Enhanced Functionality and Bio-Accessibility of Composite Pomegranate Peel Extract-Enriched “Boba Balls”
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Pomegranate Peel Extract (PPE)
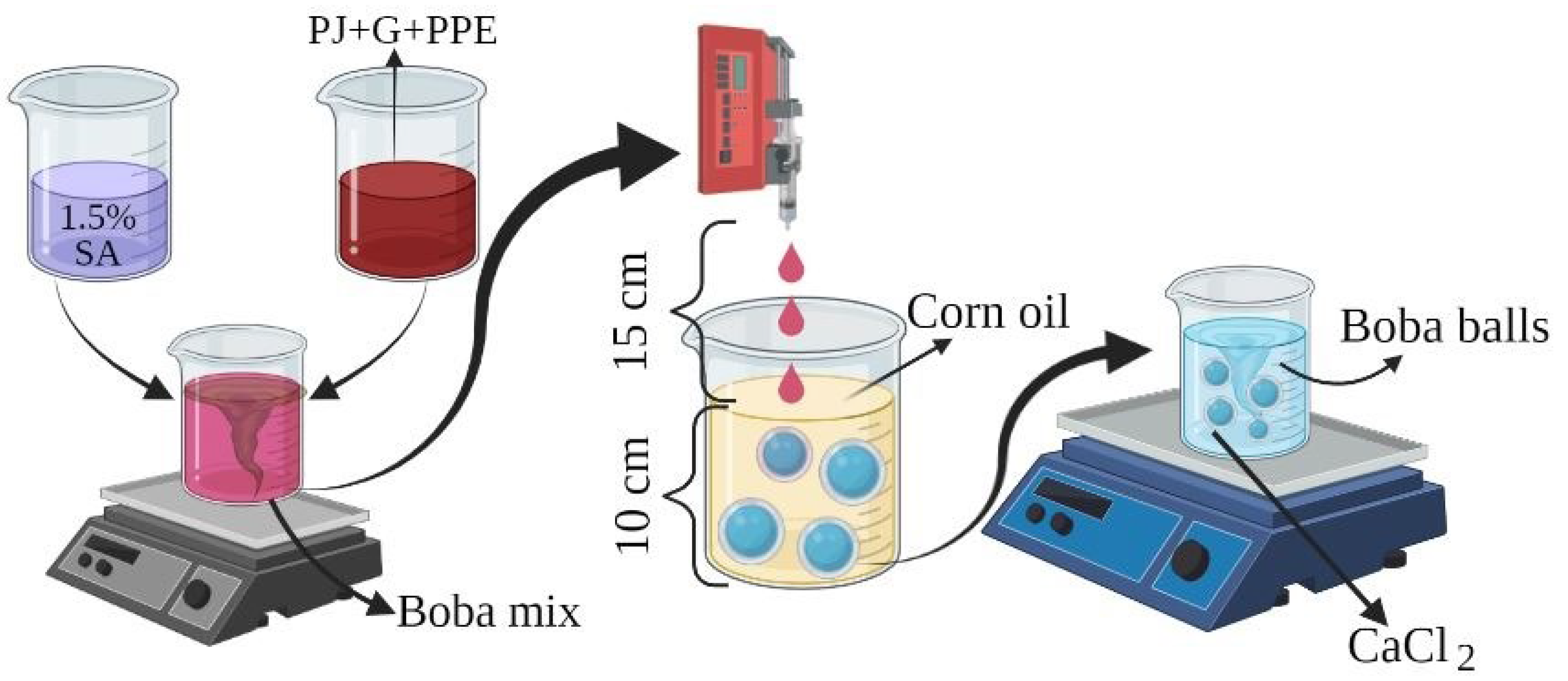
2.3. Production of “Boba Balls”
2.4. Rheological Characterization of “Boba” Mix
2.5. Characterization of “Boba Balls”
2.5.1. Total Phenolic Content (TPC), Antioxidant Capacity, and Anthocyanin Pigment Content
2.5.2. Encapsulation Efficiency (EE) of Pomegranate Peel Extract in “Boba Balls”
2.5.3. Visual Appearance and Color
2.5.4. Texture Analysis
2.5.5. FT-IR Spectrum
2.5.6. Microstructure Analysis
2.5.7. In Vitro Gastrointestinal Digestion
2.5.8. In Vitro Release Kinetic of “Boba Balls”
2.5.9. Sensory Evaluation
2.6. Statistical Analysis
3. Results and Discussion
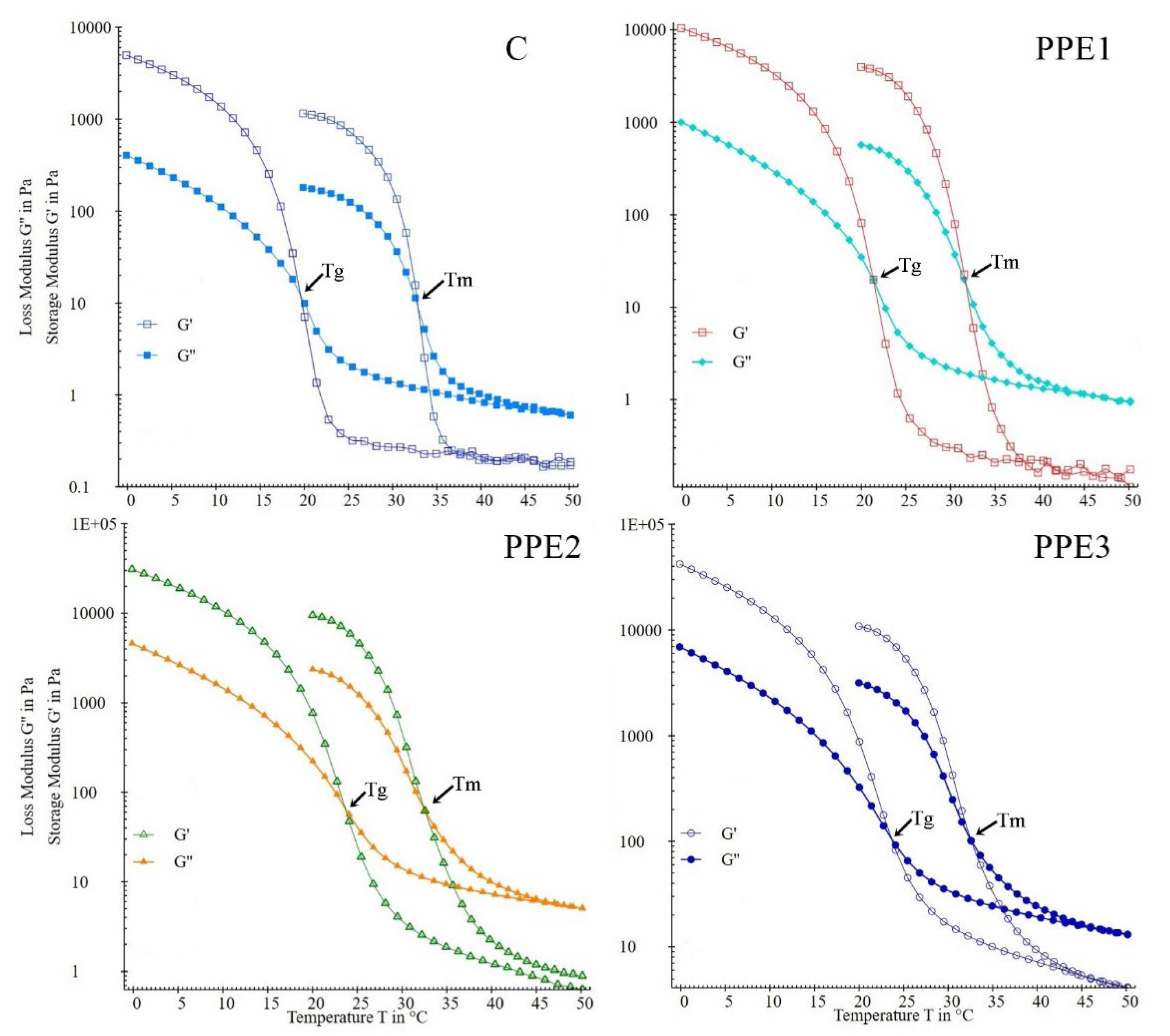
3.1. Rheological Properties of “Boba” Mix
3.2. Encapsulation Efficiency and Bioactive Properties of “Boba Balls”
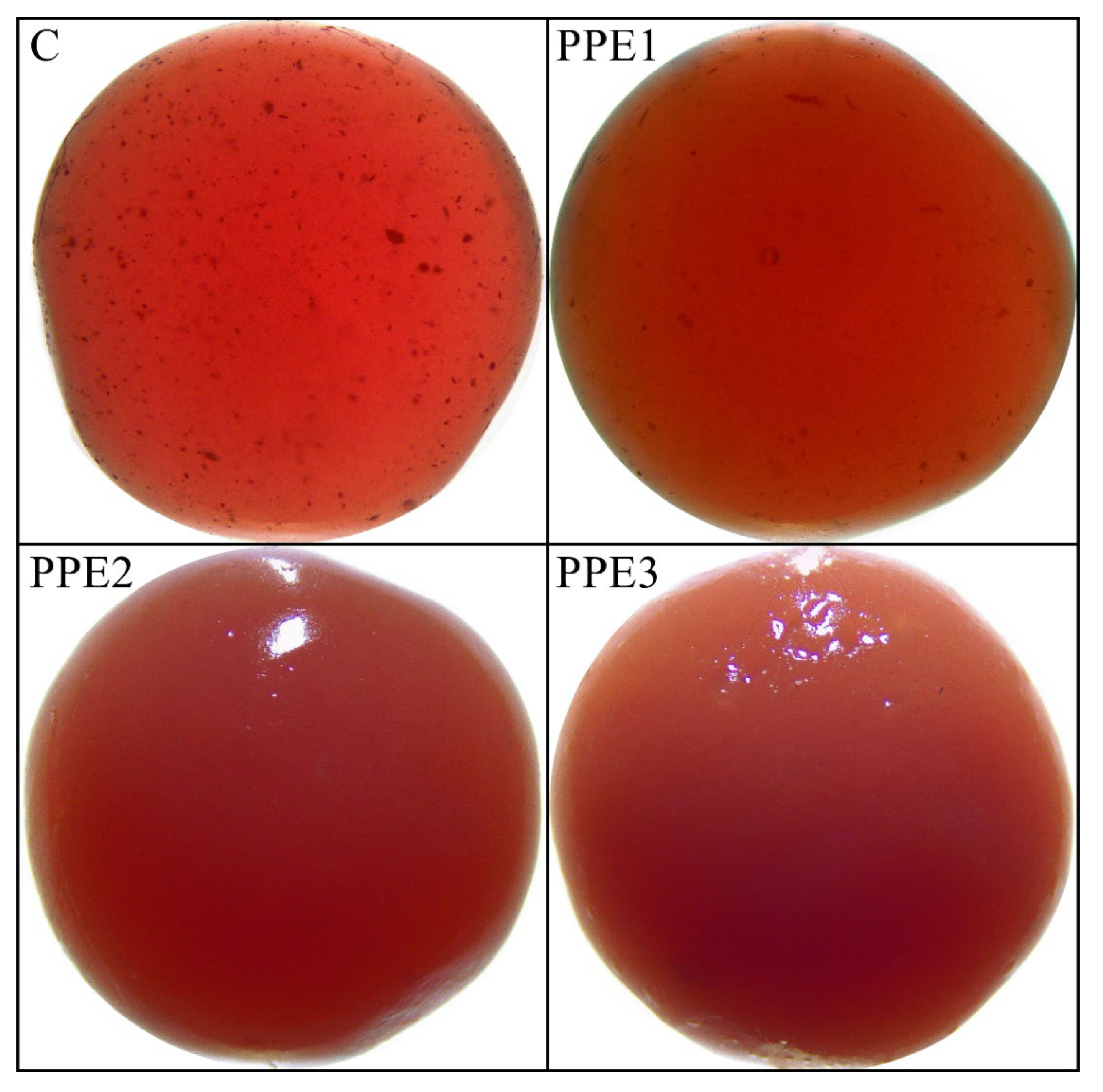
3.3. Visual Appearance and Color of “Boba Balls”
3.4. Textural Properties
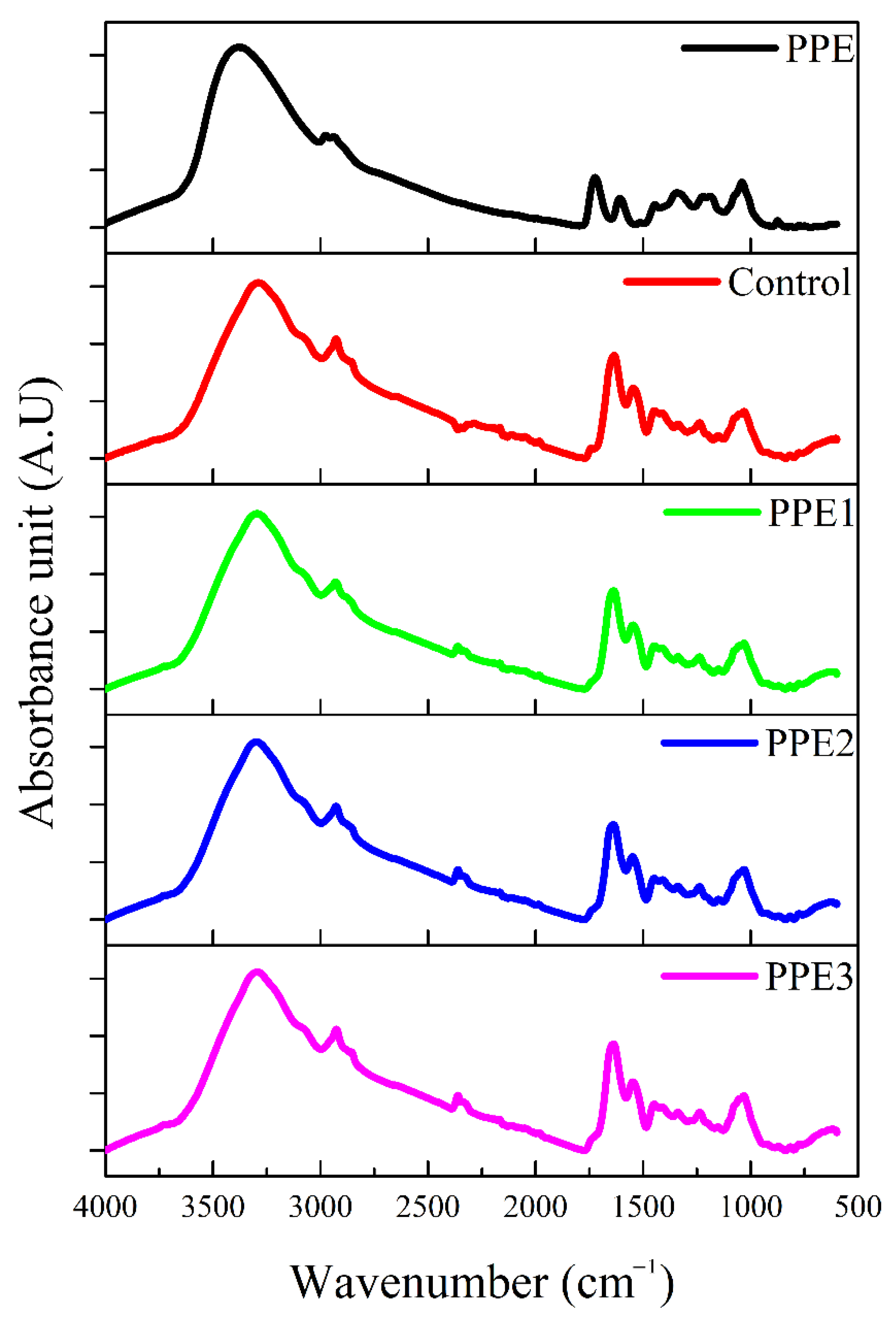
3.5. FT-IR Spectrum
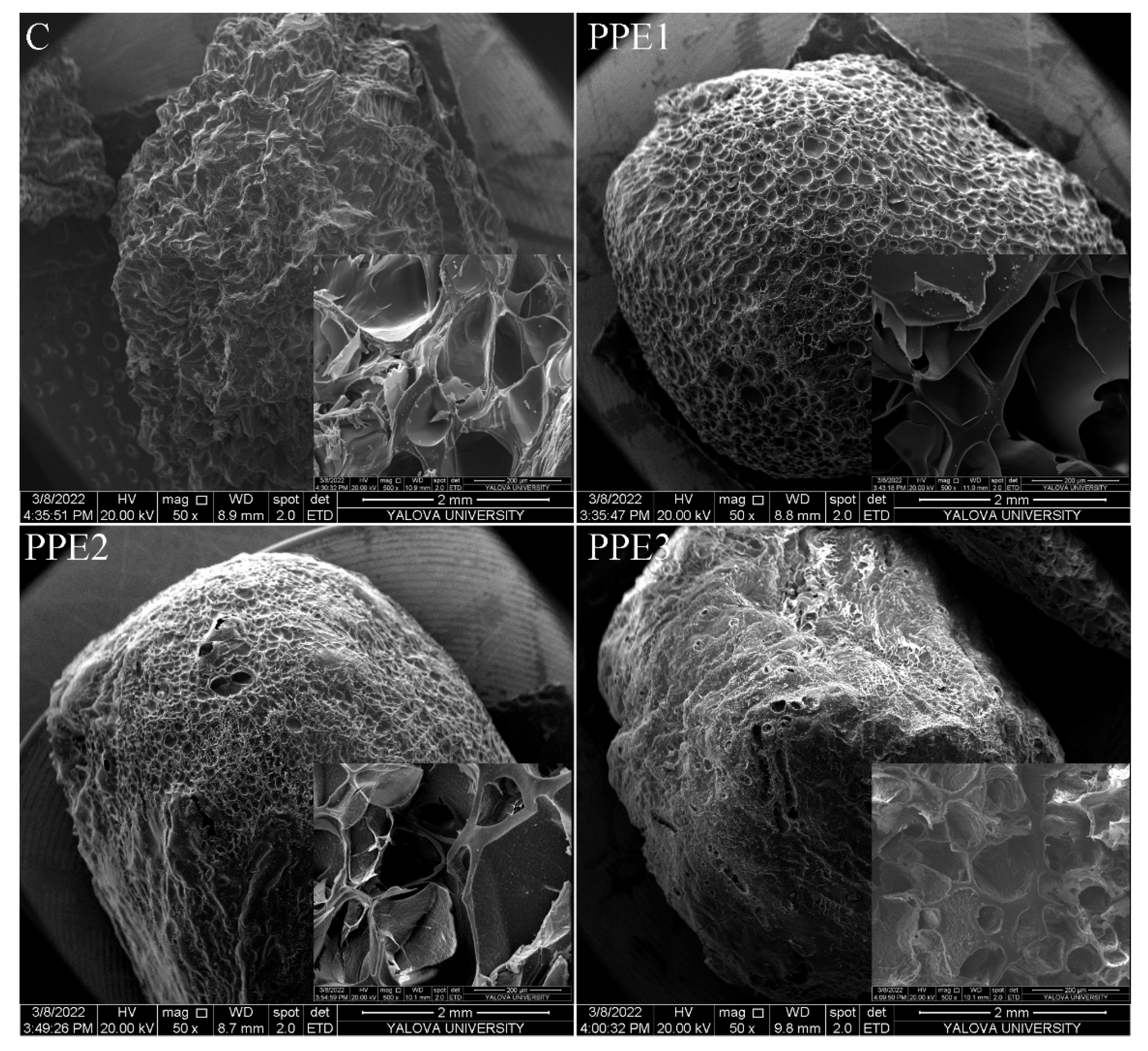
3.6. SEM Images
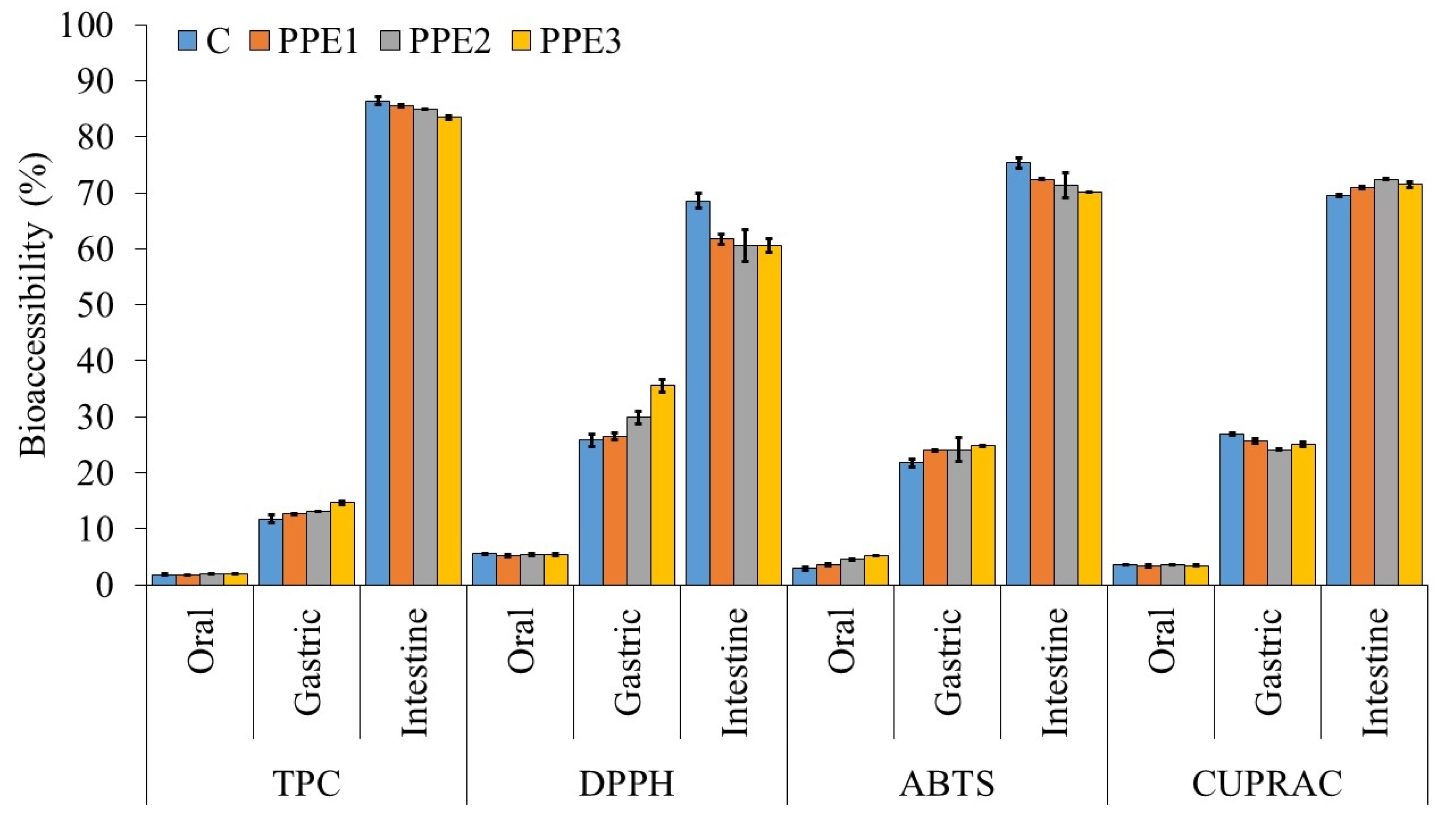
3.7. In Vitro Digestion of “Boba Balls”
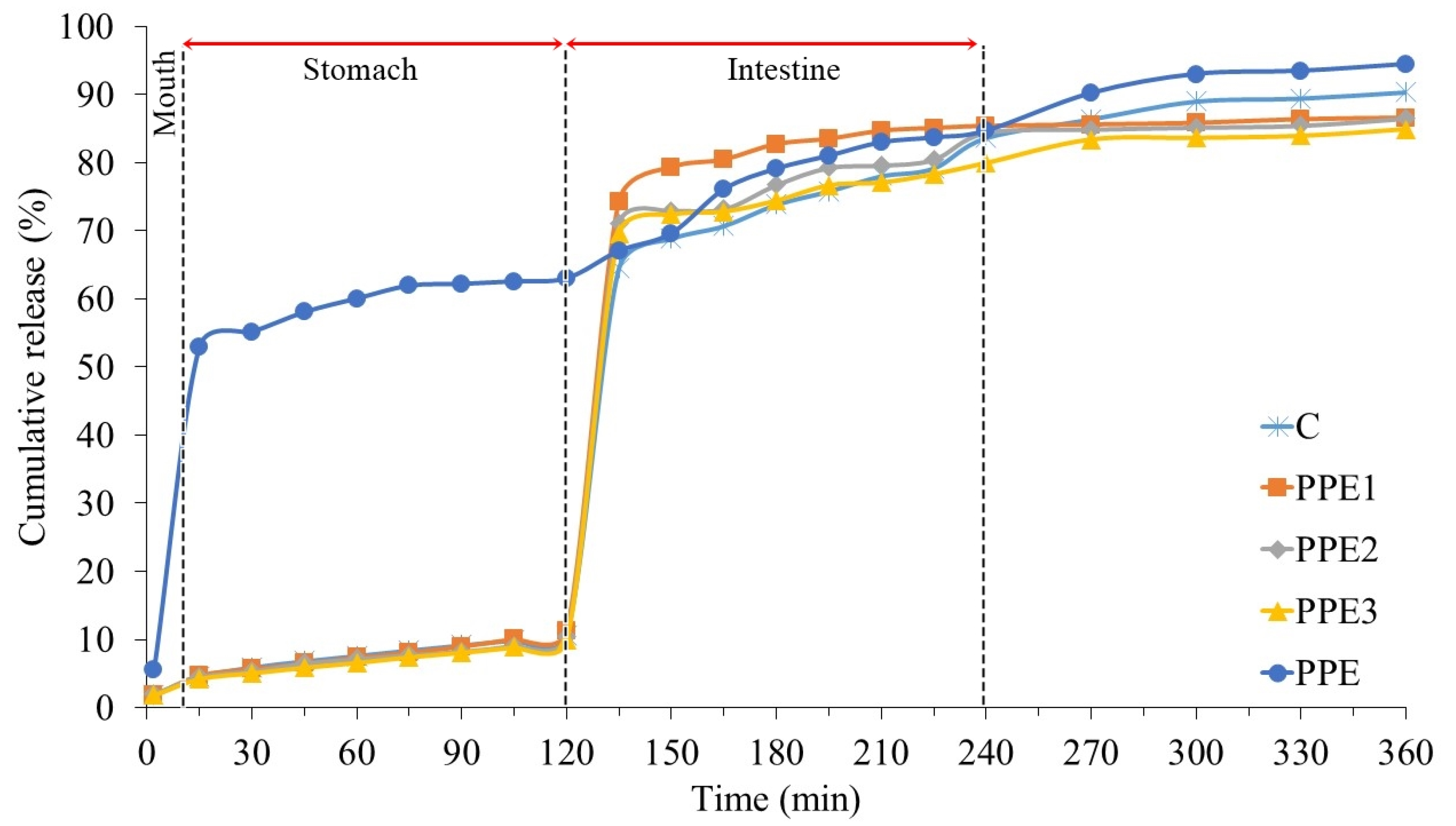
3.8. In Vitro Release Kinetic of “Boba Balls”
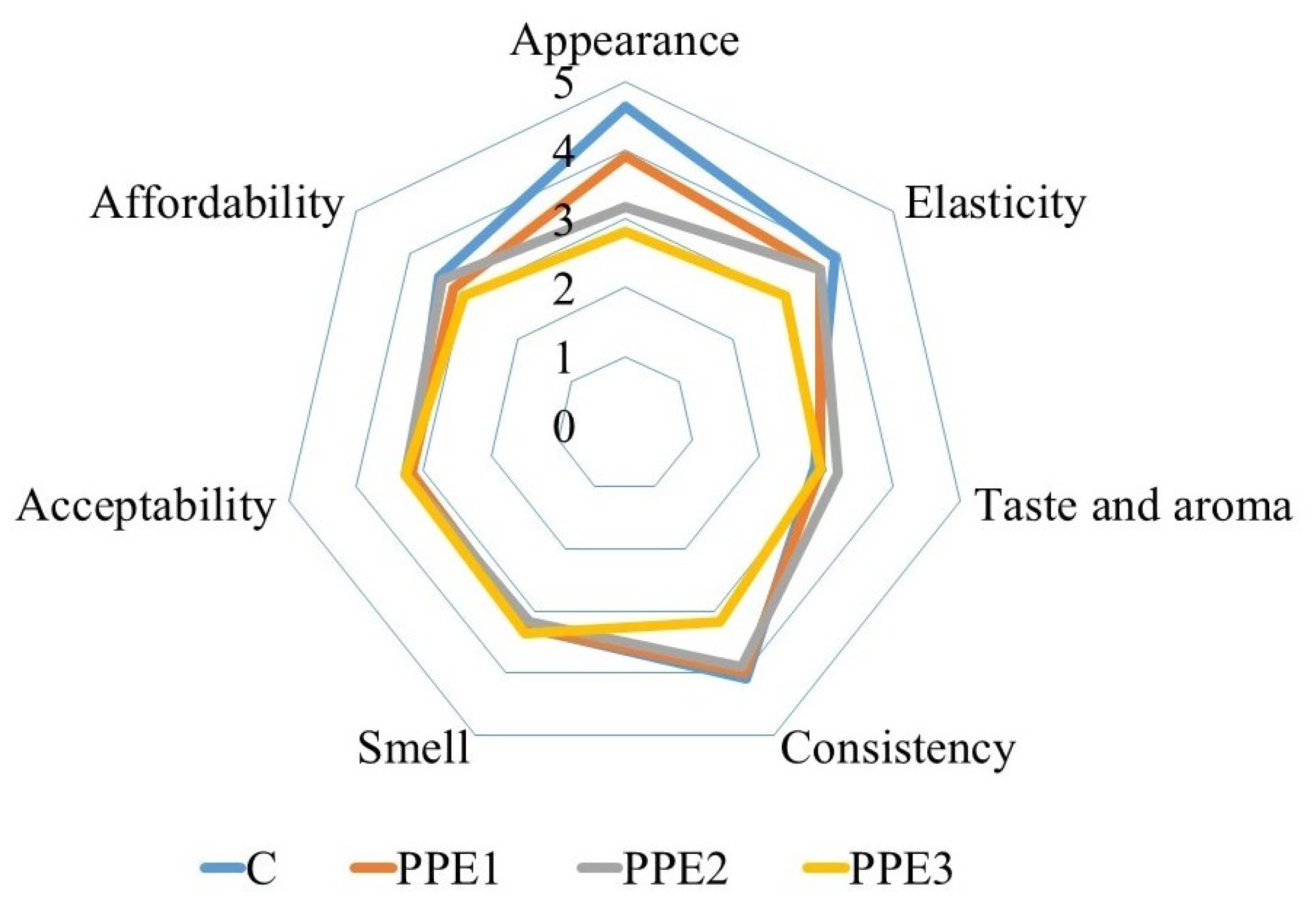
3.9. Sensory Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koay, K.Y.; Cheah, C.W. Understanding consumers’ intention to revisit bubble tea stores: An application of the theory of planned behaviour. Br. Food J. 2022; ahead-of-print. [Google Scholar] [CrossRef]
