Gas Hydrates as High-Efficiency Storage System: Perspectives and Potentialities
Abstract
:1. Introduction
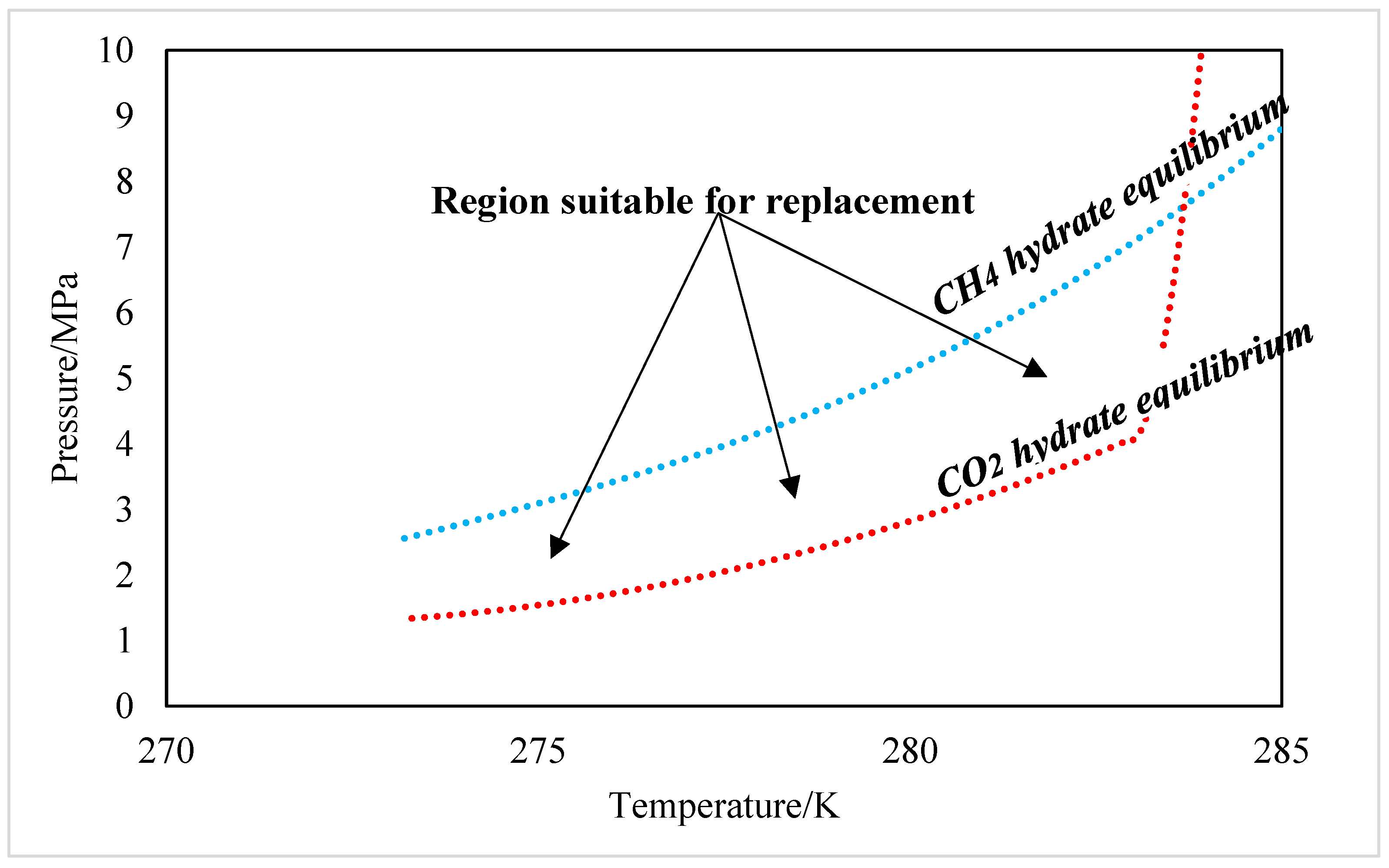
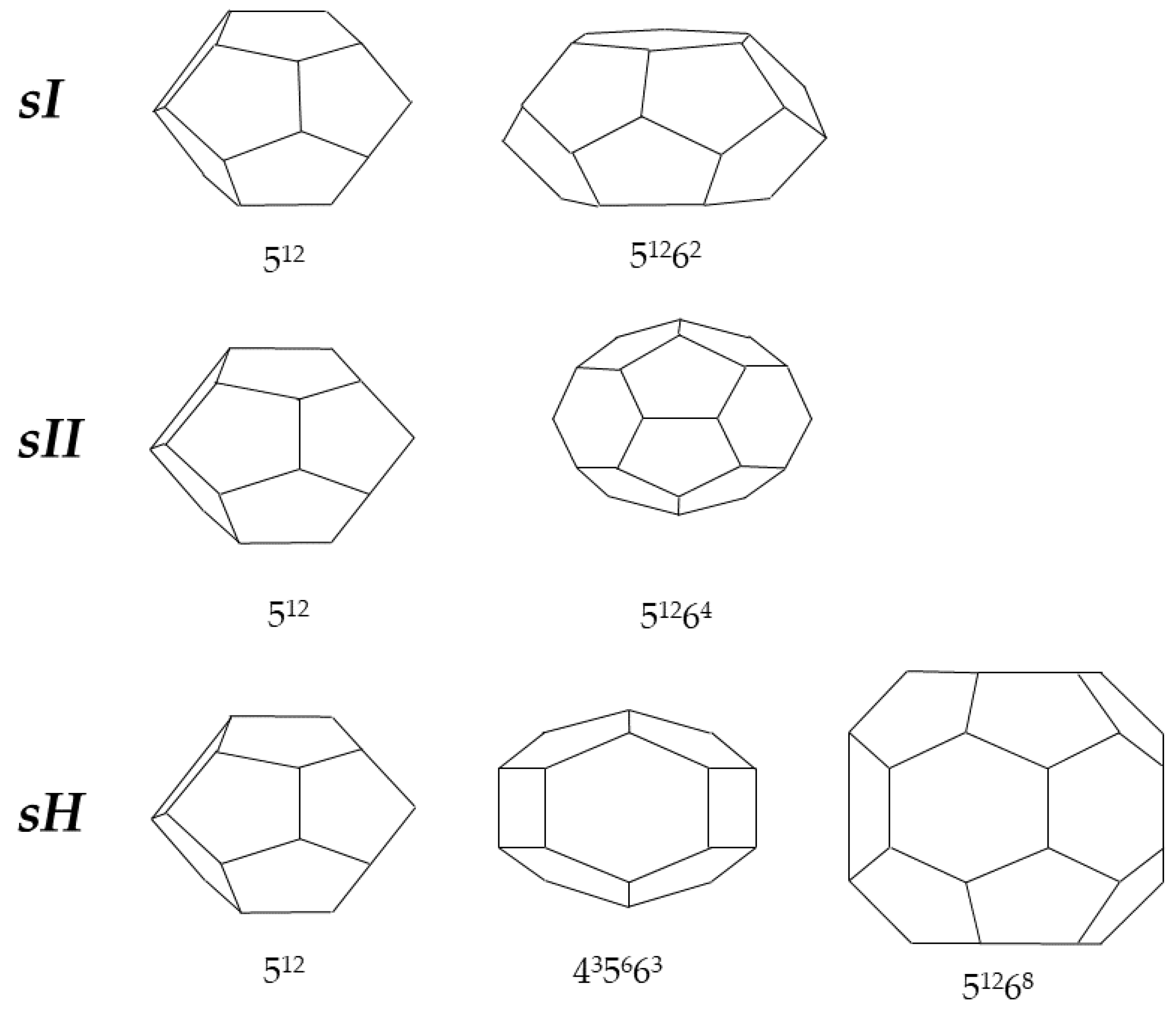
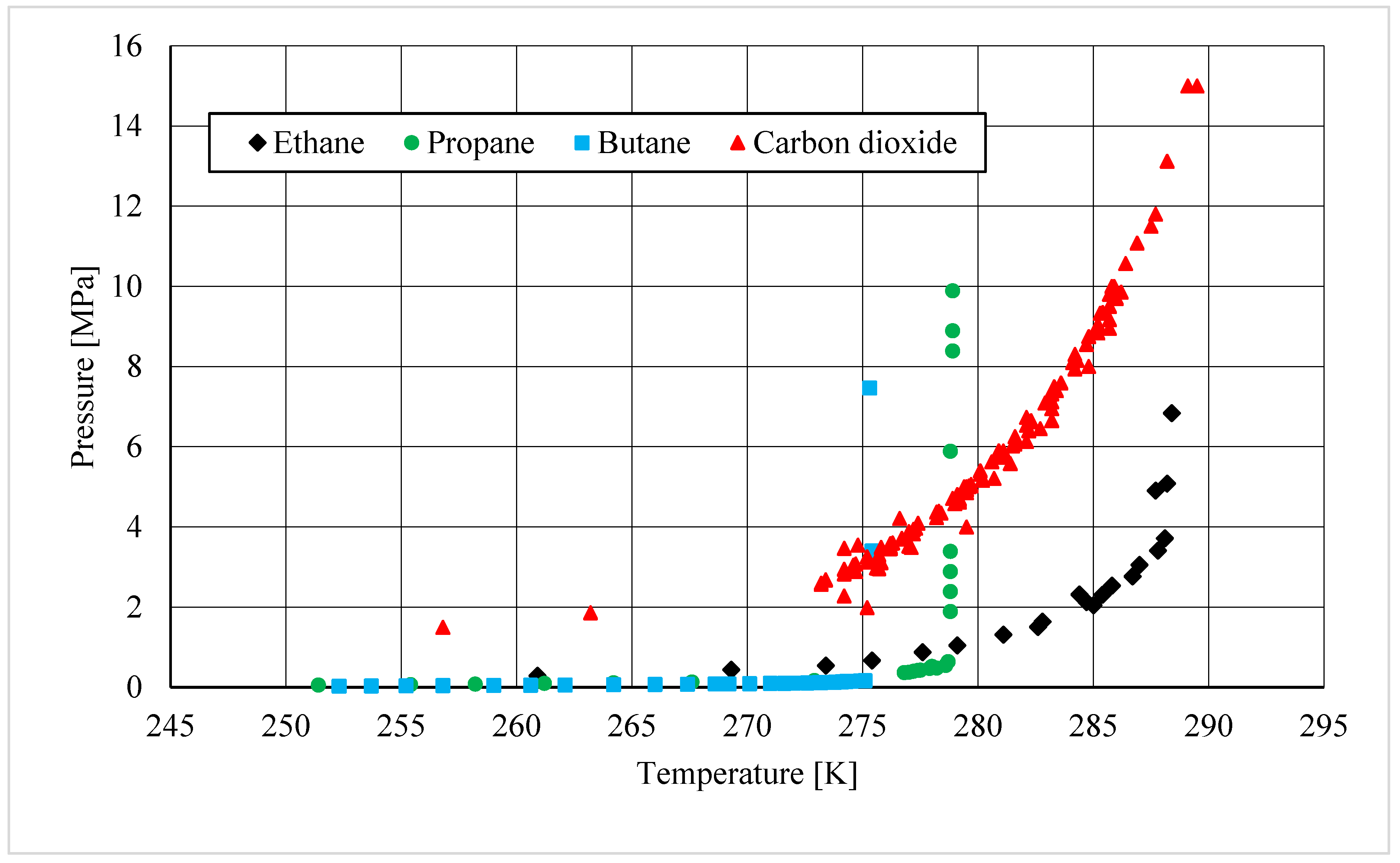
2. Hydrates as an Opportunity for Gas Storage
3. Reasons beyond the Variation of Gas Hydrate Storage Capacity
- (i)
- Water is an inexpensive material;
- (ii)
- The thermodynamic conditions required for the process are lower than those required for the traditional methods;
- (iii)
- The quantity of water per unit of volume is low, with obvious advantages in terms of weight;
- (iv)
- The solid storage makes transportation easier and less expensive;
- (v)
- The high mechanical strength ensures a high level of safety;
- (vi)
- In case of accidents, the release of gas would be extremely low, with consequent lower risks for operators and greater availability of time for solving the problem while containing the loss of gas.
