Detection of Venturia inaequalis Isolates with Multiple Resistance in Greece
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungicides
2.2. Isolates
2.3. Spore Germination and Mycelial Growth Assays
2.4. Screening for Resistance
2.5. Data Analysis
3. Results
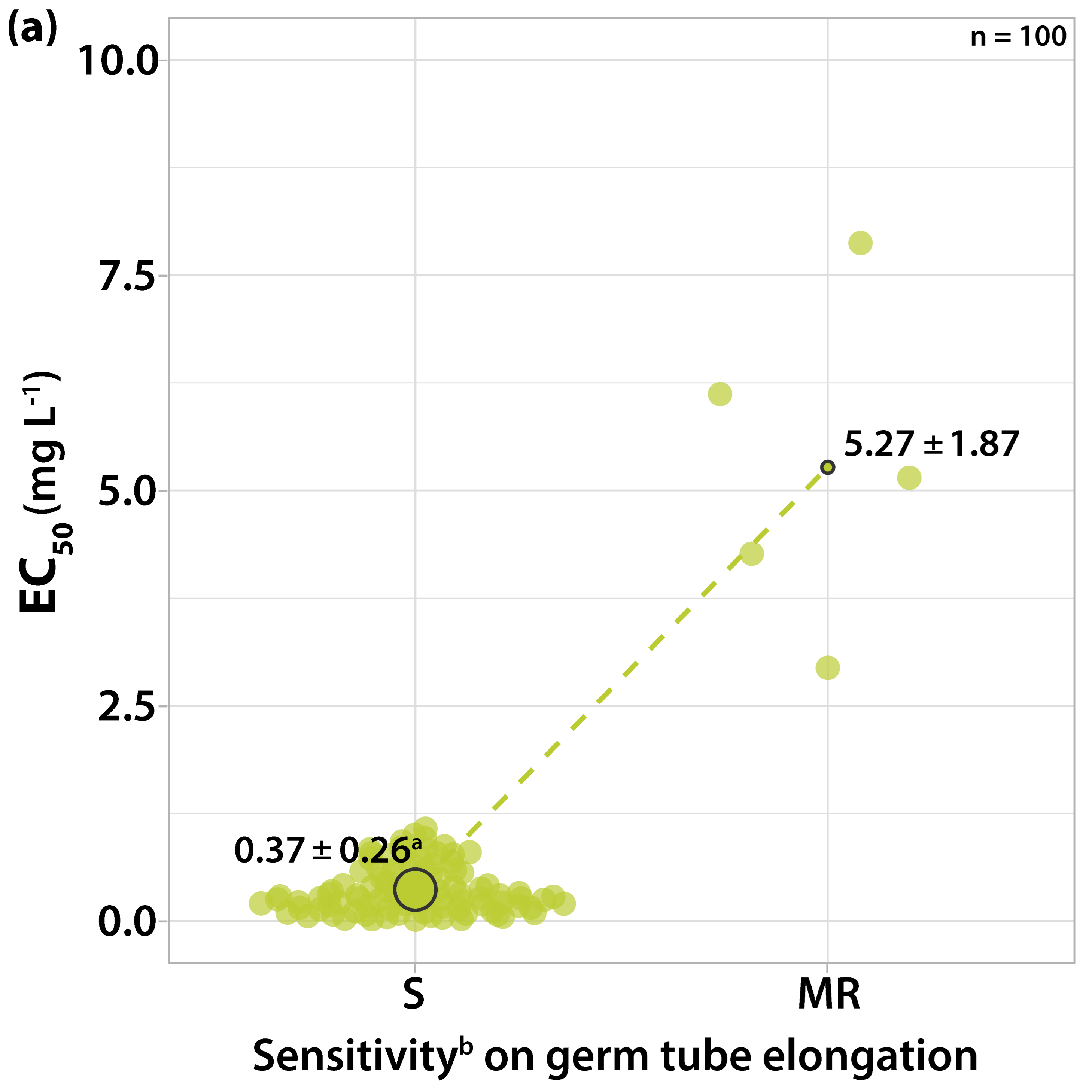
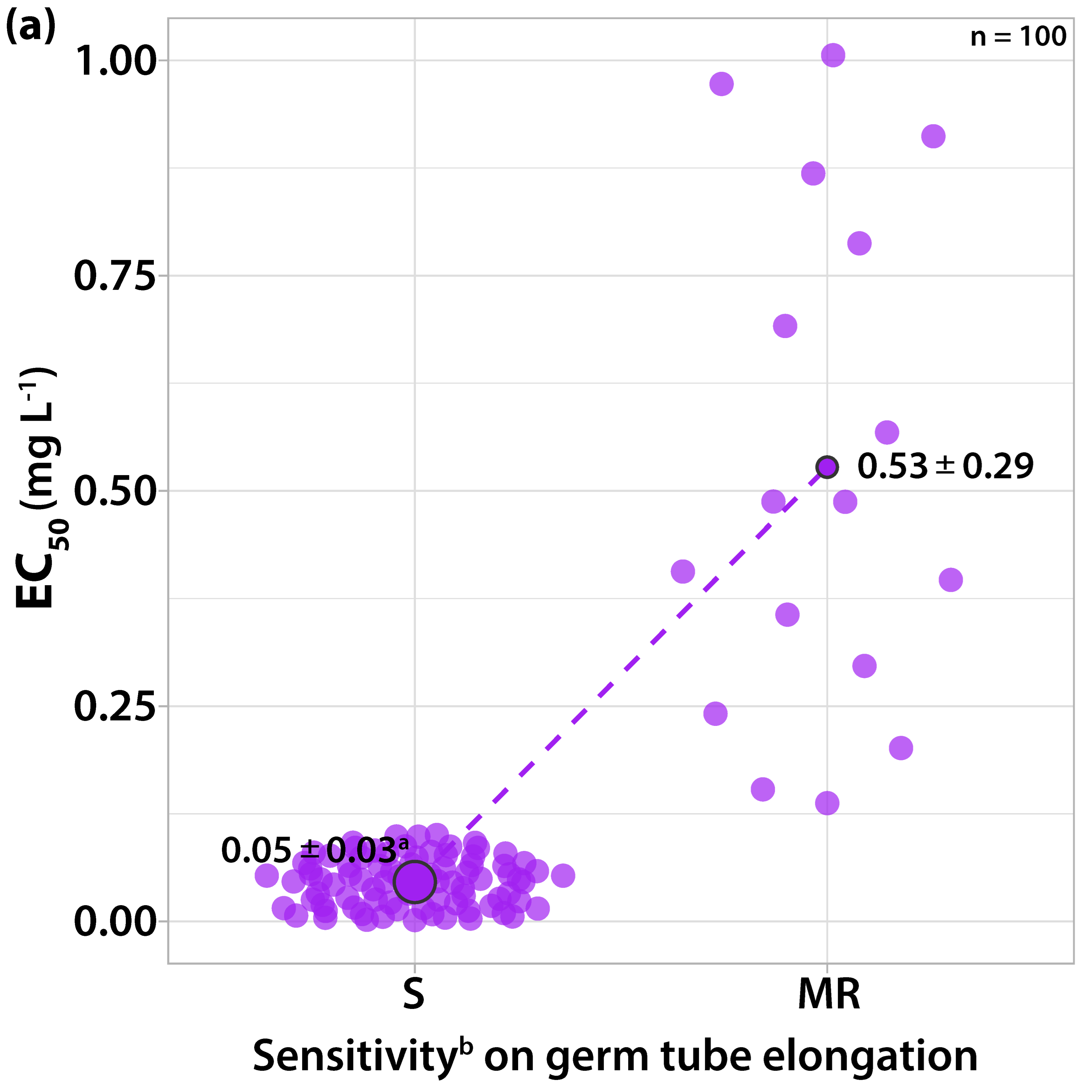
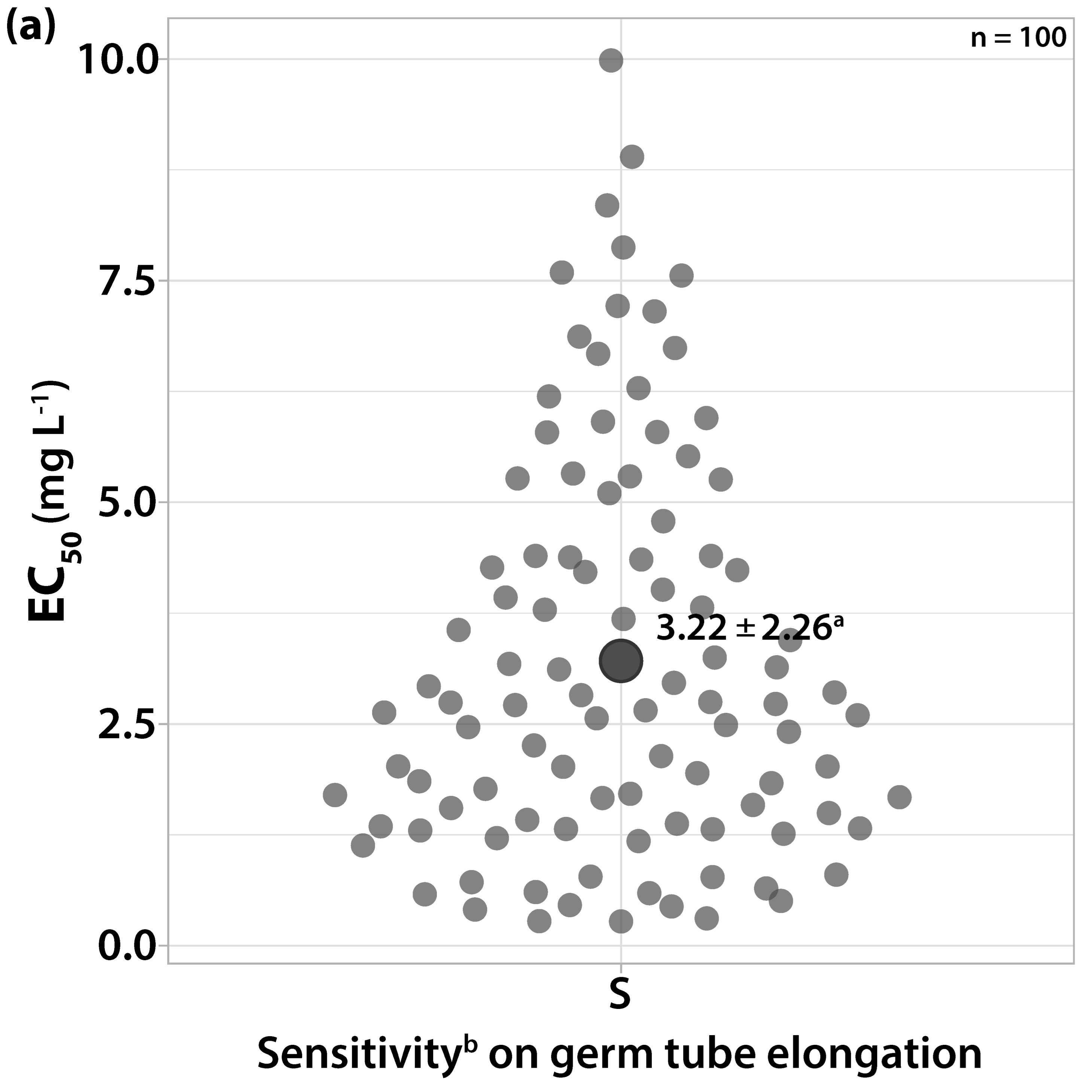
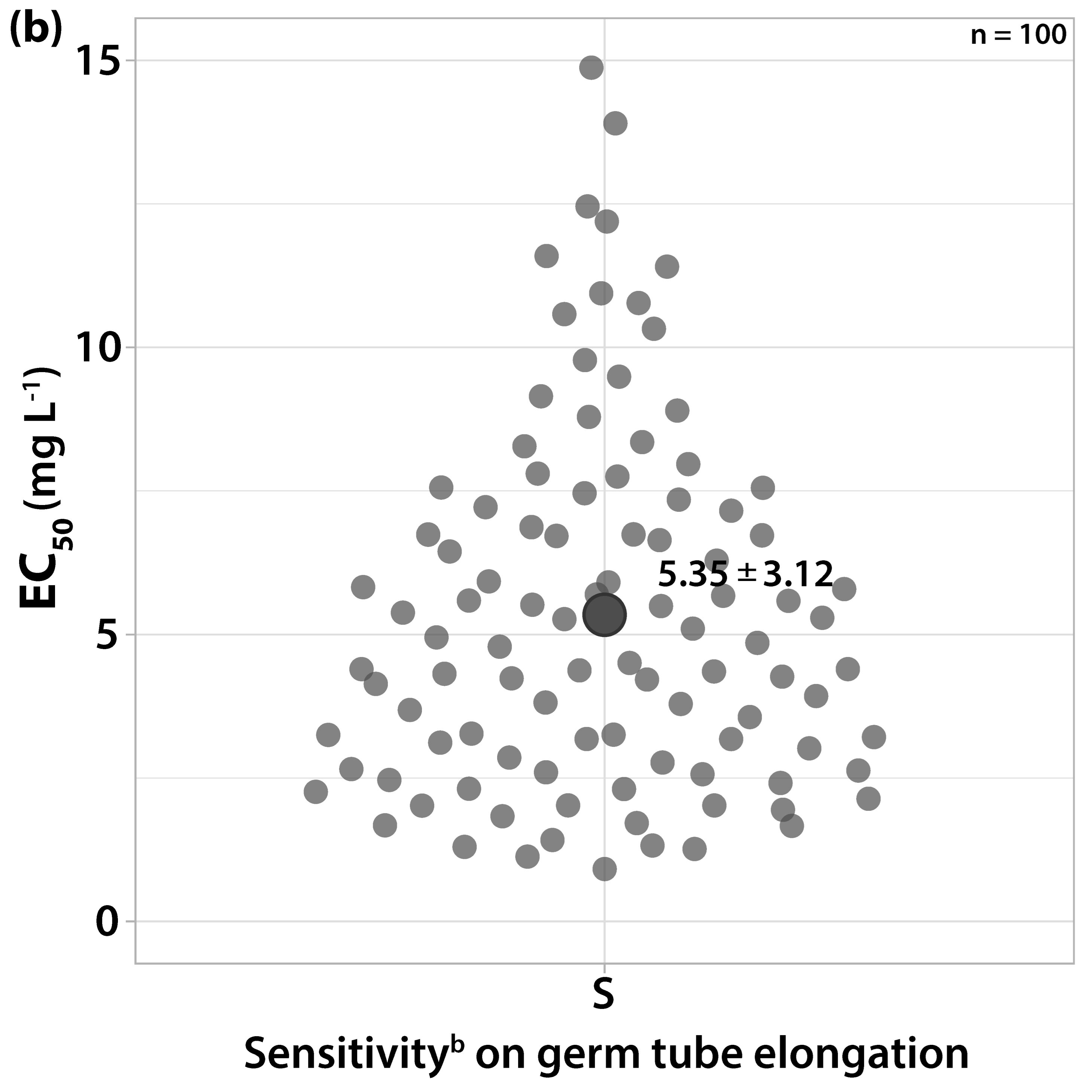
3.1. Characterization of Fungicide Sensitivity Profiles
3.1.1. Sensitivity to Trifloxystrobin
3.1.2. Sensitivity to Cyprodinil
3.1.3. Sensitivity to Dodine
3.1.4. Sensitivity to Difenoconazole
3.1.5. Sensitivity to Boscalid
3.1.6. Sensitivity to Fludioxonil
3.1.7. Sensitivity to Dithianon and Captan
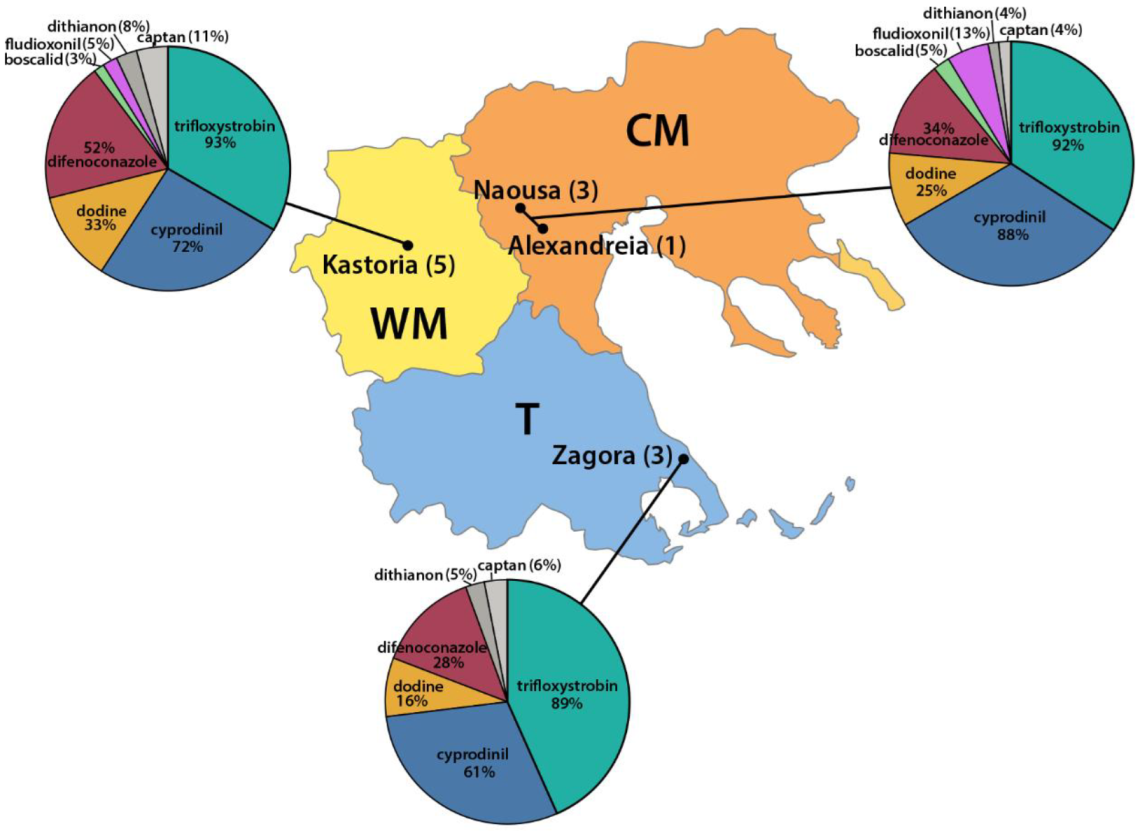
3.2. Survey on Fungicide Resistance
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chatzidimopoulos, M.; Lioliopoulou, F.; Sotiropoulos, T.; Vellios, E. Efficient control of apple scab with targeted spray applications. Agronomy 2020, 10, 217. [Google Scholar] [CrossRef]
- Schwabe, W.F.S. Changes in scab susceptibility of apple leaves as influenced by age. Phytophylactica 1979, 11, 53–56. [Google Scholar]
- Brent, K.J.; Hollomon, D.W. (Eds.) Fungicide Resistance in Crop Protection: How Can It Be Managed? 2nd ed.; FRAC Monograph No 1.; Crop Life International: Brussels, Belgium, 2007; p. 48. [Google Scholar]
- Xu, X.-M.; Murray, R.A.; Salazar, J.D.; Hyder, K. The effects of temperature, humidity and rainfall on captan decline on apple leaves and fruit in controlled environment conditions. Pest Manag. Sci. 2008, 64, 296–307. [Google Scholar] [CrossRef]
