Experimental Tests for Fluorescence LIDAR Remote Sensing of Submerged Plastic Marine Litter
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrumentation
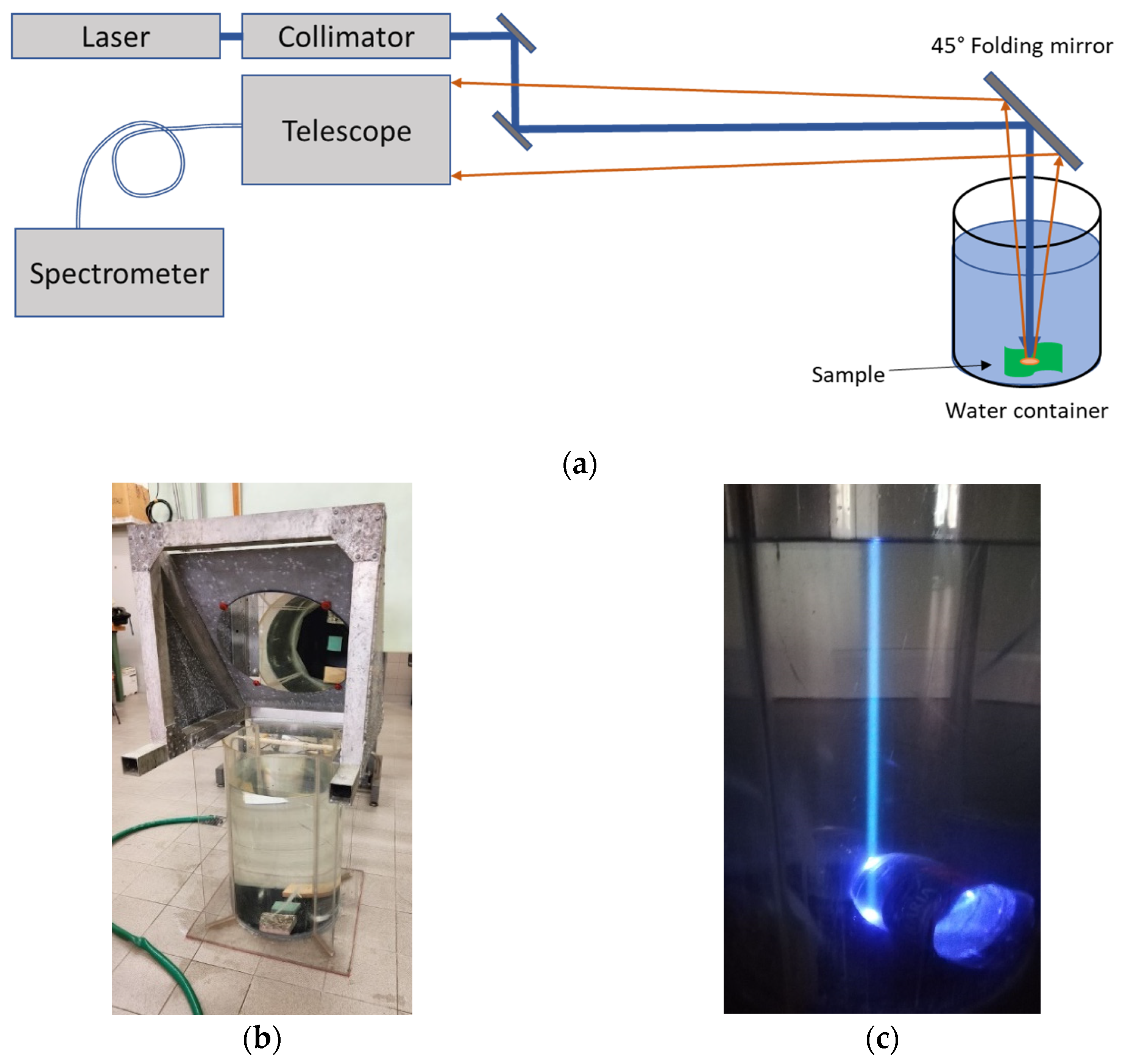
2.3. Experimental Set-Up
2.4. Data Acquisition Procedure
3. Results
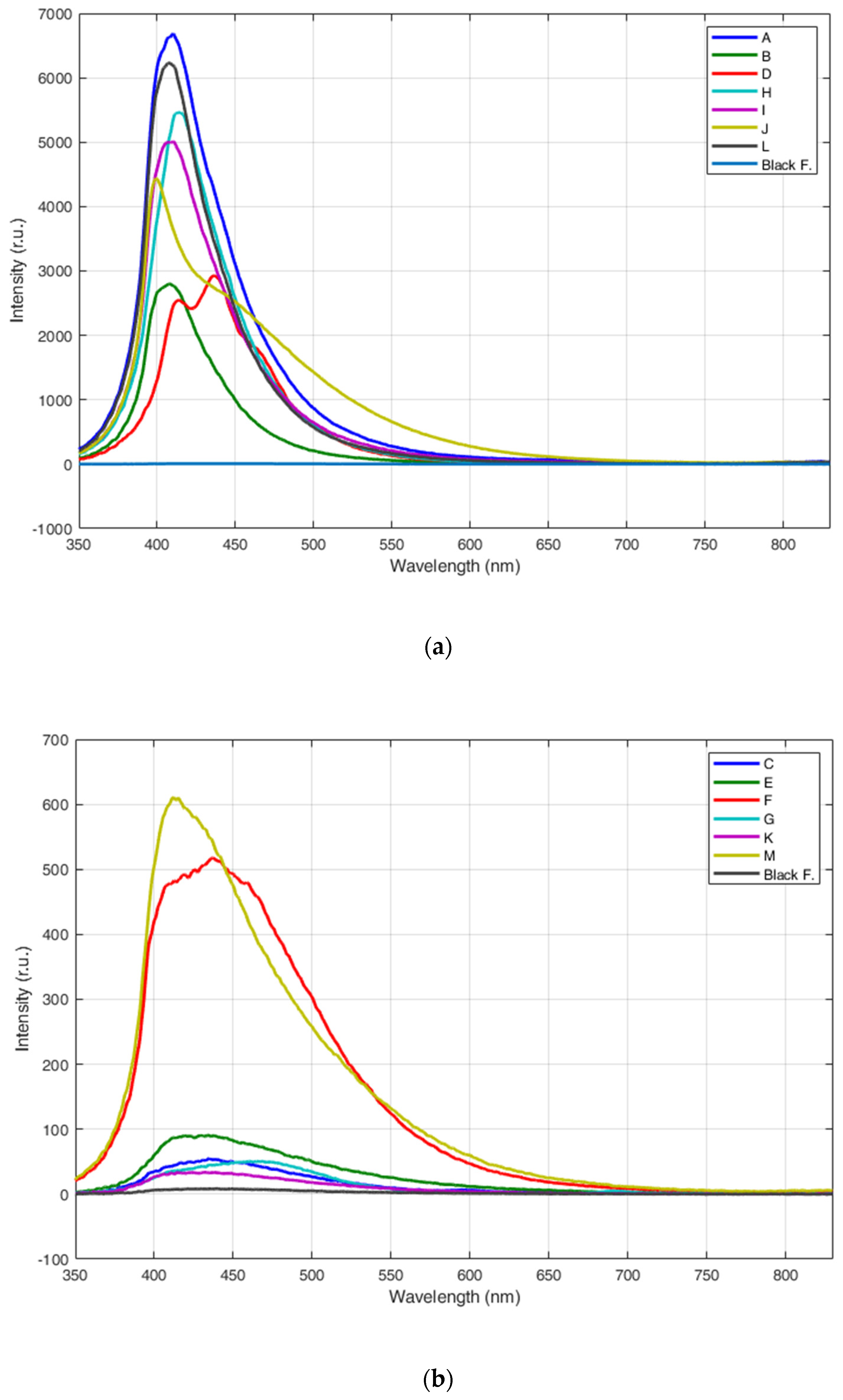
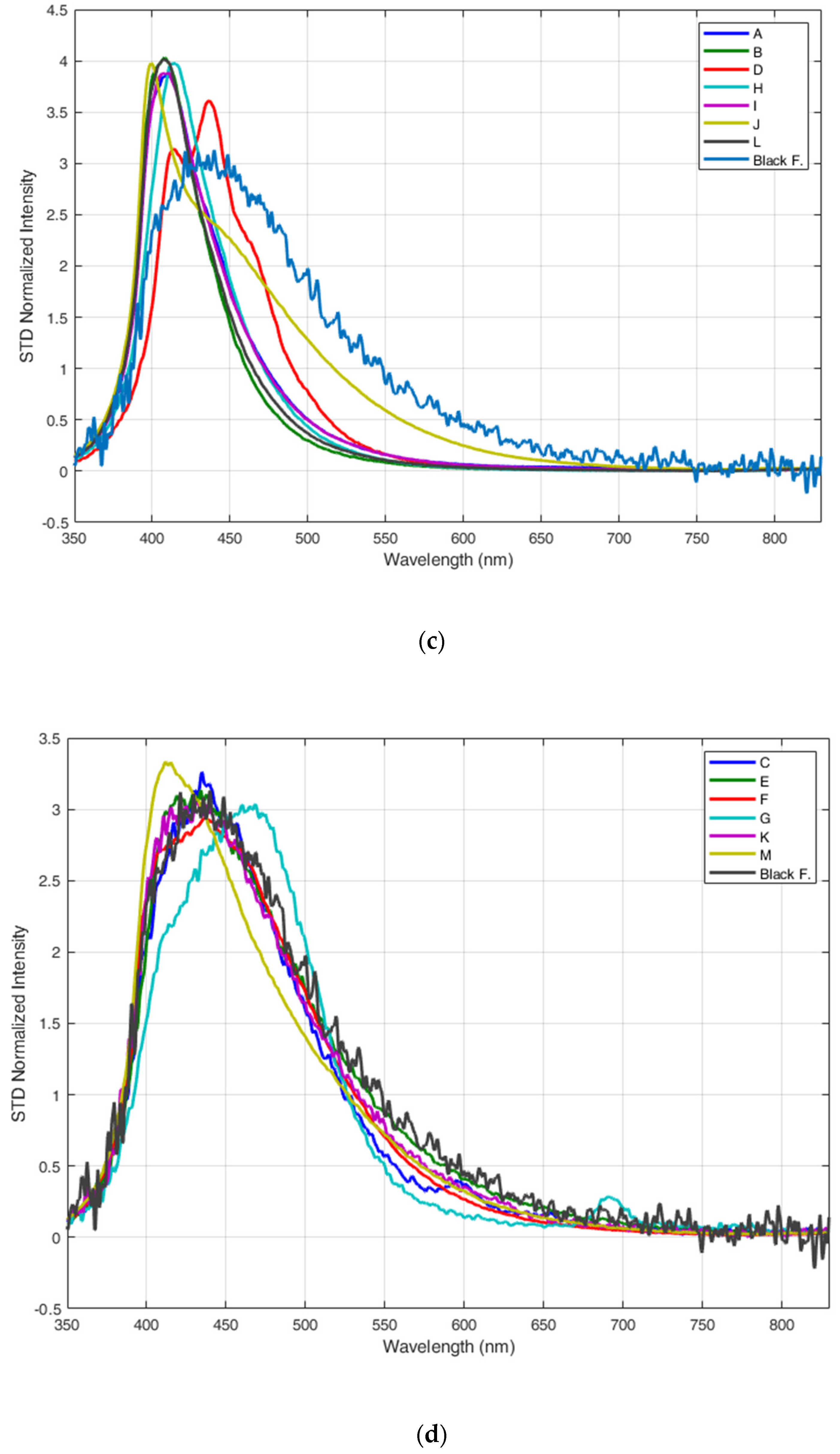
3.1. Fluorescence LIDAR Measurements of Different Types of Plastics
3.2. Fluorescence LIDAR Measurements of Submerged Plastics
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Halsband, C.; Herzke, D. Plastic Litter in the European Arctic: What Do We Know? Emerg. Contam. 2019, 5, 308–318. [Google Scholar] [CrossRef]
- Strand, K.O.; Huserbråten, M.; Dagestad, K.-F.; Mauritzen, C.; Grøsvik, B.E.; Nogueira, L.A.; Melsom, A.; Röhrs, J. Potential Sources of Marine Plastic from Survey Beaches in the Arctic and Northeast Atlantic. Sci. Total Environ. 2021, 790, 148009. [Google Scholar] [CrossRef] [PubMed]
- Mallory, M.L.; Baak, J.; Gjerdrum, C.; Mallory, O.E.; Manley, B.; Swan, C.; Provencher, J.F. Anthropogenic Litter in Marine Waters and Coastlines of Arctic Canada and West Greenland. Sci. Total Environ. 2021, 783, 146971. [Google Scholar] [CrossRef] [PubMed]
- Barnes, D.K.A.; Walters, A.; Gonçalves, L. Macroplastics at Sea around Antarctica. Mar. Environ. Res. 2010, 70, 250–252. [Google Scholar] [CrossRef] [PubMed]
