Prediction of Cervical Cancer Outcome by Identifying and Validating a NAD+ Metabolism-Derived Gene Signature
Abstract
1. Introduction
2. Materials and Methods
2.1. Dataset Processing
2.2. NAD+ Metabolic-Related Genes
2.3. Construction and Validation of the NAD+ Metabolic-Related Gene Prognosis Model
2.4. Application and Assessment of Prognosis Model
2.5. Function Enrichment Analysis
2.6. Statistical Analysis
3. Results
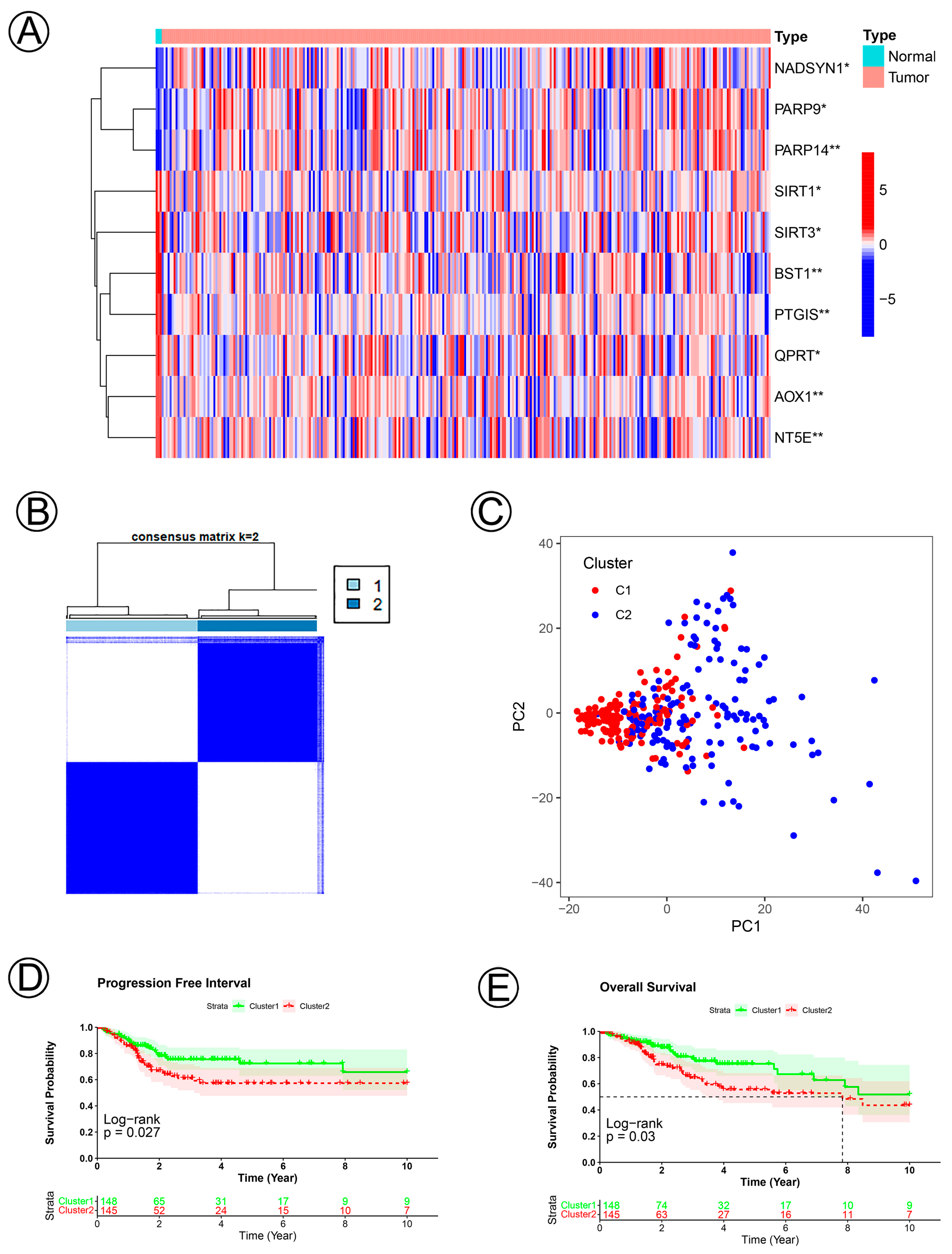
3.1. Clinical Data and Identification of NAD+ Metabolic-Related Genes in Cervical Cancer
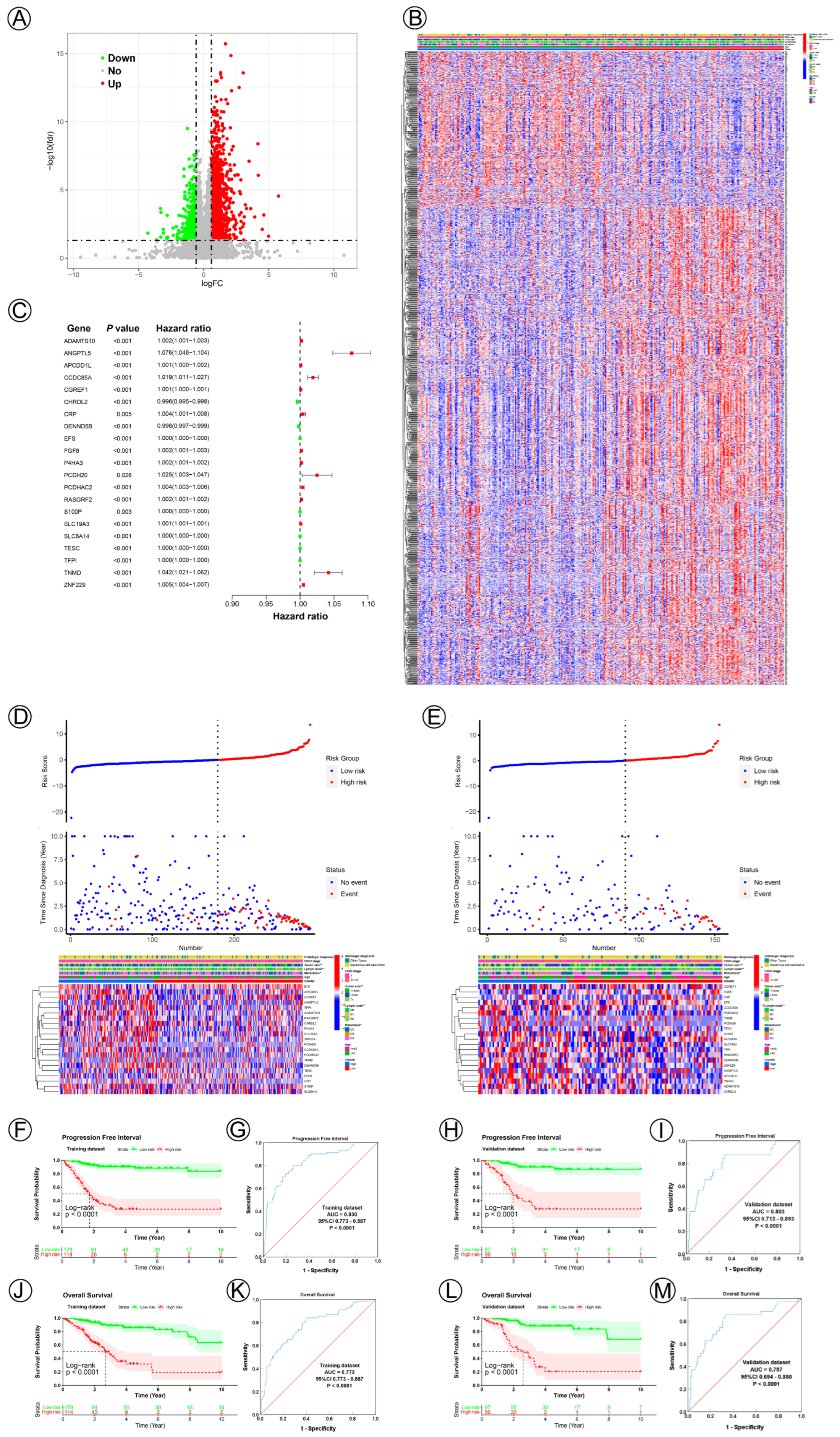
3.2. Establishment and Validation of the NAD+ Metabolic-Related Gene Signature of CC
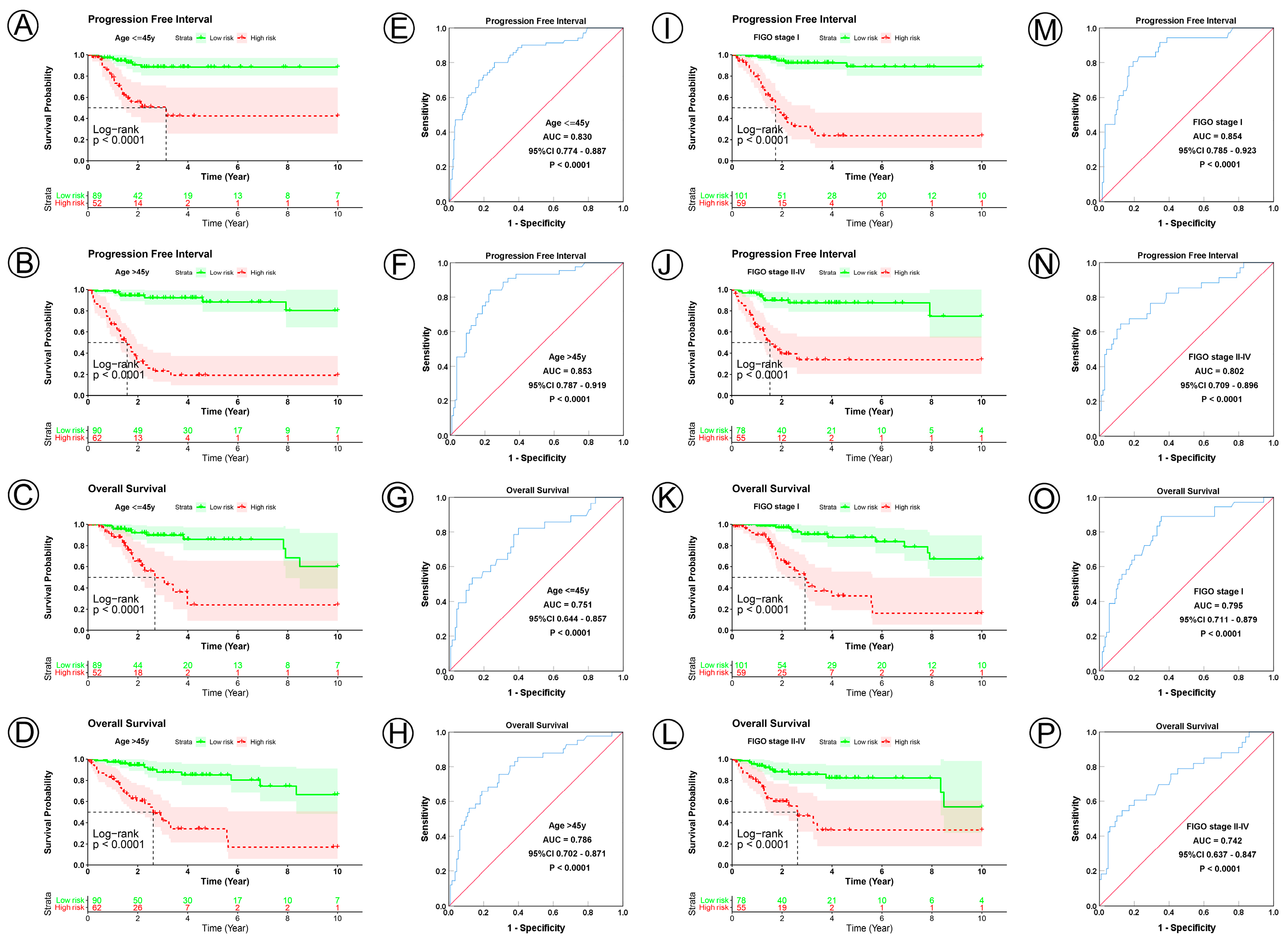
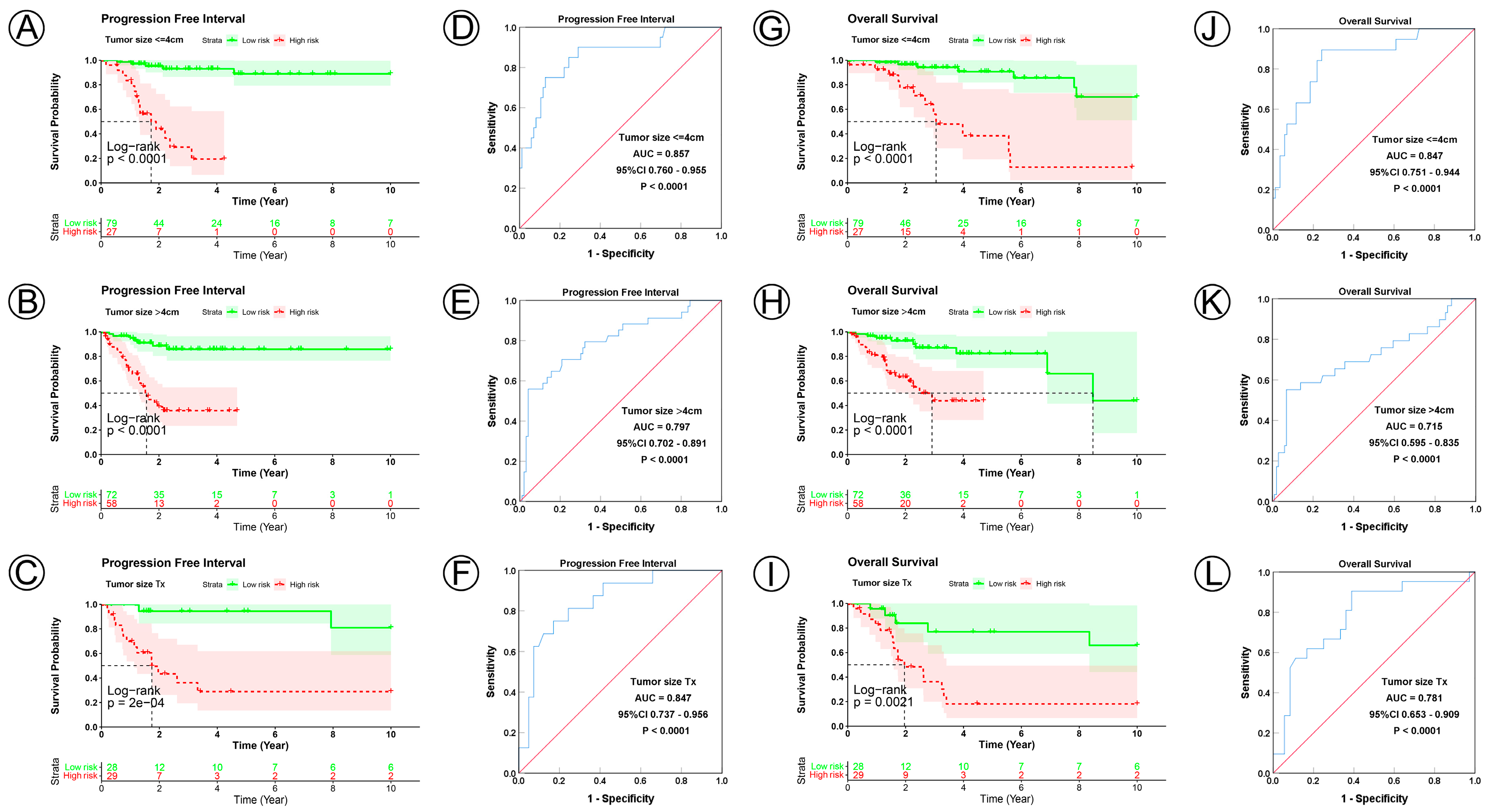
3.3. Prognostic Model Correlated with Clinicopathological Characteristics
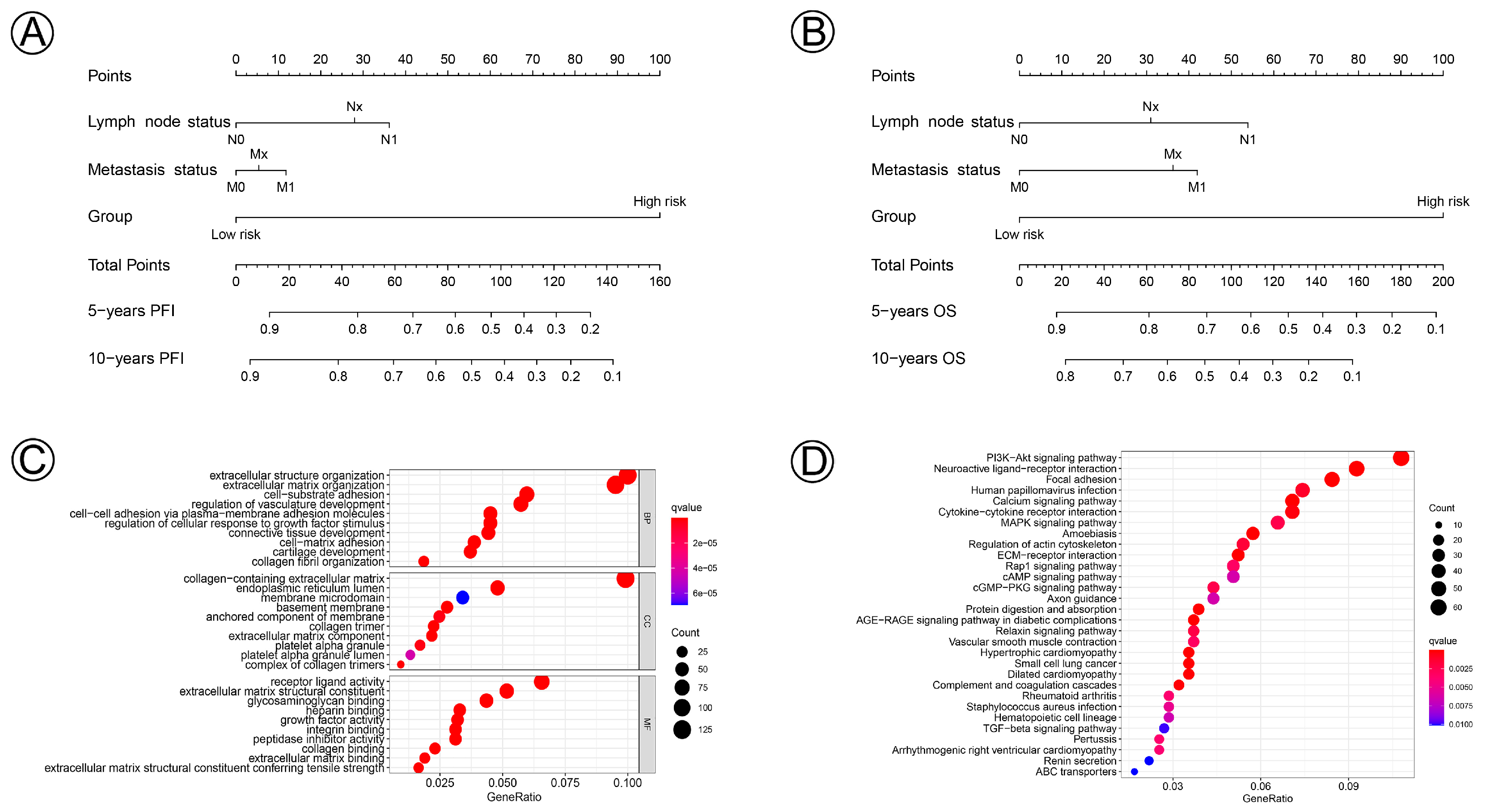
3.4. Prognostic Nomogram and Functional Enrichment Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Gardner, A.B.; Charo, L.M.; Mann, A.K.; Kapp, D.S.; Eskander, R.N.; Chan, J.K. Ovarian, uterine, and cervical cancer patients with distant metastases at diagnosis: Most common locations and outcomes. Clin. Exp. Metastasis 2020, 37, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Green, J.A.; Kirwan, J.J.; Tierney, J.; Vale, C.; Symonds, P.R.; Fresco, L.L.; Williams, C.; Collingwood, M. Concomitant chemotherapy and radiation therapy for cancer of the uterine cervix. Cochrane Database Syst. Rev. 2005, 20, CD002225. [Google Scholar] [CrossRef] [PubMed]
- Waggoner, S.E. Cervical cancer. Lancet 2003, 361, 2217–2225. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Sui, L. Metabolic reprogramming in cervical cancer and metabolomics perspectives. Nutr. Metab. 2021, 18, 93. [Google Scholar] [CrossRef]
- Shang, C.; Wang, W.; Liao, Y.; Chen, Y.; Liu, T.; Du, Q.; Huang, J.; Liang, Y.; Liu, J.; Zhao, Y.; et al. LNMICC Promotes Nodal Metastasis of Cervical Cancer by Reprogramming Fatty Acid Metabolism. Cancer Res. 2018, 78, 877–890. [Google Scholar] [CrossRef]
- Houtkooper, R.H.; Cantó, C.; Wanders, R.J.; Auwerx, J. The Secret Life of NAD+: An Old Metabolite Controlling New Metabolic Signaling Pathways. Endocr. Rev. 2010, 31, 194–223. [Google Scholar] [CrossRef]
