar] [CrossRef]
- Serna-Guerrero, R.; Belmabkhout, Y.; Sayari, A. Modeling CO2 adsorption on amine-functionalized mesoporous silica: 1. A semi-empirical equilibrium model. Chem. Eng. J. 2010, 161, 173–181. [Google Scholar] [CrossRef]
- Loganathan, S.; Tikmani, M.; Edubilli, S.; Mishra, A.; Ghoshal, A.K. CO2 adsorption kinetics on mesoporous silica under wide range of pressure and temperature. Chem. Eng. J. 2014, 256, 1–8. [Google Scholar] [CrossRef]
- Rudzinski, W.; Plazinski, W. Theoretical description of the kinetics of solute adsorption at heterogeneous solid/solution interfaces: On the possibility of distinguishing between the diffusional and the surface reaction kinetics models. Appl. Surf. Sci. 2007, 253, 5827–5840. [Google Scholar] [CrossRef]
- Weber, W.J.; Morris, J.C. Kinetics of adsorption on carbon from solution. J. Sanit. Eng. Div. 1963, 89, 31–60. [Google Scholar]
- Reichenberg, D. Properties of ion-exchange resins in relation to their structure. III. Kinetics of exchange. J. Am. Chem. Soc. 1953, 75, 589–597. [Google Scholar] [CrossRef]
- Hameed, B.H.; Tan, I.A.W.; Ahmad, A.L. Adsorption isotherm, kinetic modeling and mechanism of 2,4,6-trichlorophenol on coconut husk-based activated carbon. Chem. Eng. J. 2008, 144, 235–244. [Google Scholar] [CrossRef]
- Sharma, P.; Das, M.R. Removal of a cationic dye from aqueous solution using grapheme oxide nanosheets: Investigation of adsorption parameters. J. Chem. Eng. Data 2013, 58, 151–158. [Google Scholar] [CrossRef]
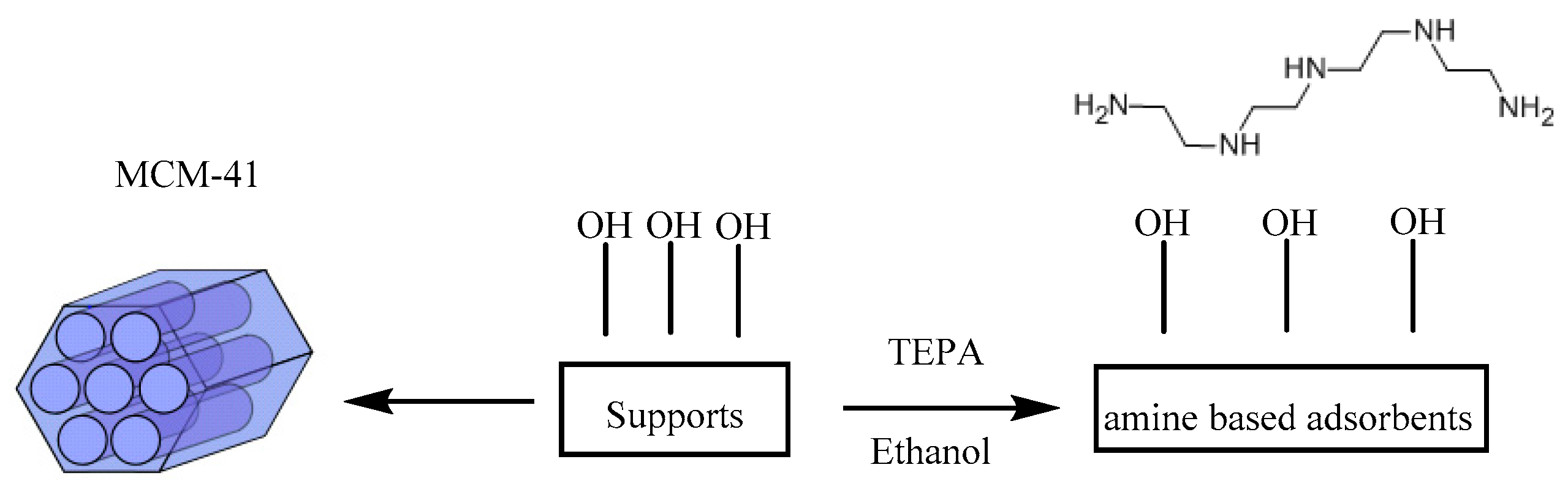
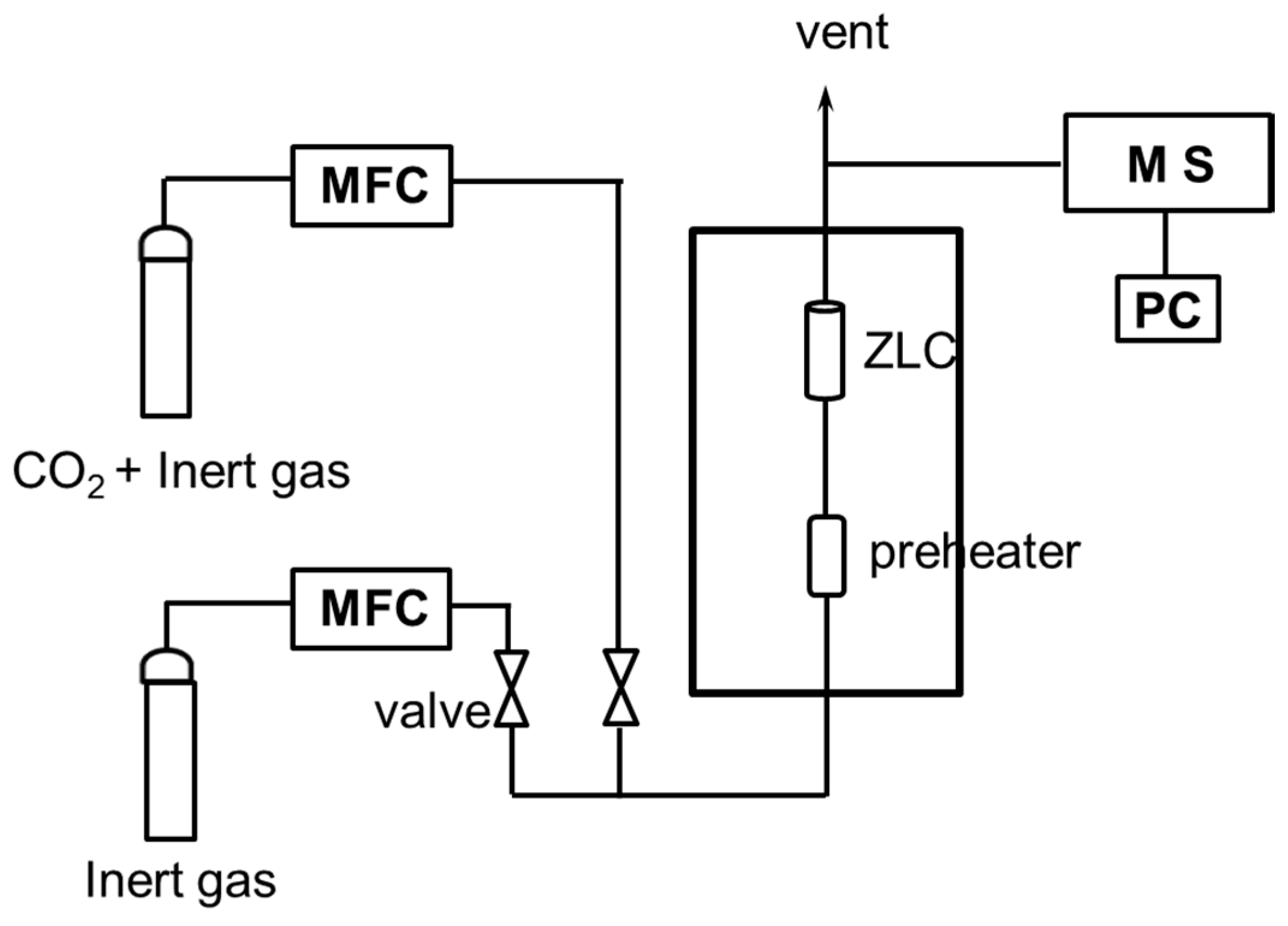
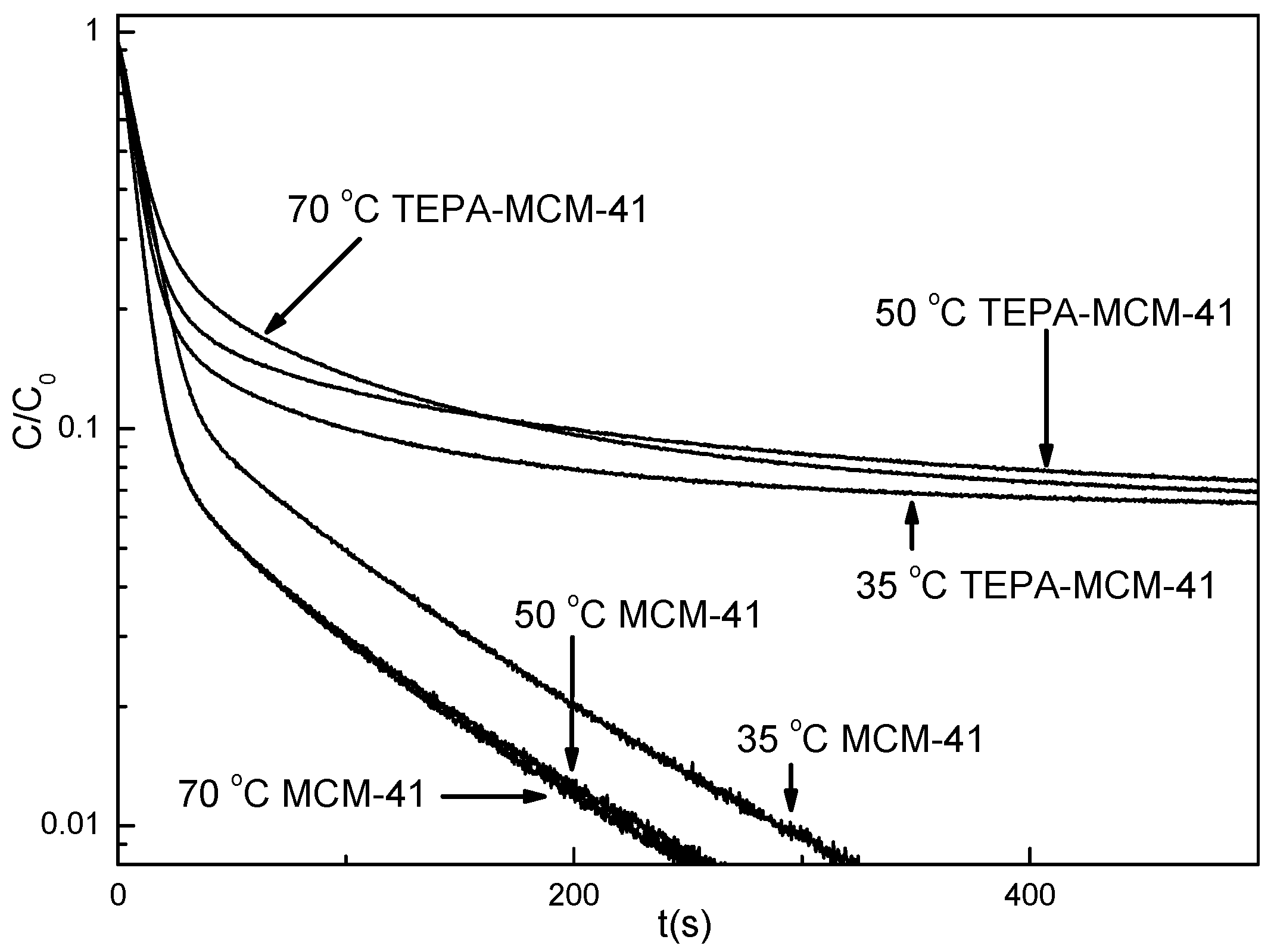
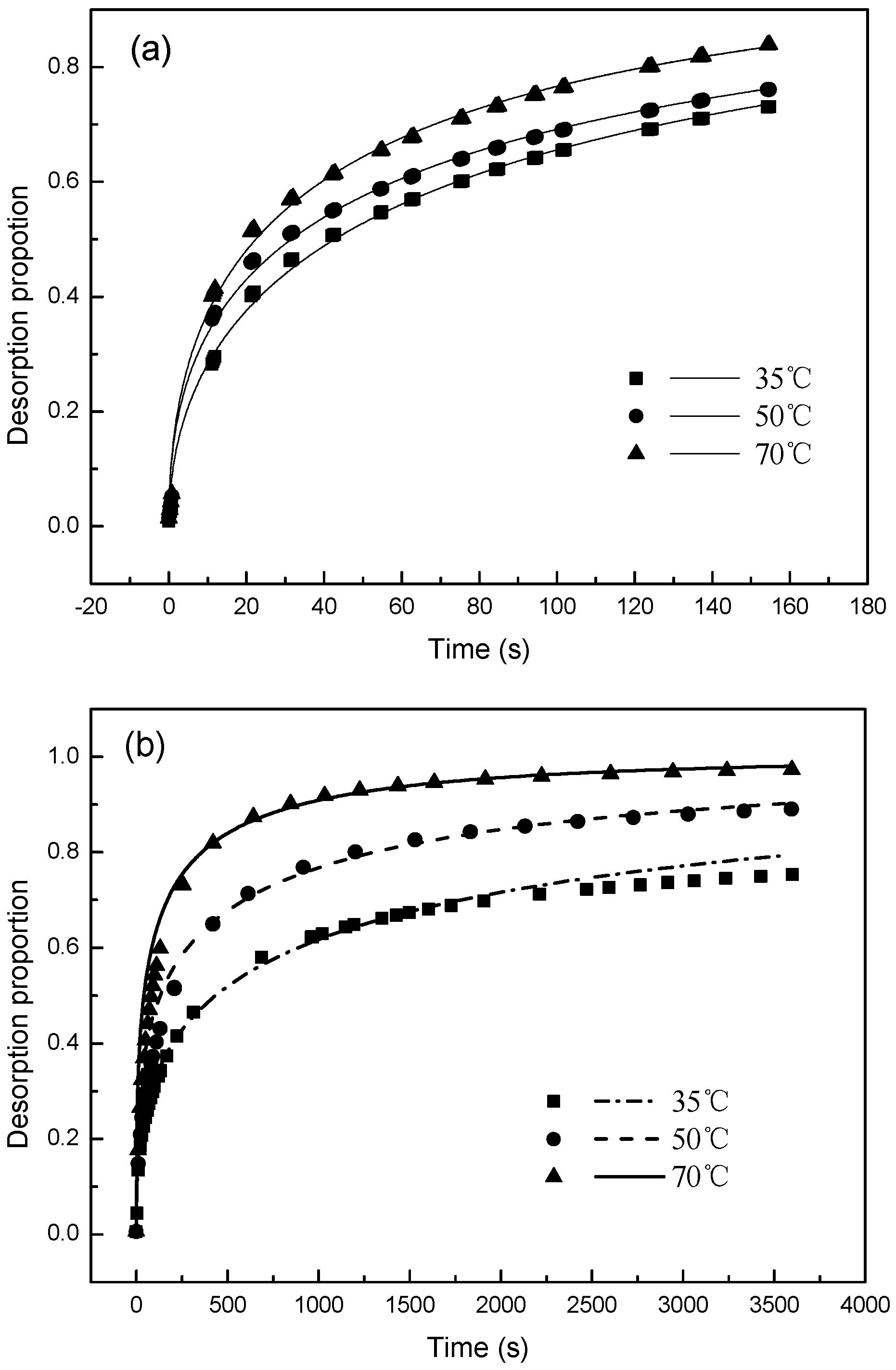

| MCM-41 | MCM-41-TEPA | ||||||
| Surface area (m2/g) | 865 | 364 | |||||