- Waller, C.L.; Griffiths, H.J.; Waluda, C.M.; Thorpe, S.E.; Loaiza, I.; Moreno, B.; Pacherres, C.O.; Hughes, K.A. Microplastics in the Antarctic Marine System: An Emerging Area of Research. Sci. Total Environ. 2017, 598, 220–227. [Google Scholar] [CrossRef] [Green Version]
- Ciappa, A.C. Marine Plastic Litter Detection Offshore Hawai’i by Sentinel-2. Mar. Pollut. Bull. 2021, 168, 112457. [Google Scholar] [CrossRef]
- MacLeod, M.; Arp, H.P.H.; Tekman, M.B.; Jahnke, A. The global threat from plastic pollution. Science 2021, 373, 61–65. [Google Scholar] [CrossRef]
- World Health Organization. Microplastics in Drinking-Water; World Health Organization: Geneva, Switzterland, 2019; ISBN 978-92-4-151619-8. [Google Scholar]
- Werner, S.; Budziak, A.; van Franeker, J.; Galgani, F.; Hanke, G.; Maes, T.; Matiddi, M.; Nilsson, P.; Oosterbaan, L.; Priestland, E.; et al. Harm caused by Marine Litter. In MSFD GES TG Marine Litter-Thematic Report; JRC Technical Report; EUR 28317 EN; Publication office of the European Union: Luxenbourg, 2016. [Google Scholar] [CrossRef]
- Haarr, M.L.; Falk-Andersson, J.; Fabres, J. Global Marine Litter Research 2015–2020: Geographical and Methodological Trends. Sci. Total Environ. 2022, 820, 153162. [Google Scholar] [CrossRef]
- PlasticsEurope: Plastics-the Facts 2021: An Analysis of European Plastics Production, Demand and Waste Data. Available online: https://plasticseurope.org/wp-content/uploads/2021/12/Plastics-the-Facts-2021-web-final.pdf (accessed on 4 August 2022).