4. Hydrogen as “Future Fuel”
5. Gas Hydrate as a Promising Solution for Hydrogen Storage
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| CCS. | carbon capture and storage |
| ni | number of edges in the specific face (related to a hydrate cavity) |
| mi | number of faces having ni edges (related to a hydrate cavity) |
| sI | Cubic Structure I |
| sII | Cubic Structure II |
| sH | Hexagonal Structure H |
| RES | renewable energy sources |
| THF | tetrahydrofuran |
| TBAB | tetrabutylammonium bromide |
References
- Sloan, E.D.; Koh, C.A. Clathrate Hydrates of Natural Gases, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Gambelli, A.M.; Presciutti, A.; Rossi, F. Review on the characteristics and advantages related to the use of flue-gas as CO2/N2 mixture for gas hydrate production. Fluid Phase Equilibr. 2021, 541, 113077. [Google Scholar] [CrossRef]
- Xu, C.G.; Zhang, W.; Yan, K.F.; Cai, J.; Chen, Z.Y.; Li, X.S. Research on micro mechanism and influence of hydrate-based methane-carbon dioxide replacement for realizing simultaneous clean energy exploitation and carbon emission reduction. Chem. Eng. Sci. 2022, 248, 117266. [Google Scholar] [CrossRef]
- Gambelli, A.M.; Tinivella, U.; Giovannetti, R.; Castellani, B.; Giustiniani, M.; Rossi, A.; Zannotti, M.; Rossi, F. Observation of the main natural parameters influencing the formation of gas hydrates. Energies 2021, 14, 1803. [Google Scholar] [CrossRef]
- Wang, P.; Teng, Y.; Zhu, J.; Bao, W.; Han, S.; Li, Y.; Zhao, Y.; Xie, H. Review on the synergistic effect between metal-organic frameworks and gas hydrates for CH4 storage and CO2 separation applications. Renew. Sust. Energ. Rev. 2022, 167, 112807. [Google Scholar] [CrossRef]
- Shi, C.; Wang, S.; Liu, H.; Zhang, L.; Yang, M.; Song, Y.; Zhao, J.; Ling, Z. Pyrolytic aerogels with tunable surface groups for efficient methane solidification storage via gas hydrates. Fuel 2023, 331, 125716. [Google Scholar] [CrossRef]
- Li, X.S.; Xu, C.G.; Zhang, Y.; Ruan, X.K.; Li, G.; Wang, Y. Investigation into gas production from natural gas hydrate: A review. Appl. Energy 2016, 172, 286–322. [Google Scholar] [CrossRef] [Green Version]
- Gambelli, A.M.; Filipponi, M.; Rossi, F. How methane release may affect carbon dioxide storage during replacement processes in natural gas hydrate reservoirs. J. Pet. Sci. Eng. 2021, 205, 108895. [Google Scholar] [CrossRef]
- He, J.; Li, X.; Chen, Z.; Li, Q.; Zhang, Y.; Wang, Y.; Xia, Z.; You, C. Combined styles of depressurization and electrical heating for methane hydrate production. Appl. Energy 2021, 282, 116112. [Google Scholar] [CrossRef]
- Gambelli, A.M.; Rossi, F. Optimization of the pressure drop produced during CO2 replacement in hydrate reservoirs: Balance between gas removal and preservation of structures. J. Petro. Sci. Eng. 2022, 217, 110936. [Google Scholar] [CrossRef]
- Wang, Z.; Duan, J.; Chen, S.; Fu, Y.; Li, X.; Wang, D.; Zhang, M.; Zhang, Z.; Liu, D.; Wang, F. A review on high-density methane storage in confined nanospace by adsorption-hydration hybrid technology. J. Energy Storage 2022, 50, 104195. [Google Scholar] [CrossRef]
- Wu, Y.; He, Y.; Tang, T.; Zhai, M. Molecular dynamic simulations of methane hydrate formation between solid surfaces: Implications for methane storage. Energy 2022, 262, 125511. [Google Scholar] [CrossRef]