- Jobin, T.; Carisse, O. Incidence of myclobutanil- and kresoxim-methyl-insensitive isolates of Venturia inaequalis in Quebec orchards. Plant Dis. 2007, 91, 1351–1358. [Google Scholar] [CrossRef][Green Version]
- Chapman, K.S.; Sundin, G.W.; Beckerman, J.L. Identification of resistance to multiple fungicides in field populations of Venturia inaequalis. Plant Dis. 2011, 95, 921–926. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, D.W.; Clough, J.M.; Godwin, J.R.; Hall, A.A.; Hamer, M.; Parr-Dobrzanski, B. The strobilurin fungicides. Pest Manag. Sci. 2002, 58, 649–662. [Google Scholar] [CrossRef]
- Cabral, J.P. Damage to the cytoplasmic membrane and cell death caused by dodine (dodecylguanidine monoacetate) in Pseudomonas syringae ATCC 12271. Antimicrob. Agents Chemother. 1991, 35, 341. [Google Scholar] [CrossRef]
- Jones, A.L.; Walker, R.J. Tolerance of Venturia inaequalis to dodine and benzimidazole fungicides in Michigan. Plant Dis. Reporter 1976, 60, 40–44. [Google Scholar]
- Mondino, P.; Casanova, L.; Celio, A.; Bentancur, O.; Leoni, C.; Alaniz, S. Sensitivity of Venturia inaequalis to trifloxystrobin and difenoconazole in Uruguay. J. Phytopathol. 2015, 163, 1–10. [Google Scholar] [CrossRef]
- Cox, K.D. Fungicide Resistance in Venturia inaequalis, the Causal Agent of Apple Scab, in the United States. In Fungicide Resistance in Plant Pathogens: Principles and a Guide to Practical Management; Ishii, H., Hollomon, D.W., Eds.; Springer: Tokyo, Japan, 2015; pp. 433–447. [Google Scholar]