- Berger, F.; Ramírez-Hernández, M.H.; Ziegler, M. The new life of a centenarian: Signalling functions of NAD(P). Trends Biochem. Sci. 2004, 29, 111–118. [Google Scholar] [CrossRef]
- Verdin, E. NAD(+) in aging, metabolism, and neurodegeneration. Science 2015, 350, 1208–1213. [Google Scholar] [CrossRef]
- Yaku, K.; Okabe, K.; Hikosaka, K.; Nakagawa, T. NAD Metabolism in Cancer Therapeutics. Front. Oncol. 2018, 8, 622. [Google Scholar] [CrossRef] [PubMed]
- Cantó, C.; Menzies, K.J.; Auwerx, J. NAD+ Metabolism and the Control of Energy Homeostasis: A Balancing Act between Mitochondria and the Nucleus. Cell Metab. 2015, 22, 31–53. [Google Scholar] [CrossRef] [PubMed]
- Ju, H.-Q.; Zhuang, Z.-N.; Li, H.; Tian, T.; Lu, Y.-X.; Fan, X.-Q.; Zhou, H.-J.; Mo, H.-Y.; Sheng, H.; Chiao, P.J.; et al. Regulation of the Nampt-mediated NAD salvage pathway and its therapeutic implications in pancreatic cancer. Cancer Lett. 2016, 379, 1–11. [Google Scholar] [CrossRef]
- Li, D.; Chen, N.N.; Cao, J.M.; Sun, W.P.; Zhou, Y.M.; Li, C.Y.; Wang, X.X. BRCA1 as a nicotinamide adenine dinucleotide (NAD)-dependent metabolic switch in ovarian cancer. Cell Cycle 2014, 13, 2564–2571. [Google Scholar] [CrossRef]
- Gujar, A.D.; Le, S.; Mao, D.D.; Dadey, D.Y.; Turski, A.; Sasaki, Y.; Aum, D.; Luo, J.; Dahiya, S.; Yuan, L.; et al. An NAD+-dependent transcriptional program governs self-renewal and radiation resistance in glioblastoma. Proc. Natl. Acad. Sci. USA 2016, 113, E8247–E8256. [Google Scholar] [CrossRef]
- Fricker, R.A.; Green, E.L.; Jenkins, S.I.; Griffin, S.M. The Influence of Nicotinamide on Health and Disease in the Central Nervous System. Int. J. Tryptophan Res. 2018, 11, 1178646918776658. [Google Scholar] [CrossRef] [PubMed]
- Maiese, K.; Chong, Z.Z. Nicotinamide: Necessary nutrient emerges as a novel cytoprotectant for the brain. Trends Pharmacol. Sci. 2003, 24, 228–232. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, Y.; Jiang, C.; Fang, Z.; Zhang, Z.; Lin, X.; Sun, L.; Jiang, W. Nicotinamide induces mitochondrial-mediated apoptosis through oxidative stress in human cervical cancer HeLa cells. Life Sci. 2017, 181, 62–69. [Google Scholar] [CrossRef]
- Vora, M.; Alattia, L.A.; Ansari, J.; Ong, M.; Cotelingam, J.; Coppola, D.; Shackelford, R. Nicotinamide Phosphoribosyl Transferase a Reliable Marker of Progression in Cervical Dysplasia. Anticancer Res. 2017, 37, 4821–4825. [Google Scholar] [CrossRef]