- Liu, S.; Xiao, J.; Feng, Y.; Zhang, M.; Li, Y.; Tu, J.; Niu, L. Anthocyanin-fortified konjac glucomannan/sodium alginate composite edible boba: Characteristics of texture, microstructure, in vitro release behaviour and antioxidant capacity. Int. J. Food Sci. Technol. 2022, 57, 1791–1803. [Google Scholar] [CrossRef]
- Gibbs, F.; Kermasha, S.; Alli, I.; Catherine, N.; Mulligan, B. Encapsulation in the food industry: A review. Int. J. Food Sci. Nutr. 1999, 50, 213–224. [Google Scholar] [CrossRef]
- Ching, S.H.; Bansal, N.; Bhandari, B. Alginate gel particles—A review of production techniques and physical properties. Crit. Rev. Food Sci. Nutr. 2017, 57, 1133–1152. [Google Scholar] [CrossRef]
- Bennacef, C.; Desobry-Banon, S.; Probst, L.; Desobry, S. Advances on alginate use for spherification to encapsulate biomolecules. Food Hydrocoll. 2021, 118, 106782. [Google Scholar] [CrossRef]
- Liu, Y.; Cheng, H.; Wu, D. Preparation of the Orange Flavoured “Boba” Ball in Milk Tea and Its Shelf-Life. Appl. Sci. 2021, 11, 200. [Google Scholar] [CrossRef]
- Kumar, N.; Daniloski, D.; Pratibha; Neeraj; D’Cunha, N.M.; Naumovski, N.; Petkoska, A.T. Pomegranate peel extract—A natural bioactive addition to novel active edible packaging. Food Res. Int. 2022, 156, 111378. [Google Scholar] [CrossRef]
- Kaderides, K.; Kyriakoudi, A.; Mourtzinos, I.; Goula, A.M. Potential of pomegranate peel extract as a natural additive in foods. Trends Food Sci. Technol. 2021, 115, 380–390. [Google Scholar] [CrossRef]
- Neslihan Dundar, A.; Ozdemir, S.; Uzuner, K.; Ekrem Parlak, M.; Irmak Sahin, O.; Fatih Dagdelen, A.; Turker Saricaoglu, F. Characterization of pomegranate peel extract loaded nanophytosomes and the enhancement of bio-accessibility and storage stability. Food Chem. 2023, 398, 133921. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Apak, R.; Güçlü, K.; Özyürek, M.; Çelik, S.E. Mechanism of antioxidant capacity assays and the CUPRAC (cupric ion reducing antioxidant capacity) assay. Microchim. Acta 2008, 160, 413–419. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Demirata, B.; Ozyürek, M.; Celik, S.E.; Bektaşoğlu, B.; Berker, K.I.; Ozyurt, D. Comparative evaluation of various total antioxidant capacity assays applied to phenolic compounds with the CUPRAC assay. Molecules 2007, 12, 1496–1547. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Determination of Total Monomeric Anthocyanin Pigment Content of Fruit Juices, Beverages, Natural Colorants, and Wines by the pH Differential Method: Collaborative Study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D. A standardised static in vitro digestion method suitable for food–an international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Wang, Q.; Cao, J.; Yu, H.; Zhang, J.; Yuan, Y.; Shen, X.; Li, C. The effects of EGCG on the mechanical, bioactivities, cross-linking and release properties of gelatin film. Food Chem. 2019, 271, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, M.A.; Bode, F.; Grillo, I.; Dreiss, C.A. Exploring the kinetics of gelation and final architecture of enzymatically cross-linked chitosan/gelatin gels. Biomacromolecules 2015, 16, 1401–1409. [Google Scholar] [CrossRef] [PubMed]
- Tau, T.; Gunasekaran, S. Thermorheological evaluation of gelation of gelatin with sugar substitutes. LWT-Food Sci. Technol. 2016, 69, 570–578. [Google Scholar] [CrossRef]