- Villani, S.M.; Biggs, A.R.; Cooley, D.R.; Raes, J.J.; Cox, K.D. Prevalence of myclobutanil resistance and difenoconazole insensitivity in populations of Venturia inaequalis. Plant Dis. 2015, 99, 1526–1536. [Google Scholar] [CrossRef]
- Mosbach, A.; Edel, D.; Farmer, A.D.; Widdison, S.; Barchietto, T.; Dietrich, R.A.; Corran, A.; Scalliet, G. Anilinopyrimidine resistance in Botrytis cinerea is linked to mitochondrial function. Front. Microbiol. 2017, 8, 2361. [Google Scholar] [CrossRef] [PubMed]
- Brandhorst, T.T.; Kean, I.R.L.; Lawry, S.M.; Wiesner, D.L.; Klein, B.S. Phenylpyrrole fungicides act on triosephosphate isomerase to induce methylglyoxal stress and alter hybrid histidine kinase activity. Sci. Rep. 2019, 9, 5047. [Google Scholar] [CrossRef] [PubMed]
- Stammler, G.; Brix, H.D.; Nave, B.; Gold, R.; Schoelf, U. Studies on the Biological Performance of Boscalid and Its Mode of Action. In Proceedings of the Modern Fungicides and Antifungal Compounds V, Friedrichroda, Germany, 6–10 May 2007; Deutsche Phytomedizinische Gesellschaft: Braunschweig, Germany, 2008; pp. 45–51. [Google Scholar]
- Köller, W.; Wilcox, W.F.; Jones, A.L. Quantification, persistence, and status of dodine resistance in New York and Michigan orchard populations of Venturia inaequalis. Plant Dis. 1999, 83, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Koenraadt, H.; Somerville, S.C.; Jones, A.L. Characterization of mutations in the beta-tubulin gene of benomyl-resistant field strains of Venturia inaequalis and other plant pathogenic fungi. Phytopathology 1992, 82, 1348–1354. [Google Scholar] [CrossRef]
- Kiebacher, H.; Hoffmann, G.M. Resistance against benzimidazole fungicides in Venturia inaequalis. J. Plant Dis. Prot. 1976, 83, 352–358. [Google Scholar]
