The Biomimetics of Mg2+-Concentration-Resolved Microenvironment for Bone and Cartilage Repairing Materials Design
Abstract
1. Introduction
2. Results
2.1. Effects of Mg2+ on Chondrocytes
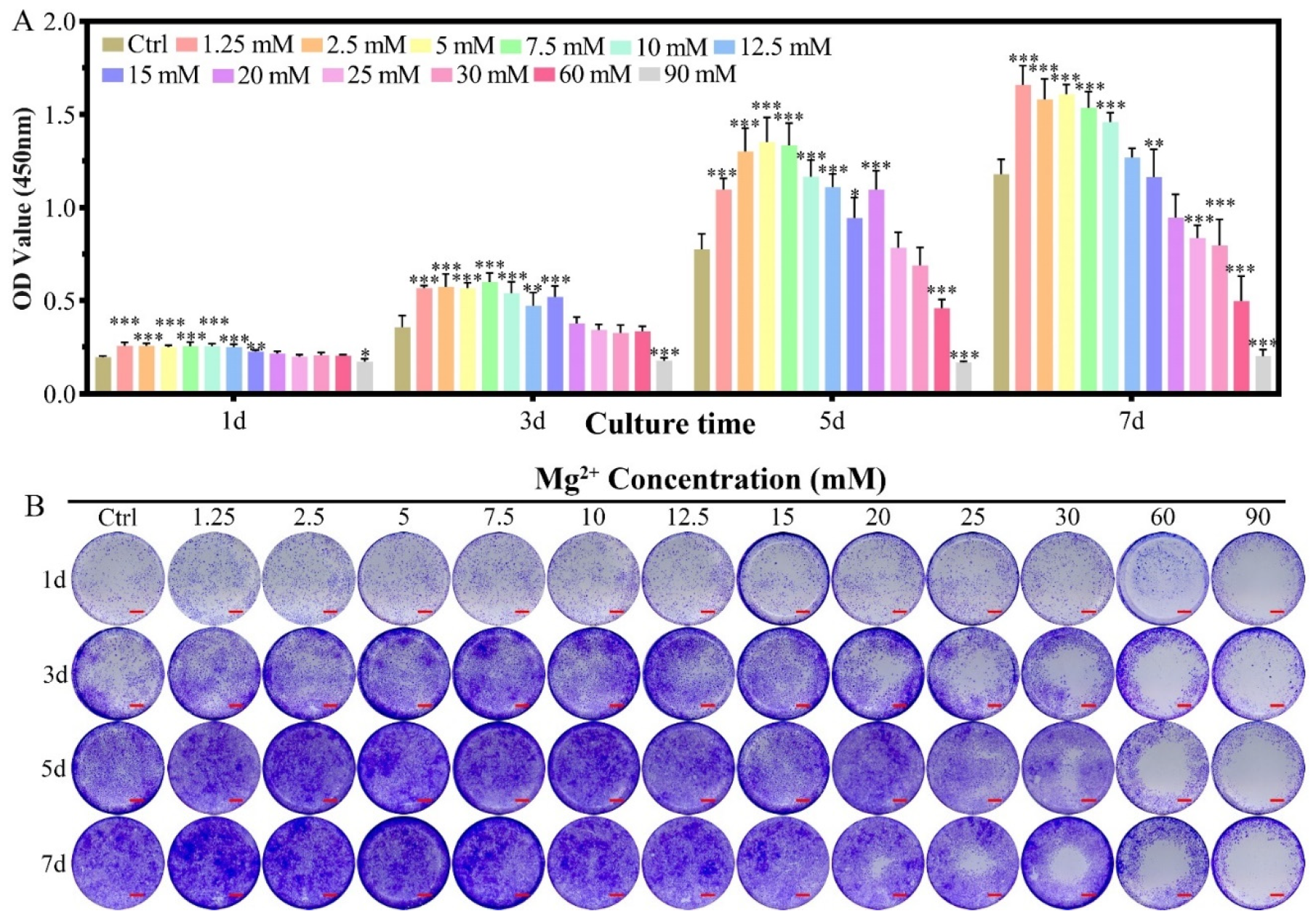
2.1.1. Cell Proliferation
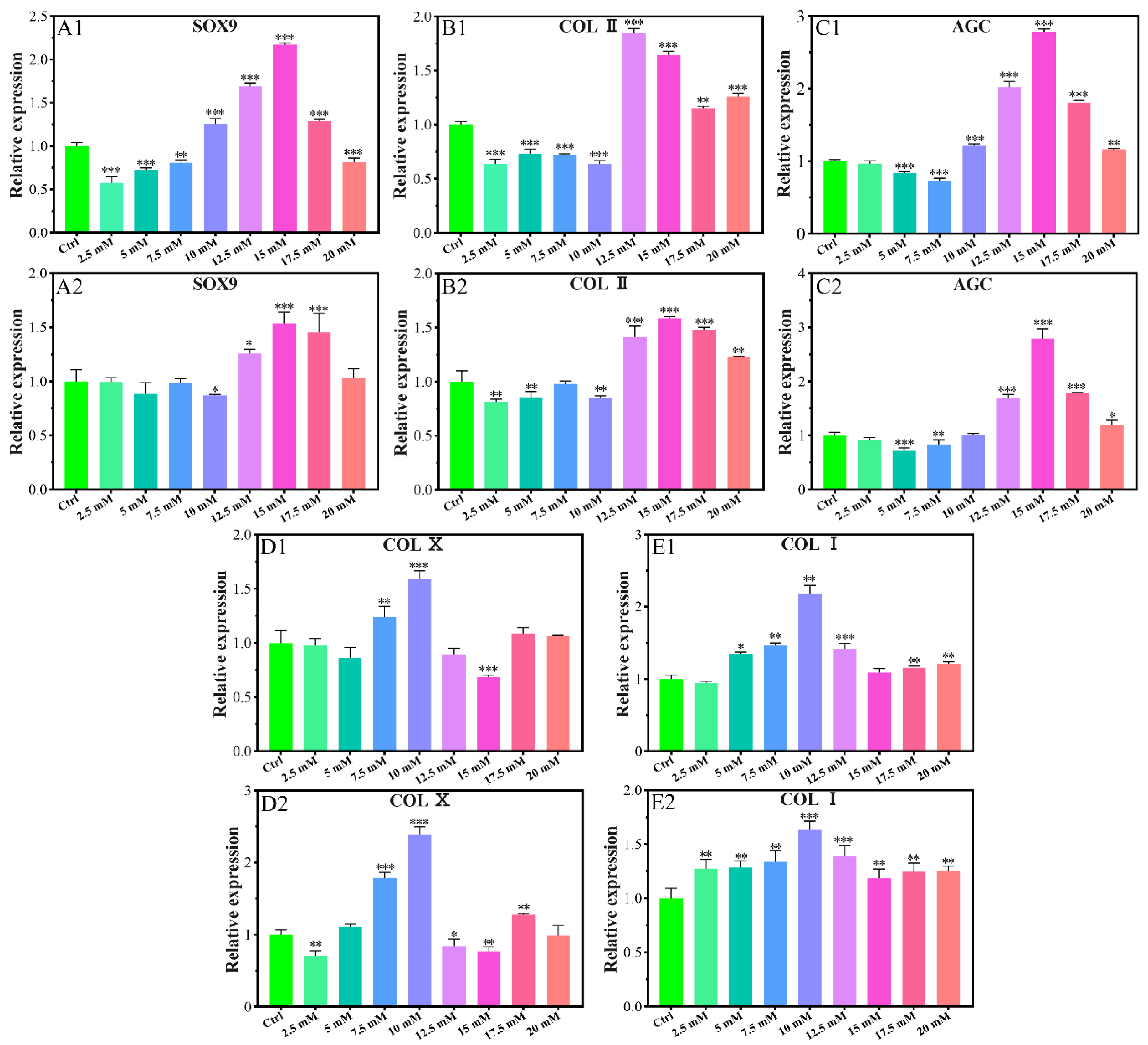
2.1.2. Chondrogenic-Related Gene Expressions
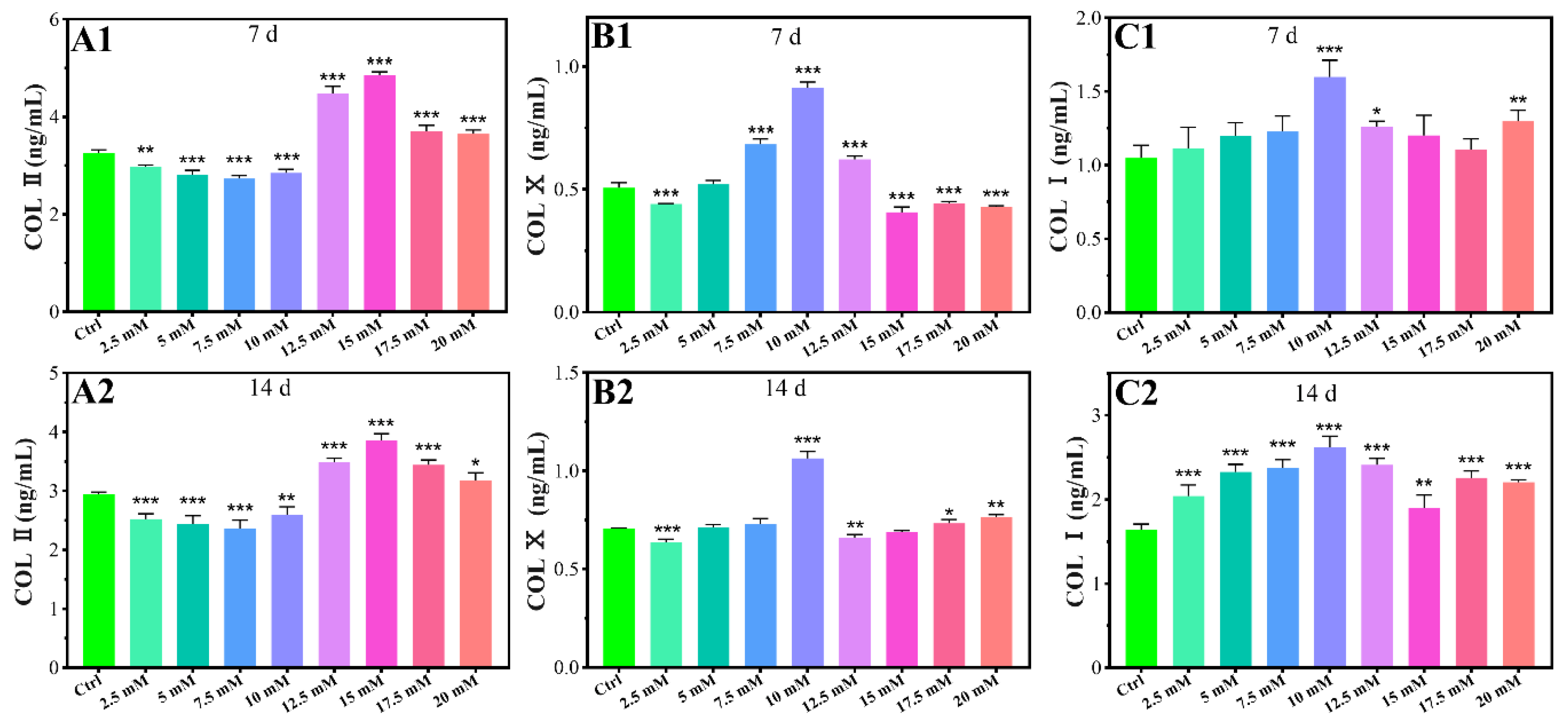
2.1.3. Chondrogenic-Related Protein Expressions
2.1.4. Inflammatory-Related Gene Expressions
2.2. Effects of Mg2+ on MC3T3-E1 Cells
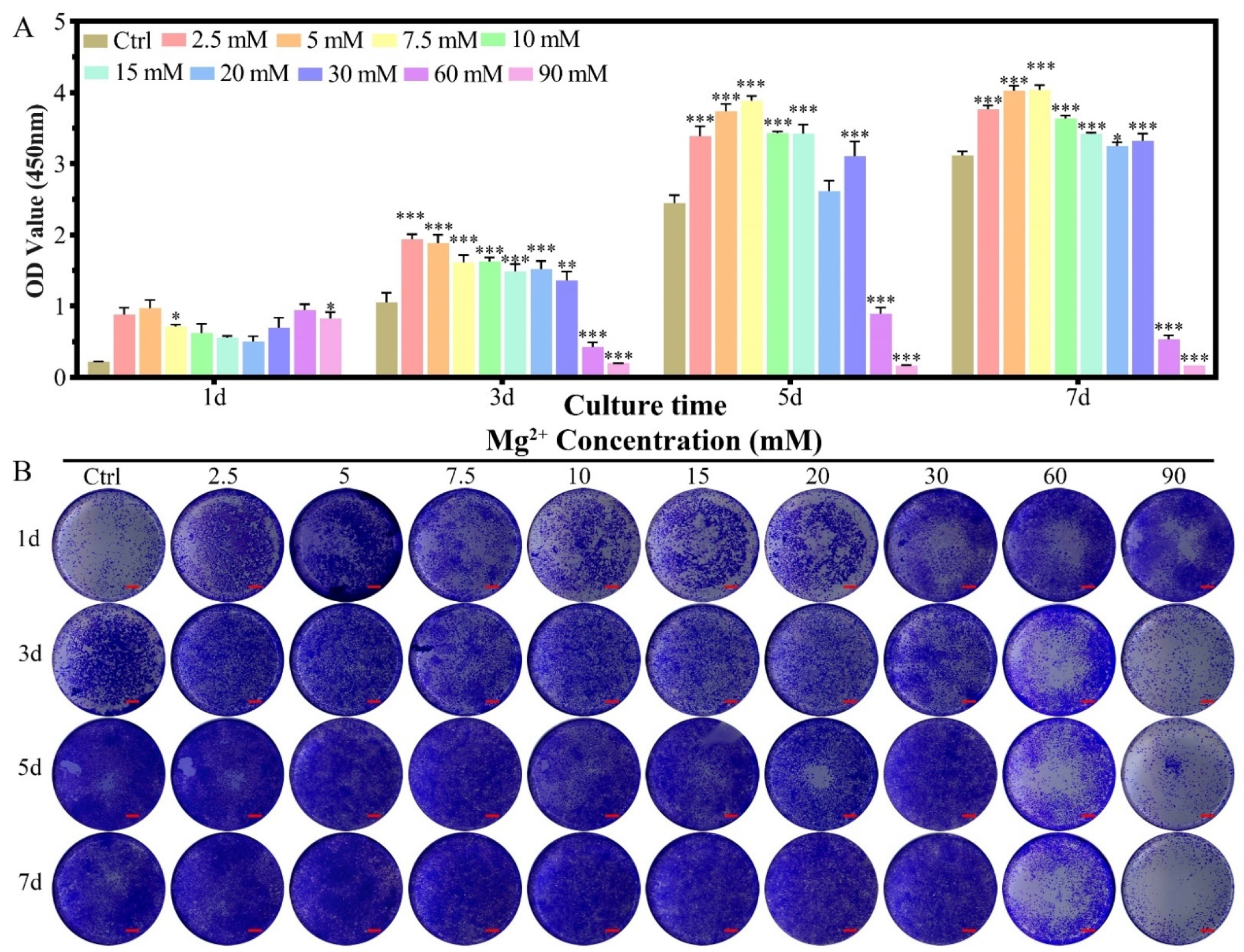
2.2.1. Cell Proliferation
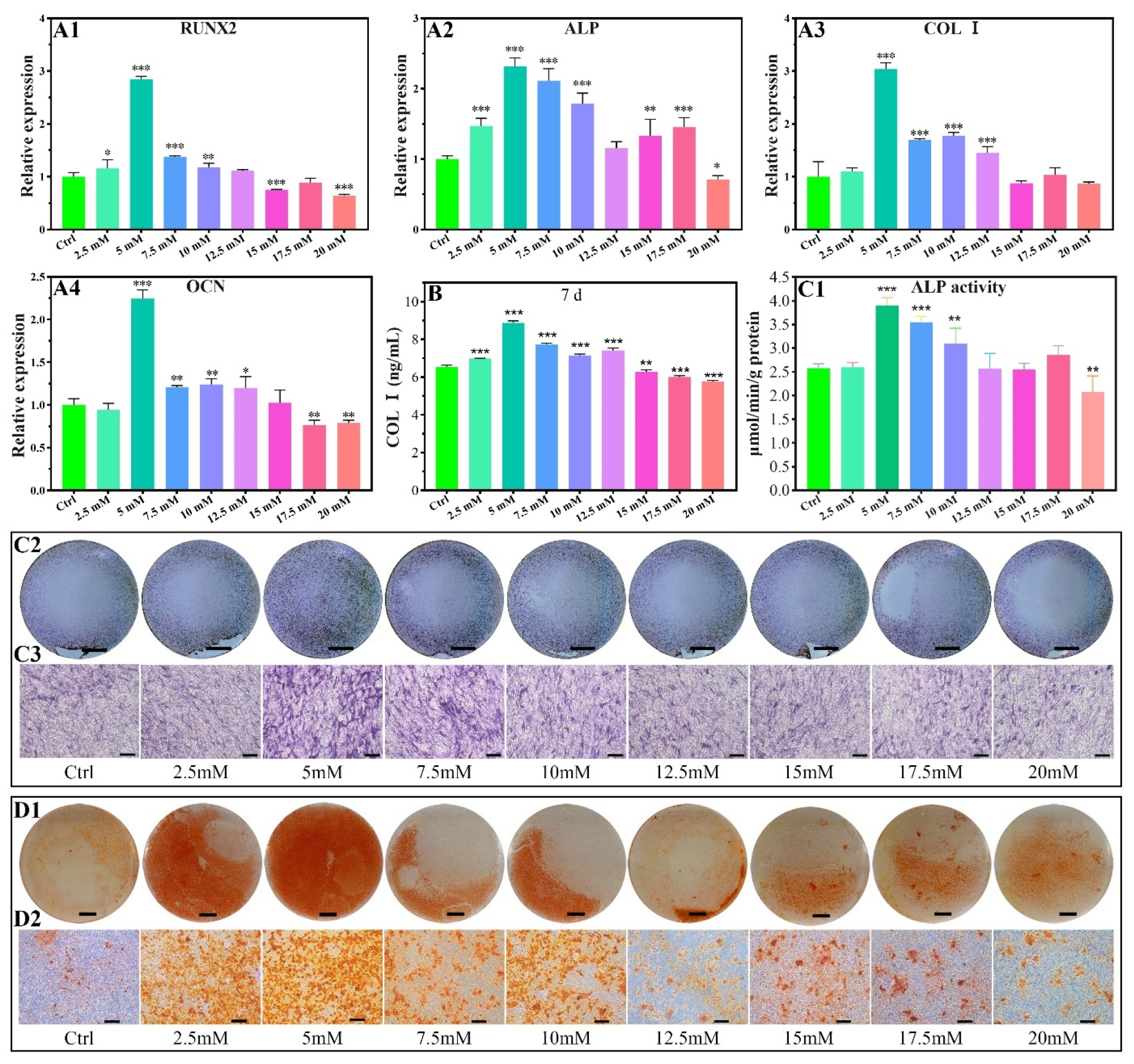
2.2.2. Osteogenic-Related Gene Expressions
2.2.3. COL I Contents
2.2.4. ALP Activity
2.2.5. Calcium Nodules Formation
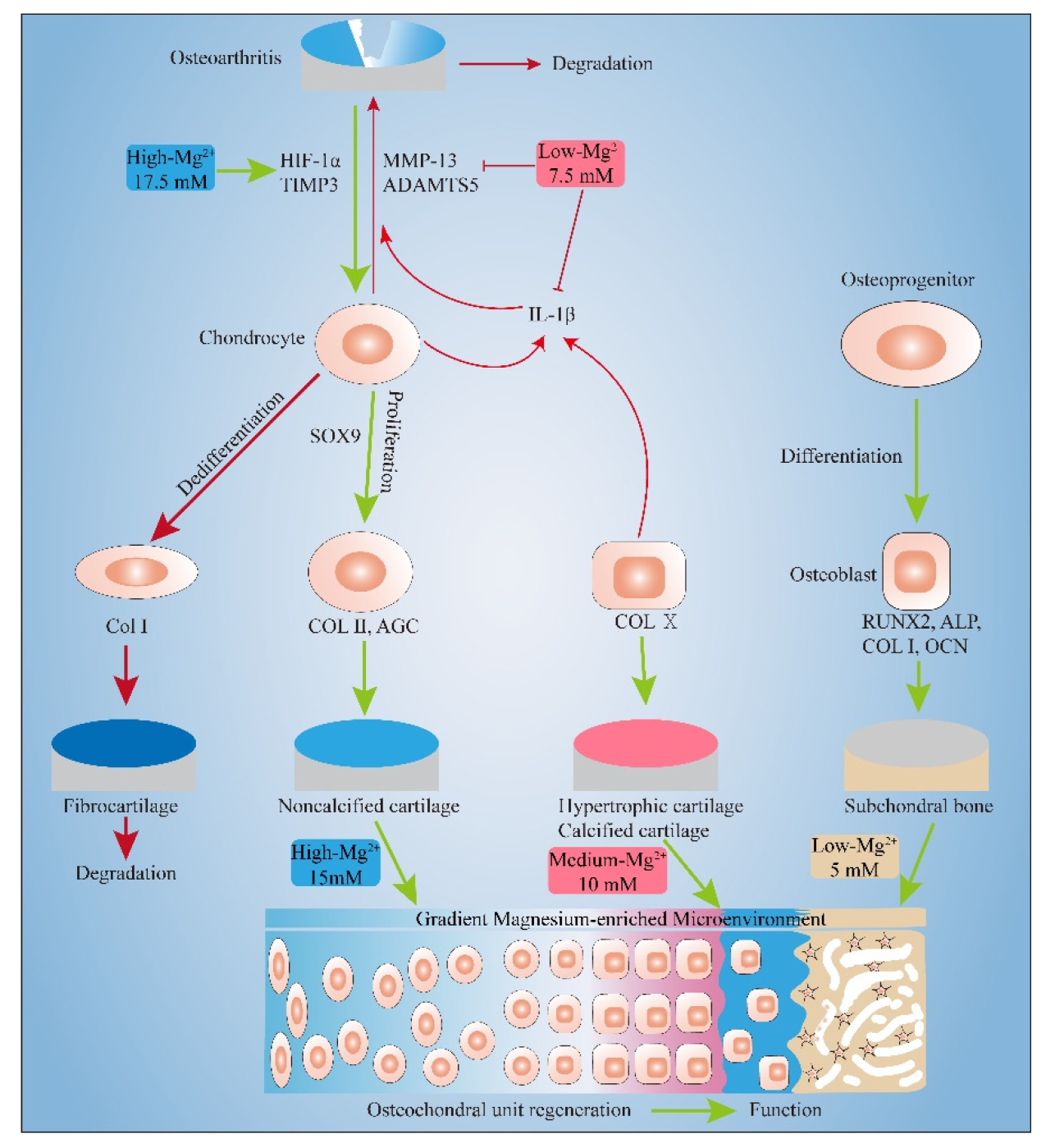
3. Discussion
3.1. Pro-Proliferation Effect of Mg2+ on Chondrocytes and MC3T3-E1 and Its Possible Mechanism
3.2. Effect of Mg2+ Concentration on Chondrogenesis of Chondrocytes and Its Mechanism
3.3. Effect of Mg2+ Concentration on Inflammation Related Gene Expressions and Its Mechanism
3.4. Effect of Mg2+ Concentration on Osteogenesis of MC3T3-E1 Cells and Its Mechanism
4. Materials and Methods
4.1. Chemicals
4.2. Chondrocytes and MC3T3-E1 Culture
4.3. Effects of Mg2+ Concentration on the Chondrocytes
4.3.1. Cell Proliferation
4.3.2. Cell Activity
4.3.3. Cell Morphology
4.3.4. Chondrogenic-Related Gene Expressions
4.3.5. Chondrogenic-Related Protein Expressions
4.3.6. Inflammatory-Related Gene Expressions
4.4. Effects of Mg2+ Concentration on the MC3T3-E1 Cells
4.4.1. Cell Proliferation
4.4.2. Cell Activity
4.4.3. Cell Morphology
4.4.4. Osteogenic-Related Gene Expressions
4.4.5. The Protein Expressions of COL Ⅰ
4.4.6. ALP Activity
4.4.7. Calcium Nodules Formation
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Gorbachova, T.; Melenevsky, Y.; Cohen, M.; Cerniglia, B.W. Osteochondral lesions of the knee: Differentiating the most common entities at MRI. Radiographics 2018, 38, 1478–1495. [Google Scholar] [CrossRef] [PubMed]
