Exosomes from Microvascular Endothelial Cells under Mechanical Unloading Inhibit Osteogenic Differentiation via miR-92b-3p/ELK4 Axis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and In Vitro Differentiation
2.2. Two-Dimensional Clinorotation
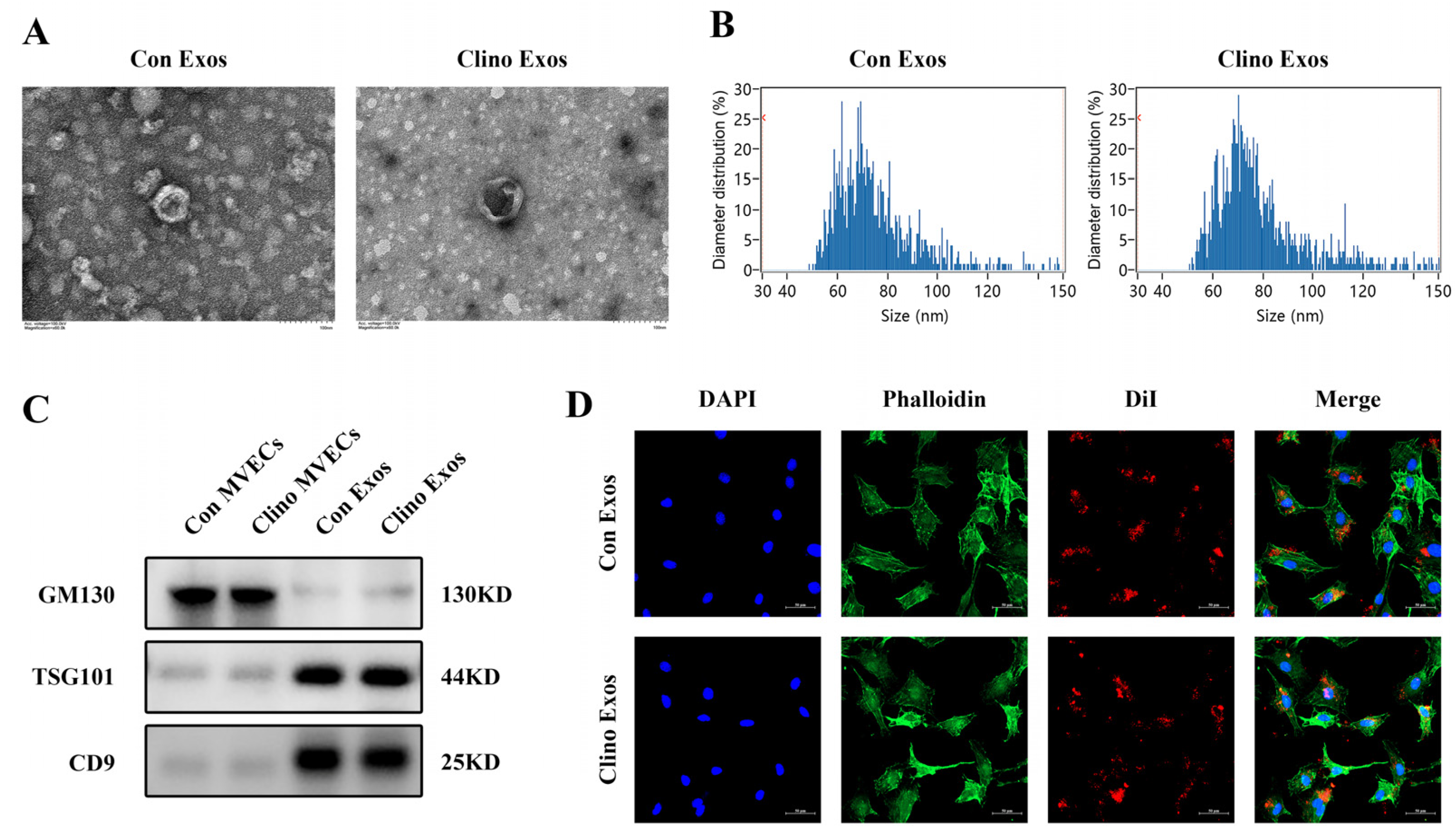
2.3. Exosome Isolation and Characterization
2.4. Exosome Internalization
2.5. miRNA Loading into Exosomes
2.6. qRT–PCR
2.7. Western Blotting Analysis
2.8. Cell Transfection
2.9. ALP Activity Assay
2.10. ALP Staining
2.11. Luciferase Assay
2.12. Statistical Analysis
3. Results
3.1. Isolation, Identification, and Internalization of MVEC-Derived Exosomes
3.2. Exosomes Derived from MVECs Cultured under Mechanical Unloading Attenuate Osteoblast Differentiation
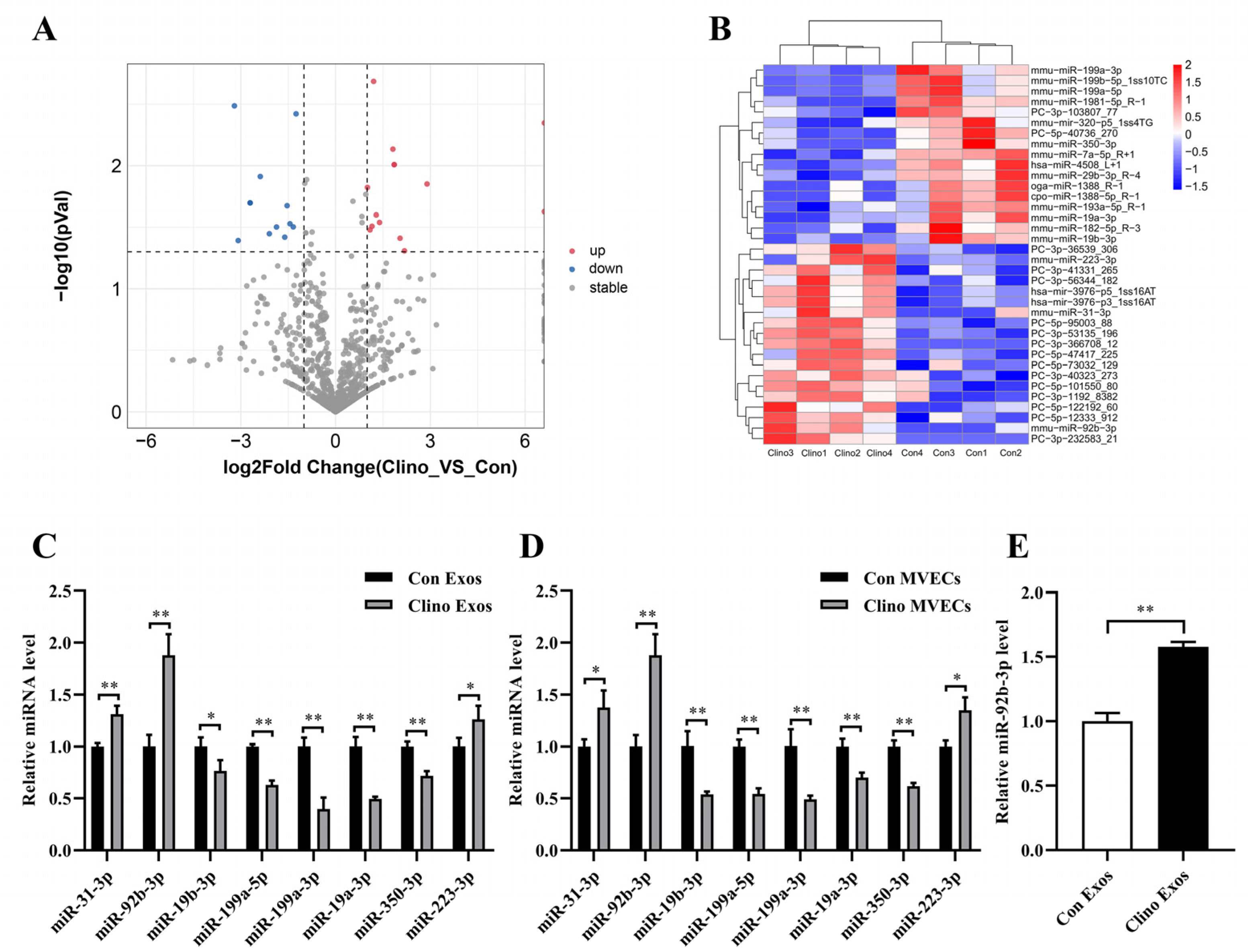
3.3. miR-92b-3p Is Elevated in MVEC-Derived Exosomes Cultured under Mechanical Unloading
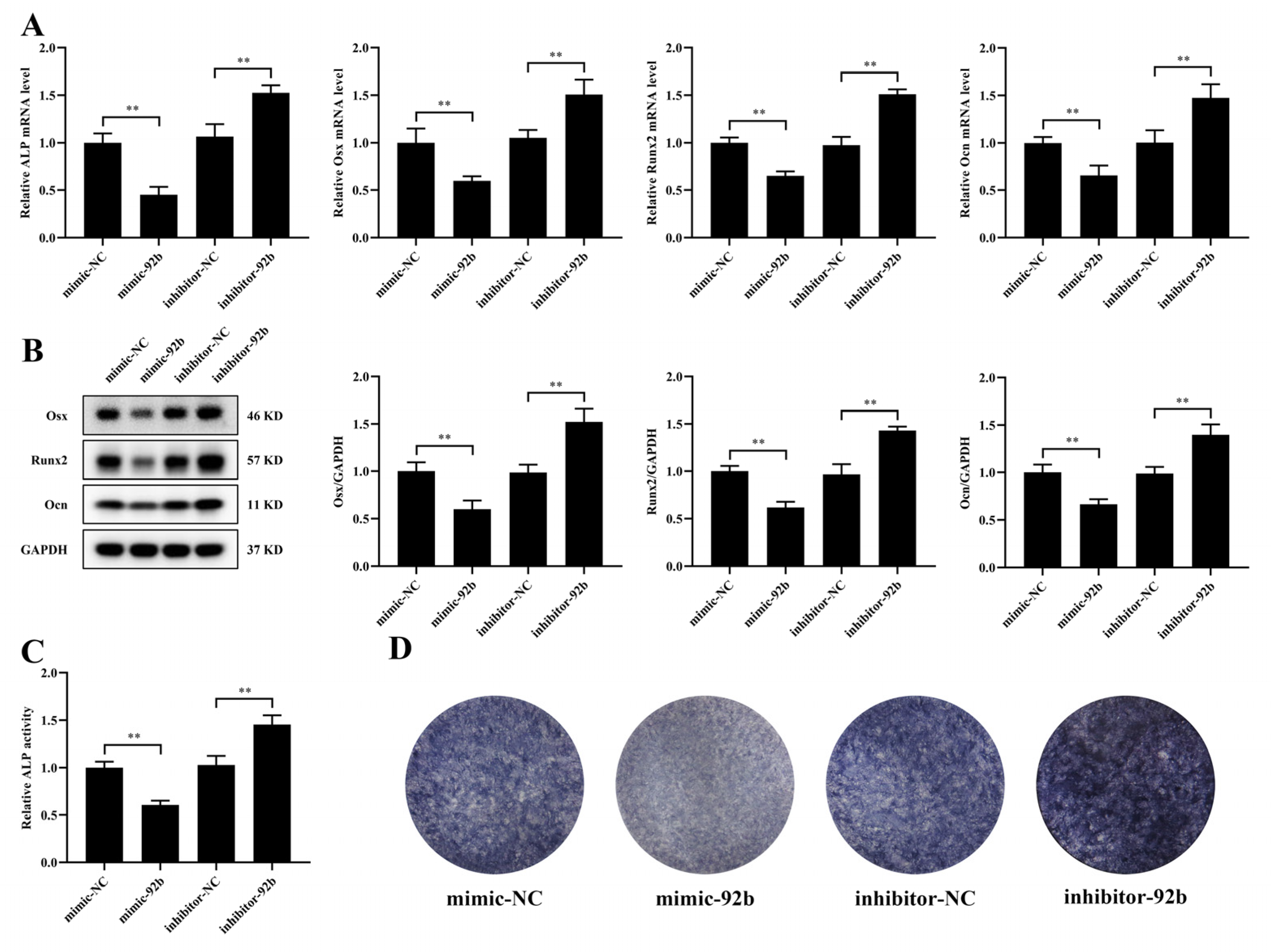
3.4. miR-92b-3p Hinders the Osteogenic Differentiation of MC3T3-E1 Cells
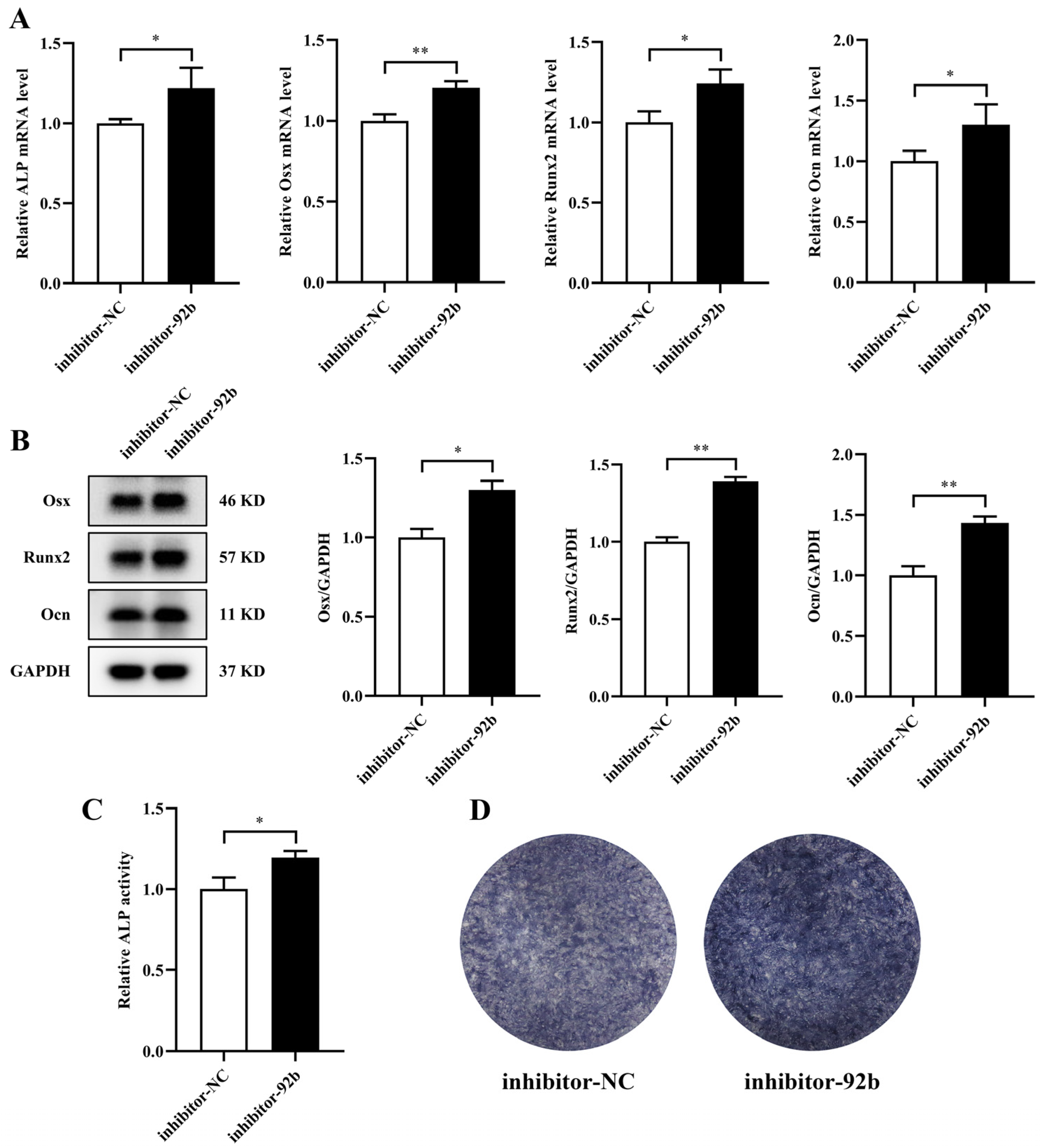
3.5. Exosomes Derived from MVECs Cultured under Mechanical Unloading Inhibit Osteogenic Differentiation by Transferring miR-92b-3p
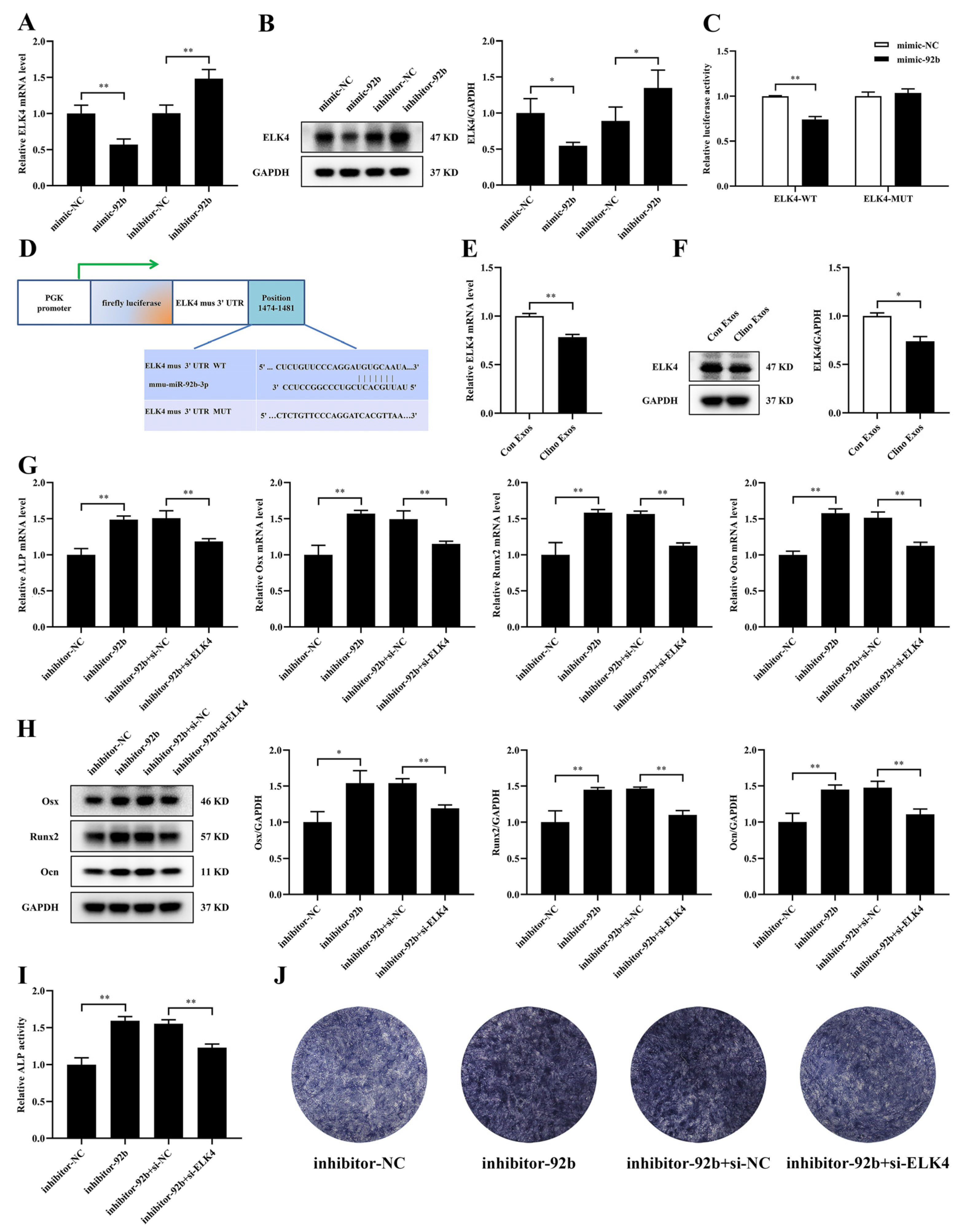
3.6. ELK4 Is a Direct Target of miR-92b-3p and Is Responsible for miR-92b-3p-Mediated Suppression of Osteogenic Differentiation in MC3T3-E1 Cells
4. Discussion
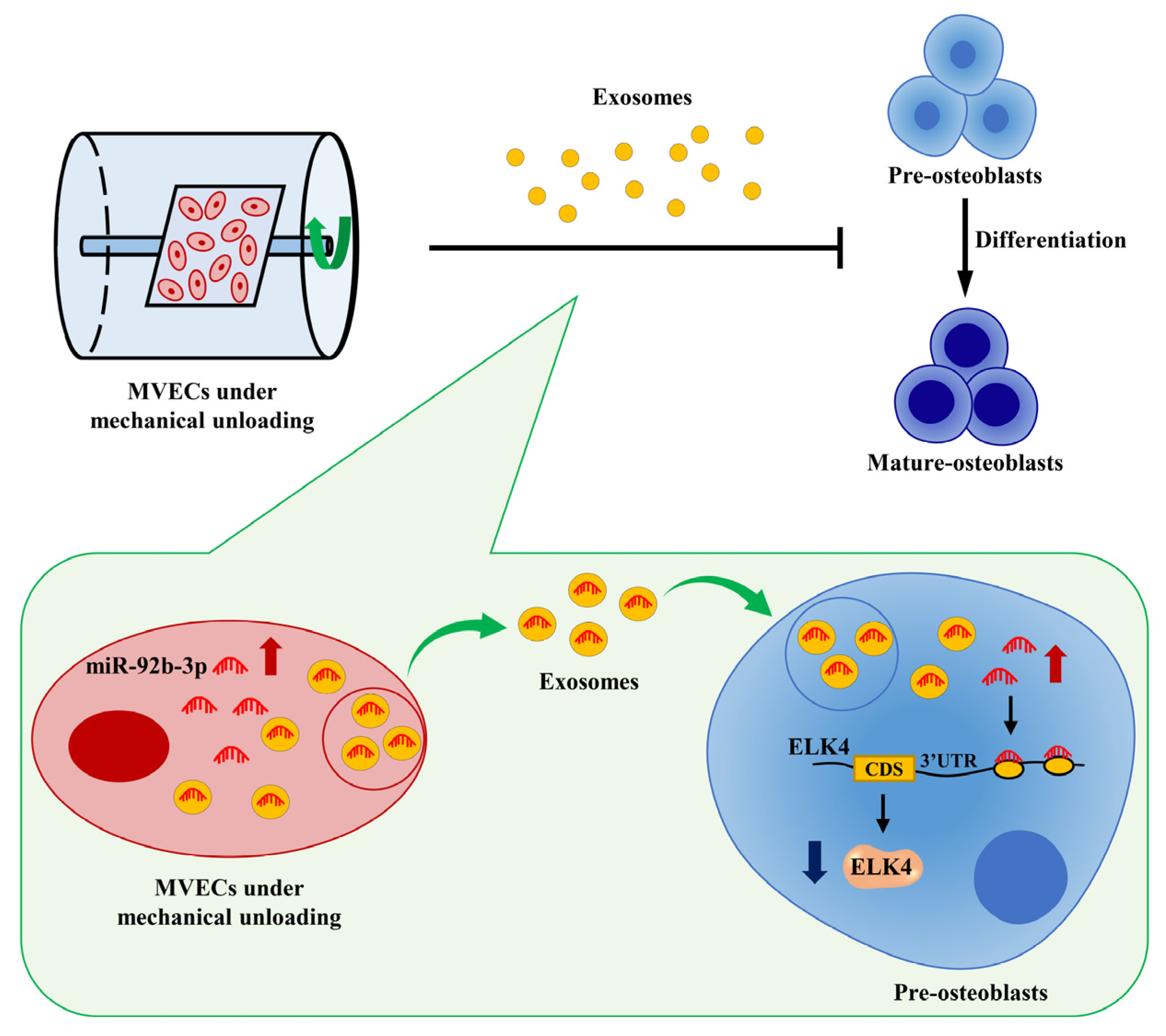
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carina, V.; Della Bella, E.; Costa, V.; Bellavia, D.; Veronesi, F.; Cepollaro, S.; Fini, M.; Giavaresi, G. Bone’s Response to Mechanical Loading in Aging and Osteoporosis: Molecular Mechanisms. Calcif. Tissue Res. 2020, 107, 301–318. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.-F.; Turner, C.H. Effects of Loading Frequency on Mechanically Induced Bone Formation. J. Bone Miner. Res. 2001, 16, 918–924. [Google Scholar] [CrossRef] [PubMed]