- Köller, W.; Wilcox, W.F.; Barnard, J.; Jones, A.L.; Braun, P.G. Detection and quantification of resistance of Venturia inaequalis populations to sterol demethylation inhibitors. Phytopathology 1997, 87, 184–190. [Google Scholar] [CrossRef]
- Polat, Z.; Bayraktar, H. Resistance of Venturia inaequalis to multiple fungicides in Turkish apple orchards. J. Phytopathol. 2021, 169, 360–368. [Google Scholar] [CrossRef]
- Li, X.; Li, H.; Yu, Z.; Gao, L.; Yang, J. Investigating the sensitivity of Venturia inaequalis isolates to difenoconazole and pyraclostrobin in apple orchards in China. Eur. J. Plant Pathol. 2021, 161, 207–217. [Google Scholar] [CrossRef]
- Köller, W.; Parker, D.M.; Turechek, W.W.; Avila-Adame, C.; Cronshaw, K. A two-phase resistance response of Venturia inaequalis populations to the QoI fungicides kresoxim-methyl and trifloxystrobin. Plant Dis. 2004, 88, 537–544. [Google Scholar] [CrossRef]
- Fiaccadori, R. In vitro, in vivo and in field sensitivity of Venturia inaequalis to anilinopyrimidine fungicides with different types of scab management and degree of control. Open Access Libr. J. 2018, 5, e5092. [Google Scholar] [CrossRef]
- Larsen, N.J.; Beresford, R.M.; Wood, P.N.; Wright, P.J.; Fisher, B.M. A synthetic agar assay for determining sensitivity of Venturia inaequalis to anilinopyrimidine fungicides in New Zealand apple orchards. New Zealand Plant Prot. 2013, 66, 293–302. [Google Scholar] [CrossRef]
- Toffolatti, S.L.; Venturini, G.; Bianco, P.A. First report of SDHI resistant strains of Venturia inaequalis from commercial orchards in northern Italy. Plant Dis. 2016, 100, 2324. [Google Scholar] [CrossRef]