- Tateiwa, D.; Yoshikawa, H.; Kaito, T. Cartilage and Bone Destruction in Arthritis: Pathogenesis and Treatment Strategy: A Literature Review. Cells 2019, 8, 818. [Google Scholar] [CrossRef] [PubMed]
- Mountziaris, P.M.; Kramer, P.R.; Mikos, A.G. Emerging intra-articular drug delivery systems for the temporomandibular joint. Methods 2009, 47, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Chui, K.; Jeys, L.; Snow, M. Knee salvage procedures: The indications, techniques and outcomes of large osteochondral allografts. World J. Orthop. 2015, 6, 340–350. [Google Scholar] [CrossRef]
- D’Ambrosi, R.; Maccario, C.; Serra, N.; Ursino, C.; Usuelli, F.G. Relationship between symptomatic osteochondral lesions of the talus and quality of life, body mass index, age, size and anatomic location. Foot Ankle Surg. 2018, 24, 365–372. [Google Scholar] [CrossRef]
- Behery, O.; Siston, R.A.; Harris, J.D.; Flanigan, D.C. Treatment of cartilage defects of the knee: Expanding on the existing algorithm. Clin. J. Sport Med. 2014, 24, 21–30. [Google Scholar] [CrossRef]
- Seo, S.G.; Kim, J.S.; Seo, D.-K.; Kim, Y.K.; Lee, S.-H.; Lee, H.S. Osteochondral lesions of the talus: Few patients require surgery. Acta Orthop. 2018, 89, 462–467. [Google Scholar] [CrossRef]
- Kan, H.S.; Chan, P.K.; Chiu, K.Y.; Yan, C.H.; Yeung, S.S.; Ng, Y.L.; Shiu, K.W.; Ho, T. Non-surgical treatment of knee osteoarthritis. Hong Kong Med. J. 2019, 25, 127–133. [Google Scholar] [CrossRef]
- Rodriguez-Merchan, E.C. The treatment of cartilage defects in the knee joint: Microfracture, mosaicplasty, and autologous chondrocyte implantation. Am. J. Orthop Belle Mead NJ 2012, 41, 236–239. [Google Scholar]
- Schinhan, M.; Gruber, M.; Dorotka, R.; Pilz, M.; Stelzeneder, D.; Chiari, C.; Rossler, N.; Windhager, R.; Nehrer, S. Matrix-associated autologous chondrocyte transplantation in a compartmentalized early stage of osteoarthritis. Osteoarthr. Cartil. 2013, 21, 217–225. [Google Scholar] [CrossRef]