- Lyons, B.P.; Cowie, W.J.; Maes, T.; Le Quesne, W.J.F. Marine Plastic Litter in the ROPME Sea Area: Current Knowledge and Recommendations. Ecotoxicol. Environ. Saf. 2020, 187, 109839. [Google Scholar] [CrossRef]
- Sabatira, F. Southeast Asia regional cooperation on tackling marine plastic litter. Lampung J. Int. Law 2020, 2, 69–84. [Google Scholar] [CrossRef]
- Lebreton, L.; Egger, M.; Slat, B. A Global Mass Budget for Positively Buoyant Macroplastic Debris in the Ocean. Sci. Rep. 2019, 9, 12922. [Google Scholar] [CrossRef] [Green Version]
- Olivelli, A.; Hardesty, B.D.; Wilcox, C. Coastal Margins and Backshores Represent a Major Sink for Marine Debris: Insights from a Continental-Scale Analysis. Environ. Res. Lett. 2020, 15, 074037. [Google Scholar] [CrossRef]
- Napper, I.E.; Thompson, R.C. Chapter 22-Marine Plastic Pollution: Other Than Microplastic. In Waste, 2nd ed.; Letcher, T.M., Vallero, D.A., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 425–442. ISBN 978-0-12-815060-3. [Google Scholar]
- Maximenko, N.; Arvesen, J.; Asner, G.; Carlton, J.; Castrence, M.; Centurioni, L.; Chao, Y.; Chapman, J.; Chirayath, V.; Corradi, P.; et al. Remote Sensing of Marine Debris to Study Dynamics, Balances and Trends. 2017. Available online: https://ecocast.arc.nasa.gov/las/Reports%20and%20Papers/Marine-Debris-Workshop-2017.pdf (accessed on 5 August 2022).