- Wilkerson, M.D.; Hayes, D.N. ConsensusClusterPlus: A class discovery tool with confidence assessments and item tracking. Bioinformatics 2010, 26, 1572–1573. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.-G.; Han, Y.; He, Q.-Y. clusterProfiler: An R Package for Comparing Biological Themes Among Gene Clusters. OMICS 2012, 16, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.; Young, D.A.; Lu, Z.-H.; Wang, T.; Meier, T.I.; Shepard, R.L.; Roth, K.; Zhai, Y.; Huss, K.; Kuo, M.-S.; et al. Pharmacological Inhibition of Nicotinamide Phosphoribosyltransferase (NAMPT), an Enzyme Essential for NAD+ Biosynthesis, in Human Cancer Cells: Metabolic Basis and Potential Clinical Implications. J. Biol. Chem. 2013, 288, 3500–3511. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Kim, L.J.; Wang, X.; Wu, Q.; Sanvoranart, T.; Hubert, C.G.; Prager, B.C.; Wallace, L.C.; Jin, X.; Mack, S.C.; et al. Nicotinamide metabolism regulates glioblastoma stem cell maintenance. JCI Insight 2017, 2, e90019. [Google Scholar] [CrossRef]
- Revollo, J.R.; Grimm, A.A.; Imai, S. The NAD biosynthesis pathway mediated by nicotinamide phosphoribosyltransferase regulates Sir2 activity in mammalian cells. J. Biol. Chem. 2004, 279, 50754–50763. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, B.E.; Sharif, T.; Martell, E.; Dai, C.; Kim, Y.; Lee, P.W.; Gujar, S.A. NAD+ salvage pathway in cancer metabolism and therapy. Pharmacol. Res. 2016, 114, 274–283. [Google Scholar] [CrossRef]
- Wang, B.; Hasan, M.K.; Alvarado, E.; Yuan, H.; Wu, H.; Chen, W.Y. NAMPT overexpression in prostate cancer and its contribution to tumor cell survival and stress response. Oncogene 2011, 30, 907–921. [Google Scholar] [CrossRef]
- Behrouzfar, K.; Alaee, M.; Nourbakhsh, M.; Gholinejad, Z.; Golestani, A. Extracellular NAMPT/visfatin causes p53 deacetylation via NAD production and SIRT1 activation in breast cancer cells. Cell Biochem. Funct. 2017, 35, 327–333. [Google Scholar] [CrossRef]
- Cantó, C.; Gerhart-Hines, Z.; Feige, J.N.; Lagouge, M.; Noriega, L.; Milne, J.C.; Elliott, P.J.; Puigserver, P.; Auwerx, J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 2009, 458, 1056–1060. [Google Scholar] [CrossRef]