- Jevsevar, D.S. Treatment of osteoarthritis of the knee: Evidence-based guideline, 2nd edition. J. Am. Acad. Orthop Surg. 2013, 21, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Armiento, A.R.; Alini, M.; Stoddart, M.J. Articular fibrocartilage—Why does hyaline cartilage fail to repair? Adv. Drug Deliv. Rev. 2019, 146, 289–305. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Zhang, T.; Chen, M.; Yao, K.; Huang, X.; Zhang, B.; Li, Y.; Liu, J.; Wang, Y.; Zhao, Z. Bone physiological microenvironment and healing mechanism: Basis for future bone-tissue engineering scaffolds. Bioact. Mater. 2021, 6, 4110–4140. [Google Scholar] [CrossRef] [PubMed]
- Bigham, A.; Foroughi, F.; Ghomi, E.R.; Rafienia, M.; Neisiany, R.E.; Ramakrishna, S. The journey of multifunctional bone scaffolds fabricated from traditional toward modern techniques. Bio. Des. Manuf. 2020, 3, 281–306. [Google Scholar] [CrossRef]
- Shields, L.B.; Raque, G.H.; Glassman, S.D.; Campbell, M.; Vitaz, T.; Harpring, J.; Shields, C.B. Adverse effects associated with high-dose recombinant human bone morphogenetic protein-2 use in anterior cervical spine fusion. Spine 2006, 31, 542–547. [Google Scholar] [CrossRef]
- Boraiah, S.; Paul, O.; Hawkes, D.; Wickham, M.; Lorich, D.G. Complications of recombinant human BMP-2 for treating complex tibial plateau fractures: A preliminary report. Clin. Orthop. Relat. Res. 2009, 467, 3257–3262. [Google Scholar] [CrossRef] [PubMed]
- Luca, L.; Rougemont, A.L.; Walpoth, B.H.; Gurny, R.; Jordan, O. The effects of carrier nature and pH on rhBMP-2-induced ectopic bone formation. J. Control. Release 2010, 147, 38–44. [Google Scholar] [CrossRef]
- van der Kraan, P.M. The changing role of TGFbeta in healthy, ageing and osteoarthritic joints. Nat. Rev. Rheumatol. 2017, 13, 155–163. [Google Scholar] [CrossRef]
- Bose, S.; Fielding, G.; Tarafder, S.; Bandyopadhyay, A. Understanding of dopant-induced osteogenesis and angiogenesis in calcium phosphate ceramics. Trends Biotechnol. 2013, 31, 594–605. [Google Scholar] [CrossRef]
- Li, M.; Yin, H.; Yan, Z.; Li, H.; Wu, J.; Wang, Y.; Wei, F.; Tian, G.; Ning, C.; Li, H.; et al. The immune microenvironment in cartilage injury and repair. Acta Biomater. 2022, 140, 23–42. [Google Scholar] [CrossRef]