- Lang, T.; Leblanc, A.; Evans, H.; Lu, Y.; Genant, H.; Yu, A. Cortical and Trabecular Bone Mineral Loss from the Spine and Hip in Long-Duration Spaceflight. J. Bone Miner. Res. 2004, 19, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- Leblanc, A.; Schneider, V.; Shackelford, L.; West, S.; Oganov, V.; Bakulin, A.; Voronin, L. Bone mineral and lean tissue loss after long duration space flight. J. Musculoskelet. Neuronal. Interact. 2000, 1, 157–160. [Google Scholar] [PubMed]
- Sambandam, Y.; Baird, K.L.; Stroebel, M.; Kowal, E.; Balasubramanian, S.; Reddy, S.V. Microgravity Induction of TRAIL Expression in Preosteoclast Cells Enhances Osteoclast Differentiation. Sci. Rep. 2016, 6, 25143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blaber, E.A.; Dvorochkin, N.; Lee, C.; Alwood, J.S.; Yousuf, R.; Pianetta, P.; Globus, R.K.; Burns, B.P.; Almeida, E.A.C. Microgravity Induces Pelvic Bone Loss through Osteoclastic Activity, Osteocytic Osteolysis, and Osteoblastic Cell Cycle Inhibition by CDKN1a/p21. PLoS ONE 2013, 8, e61372. [Google Scholar] [CrossRef] [Green Version]
- Zhu, S.; Bennett, S.; Kuek, V.; Xiang, C.; Xu, H.; Rosen, V.; Xu, J. Endothelial cells produce angiocrine factors to regulate bone and cartilage via versatile mechanisms. Theranostics 2020, 10, 5957–5965. [Google Scholar] [CrossRef]
- Laranjeira, M.S.; Fernandes, M.H.; Monteiro, F.J. Reciprocal induction of human dermal microvascular endothelial cells and human mesenchymal stem cells: Time-dependent profile in a co-culture system. Cell Prolif. 2012, 45, 320–334. [Google Scholar] [CrossRef]
