Allelopathic Potential of Sunflower Genotypes at Different Growth Stages on Lettuce
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sunflower Genotypes
2.2. Collection of Plant Material and Preparation of Water Extracts
2.3. Bioassay
2.4. Data Collection and Statistical Analysis
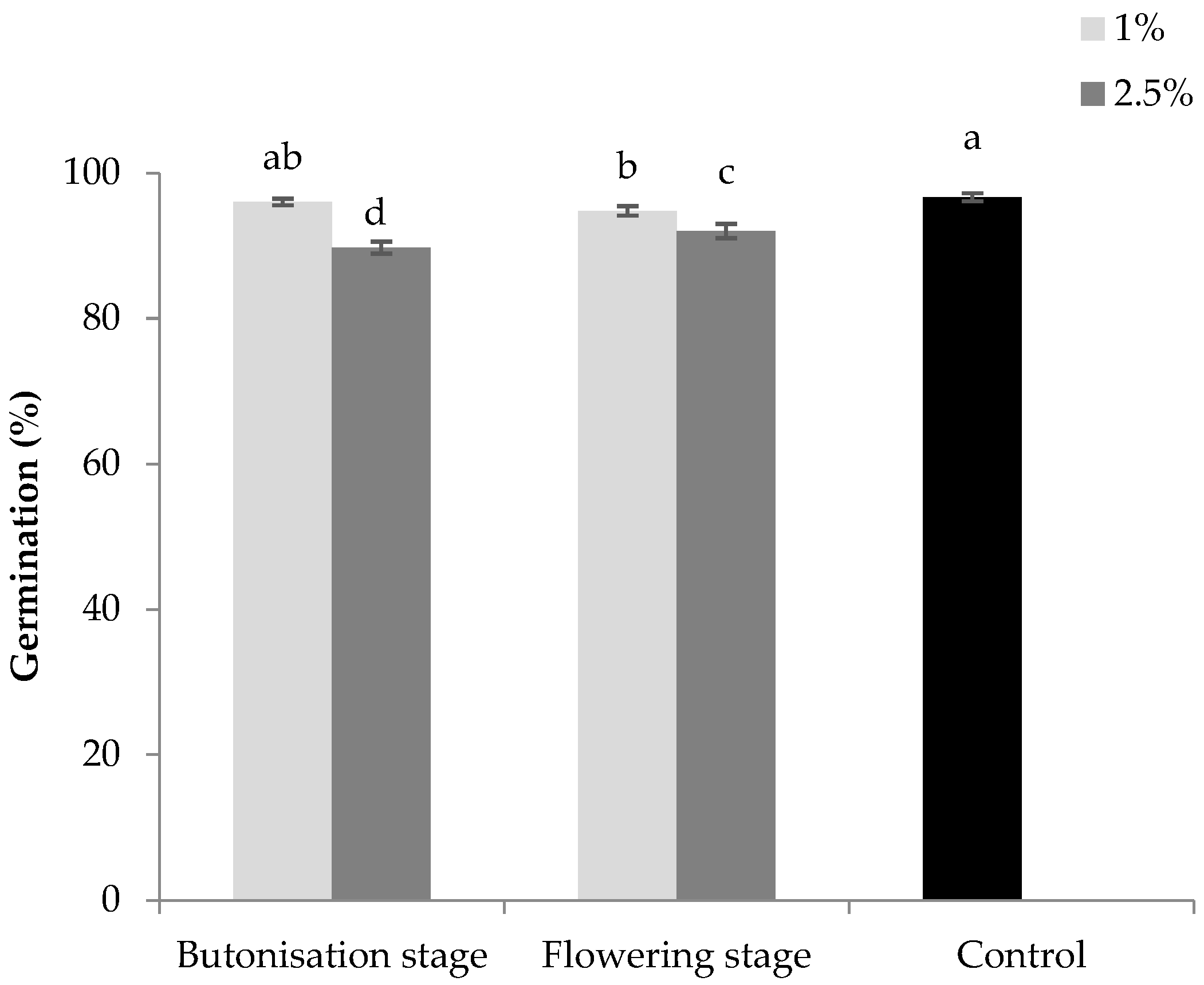
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Macías, F.A.; Marín, D.; Oliveros-Bastidas, A.; Varela, R.M.; Simonet, A.M.; Carrera, C.; Molinillo, J.M.G. Allelopathy as new strategy for sustainable ecosystems development. Biol. Sci. Space 2003, 17, 18–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhadoria, P.B.S. Allelopathy: A natural way towards weed management. Am. J. Exp. Agric. 2011, 1, 7–20. [Google Scholar] [CrossRef]
- Souto, A.L.; Sylvestre, M.; Tölke, E.D.; Tavares, J.F.; Barbosa-Filho, J.M.; Cebrián-Torrejón, G. Plant-Derived Pesticides as an Alternative to Pest Management and Sustainable Agricultural Production: Prospects, Applications and Challenges. Molecules 2021, 26, 4835. [Google Scholar] [CrossRef] [PubMed]
- Kubiak, A.; Wolna-Maruwka, A.; Niewiadomska, A.; Pilarska, A.A. The Problem of Weed Infestation of Agricultural Plantations vs. the Assumptions of the European Biodiversity Strategy. Agronomy 2022, 12, 1808. [Google Scholar] [CrossRef]
- Rice, E.L. Allelopathy, 2nd ed.; Academic Press: Orlando, FL, USA, 1984; p. 400. [Google Scholar]
- Swain, T. Secondary compounds as protective agents. Annu. Rev. Plant Physiol. 1977, 28, 479–501. [Google Scholar] [CrossRef]
- Hickman, D.T.; Rasmussen, A.; Ritz, K.; Birkett, M.A.; Neve, P. Review: Allelochemicals as multi-kingdom plant defense compounds: Towards an integrated approach. Pest Manag. Sci. 2021, 77, 1121–1131. [Google Scholar] [CrossRef]
- Alam, S.M.; Ala, S.A.; Azmi, A.R.; Khan, M.A.; Ansari, R. Allelopathy and its Role in Agriculture. J. Biol. Sci. 2001, 1, 308–315. [Google Scholar] [CrossRef]
- Dayan, F.E.; Romagni, J.G.; Duke, S.O. Investigating the mode of action of natural phytotoxins. J. Chem. Ecol. 2002, 26, 2079–2094. [Google Scholar] [CrossRef]
- Chou, C.H. Roles of allelopathy in plant biodiversity and sustainable agriculture. Crit. Rev. Plant Sci. 1999, 18, 609–636. [Google Scholar] [CrossRef]
- Singh, H.P.; Batish, D.R.; Kohli, R.K. Allelopathy in Agroecosystems. J. Crop Prod. 2001, 4, 1–41. [Google Scholar] [CrossRef]
- Alsaadawi, I.S.; Sarbout, A.K.; Al-Shamma, L.M. Differential allelopathic potential of sunflower (Helianthus annuus L.) genotypes on weeds and wheat (Triticum aestivum L.) crop. Arch. Agron. Soil Sci. 2012, 58, 1139–1148. [Google Scholar] [CrossRef]