- Tae, I.H.; Park, E.Y.; Dey, P.; Son, J.Y.; Lee, S.-Y.; Jung, J.H.; Saloni, S.; Kim, M.-H.; Kim, H.S. Novel SIRT1 inhibitor 15-deoxy-Δ12,14-prostaglandin J2 and its derivatives exhibit anticancer activity through apoptotic or autophagic cell death pathways in SKOV3 cells. Int. J. Oncol. 2018, 53, 2518–2530. [Google Scholar] [CrossRef]
- De, U.; Son, J.Y.; Sachan, R.; Park, Y.J.; Kang, D.; Yoon, K.; Lee, B.M.; Kim, I.S.; Moon, H.R.; Kim, H.S. A New Synthetic Histone Deacetylase Inhibitor, MHY2256, Induces Apoptosis and Autophagy Cell Death in Endometrial Cancer Cells via p53 Acetylation. Int. J. Mol. Sci. 2018, 19, 2743. [Google Scholar] [CrossRef]
- Park, E.Y.; Woo, Y.; Kim, S.J.; Kim, D.H.; Lee, E.K.; De, U.; Kim, K.S.; Lee, J.; Jung, J.H.; Ha, K.-T.; et al. Anticancer Effects of a New SIRT Inhibitor, MHY2256, against Human Breast Cancer MCF-7 Cells via Regulation of MDM2-p53 Binding. Int. J. Biol. Sci. 2016, 12, 1555–1567. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liu, J.; Park, J.; Rai, P.; Zhai, R.G. Subcellular compartmentalization of NAD+ and its role in cancer: A sereNADe of metabolic melodies. Pharmacol. Ther. 2019, 200, 27–41. [Google Scholar] [CrossRef]
- Liao, S.; Xiao, S.; Chen, H.; Zhang, M.; Chen, Z.; Long, Y.; Gao, L.; Zhu, G.; He, J.; Peng, S.; et al. CD38 enhances the proliferation and inhibits the apoptosis of cervical cancer cells by affecting the mitochondria functions. Mol. Carcinog. 2017, 56, 2245–2257. [Google Scholar] [CrossRef] [PubMed]
- Huo, X.; Zhou, X.; Peng, P.; Yu, M.; Zhang, Y.; Yang, J.; Cao, D.; Sun, H.; Shen, K. Identification of a Six-Gene Signature for Predicting the Overall Survival of Cervical Cancer Patients. OncoTargets Ther. 2021, 14, 809–822. [Google Scholar] [CrossRef] [PubMed]
- Tinholt, M.; Vollan, H.K.M.; Sahlberg, K.K.; Jernström, S.; Kaveh, F.; Lingjærde, O.C.; Kåresen, R.; Sauer, T.; Kristensen, V.; Børresen-Dale, A.-L.; et al. Tumor expression, plasma levels and genetic polymorphisms of the coagulation inhibitor TFPI are associated with clinicopathological parameters and survival in breast cancer, in contrast to the coagulation initiator TF. Breast Cancer Res. 2015, 17, 44. [Google Scholar] [CrossRef]
- Wu, Z.; Boonmars, T.; Nagano, I.; Boonjaraspinyo, S.; Srinontong, P.; Ratasuwan, P.; Narong, K.; Nielsen, P.S.; Maekawa, Y. Significance of S100P as a biomarker in diagnosis, prognosis and therapy of opisthorchiasis-associated cholangiocarcinoma. Int. J. Cancer 2016, 138, 396–408. [Google Scholar] [CrossRef]