- Ribeiro, V.; Garcia, M.; Oliveira, R.; Gomes, P.S.; Colaço, B.; Fernandes, M.H. Bisphosphonates induce the osteogenic gene expression in co-cultured human endothelial and mesenchymal stem cells. J. Cell. Mol. Med. 2013, 18, 27–37. [Google Scholar] [CrossRef]
- Cazzaniga, A.; Castiglioni, S.; Maier, J.A.M. Conditioned Media from Microvascular Endothelial Cells Cultured in Simulated Microgravity Inhibit Osteoblast Activity. BioMed Res. Int. 2014, 2014, 857934. [Google Scholar] [CrossRef]
- Kourembanas, S. Exosomes: Vehicles of Intercellular Signaling, Biomarkers, and Vectors of Cell Therapy. Annu. Rev. Physiol. 2015, 77, 13–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef] [PubMed]
- Fazi, F.; Nervi, C. MicroRNA: Basic mechanisms and transcriptional regulatory networks for cell fate determination. Cardiovasc. Res. 2008, 79, 553–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Z.; Wang, Y.; Sun, Z.; Wang, H.; Zhou, H.; Zhang, L.; Zhang, S.; Cao, X. miRNA-132-3p inhibits osteoblast differentiation by targeting Ep300 in simulated microgravity. Sci. Rep. 2015, 5, 18655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Wang, K.; Hu, Z.; Zhou, H.; Zhang, L.; Wang, H.; Li, G.; Zhang, S.; Cao, X.; Shi, F. MicroRNA-139-3p regulates osteoblast differentiation and apoptosis by targeting ELK1 and interacting with long noncoding RNA ODSM. Cell Death Dis. 2018, 9, 1107. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Wang, Y.; Hu, Z.; Zhang, L.; Li, G.; Dang, L.; Tan, Y.; Cao, X.; Shi, F.; Zhang, S.; et al. Bone-targeted lncRNA OGRU alleviates unloading-induced bone loss via miR-320-3p/Hoxa10 axis. Cell Death Dis. 2020, 11, 382. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, J.; Li, Z.; Jia, G. Bone Marrow Mesenchymal Stem Cell-Derived Exosomal miR-25 Regulates the Ubiquitination and Degradation of Runx2 by SMURF1 to Promote Fracture Healing in Mice. Front. Med. 2020, 7, 577578. [Google Scholar] [CrossRef]