- Gambelli, A.M.; Rossi, F. Thermodynamic and kinetic characterization of methane hydrate nucleation, growth and dissociation processes, according to the Labile Cluster Theory. Chem. Eng. J. 2021, 425, 130706. [Google Scholar] [CrossRef]
- Zhang, X.; Li, J.; Wu, Q.; Wang, C.; Nan, J. Experimental study on the effect of pore size on carbon dioxide hydrate formation and storage in porous media. J. Nat. Gas Sci. Eng. 2015, 25, 297–302. [Google Scholar] [CrossRef]
- Gambelli, A.M. Variations in terms of CO2 capture and CH4 recovery during replacement processes in gas hydrate reservoirs, associated to the “memory effect”. J. Clean. Prod. 2022, 360, 132154. [Google Scholar] [CrossRef]
- Ganteda, R.R.; Burla, S.K.; Boggu, J.M.R.; Prasad, P.S.R. Efficient storage of methane in hydrate form using soybean powder. Methane 2022, 1, 201–209. [Google Scholar] [CrossRef]
- Liu, X.; Cao, Q.; Xu, D.; Luo, S.; Guo, R. Improved methane storage capacity of methane hydrate promoted by vesicles from carboxylate surfactants and quaternary ammonium. J. Nat. Gas Sci. Eng. 2021, 93, 103990. [Google Scholar] [CrossRef]
- Kumar, S.; Kwon, H.T.; Choi, K.H.; Lim, W.; Cho, J.H.; Tak, K.; Moon, L. LNG: An eco-friendly cryogenic fuel for sustainable development. Appl. Energy 2011, 88, 4264–4273. [Google Scholar] [CrossRef]
- Veluswamy, H.P.; Wong, A.J.H.; Babu, P.; Kumar, R.; Kulprathipanja, S.; Rangsunvigit, P.; Linga, P. Rapid methane hydrate formation to develop a cost effective large scale energy storage system. Chem. Eng. J. 2016, 290, 161–173. [Google Scholar] [CrossRef]
- Wang, F.; Guo, G.; Liu, G.Q.; Luo, S.J.; Guo, R.B. Effects of surfactants micelles and surfactant-coated nanospheres on methane hydrate growth pattern. Chem. Eng. Sci. 2016, 144, 108–115. [Google Scholar] [CrossRef]
- Gambelli, A.M.; Stornelli, G.; Di Schino, A.; Rossi, F. Methane and carbon dioxide hydrates properties in presence of Inconel 718 particles: Analyses on its potential application in gas separation processes to perform efficiency improvement. J. Environ. Chem. Eng. 2021, 9, 106571. [Google Scholar] [CrossRef]
- Xie, Y.; Zhu, Y.J.; Zheng, T.; Yuan, Q.; Sun, C.Y.; Yang, L.Y.; Chen, G.J. Replacement in CH4-CO2 hydrate below freezing point based on abnormal self-preservation differences of CH4 hydrate. Chem. Eng. J. 2021, 403, 126283. [Google Scholar] [CrossRef]
- Sun, Z.G.; Wang, R.; Ma, R.; Guo, K.; Fan, S. Natural gas storage in hydrates with the presence of promoters. Energy Convers. Manag. 2003, 44, 2733–2742. [Google Scholar] [CrossRef]
- Chen, C.; Wan, J.; Li, W.; Song, Y. Water contact angles on quartz surfaces under supercritical CO2 sequestration conditions: Experimental and molecular dynamics simulation studies. Int. J. Greenh. Gas Control 2015, 42, 655–665. [Google Scholar] [CrossRef] [Green Version]
- Celia, M.A.; Bachu, S.; Nordbotten, J.M.; Bandilla, K.W. Status of CO2 storage in deep saline aquifers with emphasis on modelling approaches and practical simulations. Water Resour. Res. 2015, 51, 6846–6892. [Google Scholar] [CrossRef]