- Sarker, B.; Zehnder, T.; Rath, S.N.; Horch, R.E.; Kneser, U.; Detsch, R.; Boccaccini, A.R. Oxidized alginate-gelatin hydrogel: A favorable matrix for growth and osteogenic differentiation of adipose-derived stem cells in 3D. ACS Biomater. Sci. Eng. 2017, 3, 1730–1737. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, F.; Ren, T.; Wang, J.; Yang, M.; Yao, Y.; Chen, H. Fabrication of fish gelatin/sodium alginate double network gels for encapsulation of probiotics. J. Sci. Food Agric. 2021, 101, 4398–4408. [Google Scholar] [CrossRef]
- Moreira Mar, J.; Souza da Silva, L.; da S Rabello, M.; Moraes Biondo, M.; Ferreira Kinupp, V.; Henrique Campelo, P.; Bruginski, E.; Ramos Campos, F.; de Araújo Bezerra, J.; Aparecido Sanches, E. Development of alginate/inulin carrier systems containing non-conventional Amazonian berry extracts. Food Res. Int. 2021, 139, 109838. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic compounds as beneficial phytochemicals in pomegranate (Punica granatum L.) peel: A review. Food Chem. 2018, 261, 75–86. [Google Scholar] [CrossRef]
- Rashid, R.; Masoodi, F.A.; Wani, S.M.; Manzoor, S.; Gull, A. Ultrasound assisted extraction of bioactive compounds from pomegranate peel, their nanoencapsulation and application for improvement in shelf life extension of edible oils. Food Chem. 2022, 385, 132608. [Google Scholar] [CrossRef] [PubMed]
- Mirdehghan, S.H.; Rahemi, M. Seasonal changes of mineral nutrients and phenolics in pomegranate (Punica granatum L.) fruit. Sci. Hortic. 2007, 111, 120–127. [Google Scholar] [CrossRef]
- Azarpazhooh, E.; Sharayei, P.; Zomorodi, S.; Ramaswamy, H.S. Physicochemical and Phytochemical Characterization and Storage Stability of Freeze-dried Encapsulated Pomegranate Peel Anthocyanin and In Vitro Evaluation of Its Antioxidant Activity. Food Bioprocess Technol. 2019, 12, 199–210. [Google Scholar] [CrossRef]