- Cui, Y.; Luan, J.; Li, H.; Zhou, X.; Han, J. Exosomes derived from mineralizing osteoblasts promote ST2 cell osteogenic differentiation by alteration of microRNA expression. FEBS Lett. 2015, 590, 185–192. [Google Scholar] [CrossRef] [Green Version]
- Sun, W.; Zhao, C.; Li, Y.; Wang, L.; Nie, G.; Peng, J.; Wang, A.; Zhang, P.; Tian, W.; Li, Q.; et al. Osteoclast-derived microRNA-containing exosomes selectively inhibit osteoblast activity. Cell Discov. 2016, 2, 16015. [Google Scholar] [CrossRef]
- Li, D.; Liu, J.; Guo, B.; Liang, C.; Dang, L.; Lu, C.; He, X.; Cheung, H.Y.-S.; Xu, L.; Lu, C.; et al. Osteoclast-derived exosomal miR-214-3p inhibits osteoblastic bone formation. Nat. Commun. 2016, 7, 10872. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, X.; Li, G.; Zhang, L.; Zhang, S.; Shi, F.; Hu, Z. Inhibition of SIRT1 by miR-138-5p provides a mechanism for inhibiting osteoblast proliferation and promoting apoptosis under simulated microgravity. Life Sci. Space Res. 2022; in press. [Google Scholar] [CrossRef]
- Hara, K.; Yamada, Y.; Nakamura, S.; Umemura, E.; Ito, K.; Ueda, M. Potential Characteristics of Stem Cells from Human Exfoliated Deciduous Teeth Compared with Bone Marrow–derived Mesenchymal Stem Cells for Mineralized Tissue-forming Cell Biology. J. Endod. 2011, 37, 1647–1652. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Ling, S.; Du, R.; Li, Y.; Liu, C.; Shi, J.; Gao, J.; Sun, W.; Li, J.; Zhong, G.; et al. The coupling of reduced type H vessels with unloading-induced bone loss and the protection role of Panax quinquefolium saponin in the male mice. Bone 2020, 143, 115712. [Google Scholar] [CrossRef] [PubMed]