- Koide, H.; Shindo, Y.; Tazaki, Y.; Iijima, M.; Ito, K.; Kimura, N.; Omata, K. Deep sub-seabed disposal of CO2—The most protective storage. Energy Convers. Manag. 1997, 38, S253–S258. [Google Scholar] [CrossRef]
- House, K.Z.; Schrag, D.P.; Harvey, C.F.; Lackner, K.S. Permanent carbon dioxide storage in deep-sea sediments. Proc. Natl. Acad. Sci. USA 2006, 103, 12291–12295. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Wang, S.; Cheng, Z.; Huang, M.; Zhang, Y.; Zheng, J.; Jiang, L.; Liu, Y. Dependence of the hydrate-based CO2 storage process on the hydrate reservoir environment in high-efficiency storage methods. Chem. Eng. J. 2021, 415, 128937. [Google Scholar] [CrossRef]
- Gambelli, A.M.; Filipponi, M.; Nicolini, A.; Rossi, F. Natural gas hydrate: Effect of sodium chloride on the CO2 replacement process. Int. Multidiscip. Sci. GeoConf. Surv. Geol. Min. Ecol. Manag. (SGEM) 2019, 19, 333–343. [Google Scholar]
- Gambelli, A.M.; Rossi, F. Kinetic considerations and formation rate for carbon dioxide hydrate, formed in presence of a natural silica-based porous medium: How initial thermodynamic conditions may modify the process kinetic. Thermochim. Acta 2021, 705, 179039. [Google Scholar] [CrossRef]
- Qureshi, M.F.; Zheng, J.; Khandelwal, H.; Venkataraman, P.; Usadi, A.; Barckholtz, T.A.; Mhadeshwar, A.B.; Linga, P. Laboratory demonstration of the stability of CO2 hydrates in deep-pceanic sediments. Chem. Eng. J. 2022, 432, 134290. [Google Scholar] [CrossRef]
- Sun, Z.F.; Li, N.; Jia, S.; Cui, J.L.; Yuan, Q.; Sun, C.Y.; Chen, G.J. A novel method to enhance methane hydrate exploitation efficiency via forming impermeable overlying CO2 hydrate capture. Appl. Energy 2019, 240, 842–850. [Google Scholar] [CrossRef]
- Bhattacharjee, G.; Choudhary, N.; Kumar, A.; Chakrabarty, S.; Kumar, R. Effect of the amino acid 1-histidine on methane hydrate growth kinetics. J. Nat. Gas Sci. Eng. 2016, 35, 1453–1462. [Google Scholar] [CrossRef]
- Li, X.Y.; Li, X.S.; Wang, Y.; Liu, J.W.; Hu, H.Q. The optimization mechanism for gas hydrate dissociation by depressurization in the sediment with different water saturations and different particle sizes. Energy 2021, 215, 119129. [Google Scholar] [CrossRef]
- Rossi, F.; Gambelli, A.M. Thermodynamic phase equilibrium of single-guest hydrate and formation data of hydrate in presence of chemical additives: A review. Fluid Phase Equilibr. 2021, 536, 112958. [Google Scholar] [CrossRef]
- Gambelli, A.M. Analyses on CH4 and CO2 hydrate formation to define the optimal pressure for CO2 injection to maximize the replacement efficiency into natural gas hydrate in presence of a silica-based natural porous medium, via depressurization techniques. Chem. Eng. Process. 2021, 167, 108512. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, B.; Chen, Y.; Zhang, S.; Guo, W.; Cai, Y.; Tan, B.; Wang, W. Methane storage in a hydrated form as promoted by leucines for possible application to natural gas transportation and storage. Energy Technol. 2015, 3, 815–819. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, J.C.; Li, X.S.; Zhang, Y. Experimental investigation of optimization of well spacing for gas recovery from methane hydrate reservoir in sandy sediment by heat stimulation. Appl. Energy 2017, 207, 562–572. [Google Scholar] [CrossRef]