- Cserhalmi, Z.; Sass-Kiss, Á.; Tóth-Markus, M.; Lechner, N. Study of pulsed electric field treated citrus juices. Innov. Food Sci. Emerg. Technol. 2006, 7, 49–54. [Google Scholar] [CrossRef]
- Momosaki, R.; Abo, M.; Kobayashi, K. Swallowing Analysis for Semisolid Food Texture in Poststroke Dysphagic Patients. J. Stroke Cerebrovasc. Dis. 2013, 22, 267–270. [Google Scholar] [CrossRef]
- Lau, M.; Tang, J.; Paulson, A. Texture profile and turbidity of gellan/gelatin mixed gels. Food Res. Int. 2000, 33, 665–671. [Google Scholar] [CrossRef]
- Saadat, S.; Emam-Djomeh, Z.; Askari, G. Antibacterial and Antioxidant Gelatin Nanofiber Scaffold Containing Ethanol Extract of Pomegranate Peel: Design, Characterization and In Vitro Assay. Food Bioprocess Technol. 2021, 14, 935–944. [Google Scholar] [CrossRef]
- Hady, E.; Youssef, M.; Aljahani, A.H.; Aljumayi, H.; Ismail, K.A.; El-Damaty, E.-S.; Sami, R.; El-Sharnouby, G. Enhancement of the Stability of Encapsulated Pomegranate (Punica granatum L.) Peel Extract by Double Emulsion with Carboxymethyl Cellulose. Crystals 2022, 12, 622. [Google Scholar] [CrossRef]
- Batista, D.; De Oliveira, I.; Ribeiro, A.; Fonseca, E.; Santos-Magalhães, N.; de Sena-Filho, J.; Teodoro, A.; Grillo, L.; de Almeida, R.; Dornelas, C.B. Encapsulation and release of Beauveria bassiana from alginate–bentonite nanocomposite. RSC Adv. 2017, 7, 26468–26477. [Google Scholar] [CrossRef]
- McClements, D.J. Design of nano-laminated coatings to control bioavailability of lipophilic food components. J. Food Sci. 2010, 75, R30–R42. [Google Scholar] [CrossRef]