- Hwang, H.S.; An, J.; Kang, H.J.; Oh, B.; Oh, Y.J.; Oh, J.-H.; Kim, W.; Sung, C.O.; Shim, J.H.; Yu, E. Prognostic Molecular Indices of Resectable Hepatocellular Carcinoma: Implications of S100P for Early Recurrence. Ann. Surg. Oncol. 2021, 28, 6466–6478. [Google Scholar] [CrossRef]
- Fullár, A.; Dudás, J.; Oláh, L.; Hollósi, P.; Papp, Z.; Sobel, G.; Karászi, K.; Paku, S.; Baghy, K.; Kovalszky, I. Remodeling of extracellular matrix by normal and tumor-associated fibroblasts promotes cervical cancer progression. BMC Cancer 2015, 15, 256. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, L.; Wan, D.; Zhou, L.; Zheng, S.; Lin, S.; Qiao, Y. Extracellular matrix and its therapeutic potential for cancer treatment. Signal Transduct. Target. Ther. 2021, 6, 153. [Google Scholar] [CrossRef]
- Bahrami, A.; Hasanzadeh, M.; Hassanian, S.M.; ShahidSales, S.; Ghayour-Mobarhan, M.; Ferns, G.A.; Avan, A. The Potential Value of the PI3K/Akt/mTOR Signaling Pathway for Assessing Prognosis in Cervical Cancer and as a Target for Therapy. J. Cell. Biochem. 2017, 118, 4163–4169. [Google Scholar] [CrossRef]
- Branca, M.; Ciotti, M.; Santini, D.; Bonito, L.D.; Benedetto, A.; Giorgi, C.; Paba, P.; Favalli, C.; Costa, S.; Agarossi, A.; et al. Activation of the ERK/MAP kinase pathway in cervical intraepithelial neoplasia is related to grade of the lesion but not to high-risk human papillomavirus, virus clearance, or prognosis in cervical cancer. Am. J. Clin. Pathol. 2004, 122, 902–911. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Chen, L.; Xie, Z.; Chen, J.; Li, L.; Lin, A. Identification of NAD+ Metabolism-Derived Gene Signatures in Ovarian Cancer Prognosis and Immunotherapy. Front. Genet. 2022, 13, 905238. [Google Scholar] [CrossRef] [PubMed]
| Variable | Training Dataset | Validation Dataset | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | Risk Group | χ2 | p Value | Total | Risk Group | χ2 | p Value | |||
| Lower | Higher | Lower | Higher | |||||||
| n = 293 | n = 717 | n =308 | n = 153 | 97 | 56 | |||||
| Age, y | ||||||||||
| ≤45 | 141 | 89 | 52 | 0.470 | 0.493 | 75 | 52 | 23 | 2.233 | 0.135 |
| >45 | 152 | 90 | 62 | 78 | 45 | 33 | ||||
| FIGO stage | ||||||||||
| I | 160 | 101 | 59 | 0.613 | 0.434 | 90 | 60 | 30 | 1.006 | 0.316 |
| II–IV | 133 | 78 | 55 | 63 | 37 | 26 | ||||