- Chatzidimopoulos, M.; Ganopoulos, I.; Moraitou-Daponta, E.; Lioliopoulou, F.; Ntantali, O.; Panagiotaki, P.; Vellios, E.K. High-Resolution Melting (HRM) analysis reveals genotypic differentiation of Venturia inaequalis populations in Greece. Front. Ecol. Evol. 2019, 7, 489. [Google Scholar] [CrossRef]
- Dhingra, O.D.; Sinclair, J.B. Basic Plant Pathology Methods, 2nd ed.; CRC Press, Inc.: Boca Raton, FL, USA, 1995; p. 448. [Google Scholar]
- Pappas, A.C.; Vellios, E.K.; Mylonopoulos, I.S.; Chatzidimopoulos, M.; Vlassacoudis, A. Sensitivity of Septoria pyricola isolates to carbendazim, DMI and QoI based fungicides and to boscalid, in Greece. Phytopathol. Mediterr. 2010, 49, 227–238. [Google Scholar]
- Goedhart, J. SuperPlotsOfData—A web app for the transparent display and quantitative comparison of continuous data from different conditions. Mol. Biol. Cell 2021, 32, 470–474. [Google Scholar] [CrossRef]
- Lesniak, K.; Proffer, T.; Beckerman, J.; Sundin, G. Occurrence of QoI resistance and detection of the G143A mutation in Michigan populations of Venturia inaequalis. Plant Dis. 2011, 95, 927–934. [Google Scholar] [CrossRef]
- Frederick, Z.A.; Villani, S.M.; Cooley, D.R.; Biggs, A.R.; Raes, J.J.; Cox, K.D. Prevalence and Stability of Qualitative QoI Resistance in Populations of Venturia inaequalis in the Northeastern United States. Plant Dis. 2014, 98, 1122–1130. [Google Scholar] [CrossRef]
- Kunz, S.; Lutz, B.; Deising, H.; Mendgen, K. Assessment of sensitivities to anilinopyrimidine- and strobilurin-fungicides in populations of the apple scab fungus Venturia inaequalis. J. Phytopathol. 1998, 146, 231–238. [Google Scholar] [CrossRef]