- Cong, Z.; Shi, Y.; Wang, Y.; Wang, Y.; Chen, N.; Xue, H. A novel controlled drug delivery system based on alginate hydrogel/chitosan micelle composites. Int. J. Biol. Macromol. 2018, 107, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Foujdar, R.; Chopra, H.K.; Bera, M.B.; Batra, K. Isolation, characterization, bio-accessibility and cytotoxic effect of ellagitannins purified from peels of Punica granatum Indian var. Bhagwa. J. Food Meas. Charact. 2022, 16, 1733–1743. [Google Scholar] [CrossRef]
- Avadi, M.R.; Sadeghi, A.M.M.; Mohammadpour, N.; Abedin, S.; Atyabi, F.; Dinarvand, R.; Rafiee-Tehrani, M. Preparation and characterization of insulin nanoparticles using chitosan and Arabic gum with ionic gelation method. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Bruschi, M.L. Mathematical models of drug release. Strateg. Modify Drug Release Pharm. Syst. 2015, 5, 63–86. [Google Scholar]
- Özbilenler, C.; Altundağ, E.M.; Gazi, M. Synthesis of quercetin-encapsulated alginate beads with their antioxidant and release kinetic studies. J. Macromol. Sci. Part A 2021, 58, 22–31. [Google Scholar] [CrossRef]
- Basak, S.C.; Kumar, K.S.; Ramalingam, M. Design and release characteristics of sustained release tablet containing metformin HCl. Rev. Bras. Ciênc. Farm. 2008, 44, 477–483. [Google Scholar] [CrossRef]
- Bueno, V.B.; Bentini, R.; Catalani, L.H.; Petri, D.F.S. Synthesis and swelling behavior of xanthan-based hydrogels. Carbohydr. Polym. 2013, 92, 1091–1099. [Google Scholar] [CrossRef]
| Properties | PPE | C | PPE1 | PPE2 | PPE3 |
|---|---|---|---|---|---|
| Tm | - | 32.88 ± 1.75 | 31.70 ± 0.98 | 32.52 ± 1.57 | 32.53 ± 1.18 |
| Tg | - | 19.56 ± 0.32 c | 21.38 ± 0.45 b | 23.60 ± 0.57 a | 23.65 ± 0.86 a |
| EE | - | 75.25 ± 9.21 | 72.74 ± 3.99 | 71.59 ± 2.72 | 68.52 ± 0.51 |
| TPC | 234.18 ± 3.61 | 25.50 ± 0.01 d | 28.48 ± 0.51 c | 30.86 ± 0.02 b | 33.19 ± 0.27 a |