- Rehman, S.; Shahzad, B.; Bajwa, A.A.; Hussain, S.; Rehman, A.; Cheema, S.A.; Abbas, T.; Ali, A.; Shah, L.; Adkins, S.; et al. Utilizing the Allelopathic Potential of Brassica Species for Sustainable Crop Production: A Review. J. Plant Growth. Regul. 2019, 38, 343–356. [Google Scholar] [CrossRef]
- Šćepanović, M.; Sarić-Krsmanović, M.; Šoštarčić, V.; Brijačak, E.; Lakić, J.; Špirović Trifunović, B.; Gajić Umiljendić, J.; Radivojević, L. Inhibitory Effects of Brassicaceae Cover Crop on Ambrosia artemisiifolia Germination and Early Growth. Plants 2021, 10, 794. [Google Scholar] [CrossRef] [PubMed]
- Weston, L.A.; Duke, S.O. Weed and Crop Allelopathy. Crit. Rev. Plant Sci. 2003, 22, 367–389. [Google Scholar] [CrossRef]
- Dhima, K.V.; Vasilakoglou, I.B.; Eleftherohorinos, I.G.; Lithourgidis, A.S. Allelopathic potential of winter cereals and their cover crop mulch effect on grass weed suppression and corn development. Crop Sci. 2006, 46, 345–352. [Google Scholar] [CrossRef]
- Ravlić, M.; Baličević, R.; Tucak, M.; Mijić, M.; Stanić, L.; Stojanović, N.; Skokić, V. Allelopathic potential of alfalfa (Medicago sativa L.) on seed germination and seedling growth of vegetables. Glas. Zaštite Bilja 2021, 44, 17–22. [Google Scholar] [CrossRef]
- Amini, S.; Azizi, M.; Joharchi, M.R.; Shafei, M.N.; Moradinezhad, F.; Fujii, Y. Determination of allelopathic potential in some medicinal and wild plant species of Iran by dish pack method. Theor. Exp. Plant Physiol. 2014, 26, 189–199. [Google Scholar] [CrossRef]
- Ravlić, M.; Baličević, R.; Nikolić, M.; Sarajlić, A. Assessment of allelopathic potential of fennel, rue and sage on weed species hoary cress (Lepidium draba). Not. Bot. Horti Agrobot. Cluj Napoca 2016, 44, 48–52. [Google Scholar] [CrossRef] [Green Version]
- Qasim, M.; Fujii, Y.; Ahmed, M.Z.; Aziz, I.; Watanabe, K.N.; Khan, M.A. Phytotoxic analysis of coastal medicinal plants and quantification of phenolic compounds using HPLC. Plant Biosyst. 2019, 153, 767–774. [Google Scholar] [CrossRef]
- Norsworthy, J.K. Allelopathic potential of wild radish (Raphanus raphanistrum). Weed Technol. 2003, 17, 307–313. [Google Scholar] [CrossRef]
- Golubinova, I.; Marinov-Serafimov, P. Allelopathic potential of dodder (Cuscuta epithymum L.) on different genotypes bird’s-foot trefoil (Lotus corniculatus L.). Bulg. J. Agric. Sci. 2019, 25, 1198–1204. [Google Scholar]