- Martínez-Vicente, V.; Clark, J.R.; Corradi, P.; Aliani, S.; Arias, M.; Bochow, M.; Bonnery, G.; Cole, M.; Cózar, A.; Donnelly, R.; et al. Measuring Marine Plastic Debris from Space: Initial Assessment of Observation Requirements. Remote Sens. 2019, 11, 2443. [Google Scholar] [CrossRef] [Green Version]
- Garaba, S.P.; Aitken, J.; Slat, B.; Dierssen, H.M.; Lebreton, L.; Zielinski, O.; Reisser, J. Sensing Ocean Plastics with an Airborne Hyperspectral Shortwave Infrared Imager. Environ. Sci. Technol. 2018, 52, 11699–11707. [Google Scholar] [CrossRef]
- Tasseron, P.; van Emmerik, T.; Peller, J.; Schreyers, L.; Biermann, L. Advancing Floating Macroplastic Detection from Space Using Experimental Hyperspectral Imagery. Remote Sens. 2021, 13, 2335. [Google Scholar] [CrossRef]
- Goddijn-Murphy, L.; Peters, S.; van Sebille, E.; James, N.A.; Gibb, S. Concept for a Hyperspectral Remote Sensing Algorithm for Floating Marine Macro Plastics. Mar. Pollut. Bull. 2018, 126, 255–262. [Google Scholar] [CrossRef] [Green Version]
- Kremezi, M.; Kristollari, V.; Karathanassi, V.; Topouzelis, K.; Kolokoussis, P.; Taggio, N.; Aiello, A.; Ceriola, G.; Barbone, E.; Corradi, P. Pansharpening PRISMA Data for Marine Plastic Litter Detection Using Plastic Indexes. IEEE Access 2021, 9, 61955–61971. [Google Scholar] [CrossRef]
- Balsi, M.; Moroni, M.; Chiarabini, V.; Tanda, G. High-Resolution Aerial Detection of Marine Plastic Litter by Hyperspectral Sensing. Remote Sens. 2021, 13, 1557. [Google Scholar] [CrossRef]
- Biermann, L.; Clewley, D.; Martinez-Vicente, V.; Topouzelis, K. Finding Plastic Patches in Coastal Waters Using Optical Satellite Data. Sci. Rep. 2020, 10, 5364. [Google Scholar] [CrossRef]
- Themistocleous, K.; Papoutsa, C.; Michaelides, S.; Hadjimitsis, D. Investigating Detection of Floating Plastic Litter from Space Using Sentinel-2 Imagery. Remote Sens. 2020, 12, 2648. [Google Scholar] [CrossRef]
- Park, Y.-J.; Garaba, S.; Sainte-Rose, B. Detecting Great Pacific Garbage Patch Floating Plastic Litter Using WorldView-3 Satellite Imagery. Opt. Express 2021, 29, 35288. [Google Scholar] [CrossRef] [PubMed]
- Goddijn-Murphy, L.; Williamson, B. On Thermal Infrared Remote Sensing of Plastic Pollution in Natural Waters. Remote Sens. 2019, 11, 2159. [Google Scholar] [CrossRef] [Green Version]
- Evans, M.; Ruf, C. Toward the Detection and Imaging of Ocean Microplastics With a Spaceborne Radar. IEEE Trans. Geosci. Remote Sens. 2021, 60, 1–9. [Google Scholar] [CrossRef]
- Geraeds, M.; van Emmerik, T.; de Vries, R.; bin Ab Razak, M.S. Riverine Plastic Litter Monitoring Using Unmanned Aerial Vehicles (UAVs). Remote Sens. 2019, 11, 2045. [Google Scholar] [CrossRef] [Green Version]
- Gil, G.; Andriolo, U.; Gonçalves, L.; Sobral, P.; Bessa, F. Quantifying Marine Macro Litter Abundance on a Sandy Beach Using Unmanned Aerial Systems and Object-Oriented Machine Learning Methods. Remote Sens. 2020, 12, 2599. [Google Scholar] [CrossRef]
- Andriolo, U.; Gonçalves, G.; Bessa, F.; Sobral, P. Mapping Marine Litter on Coastal Dunes with Unmanned Aerial Systems: A Showcase on the Atlantic Coast. Sci. Total Environ. 2020, 736, 139632. [Google Scholar] [CrossRef]
- Mace, T.H. At-sea detection of marine debris: Overview of technologies, processes, issues, and options. Mar. Pollut. Bull. 2012, 65, 23–27. [Google Scholar] [CrossRef]
- Maximenko, N.; Corradi, P.; Law, K.L.; Van Sebille, E.; Garaba, S.P.; Lampitt, R.S.; Galgani, F.; Martinez-Vicente, V.; Goddijn-Murphy, L.; Veiga, J.M.; et al. Toward the Integrated Marine Debris Observing System. Front. Mar. Sci. 2019, 447. [Google Scholar] [CrossRef] [Green Version]
- Measures, R.M. Laser Remote Sensing: Fundamentals and Applications; Wiley-Interscience: New York, NY, USA, 1984. [Google Scholar]
- Rogers, S.R.; Webster, T.; Livingstone, W.; O’Driscoll, N.J. Airborne Laser-Induced Fluorescence (LIF) Light Detection and Ranging (LiDAR) for the Quantification of Dissolved Organic Matter Concentration in Natural Waters. Estuaries Coasts 2012, 35, 959–975. [Google Scholar] [CrossRef]
- Palmer, S.C.J.; Pelevin, V.V.; Goncharenko, I.; Kovács, A.W.; Zlinszky, A.; Présing, M.; Horváth, H.; Nicolás-Perea, V.; Balzter, H.; Tóth, V.R. Ultraviolet Fluorescence LiDAR (UFL) as a Measurement Tool for Water Quality Parameters in Turbid Lake Conditions. Remote Sens. 2013, 5, 4405–4422. [Google Scholar] [CrossRef]
- Churnside, J.H.; Brown, E.D.; Parker-Stetter, S.; Horne, J.K.; Hunt, G.L., Jr.; Hillgruber, N.; Sigler, M.F.; Vollenweider, J.J. Airborne Remote Sensing of a Biological Hot Spot in the Southeastern Bering Sea. Remote Sens. 2011, 3, 621–637. [Google Scholar] [CrossRef] [Green Version]
- Saito, Y.; Takano, K.; Kobayashi, F.; Kobayashi, K.; Park, H.-D. Development of a UV Laser-Induced Fluorescence Lidar for Monitoring Blue-Green Algae in Lake Suwa. Appl. Opt. 2014, 53, 7030–7036. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chen, P.; Mao, Z.; Pan, D.; He, Y. Subsurface Plankton Layers Observed from Airborne Lidar in Sanya Bay, South China Sea. Opt. Express 2018, 26, 29134–29147. [Google Scholar] [CrossRef]
- Churnside, J.H.; Thorne, R.E. Comparison of Airborne Lidar Measurements with 420 KHz Echo-Sounder Measurements of Zooplankton. Appl. Opt. 2005, 44, 5504–5511. [Google Scholar] [CrossRef]
- Raimondi, V.; Palombi, L.; Lognoli, D.; Masini, A.; Simeone, E. Experimental Tests and Radiometric Calculations for the Feasibility of Fluorescence LIDAR-Based Discrimination of Oil Spills from UAV. Int. J. Appl. Earth Obs. Geoinf. 2017, 61, 46–54. [Google Scholar] [CrossRef]
- NASA/LARC/SD/ASDC CALIPSO Lidar Level 2 Aerosol Profile, V4-20 [Data Set]; NASA Langley Atmospheric Science Data Center DAAC. 2018. Available online: https://doi.org/10.5067/CALIOP/CALIPSO/LID_L2_05KMAPRO-STANDARD-V4-20 (accessed on 7 July 2022).