- Carisse, O.; Jobin, T. Resistance to dodine in populations of Venturia inaequalis in Quebec, Canada. Plant Health Prog. 2010, 11, 17. [Google Scholar] [CrossRef]
- Beresford, R.M.; Wright, P.J.; Wood, P.N.; Park, N.M. Sensitivity of Venturia inaequalis to myclobutanil penconazole and dodine in relation to fungicide use in Hawkes Bay apple orchards. New Zealand Plant Prot. 2012, 65, 106–113. [Google Scholar] [CrossRef]
- Yoder, K.S.; Klos, E.J. Tolerance to dodine in Venturia inaequalis. Phytopathology 1976, 66, 918–923. [Google Scholar] [CrossRef]
- Stević, M.; Vukša, P.; Elezović, I. Resistance of Venturia inaequalis to demethylation inhibiting (DMI) fungicides. Zemdirb. Agric. 2010, 97, 65–72. [Google Scholar]
- Schnabel, G.; Jones, A.L. The 14alpha-demethylase (CYP51A1) gene is overexpressed in Venturia inaequalis strains resistant to myclobutanil. Phytopathology 2001, 91, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Veloukas, T.; Leroch, M.; Hahn, M.; Karaoglanidis, G.S. Detection and molecular characterization of boscalid-resistant Botrytis cinerea isolates from strawberry. Plant Dis. 2011, 95, 1302–1307. [Google Scholar] [CrossRef]
- Kilani, J.; Fillinger, S. Phenylpyrroles: 30 Years, two molecules and (nearly) no resistance. Front. Microbiol. 2016, 7, 2014. [Google Scholar] [CrossRef]
- Kretschmer, M.; Leroch, M.; Mosbach, A.; Walker, A.S.; Fillinger, S.; Mernke, D.; Schoonbeek, H.J.; Pradier, J.M.; Leroux, P.; De Waard, M.A.; et al. Fungicide-driven evolution and molecular basis of multidrug resistance in field populations of the grey mould fungus Botrytis cinerea. PLoS Pathog. 2009, 5, e1000696. [Google Scholar] [CrossRef]