- Rys, M.; Saja-Garbarz, D.; Skoczowski, A. Phytotoxic Effects of Selected Herbal Extracts on the Germination, Growth and Metabolism of Mustard and Oilseed Rape. Agronomy 2022, 12, 110. [Google Scholar] [CrossRef]
- Scavo, A.; Pandino, G.; Restuccia, A.; Caruso, P.; Lombardo, S.; Mauromicale, G. Allelopathy in Durum Wheat Landraces as Affected by Genotype and Plant Part. Plants 2022, 11, 1021. [Google Scholar] [CrossRef] [PubMed]
- Appiah, K.S.; Omari, R.A.; Onwona-Agyeman, S.; Amoatey, C.A.; Ofosu-Anim, J.; Smaoui, A.; Arfa, A.B.; Suzuki, Y.; Oikawa, Y.; Okazaki, S.; et al. Seasonal Changes in the Plant Growth-Inhibitory Effects of Rosemary Leaves on Lettuce Seedlings. Plants 2022, 11, 673. [Google Scholar] [CrossRef] [PubMed]
- Zribi, I.; Omezzine, F.; Haouala, R. Variation in phytochemical constituents and allelopathic potential of Nigella sativa with developmental stages. S. Afr. J. Bot. 2014, 94, 255–262. [Google Scholar] [CrossRef] [Green Version]
- de Sousa, M.V.; de Farias, S.G.G.; de Castro, D.P.; e Silva, R.B.; de Oliveira Silva, D.Y.B.O.; Souto Dias, B.A.S.; da Silva, A.F.; dos Santos, G.N.L.; de Matos, D.C.P.; de Almada Oliveira, C.V.A. Allelopathy of the Leaf Extract of Eucalyptus Genetic Material on the Physiological Performance of Millet Seeds. Am. J. Plant Sci. 2018, 9, 34–45. [Google Scholar] [CrossRef] [Green Version]
- Omezzine, F.; Haouala, R. Effect of Trigonella foenum-graecum L. development stages on some phytochemicals content and allelopathic potential. Sci. Hortic. 2013, 160, 335–344. [Google Scholar] [CrossRef]
- Wu, H.; Pratley, J.; Lemerle, D.; Haig, T. Crop cultivars with allelopathic capability. Weed Res. 1999, 39, 171–180. [Google Scholar] [CrossRef]
- Zubair, H.M.; Pratley, J.E.; Sandral, G.A.; Humphries, A. Allelopathic interference of alfalfa (Medicago sativa L.) genotypes to annual ryegrass (Lolium rigidum). J. Plant Res. 2017, 130, 647–658. [Google Scholar] [CrossRef]
- Khaliq, A.; Matloob, A.; Cheema, Z.A.; Farooq, M. Allelopathic activity of crop residue incorporation alone or mixed against rice and its associated grass weed jungle rice (Echinochloa colona (L.) Link). Chil. J. Agric. Res. 2011, 71, 418–423. [Google Scholar] [CrossRef] [Green Version]
- Kamal, J. Impact of allelopathy of sunflower (Helianthus annuus L.) roots extract on physiology of wheat (Triticum aestivum L.). Afr. J. Biotechnol. 2011, 10, 14465–14477. [Google Scholar] [CrossRef]
- Bogatek, R.; Gniazdowska, A.; Zakrzewska, W.; Oracz, K.; Gawroński, S.W. Allelopathic effect of sunflower extracts on mustard seed germination and seedling growth. Biol. Plant. 2006, 50, 156–158. [Google Scholar] [CrossRef]