- Grober, U.; Schmidt, J.; Kisters, K. Magnesium in Prevention and Therapy. Nutrients 2015, 7, 8199–8226. [Google Scholar] [CrossRef]
- Rondanelli, M.; Faliva, M.A.; Tartara, A.; Gasparri, C.; Perna, S.; Infantino, V.; Riva, A.; Petrangolini, G.; Peroni, G. An update on magnesium and bone health. Biometals 2021, 34, 715–736. [Google Scholar] [CrossRef]
- Yao, H.; Xu, J.K.; Zheng, N.Y.; Wang, J.L.; Mok, S.W.; Lee, Y.W.; Shi, L.; Wang, J.Y.; Yue, J.; Yung, S.H.; et al. Intra-articular injection of magnesium chloride attenuates osteoarthritis progression in rats. Osteoarthr. Cartil. 2019, 27, 1811–1821. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, J.; Qin, L.; Jiang, Q. Magnesium and osteoarthritis: From a new perspective. Ann. Jt. 2016, 1, 1–13. [Google Scholar] [CrossRef]
- Gruber, H.E.; Ingram, J.; Norton, H.J.; Wei, L.Y.; Frausto, A.; Mills, B.G.; Rude, R.K. Alterations in growth plate and articular cartilage morphology are associated with reduced SOX9 localization in the magnesium-deficient rat. Biotech Histochem. 2004, 79, 45–52. [Google Scholar] [CrossRef]
- Medzhitov, R. The spectrum of inflammatory responses. Science 2021, 374, 1070–1075. [Google Scholar] [CrossRef]
- Feyerabend, F.; Witte, F.; Kammal, M.; Willumeit, R. Unphysiologically high magnesium concentrations support chondrocyte proliferation and redifferentiation. Tissue Eng. 2006, 12, 3545–3556. [Google Scholar] [CrossRef]
- Dou, Y.; Li, N.; Zheng, Y.; Ge, Z. Effects of fluctuant magnesium concentration on phenotype of the primary chondrocytes. J. Biomed. Mater. Res. A 2014, 102, 4455–4463. [Google Scholar] [CrossRef]
- Kim, J.A.; Yun, H.-s.; Choi, Y.-A.; Kim, J.-E.; Choi, S.-Y.; Kwon, T.-G.; Kim, Y.K.; Kwon, T.-Y.; Bae, M.A.; Kim, N.J. Magnesium phosphate ceramics incorporating a novel indene compound promote osteoblast differentiation in vitro and bone regeneration in vivo. Biomaterials 2018, 157, 51–61. [Google Scholar] [CrossRef]