- Straume, A.G.; Elfving, A.; Wernham, D.; de Bruin, F.; Kanitz, T.; Schuettemeyer, D.; von Bismarck, J.; Buscaglione, F.; Lecrenier, O.; McGoldrick, P. ESA’s Spaceborne Lidar Mission ADM-Aeolus; Project Status and Preparations for Launch. EPJ Web Conf. 2018, 176, 04007. [Google Scholar] [CrossRef] [Green Version]
- Churnside, J.H. LIDAR Detection of Plankton in the Ocean. In Proceedings of the 2007 IEEE International Geoscience and Remote Sensing Symposium, Barcelona, Spain, 23–28 July 2007; IEEE: Barcelona, Spain, 2007; pp. 3174–3177. [Google Scholar]
- Behrenfeld, M.J.; Hu, Y.; Hostetler, C.A.; Dall’Olmo, G.; Rodier, S.D.; Hair, J.W.; Trepte, C.R. Space-Based Lidar Measurements of Global Ocean Carbon Stocks. Geophys. Res. Lett. 2013, 40, 4355–4360. [Google Scholar] [CrossRef]
- Hostetler, C.A.; Behrenfeld, M.J.; Hu, Y.; Hair, J.W.; Schulien, J.A. Spaceborne Lidar in the Study of Marine Systems. Annu. Rev. Mar. Sci. 2018, 10, 121–147. [Google Scholar] [CrossRef] [Green Version]
- Jamet, C.; Ibrahim, A.; Ahmad, Z.; Angelini, F.; Babin, M.; Behrenfeld, M.J.; Boss, E.; Cairns, B.; Churnside, J.; Chowdhary, J.; et al. Going Beyond Standard Ocean Color Observations: Lidar and Polarimetry. Front. Mar. Sci. 2019, 6, 251. [Google Scholar] [CrossRef]
- Pichel, W.G.; Veenstra, T.S.; Churnside, J.H.; Arabini, E.; Friedman, K.S.; Foley, D.G.; Brainard, R.E.; Kiefer, D.; Ogle, S.; Clemente-Colón, P.; et al. GhostNet Marine Debris Survey in the Gulf of Alaska–Satellite Guidance and Aircraft Observations. Mar. Pollut. Bull. 2012, 65, 28–41. [Google Scholar] [CrossRef]
- Ge, Z.; Shi, H.; Mei, X.; Dai, Z.; Li, D. Semi-Automatic Recognition of Marine Debris on Beaches. Sci. Rep. 2016, 6, 25759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merlino, S.; Paterni, M.; Berton, A.; Massetti, L. Unmanned Aerial Vehicles for Debris Survey in Coastal Areas: Long-Term Monitoring Programme to Study Spatial and Temporal Accumulation of the Dynamics of Beached Marine Litter. Remote Sens. 2020, 12, 1260. [Google Scholar] [CrossRef] [Green Version]