- Gordon, E.B. Captan and Folpet. In Hayes’ Handbook of Pesticide Toxicology; Elsevier: Amsterdam, The Netherlands, 2010; pp. 1915–1949. [Google Scholar]
- Fiaccadori, R. Research on dithianon to evaluate suspects of reduced sensitivity of Venturia inaequalis with different methodologies in Italian apple areas. Am. J. Plant Sci. 2021, 12, 406–416. [Google Scholar] [CrossRef]
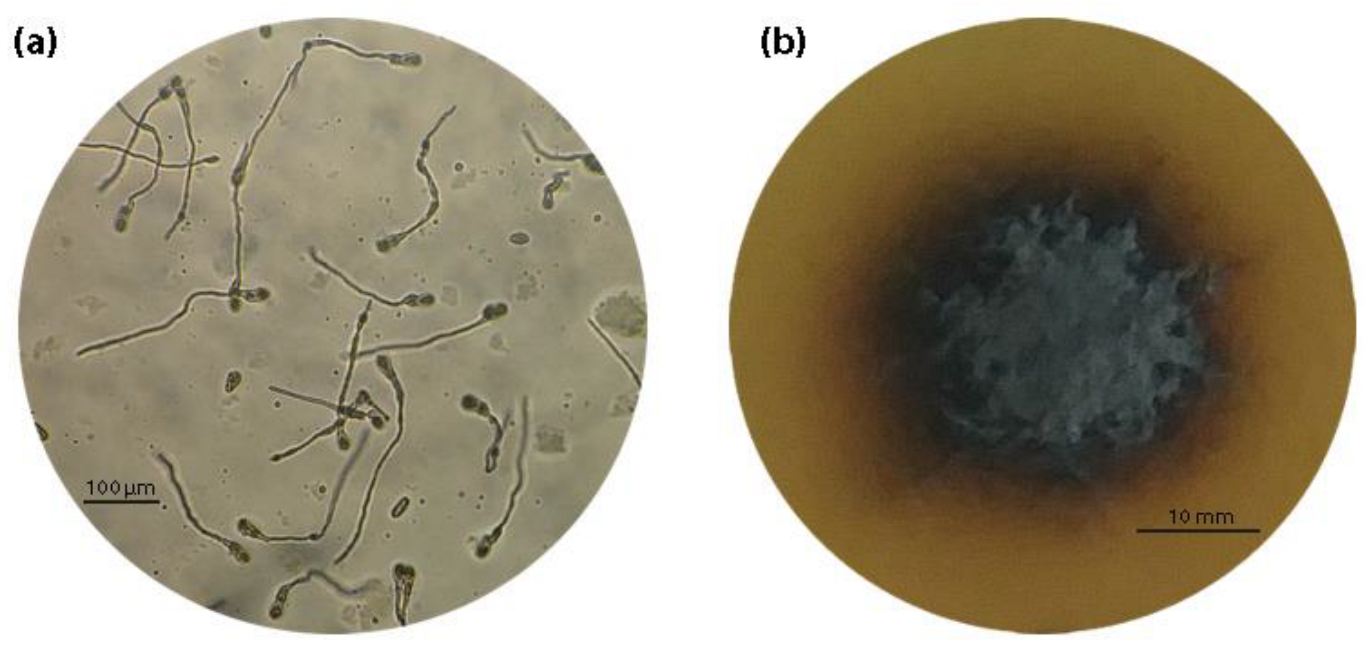

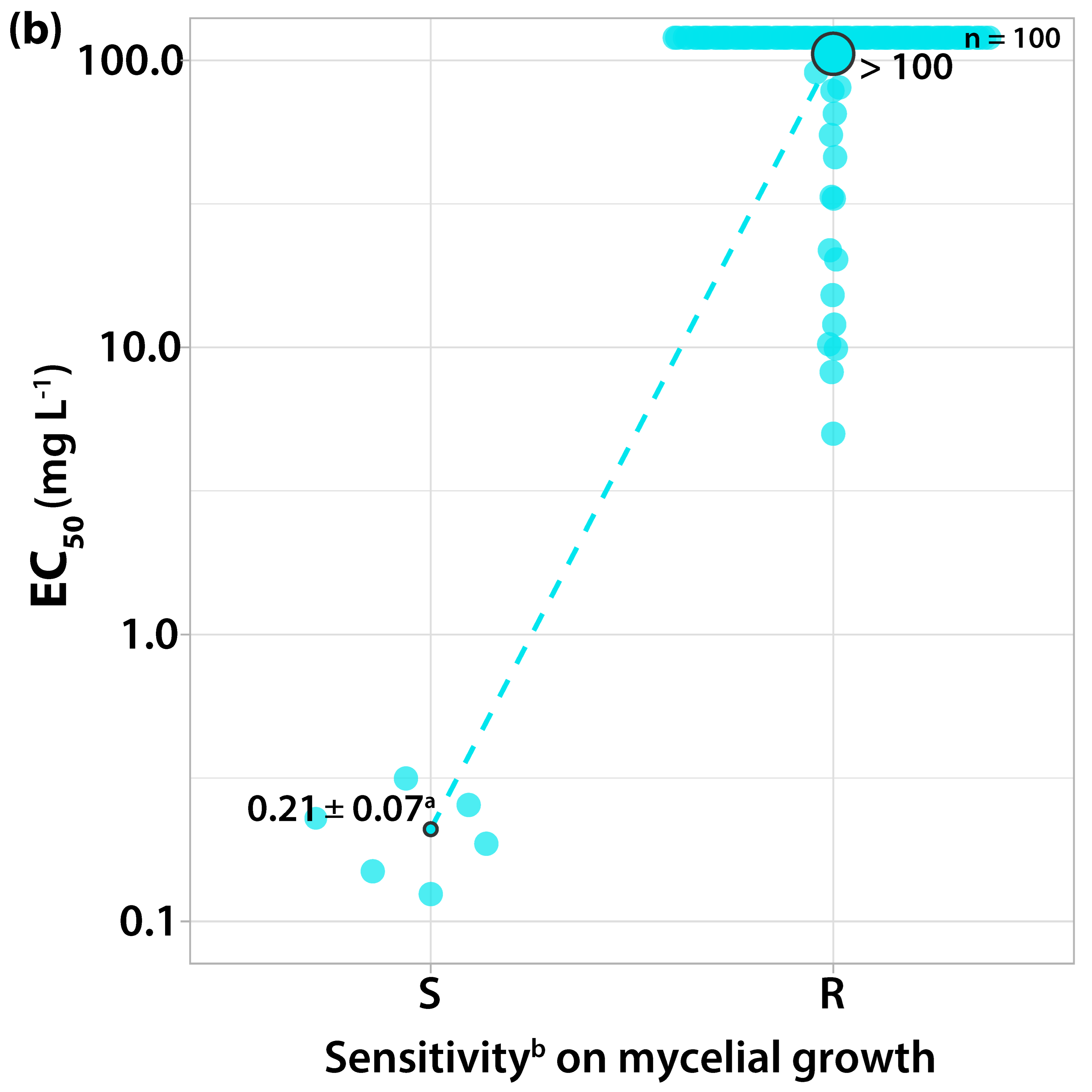
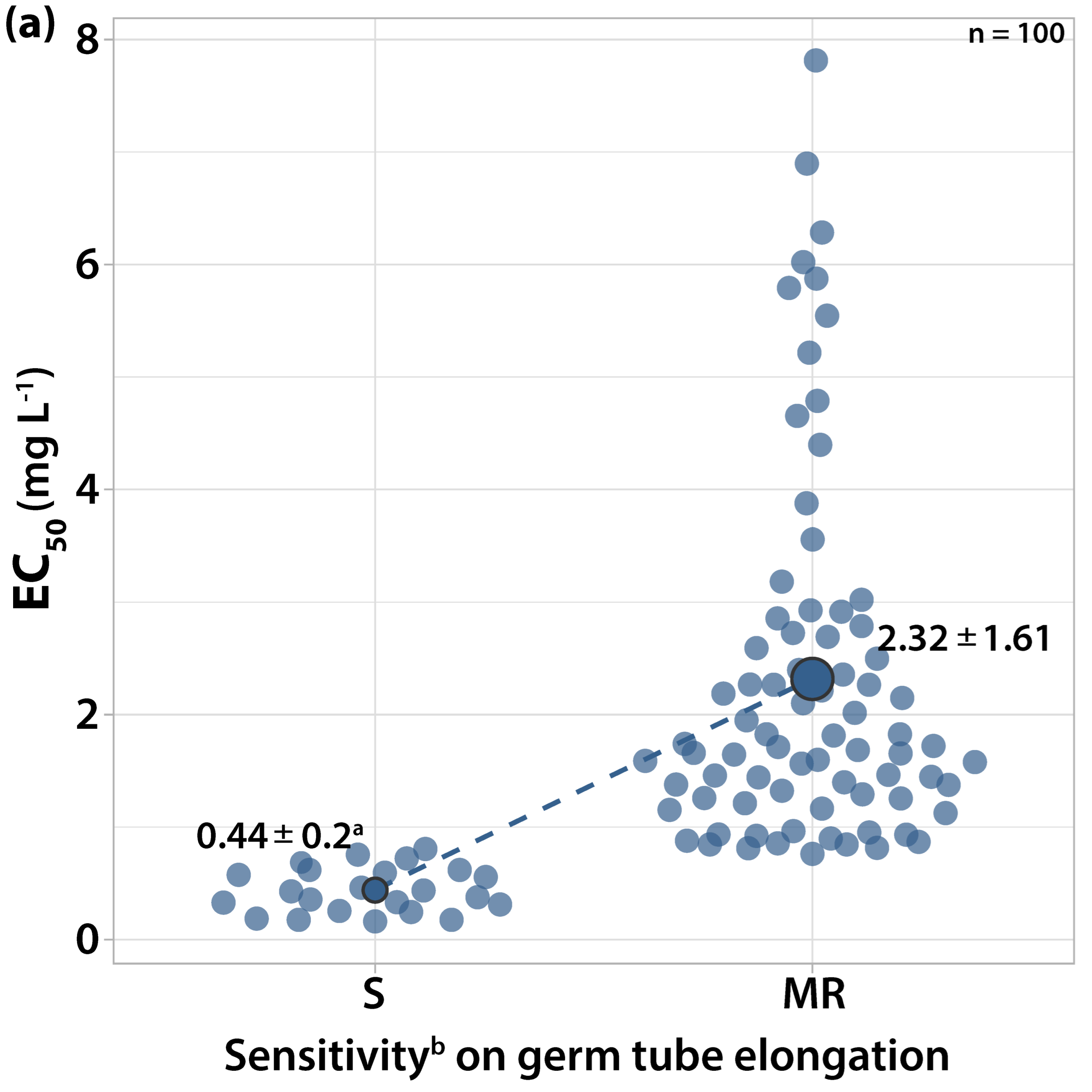
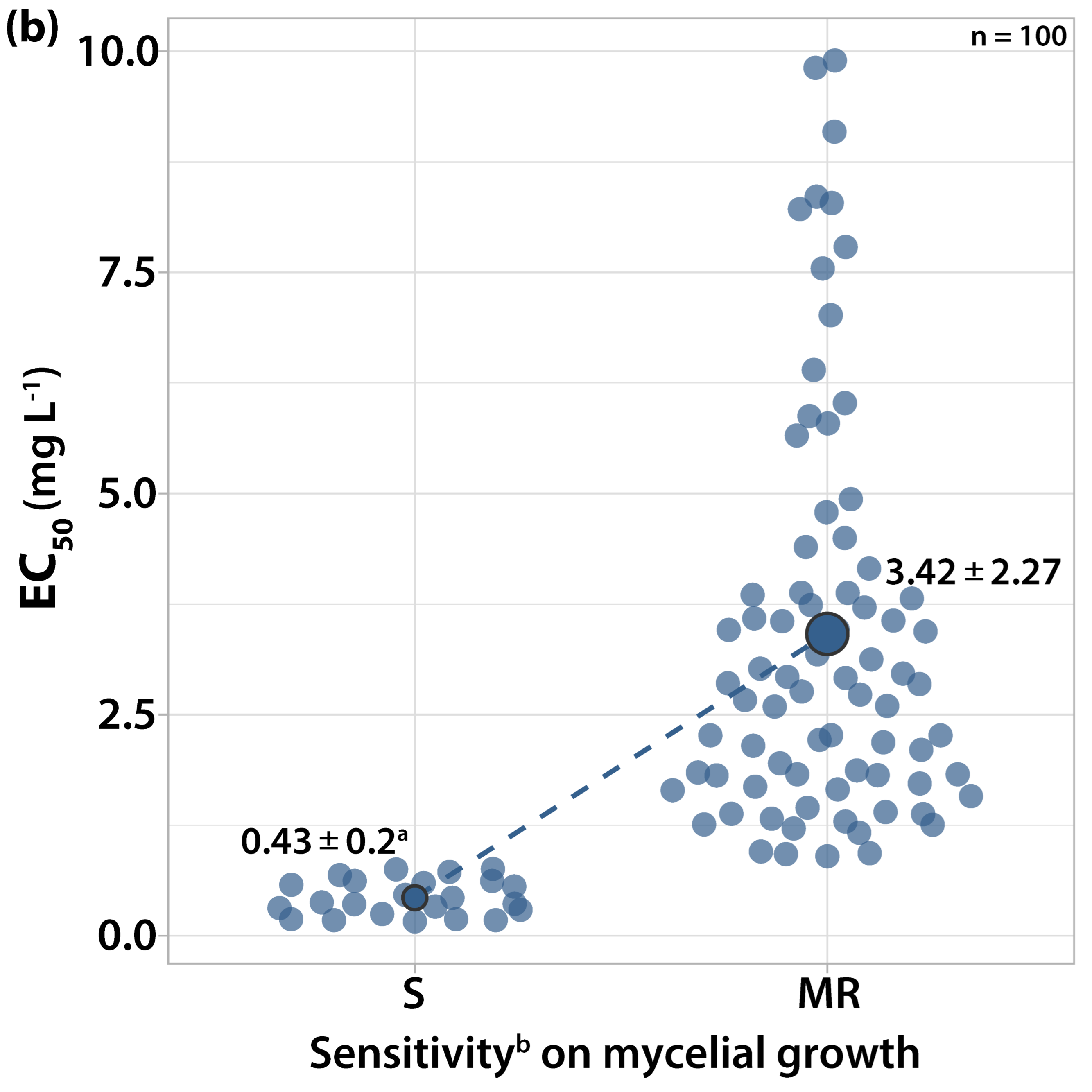
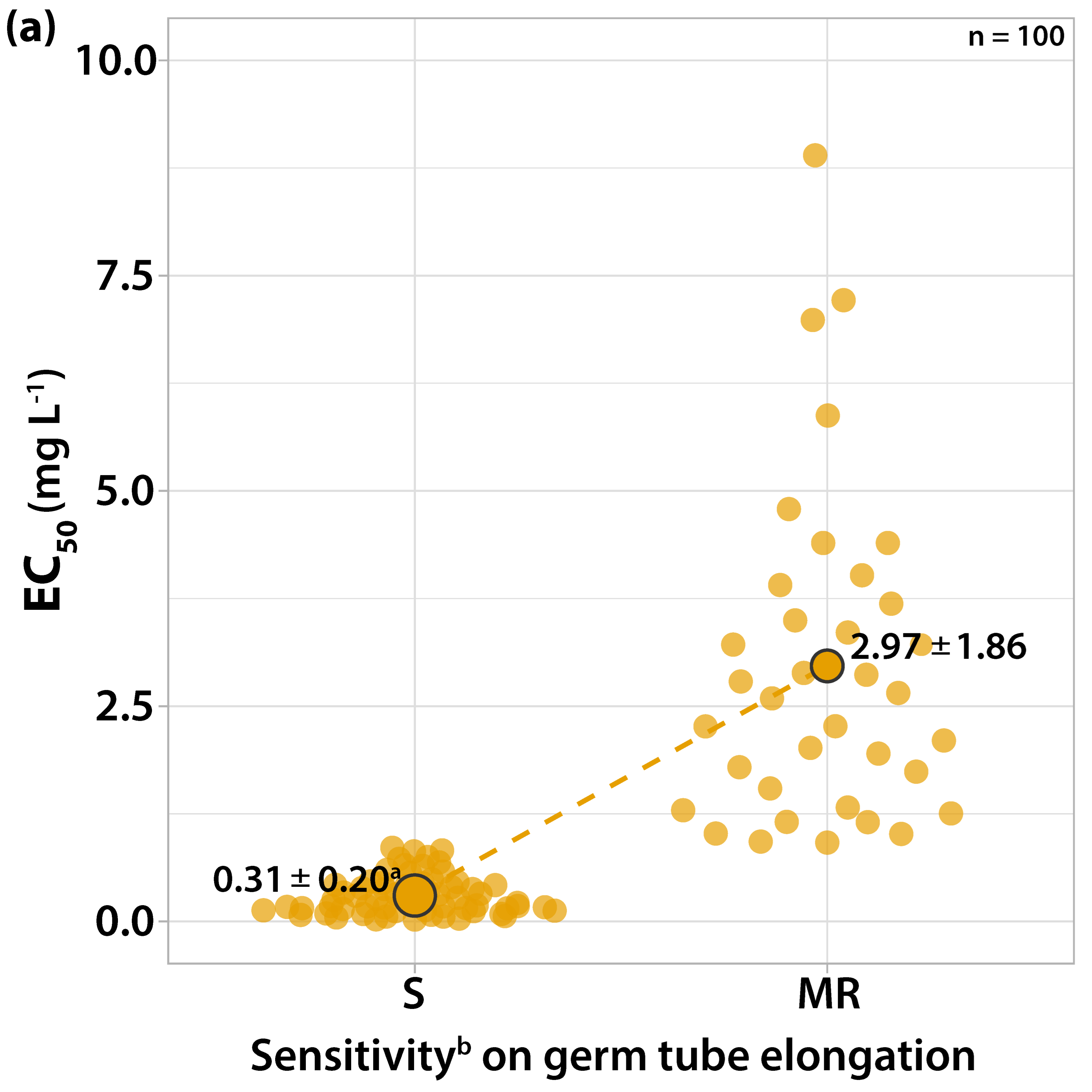
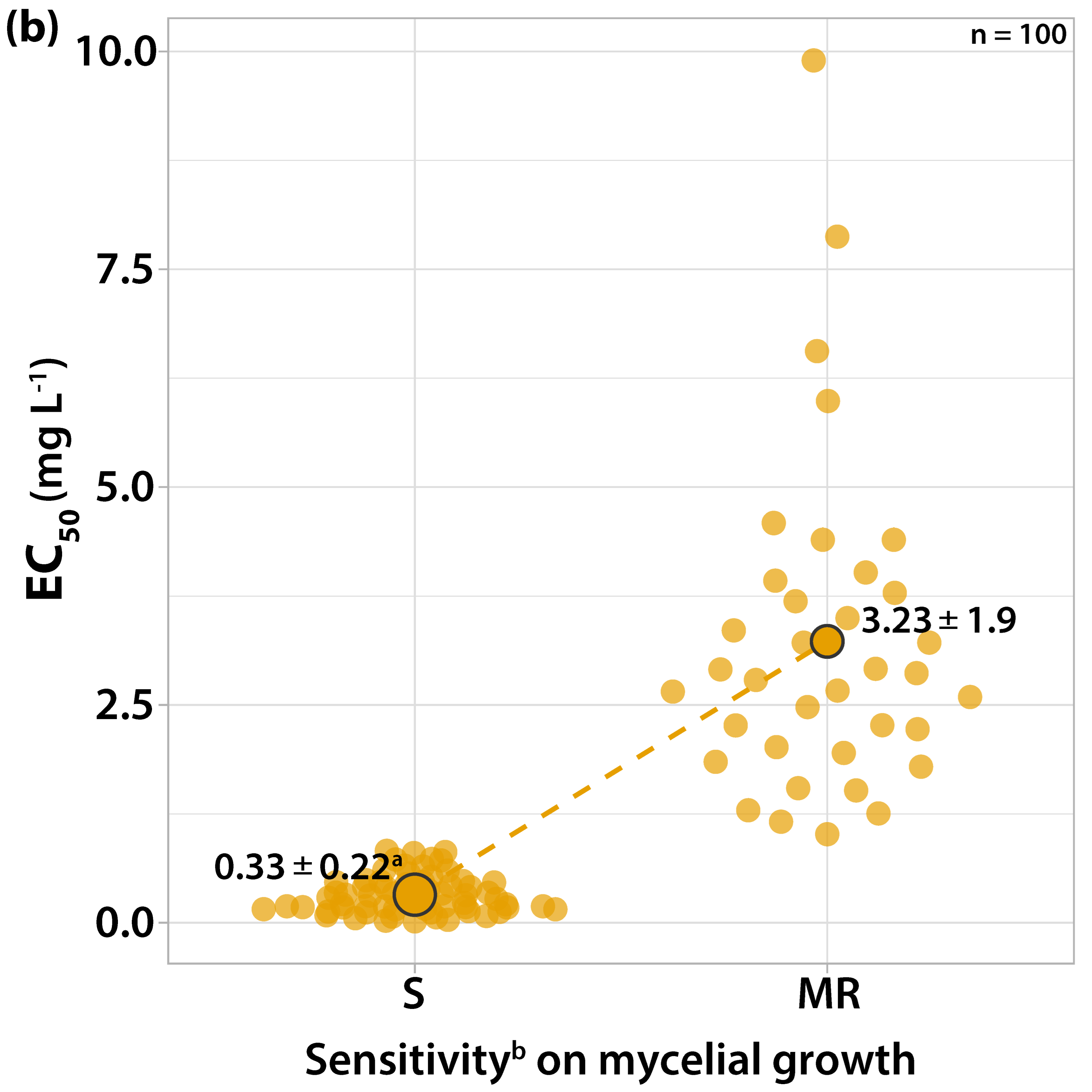
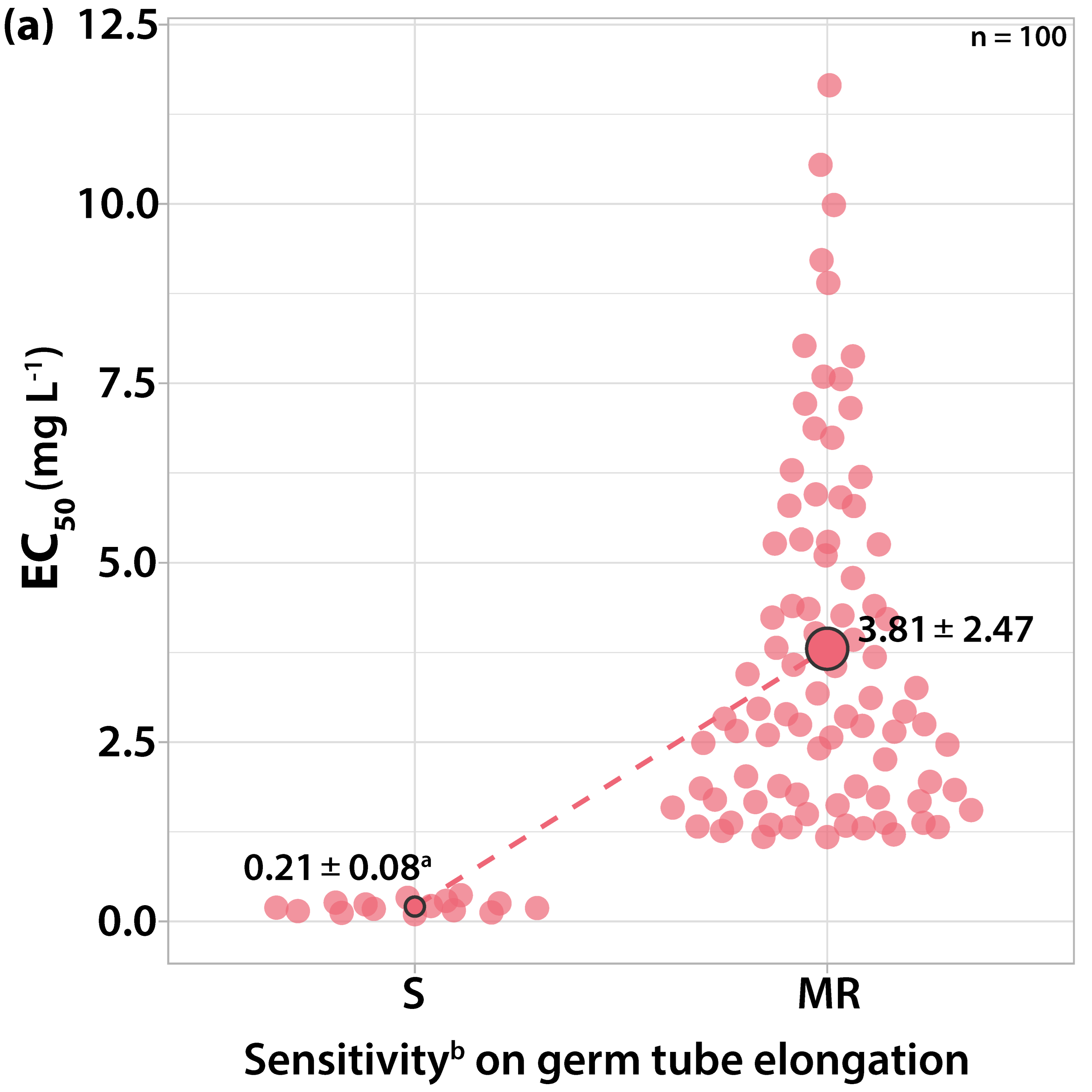
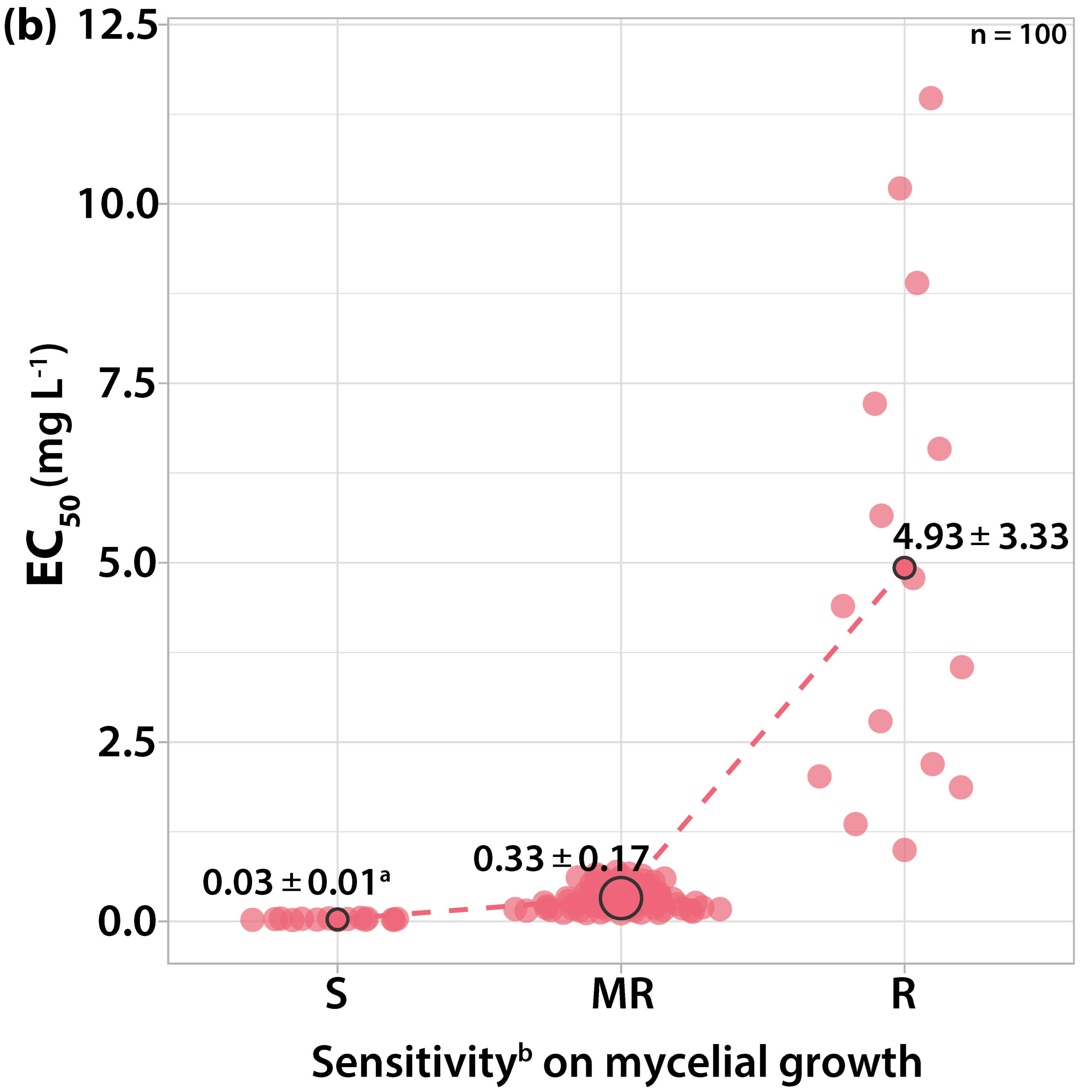


| Orchard Number | Site (Region a) | Apple Cultivar | Number and Code of Isolates |
|---|---|---|---|
| 1 | Kastoria (WM) | Gala SchniCo Red | 48 (KG) |
| 2 | Kastoria (WM) | Super Chief | 57 (KT 1-57) |
| 3 | Kastoria (WM) | Super Chief | 36 (KT 58-93) |
| 4 | Kastoria (WM) | Fuji Kiku | 29 (KF 1-29) |
| 5 | Kastoria (WM) | Fuji Kiku | 51 (KF 30-80) |
| 6 | Naousa (CM) | Scarlet spur | 35 (NT) |
| 7 | Naousa (CM) | Super Chief | 30 (GT) |
| 8 | Naousa (CM) | Fuji Kiku | 27 (RT) |
| 9 | Alexandreia (CM) | Pink Lady | 41 (VT) |
| 10 | Zagora (T) | Scarlet spur | 19 (ZG 1-19) |
| 11 | Zagora (T) | Scarlet spur | 25 (ZG 20-44) |
| 12 | Zagora (T) | Scarlet spur | 20 (ZG 45-64) |
| EC50 for Germ Tube Elongation | EC50 for Mycelial Growth | |||||
|---|---|---|---|---|---|---|