- Rubin, A.; Terasaki, M.; Sanui, H. Major intracellular cations and growth control: Correspondence among magnesium content, protein synthesis, and the onset of DNA synthesis in BALB/c3T3 cells. Proc. Natl. Acad. Sci. USA 1979, 76, 3917–3921. [Google Scholar] [CrossRef]
- Rubin, H. Central roles of Mg2+ and MgATP2- in the regulation of protein synthesis and cell proliferation: Significance for neoplastic transformation. Adv. Cancer Res. 2005, 93, 1–58. [Google Scholar] [CrossRef] [PubMed]
- Wolf, F.I.; Cittadini, A. Magnesium in cell proliferation and differentiation. Front. Biosci. 1999, 4, 607–617. [Google Scholar] [CrossRef] [PubMed]
- de Baaij, J.H.; Hoenderop, J.G.; Bindels, R.J. Magnesium in man: Implications for health and disease. Physiol. Rev. 2015, 95, 1–46. [Google Scholar] [CrossRef] [PubMed]
- Zhen, Z.; Liu, X.; Huang, T.; Xi, T.; Zheng, Y. Hemolysis and cytotoxicity mechanisms of biodegradable magnesium and its alloys. Mater Sci. Eng. C Mater Biol. Appl. 2015, 46, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Bush, P.G.; Parisinos, C.A.; Hall, A.C. The osmotic sensitivity of rat growth plate chondrocytes in situ; clarifying the mechanisms of hypertrophy. J. Cell Physiol. 2008, 214, 621–629. [Google Scholar] [CrossRef]
- Bell, D.M.; Leung, K.K.; Wheatley, S.C.; Ng, L.J.; Zhou, S.; Ling, K.W.; Sham, M.H.; Koopman, P.; Tam, P.P.; Cheah, K.S. SOX9 directly regulates the type-II collagen gene. Nat. Genet. 1997, 16, 174–178. [Google Scholar] [CrossRef]
- Ono, N.; Ono, W.; Nagasawa, T.; Kronenberg, H.M. A subset of chondrogenic cells provides early mesenchymal progenitors in growing bones. Nat. Cell Biol. 2014, 16, 1157–1167. [Google Scholar] [CrossRef]
- Bielajew, B.J.; Hu, J.C.; Athanasiou, K.A. Collagen: Quantification, biomechanics, and role of minor subtypes in cartilage. Nat. Rev. Mater. 2020, 5, 730–747. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.; Jin, S.; Gu, S.; Sun, R.; Liang, Q. High concentration magnesium inhibits extracellular matrix calcification and protects articular cartilage via Erk/autophagy pathway. J. Cell Physiol. 2019, 234, 23190–23201. [Google Scholar] [CrossRef]