- Allen, N.S.; Homer, J.; McKellar, J.F. The Use of Luminescence Spectroscopy in Aiding the Identification of Commercial Polymers. Analyst 1976, 101, 260. [Google Scholar] [CrossRef]
- Ahmad, S.R. UV Laser Induced Fluorescence in High-Density Polyethylene. J. Phys. D Appl. Phys. 1983, 16, L137–L144. [Google Scholar] [CrossRef]
- Piruska, A.; Nikcevic, I.; Lee, S.H.; Ahn, C.; Heineman, W.R.; Limbach, P.A.; Seliskar, C.J. The Autofluorescence of Plastic Materials and Chips Measured under Laser Irradiation. Lab Chip 2005, 5, 1348. [Google Scholar] [CrossRef]
- Htun, M.T. Characterization of High-Density Polyethylene Using Laser-Induced Fluorescence (LIF). J. Polym. Res. 2012, 19, 9823. [Google Scholar] [CrossRef]
- Langhals, H.; Zgela, D.; Schlücker, T. High Performance Recycling of Polymers by Means of Their Fluorescence Lifetimes. GSC 2014, 4, 144–150. [Google Scholar] [CrossRef] [Green Version]
- Spizzichino, V.; Caneve, L.; Colao, F.; Ruggiero, L. Characterization and Discrimination of Plastic Materials Using Laser-Induced Fluorescence. Appl. Spectrosc. 2016, 70, 1001–1008. [Google Scholar] [CrossRef]
- Monteleone, A.; Wenzel, F.; Langhals, H.; Dietrich, D. New Application for the Identification and Differentiation of Microplastics Based on Fluorescence Lifetime Imaging Microscopy (FLIM). J. Environ. Chem. Eng. 2021, 9, 104769. [Google Scholar] [CrossRef]
- Palombi, L.; Alderighi, D.; Cecchi, G.; Raimondi, V.; Toci, G.; Lognoli, D. A Fluorescence LIDAR Sensor for Hyper-Spectral Time-Resolved Remote Sensing and Mapping. Opt. Express 2013, 21, 14736–14746. [Google Scholar] [CrossRef]