| Fungicides | Wild-Type Isolate KT10 | Multiple Resistant Isolate VT18 | RF | Wild-Type Isolate KT10 | Multiple Resistant Isolate VT18 | RF |
| Trifloxystrobin 1 | 0.1495 | >100 | >668.90 | 0.3219 | >100 | >310.66 |
| Cyprodinil | 0.1727 | 6.8340 | 39.57 | 0.2919 | 9.6899 | 33.20 |
| Dodine | 0.048 | 5.9083 | 123.09 | 0.0558 | 4.3968 | 78.80 |
| Difenoconazole | 0.1248 | 5.7317 | 45.93 | 0.0183 | 1.8744 | 102.43 |
| Boscalid | 0.0849 | 2.1692 | 25.55 | 0.8954 | 30.62 | 34.20 |
| Fludioxonil | 0.0033 | 0.2532 | 76.72 | 0.4586 | 25.96 | 56.61 |
| Dithianon | 0.4572 | 8.8974 | 19.46 | >100 | >100 | - |
| Captan | 1.2987 | 10.7727 | 8.29 | >100 | >100 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chatzidimopoulos, M.; Zambounis, A.; Lioliopoulou, F.; Vellios, E. Detection of Venturia inaequalis Isolates with Multiple Resistance in Greece. Microorganisms 2022, 10, 2354. https://doi.org/10.3390/microorganisms10122354
Chatzidimopoulos M, Zambounis A, Lioliopoulou F, Vellios E. Detection of Venturia inaequalis Isolates with Multiple Resistance in Greece. Microorganisms. 2022; 10(12):2354. https://doi.org/10.3390/microorganisms10122354
Chicago/Turabian StyleChatzidimopoulos, Michael, Antonios Zambounis, Fenia Lioliopoulou, and Evangelos Vellios. 2022. "Detection of Venturia inaequalis Isolates with Multiple Resistance in Greece" Microorganisms 10, no. 12: 2354. https://doi.org/10.3390/microorganisms10122354
APA StyleChatzidimopoulos, M., Zambounis, A., Lioliopoulou, F., & Vellios, E. (2022). Detection of Venturia inaequalis Isolates with Multiple Resistance in Greece. Microorganisms, 10(12), 2354. https://doi.org/10.3390/microorganisms10122354