| Tumor size status | ||||||||||
| ≤4 cm | 106 | 79 | 27 | 13.268 | 0.001 | 58 | 49 | 9 | 19.358 | <0.001 |
| >4 cm | 130 | 72 | 58 | 64 | 35 | 29 | ||||
| Tx | 57 | 28 | 29 | 31 | 13 | 18 | ||||
| Lymph node status | ||||||||||
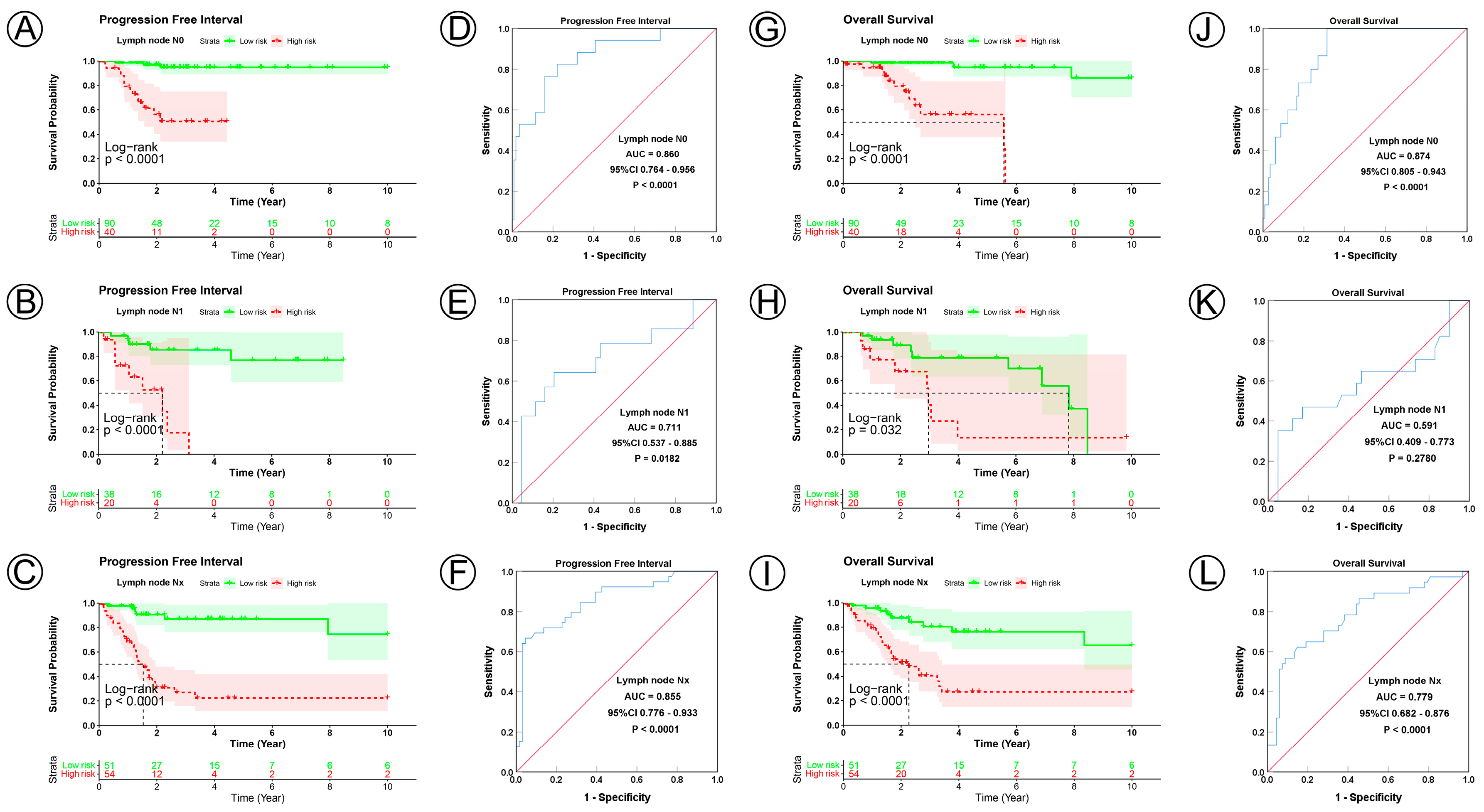
| N0 | 130 | 90 | 40 | 11.026 | 0.004 | 66 | 51 | 15 | 12.441 | 0.002 |
| N1 | 58 | 38 | 20 | 31 | 20 | 11 | ||||
| Nx | 105 | 51 | 54 | 56 | 26 | 30 | ||||
| Metastasis status | ||||||||||
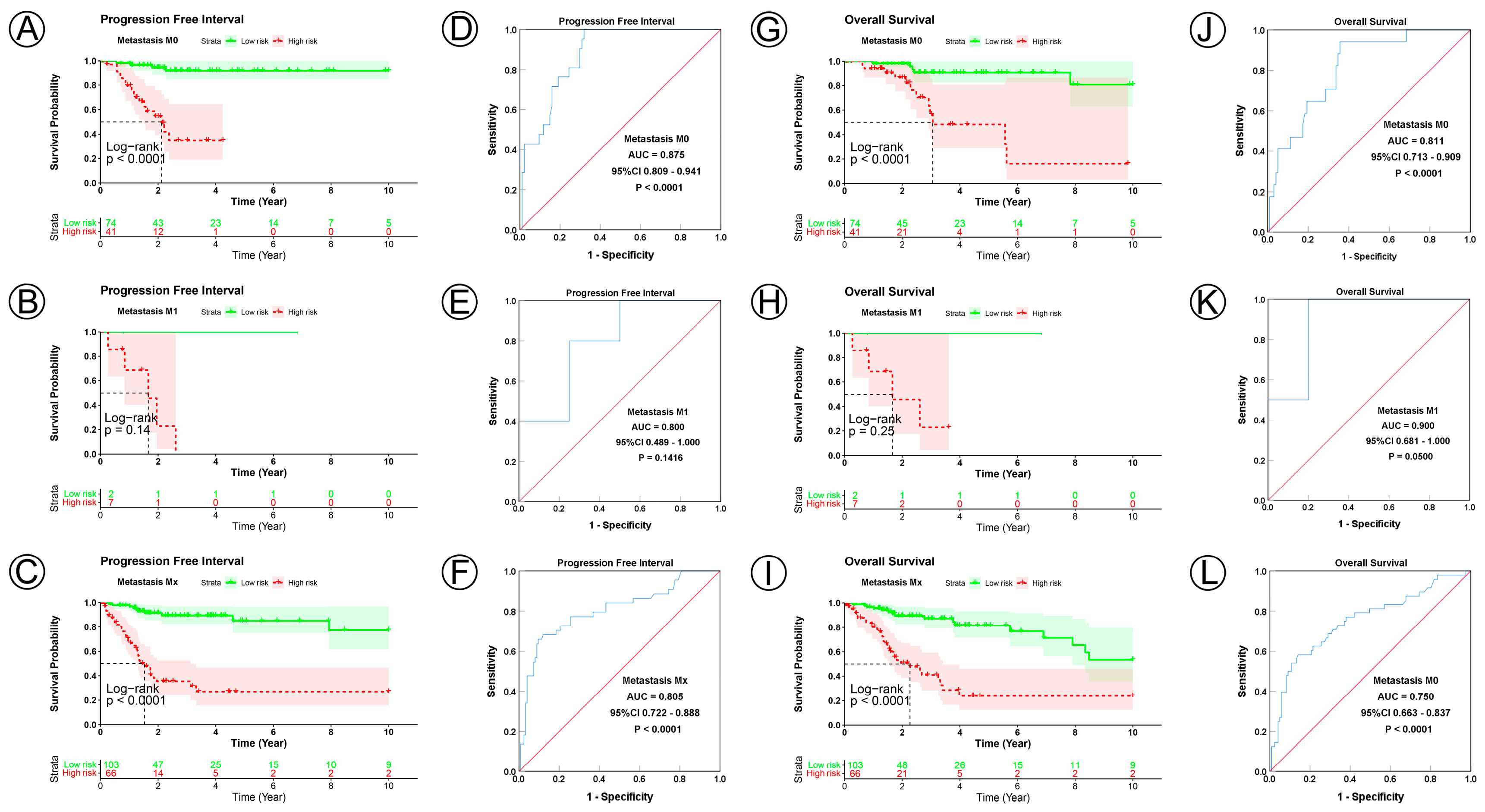
| M0 | 115 | 72 | 41 | 6.235 | 0.044 | 57 | 40 | 17 | 6.772 a | 0.034 |
| M1 | 9 | 2 | 7 | 6 | 1 | 5 | ||||
| Mx | 169 | 103 | 66 | 90 | 56 | 34 | ||||
| Variables | Progression Free Interval | Overall Survival | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | ||||||||||||
| HR | 95%CI | p Value | HR | 95%CI | p Value | HR | 95%CI | p Value | HR | 95%CI | p Value | ||||
| Age | |||||||||||||||
| >45 y | 1.541 | 0.949–2.503 | 0.081 | 1.230 | 0.760–1.989 | 0.400 | |||||||||
| FIGO stage | |||||||||||||||
| II–IV | 1.255 | 0.785–2.006 | 0.343 | 1.315 | 0.819–2.112 | 0.257 | |||||||||
| Tumor size | |||||||||||||||
| >4 cm | 1.736 | 0.998–3.018 | 0.051 | 0.890 | 0.479–1.652 | 0.712 | 1.828 | 1.021–3.272 | 0.042 | 1.318 | 0.689–2.520 | 0.404 | |||
| Tx | 1.788 | 0.926–3.456 | 0.084 | 0.536 | 0.232–1.237 | 0.144 | 2.443 | 1.309–4.560 | 0.005 | 1.097 | 0.464–2.592 | 0.834 | |||
| Lymph node status | |||||||||||||||
| N1 | 2.174 | 1.071–4.412 | 0.032 | 2.213 | 1.082–4.526 | 0.030 | 2.855 | 1.424–5.721 | 0.003 | 2.505 | 1.239–5.062 | 0.011 | |||
| Nx | 3.016 | 1.706–5.334 | <0.001 | 2.352 | 1.126–4.916 | 0.023 | 3.307 | 1.814–6.029 | <0.001 | 1.628 | 0.735–3.607 | 0.230 | |||
| Metastasis status | |||||||||||||||
| M1 | 3.640 | 1.370–9.674 | 0.001 | 1.394 | 0.486–3.997 | 0.536 | 4.163 | 1.396–12.413 | 0.011 | 1.903 | 0.592–6.114 | 0.280 | |||
| Mx | 1.550 | 0.921–2.608 | 0.099 | 1.235 | 0.669–2.279 | 0.501 | 2.159 | 1.241–3.756 | 0.006 | 1.871 | 0.970–3.608 | 0.062 | |||
| Risk group | |||||||||||||||
| High risk | 10.256 | 5.647–18.625 | <0.001 | 9.794 | 5.312–18.056 | <0.001 | 5.980 | 3.528–10.134 | <0.001 | 5.681 | 3.298–9.785 | <0.001 | |||
| Regrouping Factors | Subgroup | Sample Size | Progression Free Interval | Overall Survival | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Kaplan-Meier | ROC | Kaplan-Meier | ROC | |||||||