- Schmid, T.M.; Linsenmayer, T.F. Immunohistochemical localization of short chain cartilage collagen (type X) in avian tissues. J. Cell Biol. 1985, 100, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.; Jacenko, O. Phenotypic and biochemical consequences of collagen X mutations in mice and humans. Matrix Biol. 1998, 17, 169–184. [Google Scholar] [CrossRef]
- Kirsch, T.; Harrison, G.; Golub, E.E.; Nah, H.D. The roles of annexins and types II and X collagen in matrix vesicle-mediated mineralization of growth plate cartilage. J. Biol. Chem. 2000, 275, 35577–35583. [Google Scholar] [CrossRef] [PubMed]
- Muir, H. The chondrocyte, architect of cartilage. Biomechanics, structure, function and molecular biology of cartilage matrix macromolecules. Bioessays 1995, 17, 1039–1048. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.Z.; Kim, H.W. Efficacy of collagen and alginate hydrogels for the prevention of rat chondrocyte dedifferentiation. J. Tissue Eng. 2018, 9, 2041731418802438. [Google Scholar] [CrossRef] [PubMed]
- Charlier, E.; Deroyer, C.; Ciregia, F.; Malaise, O.; Neuville, S.; Plener, Z.; Malaise, M.; de Seny, D. Chondrocyte dedifferentiation and osteoarthritis (OA). Biochem. Pharmacol. 2019, 165, 49–65. [Google Scholar] [CrossRef]
- Hao, Z.Y.; Wang, S.; Nie, J.C.; Li, D.C.; Fang, A.O.; Kang, J.F.; Liu, C.Z.; Wang, L. Effects of bionic mechanical stimulation on the properties of engineered cartilage tissue. Bio. Des. Manuf. 2021, 4, 33–43. [Google Scholar] [CrossRef]
- Xu, H.Y.; Huang, H.; Zou, X.J.; Xia, P.C.; Foon, W.A.L.S.; Wang, J.W. A novel bio-active microsphere for meniscus regeneration via inducing cell migration and chondrocyte differentiation. Bio. Des. Manuf. 2021, 4, 203–221. [Google Scholar] [CrossRef]
- Jin, J.; Lv, X.; Wang, B.; Ren, C.; Jiang, J.; Chen, H.; Chen, X.; Gu, M.; Pan, Z.; Tian, N.; et al. Limonin Inhibits IL-1beta-Induced Inflammation and Catabolism in Chondrocytes and Ameliorates Osteoarthritis by Activating Nrf2. Oxid Med. Cell Longev. 2021, 2021, 7292512. [Google Scholar] [CrossRef]