- Cecchi, G.; Palombi, L.; Mochi, I.; Lognoli, D.; Raimondi, V.; Tirelli, D.; Trambusti, M.; Breschi, B. LIDAR Measurement of the Attenuation Coefficient of Natural Waters. In Proceedings of the 22nd International Laser Radar Conference, Paris, France, 12–16 July 2004; European Space Agency: Paris, France, 2004; Volume 827. [Google Scholar]
- Donaldson, L. Softwood and hardwood lignin fluorescence spectra of wood cell walls in different mounting media. IAWA J. 2013, 34, 3–19. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Rinderle, U. The Role of Chlorophyll Fluorescence in The Detection of Stress Conditions in Plants. CRC Crit. Rev. Anal. Chem. 1988, 19, S29–S85. [Google Scholar] [CrossRef]
- Palombi, L.; Cecchi, G.; Lognoli, D.; Raimondi, V.; Toci, G.; Agati, G. A Retrieval Algorithm to Evaluate the Photosystem I and Photosystem II Spectral Contributions to Leaf Chlorophyll Fluorescence at Physiological Temperatures. Photosynth. Res. 2011, 108, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Falkowski, P.G.; Kolber, Z. Variations in Chlorophyll Fluorescence Yields in Phytoplankton in the World Oceans. Funct. Plant Biol. 1995, 22, 341–355. [Google Scholar] [CrossRef]
- Lionetto, F.; Lionetto, M.G.; Mele, C.; Corcione, C.E.; Bagheri, S.; Udayan, G.; Maffezzoli, A. Autofluorescence of Model Polyethylene Terephthalate Nanoplastics for Cell Interaction Studies. Nanomaterials 2022, 12, 1560. [Google Scholar] [CrossRef]
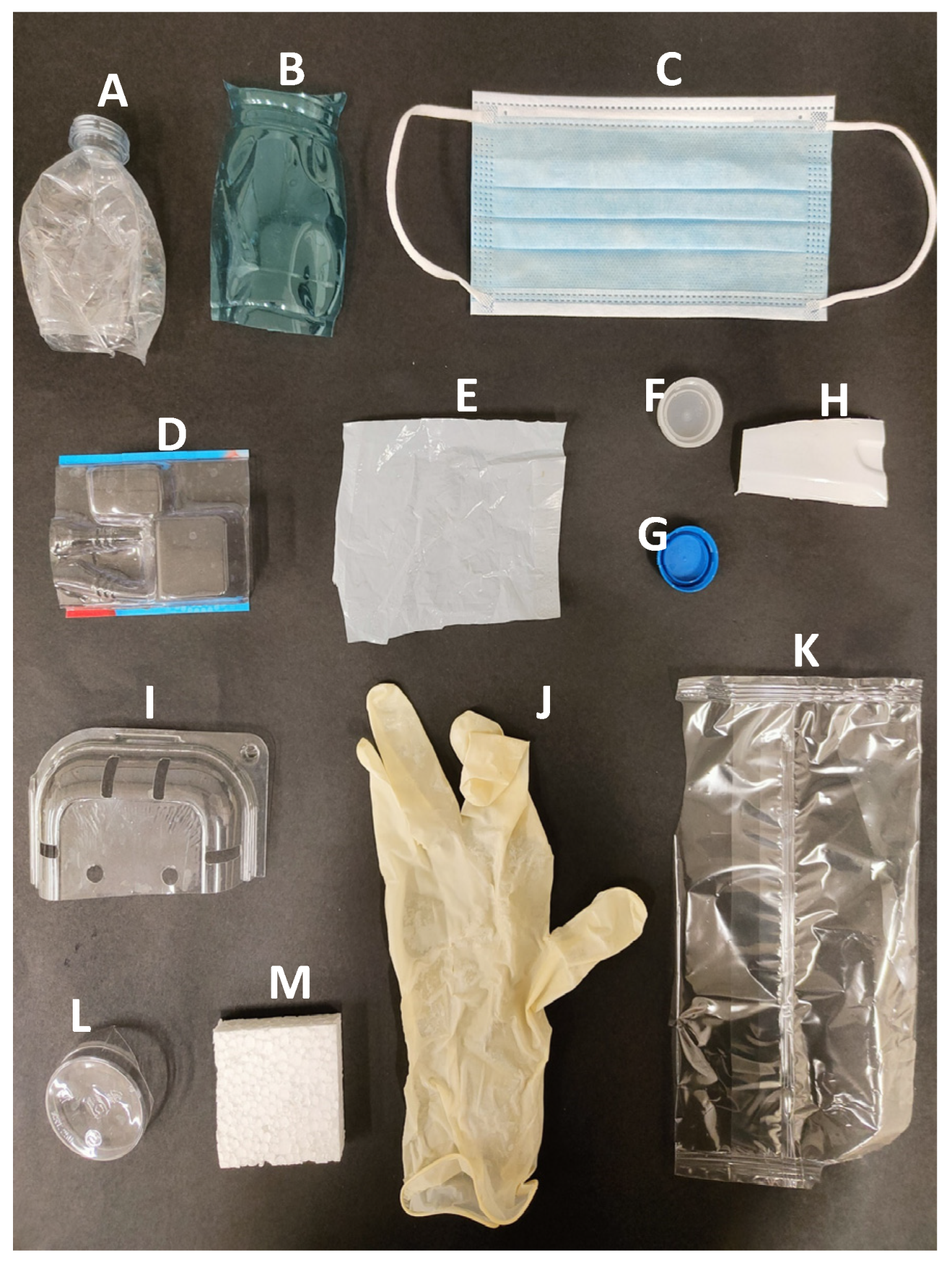
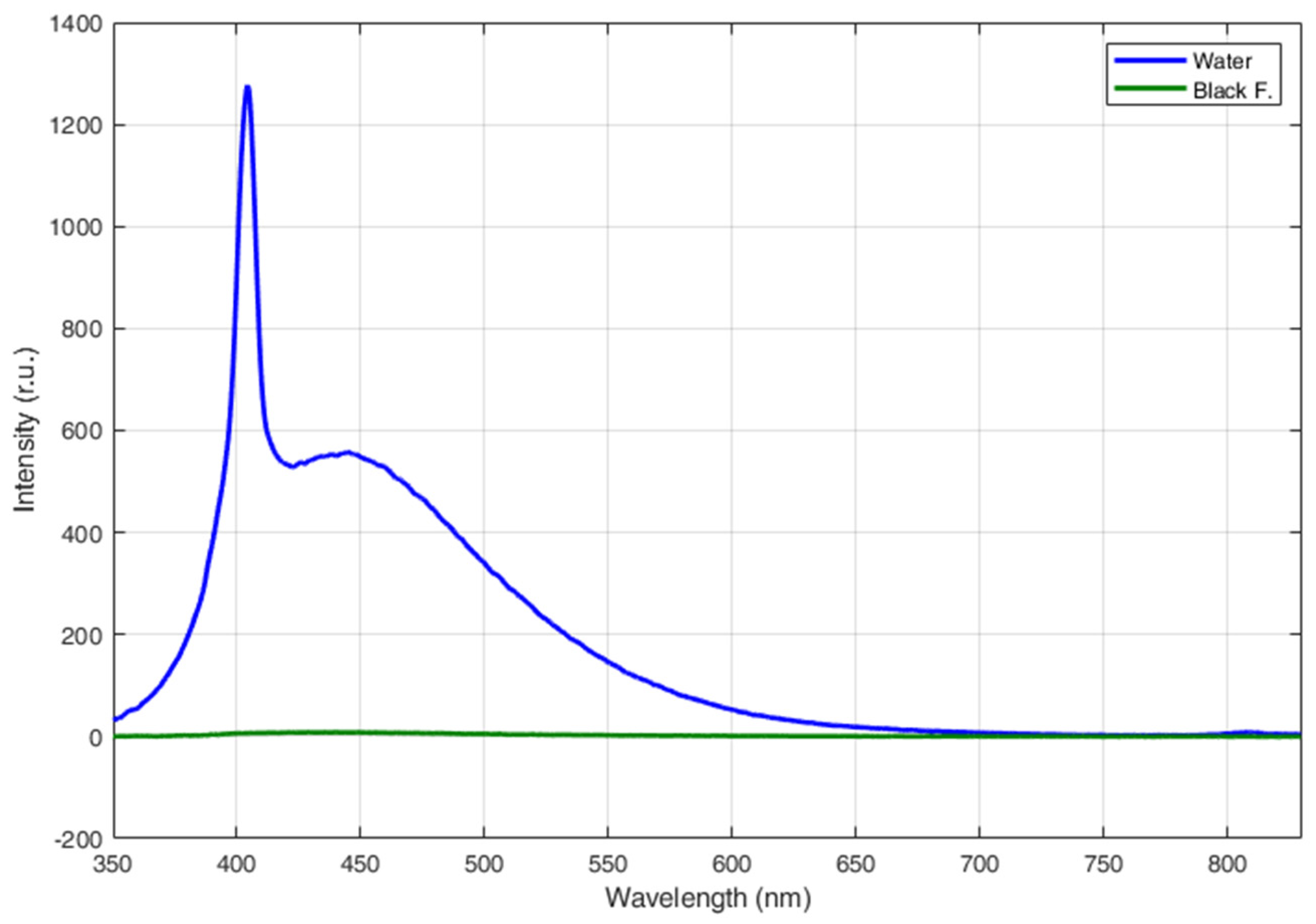
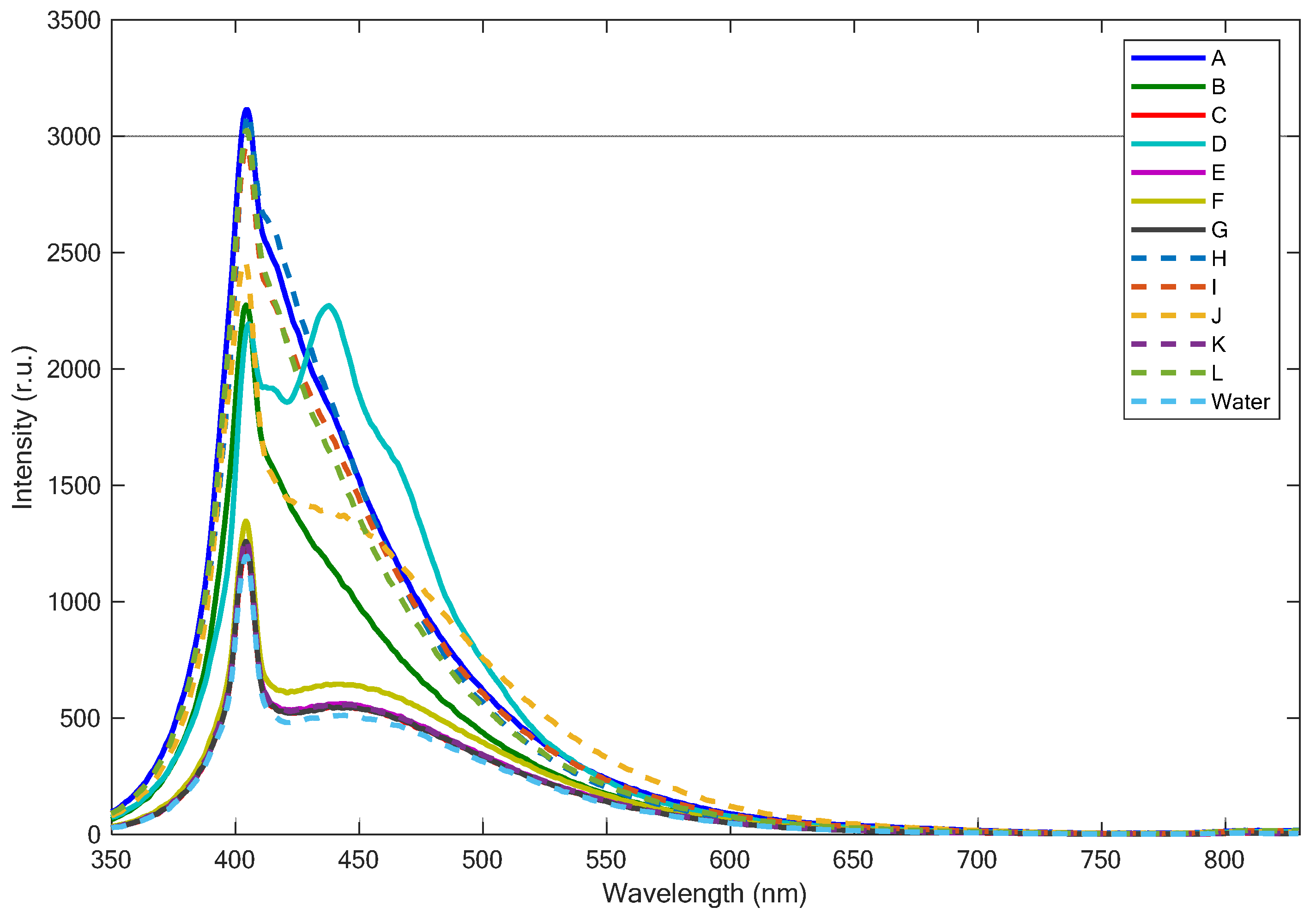

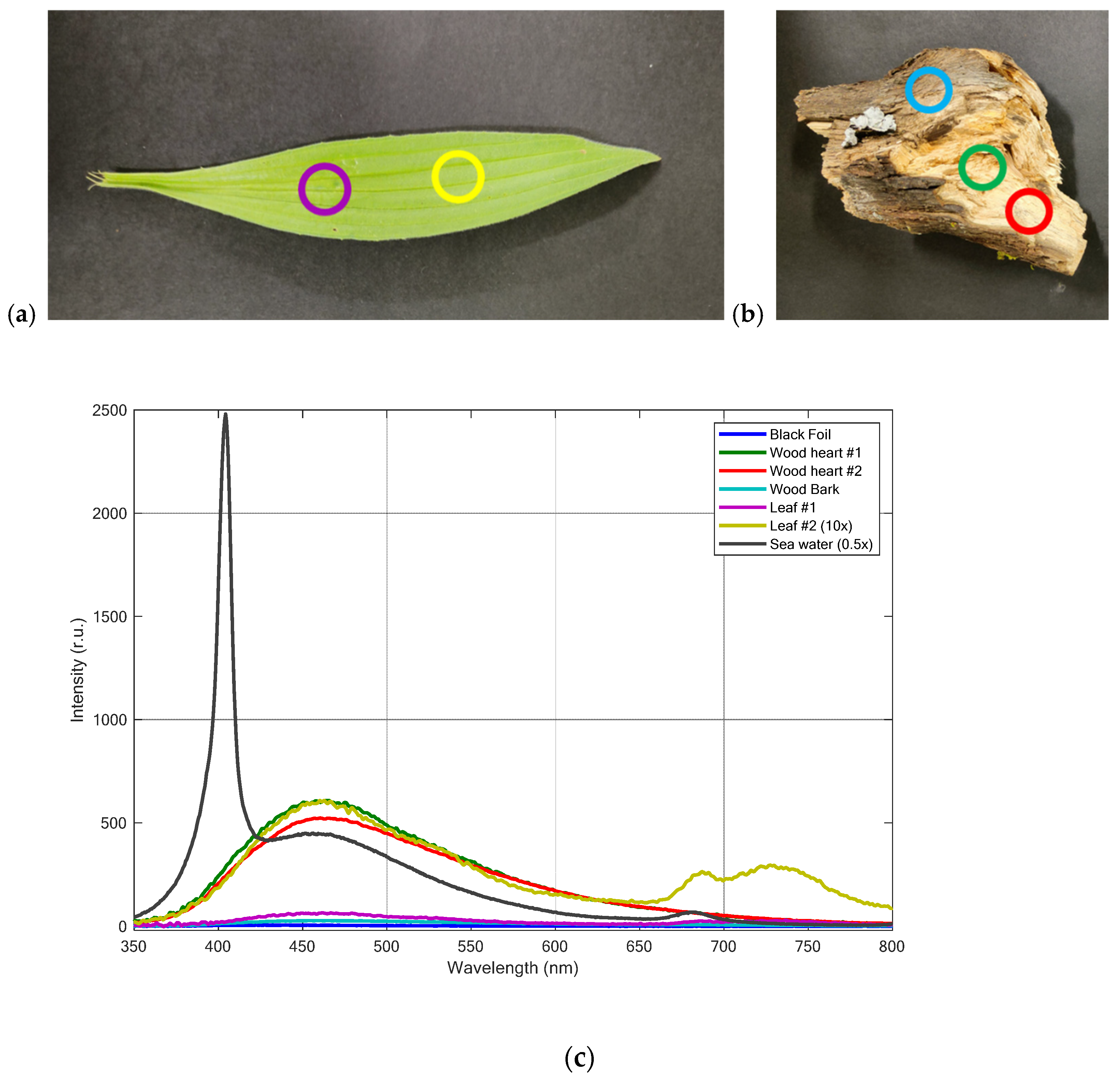
| Name | Description | Material | Thickness (µm) |
|---|---|---|---|
| A | Transparent Bottle | Polyethylene terephthalate (PET) | 140 |
| B | Bluish Bottle | Polyethylene terephthalate (PET) | 180 |
| C | Surgical Mask | Polypropylene (PP) | 300 |