- Silva, H.L.; Trezzi, M.M.; Marchese, J.A.; Buzzello, G.; Miotto, E., Jr.; Patel, F.; Debastiani, F.; e Fiorese, J. Determination of Indicative Species and Comparison of Sunflower Genotypes as to their Allelopathic Potential. Planta Daninha 2009, 27, 655–663. [Google Scholar] [CrossRef] [Green Version]
- Dilipkumar, M.; Adzemi, M.A.; Chuah, T.S. Effects of Soil Types on Phytotoxic Activity of Pretilachlor in Combination with Sunflower Leaf Extracts on Barnyardgrass (Echinochloa crus-galli). Weed Sci. 2012, 60, 126–132. [Google Scholar] [CrossRef]
- Díaz de Villegas, M.E.; Delgado, G.; Rivas, M.; Torres, E.; Saura, M. Implementation of an in vitro bioassay as an indicator of the bionutrient FitoMas E. Cienc. Investig. Agrar. 2011, 38, 205–210. [Google Scholar] [CrossRef]
- Batish, D.R.; Tung, P.; Singh, H.P.; Kohli, R.K. Phytotoxicity of Sunflower Residues against Some Summer Season Crops. J. Agron. Crop Sci. 2002, 188, 19–24. [Google Scholar] [CrossRef]
- Tian, M.; Li, Q.; Zhao, W.; Qiao, B.; Shi, S.; Yu, M.; Li, X.; Li, C.; Zhao, C. Potential Allelopathic Interference of Abutilon theophrasti Medik. Powder/Extract on Seed Germination, Seedling Growth and Root System Activity of Maize, Wheat and Soybean. Agronomy 2022, 12, 844. [Google Scholar] [CrossRef]
- Macías, F.A.; Oliva, R.M.; Varela, R.M.; Torres, A.; Molinillo, J.M.G. Allelochemicals from sunflower leaves cv. Peredovick. Phytochemisty 1999, 52, 613–621. [Google Scholar] [CrossRef]
- Pannacci, E.; Masi, M.; Farneselli, M.; Tei, F. Evaluation of Mugwort (Artemisia vulgaris L.) Aqueous Extract as a Potential Bioherbicide to Control Amaranthus retroflexus L. in Maize. Agriculture 2020, 10, 642. [Google Scholar] [CrossRef]
- Ebana, K.; Yan, W.; Dilday, R.H.; Namai, H.; Okuno, K. Variation in Allelopathic Effect of Rice with Water Soluble Extracts. Agron. J. 2001, 93, 12–16. [Google Scholar] [CrossRef] [Green Version]
- Fernandez, C.; Monnier, Y.; Ormeño, E.; Baldy, V.; Greff, S.; Pasqualini, V.; Mévy, J.P.; Bousquet-Mélou, A. Variations in Allelochemical Composition of Leachates of Different Organs and Maturity Stages of Pinus halepensis. J. Chem. Ecol. 2009, 35, 970–979. [Google Scholar] [CrossRef] [PubMed]
- Đikić, M.; Gadžo, D.; Šarić, T.; Gavrić, T.; Muminović, Š. Investigation of allelopathic potential of buckwheat. Herbologia 2008, 9, 59–71. [Google Scholar]