- Chen, R.; Zhou, X.; Yin, S.; Lu, Z.; Nie, J.; Zhou, W.; Liu, X. Study on the protective mechanism of autophagy on cartilage by magnesium sulfate. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2018, 32, 1340–1345. [Google Scholar] [CrossRef]
- Zan, R.; Ji, W.; Qiao, S.; Wu, H.; Wang, W.; Ji, T.; Yang, B.; Zhang, S.; Luo, C.; Song, Y. Biodegradable magnesium implants: A potential scaffold for bone tumor patients. Sci. China Mater. 2021, 64, 1007–1020. [Google Scholar] [CrossRef]
- Yamamoto, K.; Okano, H.; Miyagawa, W.; Visse, R.; Shitomi, Y.; Santamaria, S.; Dudhia, J.; Troeberg, L.; Strickland, D.K.; Hirohata, S.; et al. MMP-13 is constitutively produced in human chondrocytes and co-endocytosed with ADAMTS-5 and TIMP-3 by the endocytic receptor LRP1. Matrix Biol. 2016, 56, 57–73. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Hasegawa, M.; Matsui, Y.; Unno, H.; Iino, T.; Yoshida, T.; Sudo, A. Intra-articular injection of rebamipide prevents articular cartilage degeneration in murine post-traumatic osteoarthritis models. Mod. Rheumatol. 2020, 30, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y. Activation of Hypoxia Inducible Factor-1α Enhances Articular Cartilage Regeneration; The Chinese University of Hong Kong: Hong Kong, 2016. [Google Scholar]
- Zhang, C.; Yang, F.; Cornelia, R.; Tang, W.; Swisher, S.; Kim, H. Hypoxia-inducible factor-1 is a positive regulator of Sox9 activity in femoral head osteonecrosis. Bone 2011, 48, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Hu, N.; Liao, J.-Y.; Lin, L.-B.; Zhao, C.; Si, W.-K.; Yang, Z.; Yi, S.-X.; Fan, T.-X.; Bao, W. HIF-1α as a regulator of BMP2-induced chondrogenic differentiation, osteogenic differentiation, and endochondral ossification in stem cells. Cell. Physiol. Biochem. 2015, 36, 44–60. [Google Scholar] [CrossRef]
- Lu, J.; Peng, Y.; Zou, J.; Wang, J.; Lu, S.; Fu, T.; Jiang, L.; Zhang, C.; Zhang, J. Hypoxia Inducible Factor-1alpha Is a Regulator of Autophagy in Osteoarthritic Chondrocytes. Cartilage 2021, 13, 1030S–1040S. [Google Scholar] [CrossRef]