- Ducy, P.; Karsenty, G. Two distinct osteoblast-specific cis-acting elements control expression of a mouse osteocalcin gene. Mol. Cell. Biol. 1995, 15, 1858–1869. [Google Scholar] [CrossRef] [Green Version]
- Ducy, P.; Zhang, R.; Geoffroy, V.; Ridall, A.L.; Karsenty, G. Osf2/Cbfa1: A Transcriptional Activator of Osteoblast Differentiation. Cell 1997, 89, 747–754. [Google Scholar] [CrossRef] [Green Version]
- Nakashima, K.; Zhou, X.; Kunkel, G.; Zhang, Z.; Deng, J.M.; Behringer, R.R.; de Crombrugghe, B. The Novel Zinc Finger-Containing Transcription Factor Osterix Is Required for Osteoblast Differentiation and Bone Formation. Cell 2002, 108, 17–29. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Zhang, W.; Dai, J.; Wang, X.; Shen, S.G. Overexpression of Dlx2 enhances osteogenic differentiation of BMSCs and MC3T3-E1 cells via direct upregulation of Osteocalcin and Alp. Int. J. Oral. Sci. 2019, 11, 12. [Google Scholar] [CrossRef] [Green Version]
- Gundberg, C.M. Biochemical Markers of Bone Formation. Clin. Lab. Med. 2000, 20, 489–502. [Google Scholar] [CrossRef]
- Pegtel, D.M.; Gould, S.J. Exosomes. Annu. Rev. Biochem. 2019, 88, 487–514. [Google Scholar] [CrossRef]
- Xie, X.; Xiong, Y.; Panayi, A.C.; Hu, L.; Zhou, W.; Xue, H.; Lin, Z.; Chen, L.; Yan, C.; Mi, B.; et al. Exosomes as a Novel Approach to Reverse Osteoporosis: A Review of the Literature. Front. Bioeng. Biotechnol. 2020, 8, 594247. [Google Scholar] [CrossRef]
- Kobayashi, M.; Sawada, K.; Miyamoto, M.; Shimizu, A.; Yamamoto, M.; Kinose, Y.; Nakamura, K.; Kawano, M.; Kodama, M.; Hashimoto, K.; et al. Exploring the potential of engineered exosomes as delivery systems for tumor-suppressor microRNA replacement therapy in ovarian cancer. Biochem. Biophys. Res. Commun. 