- Jafariehyazdi, E.; Javidfar, F. Comparison of allelopathic effects of some brassica species in two growth stages on germination and growth of sunflower. Plant Soil Environ. 2011, 57, 52–56. [Google Scholar] [CrossRef] [Green Version]
- Macías, F.A.; Varela, R.M.; Torres, A.; Molinillo, J.M.G. Potential of Cultivar Sunflowers (Helianthus annuus L.) as a Source of Natural Herbicide Template. In Principles and Practices in Plant Ecology: Allelochemical Interactions; Inderjit, Dakshini, K.M.M., Foy, C.L., Eds.; CRC Press: Boca Raton, FL, USA, 1999; pp. 531–550. [Google Scholar]
- Zucareli, V.; Coelho, E.M.P.; Fernandes, W.V.; Peres, E.M.; Stracieri, J. Allelopathic potential of Sorghum bicolor at different phenological stages. Planta Daninha 2019, 37, e019184017. [Google Scholar] [CrossRef]


| Genotype Code | Genotype Name | Status |
|---|---|---|
| Genotype 1 | Luka | Hybrid |
| Genotype 2 | G cms | Female (CMS) line |
| Genotype 3 | PIO-2 | Male (restorer) line |
| Genotype 4 | Matej | Hybrid |
| Genotype 5 | L-10 A | Female (CMS) line |
| Genotype 6 | RF-56/07 | Male (restorer) line |
| Genotype 7 | OS-H-17 | Hybrid |
| Genotype 8 | L-13 cms | Female (CMS) line |
| Genotype 9 | I5-25/14 | Male (restorer) line |
| Source of Variation | Degree of Freedom | Germination | Root Length | Shoot Length | Fresh Weight |
|---|---|---|---|---|---|
| Growth stage (S) | 1 | 7 | 0.05 | 0.64 * | 1.03 |
| Concentration (C) | 2 | 667 * | 83.22 * | 23.76 * | 791.24 * |
| Genotype (G) | 8 | 27 | 0.24 * | 0.14 | 9.10 * |
| S × C | 2 | 57* | 0.21 * | 0.17 | 1.26 |
| S × G | 8 | 26 | 0.42 * | 0.21* | 3.08 * |
| C × G | 16 | 11 | 0.16 * | 0.11 | 4.02 * |
| S ×C × G | 16 | 23 | 0.20 * | 0.07 | 2.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ravlić, M.; Markulj Kulundžić, A.; Baličević, R.; Marković, M.; Viljevac Vuletić, M.; Kranjac, D.; Sarajlić, A. Allelopathic Potential of Sunflower Genotypes at Different Growth Stages on Lettuce. Appl. Sci. 2022, 12, 12568. https://doi.org/10.3390/app122412568
Ravlić M, Markulj Kulundžić A, Baličević R, Marković M, Viljevac Vuletić M, Kranjac D, Sarajlić A. Allelopathic Potential of Sunflower Genotypes at Different Growth Stages on Lettuce. Applied Sciences. 2022; 12(24):12568. https://doi.org/10.3390/app122412568
Chicago/Turabian StyleRavlić, Marija, Antonela Markulj Kulundžić, Renata Baličević, Monika Marković, Marija Viljevac Vuletić, David Kranjac, and Ankica Sarajlić. 2022. "Allelopathic Potential of Sunflower Genotypes at Different Growth Stages on Lettuce" Applied Sciences 12, no. 24: 12568. https://doi.org/10.3390/app122412568