- Bouaziz, W.; Sigaux, J.; Modrowski, D.; Devignes, C.S.; Funck-Brentano, T.; Richette, P.; Ea, H.K.; Provot, S.; Cohen-Solal, M.; Hay, E. Interaction of HIF1alpha and beta-catenin inhibits matrix metalloproteinase 13 expression and prevents cartilage damage in mice. Proc. Natl. Acad. Sci. USA 2016, 113, 5453–5458. [Google Scholar] [CrossRef]
- Komori, T. Molecular mechanism of Runx2-dependent bone development. Mol. Cells 2020, 43, 168. [Google Scholar]
- Li, Z.; Liu, P.; Yang, T.; Sun, Y.; You, Q.; Li, J.; Wang, Z.; Han, B. Composite poly(l-lactic-acid)/silk fibroin scaffold prepared by electrospinning promotes chondrogenesis for cartilage tissue engineering. J. Biomater. Appl. 2016, 30, 1552–1565. [Google Scholar] [CrossRef]
- Zhao, Y.; Huang, Y.; Jia, L.; Wang, R.; Tan, K.; Li, W. A Novel Tension Machine Promotes Bone Marrow Mesenchymal Stem Cell Osteoblastic and Fibroblastic Differentiation by Applying Cyclic Tension. Stem. Cells Int. 2021, 2021, 6647651. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Zheng, X.; Wang, Y.; Tao, T.; Wang, Z.; Yuan, L.; Han, B. The Biomimetics of Mg2+-Concentration-Resolved Microenvironment for Bone and Cartilage Repairing Materials Design. Biomimetics 2022, 7, 227. https://doi.org/10.3390/biomimetics7040227
Li Z, Zheng X, Wang Y, Tao T, Wang Z, Yuan L, Han B. The Biomimetics of Mg2+-Concentration-Resolved Microenvironment for Bone and Cartilage Repairing Materials Design. Biomimetics. 2022; 7(4):227. https://doi.org/10.3390/biomimetics7040227
Chicago/Turabian StyleLi, Zhengqiang, Xiaoxue Zheng, Yixing Wang, Tianyi Tao, Zilin Wang, Long Yuan, and Bing Han. 2022. "The Biomimetics of Mg2+-Concentration-Resolved Microenvironment for Bone and Cartilage Repairing Materials Design" Biomimetics 7, no. 4: 227. https://doi.org/10.3390/biomimetics7040227
APA StyleLi, Z., Zheng, X., Wang, Y., Tao, T., Wang, Z., Yuan, L., & Han, B. (2022). The Biomimetics of Mg2+-Concentration-Resolved Microenvironment for Bone and Cartilage Repairing Materials Design. Biomimetics, 7(4), 227. https://doi.org/10.3390/biomimetics7040227