| p Value | AUC | 95%CI | p Value | p Value | AUC | 95%CI | p Value | |||
| Age, y | ||||||||||
| ≤45 | 141 | <0.0001 | 0.830 | 0.774–0.887 | <0.0001 | <0.0001 | 0.751 | 0.644–0.857 | <0.0001 | |
| >45 | 152 | <0.0001 | 0.853 | 0.787–0.919 | <0.0001 | <0.0001 | 0.786 | 0.702–0.871 | <0.0001 | |
| FIGO stage | ||||||||||
| I | 160 | <0.0001 | 0.854 | 0.785–0.923 | <0.0001 | <0.0001 | 0.795 | 0.711–0.879 | <0.0001 | |
| II–IV | 133 | <0.0001 | 0.802 | 0.709–0.896 | <0.0001 | <0.0001 | 0.742 | 0.637–0.847 | <0.0001 | |
| Tumor size status | ||||||||||
| ≤4 cm | 106 | <0.0001 | 0.857 | 0.760–0.955 | <0.0001 | <0.0001 | 0.847 | 0.751–0.944 | <0.0001 | |
| >4 cm | 130 | <0.0001 | 0.797 | 0.702–0.891 | <0.0001 | <0.0001 | 0.715 | 0.595–0.835 | <0.0001 | |
| Tx | 57 | 0.0002 | 0.847 | 0.737–0.956 | <0.0001 | 0.0021 | 0.781 | 0.653–0.909 | <0.0001 | |
| Lymph node status | ||||||||||
| N0 | 130 | <0.0001 | 0.860 | 0.764–0.956 | <0.0001 | <0.0001 | 0.874 | 0.805–0.943 | <0.0001 | |
| N1 | 58 | <0.0001 | 0.711 | 0.537–0.885 | 0.018 | 0.0320 | 0.591 | 0.409–0.773 | 0.2780 | |
| Nx | 105 | <0.0001 | 0.855 | 0.776–0.933 | <0.0001 | <0.0001 | 0.779 | 0.682–0.876 | <0.0001 | |
| Metastasis status | ||||||||||
| M0 | 115 | <0.0001 | 0.875 | 0.809–0.941 | <0.0001 | <0.0001 | 0.811 | 0.713–0.909 | <0.0001 | |
| M1 | 9 | 0.1400 | 0.800 | 0.489–1.000 | 0.1416 | 0.2500 | 0.900 | 0.681–1.00 | 0.0500 | |
| Mx | 169 | <0.0001 | 0.805 | 0.722–0.888 | <0.0001 | <0.0001 | 0.750 | 0.663–0.837 | <0.0001 | |
| Gene | Mean Expression | logFC | p Value | FDR | Expression Regulation | Biological Function | Reference | |
|---|---|---|---|---|---|---|---|---|
| Cluster1 | Cluster2 | |||||||
| AMIGO2 | 656.03 | 2089.59 | 1.67 | 4.48E-20 | 1.93E-16 | Up | Malignant Progression | [1,2,3,4] |
| TGM2 | 5220.71 | 22,242.27 | 2.09 | 4.43E-19 | 1.43E-15 | Up | Malignant Progression | [5,6] |
| SAMD4A | 431.82 | 1061.23 | 1.30 | 9.76E-18 | 2.52E-14 | Up | Inhibit Angiogenesis | [7] |
| NT5E | 355.48 | 2900.12 | 3.03 | 2.04E-18 | 2.63E-14 | Up | Immunotherapy targeter | [8,9] |
| ANTXR2 | 733.58 | 1809.17 | 1.30 | 1.53E-17 | 3.29E-14 | Up | Malignant Progression | [10,11] |
| PRSS23 | 2982.48 | 7634.59 | 1.36 | 3.11E-17 | 5.73E-14 | Up | Malignant Progression | [12,13] |
| ARHGAP29 | 855.33 | 1612.31 | 0.91 | 7.28E-17 | 1.04E-13 | Up | Malignant Progression | [14,15] |