2020, 527, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Li, X.; Zhao, Z.; Qian, J.; Wang, Y.; Cui, J.; Weng, W.; Cao, L.; Chen, X.; Hu, Y.; et al. Reversal of Osteoporotic Activity by Endothelial Cell-Secreted Bone Targeting and Biocompatible Exosomes. Nano Lett. 2019, 19, 3040–3048. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Cheng, B.; Jia, R.; Tan, B.; Liu, W. Altered expression of microRNA-92b-3p predicts survival outcomes of patients with prostate cancer and functions as an oncogene in tumor progression. Oncol. Lett. 2020, 21, 4. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wang, B.; Hui, K.; Zeng, J.; Fan, J.; Wang, X.; Hsieh, J.-T.; He, D.; Wu, K. miR-92b targets DAB2IP to promote EMT in bladder cancer migration and invasion. Oncol. Rep. 2016, 36, 1693–1701. [Google Scholar] [CrossRef] [Green Version]
- Long, M.; Zhan, M.; Xu, S.; Yang, R.; Chen, W.; Zhang, S.; Shi, Y.; Yongheng, S.; Mohan, M.; Liu, Q.; et al. miR-92b-3p acts as a tumor suppressor by targeting Gabra3 in pancreatic cancer. Mol. Cancer 2017, 16, 167. [Google Scholar] [CrossRef] [Green Version]
- Xu, T.; Wang, H.; Jiang, M.; Yan, Y.; Li, W.; Xu, H.; Huang, Q.; Lu, Y.; Chen, J. The E3 ubiquitin ligase CHIP/miR-92b/PTEN regulatory network contributes to tumorigenesis of glioblastoma. Am. J. Cancer Res. 2017, 7, 289–300. [Google Scholar]
- Huang, P.; Hu, Y.; Duan, Y. TGF-β2-induced circ-PRDM5 regulates migration, invasion, and EMT through the miR-92b-3p/COL1A2 pathway in human lens epithelial cells. Histochem. J. 2022, 53, 309–320. [Google Scholar] [CrossRef]
- Li, M.; Luo, R.; Yang, W.; Zhou, Z.; Li, C. miR-363-3p is activated by MYB and regulates osteoporosis pathogenesis via PTEN/PI3K/AKT signaling pathway. Vitr. Cell. Dev. Biol. Anim. 2019, 55, 376–386. [Google Scholar] [CrossRef]