- Gambelli, A.M. An experimental description of the double positive effect of CO2 injection in methane hydrate deposits in terms of climate change mitigation. Chem. Eng. Sci. 2021, 233, 116430. [Google Scholar] [CrossRef]
- Aregba, A.G. Gas hydrate-properties, formation and benefits. Open. J. Yangtze Gas Oil 2017, 2, 27–44. [Google Scholar] [CrossRef] [Green Version]
- Jeffrey, G.A. Hydrate inclusion compounds. J. Incl. Phenom. 1984, 1, 211–222. [Google Scholar] [CrossRef]
- Pauling, L.; Marsh, R.E. The structure of chlorine hydrate. Proc. Natl. Acad. Sci. USA 1952, 38, 112–118. [Google Scholar] [CrossRef]
- Claussen, W.F. A second water structure for inert gas hydrates. J. Chem. Phys. 1951, 19, 1425–1426. [Google Scholar] [CrossRef]
- Ripmeester, J.A.; Tse, J.S.; Ratcliffe, C.I.; Powell, B.M. A new clathrate hydrate structure. Nature 1987, 325, 135–136. [Google Scholar] [CrossRef]
- Gambelli, A.M.; Rossi, F. Experimental characterization of memory effect, anomalous self-preservation and ice-hydrate competition, during methane-hydrates formation and dissociation in a lab-scale apparatus. Sustainability 2022, 14, 4807. [Google Scholar] [CrossRef]
- Park, Y.; Kim, D.Y.; Lee, J.W.; Huh, D.G.; Park, K.P.; Lee, J.; Lee, H. Sequestering carbon dioxide into complex structures of naturally occurring gas hydrates. Proc. Natl. Acad. Sci. USA 2006, 103, 12690–12694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, D.; Ro, H.; Seo, Y.; Seo, Y.J.; Lee, J.Y.; Kim, S.J.; Lee, J.; Lee, H. Thermodynamic stability and guest distribution of CH4/N2/CO2 mixed hydrates for methane hydrate production using N2/CO2 injection. J. Chem. Thermodyn. 2017, 106, 16–21. [Google Scholar] [CrossRef]
- Durham, W.B.; Kirby, S.H.; Stern, L.A.; Zhang, W.U. The strength and rheology of methane clathrate hydrate. J. Geophys. Res. 2003, 108, 2182. [Google Scholar] [CrossRef] [Green Version]
- Gambelli, A.M.; Li, Y.; Rossi, F. Influence of different proportion of CO2/N2 binary gas mixture on methane recovery through replacement processes in natural gas hydrate. Chem. Eng. Process. 2022, 175, 108932. [Google Scholar] [CrossRef]
- Li, Y.; Gambelli, A.M.; Rossi, F.; Mei, S. Effect of promoters on CO2 hydrate formation: Thermodynamic assessment and microscale Raman spectroscopy/hydrate crystal morphology characterization analysis. Fluid Phase Equilibr. 2021, 550, 113218. [Google Scholar] [CrossRef]
- Li, Y.; Chen, J.; Gambelli, A.M.; Zhao, X.; Gao, Y.; Rossi, F.; Mei, S. In situ experimental study on the effect of mixed inhibitors on the phase equilibrium of carbon dioxide hydrate. Chem. Eng. Sci. 2022, 248, 117230. [Google Scholar] [CrossRef]
- Acar, C.; Dincer, I. Review and evaluation of hydrogen production options for better environment. J. Clean. Prod. 2019, 218, 835–849. [Google Scholar] [CrossRef]
- Jain, I.P.; Lal, C.; Jain, A. Hydrogen storage in Mg: A most promising material. Int. J. Hydrogen Energy 2010, 35, 5133–5144. [Google Scholar] [CrossRef]
- Saadi, A.; Becherif, M.; Ramadan, H.S. Hydrogen production horizon using solar energy in Biskra, Algeria. Int. J. Hydrogen Energy 2016, 41, 21899–21912. [Google Scholar] [CrossRef]