| MICAL2 | 1510.53 | 3084.34 | 1.03 | 6.50E-17 | 1.04E-13 | Up | Malignant Progression | [16,17] |
| AOX1 | 42.78 | 282.14 | 2.72 | 4.79E-17 | 3.09E-13 | Up | Tumor Suppressor | [18,19] |
| HRCT1 | 27.36 | 120.47 | 2.14 | 4.17E-16 | 5.38E-13 | Up | Component of Membrane | [20] |
| BCL11A | 2624.57 | 1097.93 | −1.26 | 9.50E-13 | 3.14E-10 | Down | Malignant Progression | [21,22] |
| NMNAT3 | 769.70 | 457.02 | −0.75 | 1.98E-10 | 2.63E-08 | Down | NAD+ Metabolism | [23,24] |
| EFS | 4077.12 | 2548.44 | −0.68 | 2.50E-10 | 3.26E-08 | Down | Malignant Progression | [25,26] |
| PRIMA1 | 1264.97 | 582.95 | −1.12 | 4.68E-10 | 5.39E-08 | Down | Target for Mutant p53 | [27,28] |
| FAM117B | 1611.53 | 977.46 | −0.72 | 6.03E-10 | 6.59E-08 | Down | Small Vessel Disease | [29,30] |
| RGMA | 2284.31 | 1224.47 | −0.90 | 2.05E-09 | 1.73E-07 | Down | Malignant Progression | [31,32] |
| BNIPL | 1857.96 | 880.08 | −1.08 | 2.53E-09 | 2.06E-07 | Down | Malignant Progression | [33,34] |
| PRKX | 5039.73 | 3275.11 | −0.62 | 2.82E-09 | 2.23E-07 | Down | Malignant Progression | [35,36] |
| CALML5 | 13,974.56 | 4872.76 | −1.52 | 3.80E-09 | 2.92E-07 | Down | Tumor Suppressor | [37] |
| C3orf58 | 3176.54 | 1993.21 | −0.67 | 5.07E-09 | 3.59E-07 | Down | Mesenchymal Differentiation | [38,39] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, A.; Jing, W.; Qiu, J.; Zhang, R. Prediction of Cervical Cancer Outcome by Identifying and Validating a NAD+ Metabolism-Derived Gene Signature. J. Pers. Med. 2022, 12, 2031. https://doi.org/10.3390/jpm12122031
Chen A, Jing W, Qiu J, Zhang R. Prediction of Cervical Cancer Outcome by Identifying and Validating a NAD+ Metabolism-Derived Gene Signature. Journal of Personalized Medicine. 2022; 12(12):2031. https://doi.org/10.3390/jpm12122031
Chicago/Turabian StyleChen, Aozheng, Wanling Jing, Jin Qiu, and Runjie Zhang. 2022. "Prediction of Cervical Cancer Outcome by Identifying and Validating a NAD+ Metabolism-Derived Gene Signature" Journal of Personalized Medicine 12, no. 12: 2031. https://doi.org/10.3390/jpm12122031
APA StyleChen, A., Jing, W., Qiu, J., & Zhang, R. (2022). Prediction of Cervical Cancer Outcome by Identifying and Validating a NAD+ Metabolism-Derived Gene Signature. Journal of Personalized Medicine, 12(12), 2031. https://doi.org/10.3390/jpm12122031