- Wang, D.; Cai, G.; Wang, H.; He, J. TRAF3, a Target of MicroRNA-363-3p, Suppresses Senescence and Regulates the Balance between Osteoblastic and Adipocytic Differentiation of Rat Bone Marrow-Derived Mesenchymal Stem Cells. Stem Cells Dev. 2020, 29, 737–745. [Google Scholar] [CrossRef]
- Chen, C.; Li, Y.; Lu, H.; Liu, K.; Jiang, W.; Zhang, Z.; Qin, X. Curcumin attenuates vascular calcification via the exosomal miR-92b-3p/KLF4 axis. Exp. Biol. Med. 2022, 247, 1420–1432. [Google Scholar] [CrossRef]
- Zheng, L.; Xu, H.; Di, Y.; Chen, L.; Liu, J.; Kang, L.; Gao, L. ELK4 promotes the development of gastric cancer by inducing M2 polarization of macrophages through regulation of the KDM5A-PJA2-KSR1 axis. J. Transl. Med. 2021, 19, 342. [Google Scholar] [CrossRef] [PubMed]
- Wernert, N.; Shaikhibrahim, Z.; Lindstrot, A.; Langer, B.; Buettner, R. Differential expression of ETS family members in prostate cancer tissues and androgen-sensitive and insensitive prostate cancer cell lines. Int. J. Mol. Med. 2011, 28, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Day, B.W.; Stringer, B.W.; Spanevello, M.D.; Charmsaz, S.; Jamieson, P.R.; Ensbey, K.S.; Carter, J.C.; Cox, J.M.; Ellis, V.J.; Brown, C.L.; et al. ELK4 neutralization sensitizes glioblastoma to apoptosis through downregulation of the anti-apoptotic protein Mcl-1. Neuro-Oncology 2011, 13, 1202–1212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, W.; Gao, K.; Qian, Y.; Huang, Y.; Xiang, Q.; Chen, C.; Chen, Q.; Wang, Y.; Fang, F.; He, Q.; et al. A novel tRNA-derived fragment AS-tDR-007333 promotes the malignancy of NSCLC via the HSPB1/MED29 and ELK4/MED29 axes. J. Hematol. Oncol. 2022, 15, 53. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Yao, F.; Qiu, H.; Zhang, G.; Xu, H.; Xu, J. Coupling factors and exosomal packaging microRNAs involved in the regulation of bone remodelling. Biol. Rev. 2017, 93, 469–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramasamy, S.K.; Kusumbe, A.P.; Itkin, T.; Gur-Cohen, S.; Lapidot, T.; Adams, R.H. Regulation of Hematopoiesis and Osteogenesis by Blood Vessel–Derived Signals. Annu. Rev. Cell Dev. Biol. 2016, 32, 649–675. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Zhang, L.; Xu, L.; Li, G.; Wang, K.; Xue, T.; Sun, Q.; Tang, H.; Cao, X.; Hu, Z.; et al. Exosomes from Microvascular Endothelial Cells under Mechanical Unloading Inhibit Osteogenic Differentiation via miR-92b-3p/ELK4 Axis. J. Pers. Med. 2022, 12, 2030. https://doi.org/10.3390/jpm12122030
Zhang X, Zhang L, Xu L, Li G, Wang K, Xue T, Sun Q, Tang H, Cao X, Hu Z, et al. Exosomes from Microvascular Endothelial Cells under Mechanical Unloading Inhibit Osteogenic Differentiation via miR-92b-3p/ELK4 Axis. Journal of Personalized Medicine. 2022; 12(12):2030. https://doi.org/10.3390/jpm12122030
Chicago/Turabian StyleZhang, Xiaoyan, Lijun Zhang, Liqun Xu, Gaozhi Li, Ke Wang, Tong Xue, Quan Sun, Hao Tang, Xinsheng Cao, Zebing Hu, and et al. 2022. "Exosomes from Microvascular Endothelial Cells under Mechanical Unloading Inhibit Osteogenic Differentiation via miR-92b-3p/ELK4 Axis" Journal of Personalized Medicine 12, no. 12: 2030. https://doi.org/10.3390/jpm12122030
APA StyleZhang, X., Zhang, L., Xu, L., Li, G., Wang, K., Xue, T., Sun, Q., Tang, H., Cao, X., Hu, Z., Zhang, S., & Shi, F. (2022). Exosomes from Microvascular Endothelial Cells under Mechanical Unloading Inhibit Osteogenic Differentiation via miR-92b-3p/ELK4 Axis. Journal of Personalized Medicine, 12(12), 2030. https://doi.org/10.3390/jpm12122030