| ABTS | 1465.46 ± 26.71 | 7.96 ± 0.32 d | 11.69 ± 0.99 c | 27.56 ± 0.01 b | 50.49 ± 1.49 a |
| CUPRAC | 3215.66 ± 48.69 | 7.45 ± 0.25 d | 16.43 ± 0.76 c | 22.52 ± 0.67 b | 36.52 ± 0.58 a |
| DPPH | 458.74 ± 33.25 | 8.15 ± 0.31 d | 18.66 ± 0.15 c | 32.75 ± 1.09 b | 35.54 ± 1.13 a |
| TMA | 80.54 ± 0.06 | 34.63 ± 0.04 d | 41.39 ± 0.15 c | 49.10 ± 0.04 b | 54.35 ± 0.17 a |
| C | PPE1 | PPE2 | PPE3 | |
|---|---|---|---|---|
| L* | 20.96 ± 0.37 c | 16.97 ± 0.49 d | 26.24 ± 3.10 b | 29.72 ± 1.78 a |
| a* | 28.39 ± 1.88 | 26.39 ± 0.44 | 31.48 ± 2.51 | 28.47 ± 1.94 |
| b* | 12.25 ± 0.19 b | 10.51 ± 0.31 b | 12.48 ± 1.37 b | 18.04 ± 1.89 a |
| ΔE | - | 4.60 ± 0.27 b | 5.02 ± 0.26 b | 6.97 ± 0.98 a |
| Hardness (g) | 424.18 ± 95.13 a | 398.76 ± 50.66 a | 275.88 ± 48.22 b | 116.53 ± 25.74 c |
| Springiness | 0.98 ± 0.01 b | 0.98 ± 0.01 b | 0.98 ± 0.01 b | 1.03 ± 0.04 a |
| Cohesiveness | 0.80 ± 0.01 a | 0.82 ± 0.01 a | 0.83 ± 0.02 a | 0.50 ± 0.09 b |
| Gumminess (g) | 340.22 ± 79.42 a | 325.2 ± 42.06 a | 228.33 ± 42.62 b | 60.41 ± 21.52 c |
| Chewiness (g) | 334.17 ± 77.91 a | 319.15 ± 41.1 a | 224.36 ± 41.46 b | 61.37 ± 20.38 c |
| Resilience | 0.88 ± 0.02 a | 0.84 ± 0.02 bc | 0.87 ± 0.03 ab | 0.81 ± 0.06 c |
| Medium | Model | Coefficients | C | PPE1 | PPE2 | PPE3 |
|---|---|---|---|---|---|---|
| Gastric conditions | Korsmeyer–Peppas | k | 1.885 | 1.402 | 1.400 | 1.490 |
| n | 0.351 | 0.422 | 0.410 | 0.378 | ||
| R2 | 0.9797 | 0.9647 | 0.9609 | 0.9742 | ||
| Peppas–Sahlin | k1 | 1.451 | 1.248 | 1.289 | 1.289 | |
| k2 | 0.657 | 0.427 | 0.371 | 0.426 | ||
| m | 0.227 | 0.274 | 0.274 | 0.252 | ||
| R2 | 0.9821 | 0.9677 | 0.9656 | 0.9782 | ||
| Makoid–Banakar | KMB | 1.677 | 1.324 | 1.868 | 1.747 | |
| n | 0.385 | 0.439 | 0.328 | 0.332 | ||
| c | 0.000 | 0.000 | −0.001 | 0.000 | ||