| Pore volume (cm3/g) | 1.00 | 0.4 | |||||
| Pore size (nm) | 4.64 | 3.62 | |||||
| Elemental Analysis | Desorption Capacity (mmol/g) | ||||||
| Samples | C% | H% | S% | N% | 35 °C | 50 °C | 70 °C |
| MCM-41 | 2.01 | 0.56 | 0.07 | 0.26 | 0.15 | 0.10 | 0.08 |
| MCM-41-TEPA | 19.32 | 4.81 | 0.10 | 10.22 | 1.35 | 1.10 | 0.80 |
| Parameters | Physical Desorption | Chemical Desorption | ||||||
| 35 °C | 50 °C | 70 °C | 35 °C | 50 °C | 70 °C | |||
| kA | 0.011 | 0.014 | 0.021 | 9.04 × 10−4 | 0.00282 | 0.00954 | ||
| nA | 0.507 | 0.459 | 0.495 | 0.391 | 0.366 | 0.388 | ||
| R2 | 0.995 | 0.993 | 0.994 | 0.993 | 0.983 | 0.984 | ||
| Parameters of Arrhenius Equation | Desorption Enthaply ΔH (kJ/mol) | |||||||
| A (s−1) | 5.4517 | 4.85 × 106 | MCM-41 | MCM-41-TEPA | ||||
| Ea (kJ/mol) | 15.864 | 57.152 | 25.42 | 70.56 | ||||
| R2 | 0.9768 | 0.9997 | ||||||
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teng, Y.; Liu, Z.; Xu, G.; Zhang, K. Desorption Kinetics and Mechanisms of CO2 on Amine-Based Mesoporous Silica Materials. Energies 2017, 10, 115. https://doi.org/10.3390/en10010115
Teng Y, Liu Z, Xu G, Zhang K. Desorption Kinetics and Mechanisms of CO2 on Amine-Based Mesoporous Silica Materials. Energies. 2017; 10(1):115. https://doi.org/10.3390/en10010115
Chicago/Turabian StyleTeng, Yang, Zhilin Liu, Gang Xu, and Kai Zhang. 2017. "Desorption Kinetics and Mechanisms of CO2 on Amine-Based Mesoporous Silica Materials" Energies 10, no. 1: 115. https://doi.org/10.3390/en10010115
APA StyleTeng, Y., Liu, Z., Xu, G., & Zhang, K. (2017). Desorption Kinetics and Mechanisms of CO2 on Amine-Based Mesoporous Silica Materials. Energies, 10(1), 115. https://doi.org/10.3390/en10010115