| D | Blister | Polyvinyl chloride (PVC) | 160 |
| E | Plastic Bag | n.a. | 30 |
| F | White Bottle Cap | High-density polyethylene (HDPE) | 550 |
| G | Blue Bottle Cap | High-density polyethylene (HDPE) | 700 |
| H | White Bottle | Polyethylene terephthalate (PET) | 600 |
| I | Fresh Fruit Container | Recycled polyethylene terephthalate (R-PET) | 260 |
| J | Surgical Glove | LATEX | 230 |
| K | Transparent Packaging | Polypropylene (PP) | 60 |
| L | Transparent Plastic Glass | Polyethylene terephthalate (PET) | 430 |
| M | Polystyrene Slab | Expanded polystyrene (EPS) | 10,000 |
| Parameter | Value |
|---|---|
| Laser source | 3ω Nd:YAG, Q-Switched (355 nm, 12 mJ@20 Hz, 5 ns) |
| Telescope | Newtonian (1000-mm focal length, f/4) |
| Operative distance range | from 4 m to 100 m |
| IFOV | 1 mrad |
| Spectrometer | 300 mm focal length, f/3.9 |
| Spectral range | 350–830 nm |
| Spectral resolution | 0.5 nm, 0.1 nm or 0.02 nm depending on the used grating (150 gg/mm, 600 gg/mm, 2400 gg/mm) |
| Pointing field of view | Full solid angle |
| Pointing accuracy | 25 µrad |
| Size | 100 cm × 85 cm × 75 cm (operative) |
| Weight | 35 kg + spectrometer module |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palombi, L.; Raimondi, V. Experimental Tests for Fluorescence LIDAR Remote Sensing of Submerged Plastic Marine Litter. Remote Sens. 2022, 14, 5914. https://doi.org/10.3390/rs14235914
Palombi L, Raimondi V. Experimental Tests for Fluorescence LIDAR Remote Sensing of Submerged Plastic Marine Litter. Remote Sensing. 2022; 14(23):5914. https://doi.org/10.3390/rs14235914
Chicago/Turabian StylePalombi, Lorenzo, and Valentina Raimondi. 2022. "Experimental Tests for Fluorescence LIDAR Remote Sensing of Submerged Plastic Marine Litter" Remote Sensing 14, no. 23: 5914. https://doi.org/10.3390/rs14235914