| R2 | 0.9801 | 0.9648 | 0.9625 | 0.9748 | ||
| Intestinal conditions | Korsmeyer–Peppas | k | 34.639 | 43.597 | 39.809 | 39.718 |
| n | 0.182 | 0.141 | 0.153 | 0.148 | ||
| R2 | 0.9547 | 0.8411 | 0.8983 | 0.9036 | ||
| Peppas–Sahlin | k1 | 33.307 | 40.527 | 38.244 | 39.552 | |
| k2 | −3.048 | −4.706 | −4.302 | −4.712 | ||
| m | 0.285 | 0.301 | 0.286 | 0.271 | ||
| R2 | 0.9798 | 0.9738 | 0.9701 | 0.9688 | ||
| Makoid–Banakar | KMB | 30.543 | 35.333 | 33.768 | 34.640 | |
| n | 0.232 | 0.239 | 0.226 | 0.211 | ||
| c | 0.001 | 0.002 | 0.001 | 0.001 | ||
| R2 | 0.9722 | 0.9569 | 0.9563 | 0.9534 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dundar, A.N.; Uzuner, K.; Parlak, M.E.; Sahin, O.I.; Saricaoglu, F.T.; Simsek, S. Enhanced Functionality and Bio-Accessibility of Composite Pomegranate Peel Extract-Enriched “Boba Balls”. Foods 2022, 11, 3785. https://doi.org/10.3390/foods11233785
Dundar AN, Uzuner K, Parlak ME, Sahin OI, Saricaoglu FT, Simsek S. Enhanced Functionality and Bio-Accessibility of Composite Pomegranate Peel Extract-Enriched “Boba Balls”. Foods. 2022; 11(23):3785. https://doi.org/10.3390/foods11233785
Chicago/Turabian StyleDundar, Ayse Neslihan, Kubra Uzuner, Mahmud Ekrem Parlak, Oya Irmak Sahin, Furkan Turker Saricaoglu, and Senay Simsek. 2022. "Enhanced Functionality and Bio-Accessibility of Composite Pomegranate Peel Extract-Enriched “Boba Balls”" Foods 11, no. 23: 3785. https://doi.org/10.3390/foods11233785
APA StyleDundar, A. N., Uzuner, K., Parlak, M. E., Sahin, O. I., Saricaoglu, F. T., & Simsek, S. (2022). Enhanced Functionality and Bio-Accessibility of Composite Pomegranate Peel Extract-Enriched “Boba Balls”. Foods, 11(23), 3785. https://doi.org/10.3390/foods11233785