- Davoodabadi, A.; Mahmoudi, A.; Ghasemi, H. The potential of hydrogen hydrate as a future storage medium. iScience 2020, 24, 101907. [Google Scholar] [CrossRef] [PubMed]
- Cipriani, G.; Genduso, F.; La Cascia, D.; Liga, R.; Miceli, R.; Ricco Galluzzo, G. Perspective on hydrogen energy carrier and its automotive applications. Int. J. Hydrogen Energy 2014, 39, 8482–8494. [Google Scholar] [CrossRef]
- Singh, A.K.; Singh, S.; Kumar, A. Hydrogen energy future with formic acid: A renewable chemical hydrogen storage system. Catal. Sci. Technol. 2016, 1, 12–40. [Google Scholar] [CrossRef]
- Uyar, T.S.; Besikci, D. Integration of hydrogen energy systems into renewable energy system for better design of 100% renewable energy communities. Int. J. Hydrogen Energy 2017, 42, 2453–2456. [Google Scholar] [CrossRef]
- Hua, T.Q.; Ahluwalia, R.K.; Peng, J.K.; Kromer, M.; Lasher, S.; McKenney, K.; Law, K.; Sinha, J. Technical assessment compressed hydrogen storage tank systems for automotive applications. Int. J. Hydrogen Storage 2011, 36, 3037–3049. [Google Scholar] [CrossRef] [Green Version]
- Sethia, G.; Sayari, A. Activated carbon with optimum pore size distribution for hydrogen storage. Carbon 2016, 99, 289–294. [Google Scholar] [CrossRef]
- Farha, O.K.; Hupp, J.T. Rational design, synthesis, purification, and activation of metal-organic framework materials. Acc. Chem. Res. 2010, 43, 1166–1175. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Kobayashi, H.; Taylor, J.M.; Ikeda, R.; Kubota, Y.; Kato, K.; Takata, M.; Yamamoto, T.; Toh, S.; Matsumura, S.; et al. Hydrogen storage in Pd nanocrystals covered with a metal organic framework. Nat. Mater. 2014, 13, 802–806. [Google Scholar] [CrossRef]
- Tong, L.; Xiao, J.; Bénard, P.; Chanine, R. Thermal management of metal hydride hydrogen storage reservoir using phase change materials. Int. J. Hydrogen Energy 2019, 44, 21055–21066. [Google Scholar] [CrossRef]
- Yanxing, Z.; Maoqiong, G.; Yuan, Z.; Xueqiang, D.; Jun, S. Thermodynamics analysis of hydrogen storage based on compressed gaseous hydrogen, liquid hydrogen and cryo-compressed hydrogen. Int. J. Hydrogen Energy 2019, 22, 16833–16840. [Google Scholar] [CrossRef]
- Di Profio, P.; Arca, S.; Rossi, F.; Filipponi, M. Reverse micelles enhance the formation of clathrate hydrates of hydrogen. J. Colloid Interf. Sci. 2018, 516, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, S.W. Hydrogen storage tanks for vehicles: Recent progress and current status. Curr. Opin. Solid State Mater. Sci. 2011, 15, 39–43. [Google Scholar] [CrossRef]
- Dagdougui, H.; Sacile, R.; Bersani, C.; Ouammi, A. Chapter 4—Hydrogen storage and distribution: Implementation scenarios. Hydrog. Infrastruct. Energy Appl. 2018, 4, 37–52. [Google Scholar]
- Luo, Y.; Sun, L.; Xu, F.; Liu, Z. Improved hydrogen storage of LiBH4 and NH3BH3 by catalysts. J. Mater. Chem. A 2018, 17, 7293–7309. [Google Scholar] [CrossRef]
- Stamatakis, E.; Zoulias, E.; Tzamalis, G.; Massina, Z.; Analytis, V.; Christodoulou, C.; Stubos, A. Metal hydride hydrogen compressors: Current developments and early markets. Renew. Energy 2018, 127, 850–862. [Google Scholar] [CrossRef]
- Zohuri, B.; Zohuri, B. Cryogenics and liquid hydrogen storage. In Hydrogen Energy; Springer: Cham, Switzerland, 2019; pp. 121–139. [Google Scholar]
- Cha, I.; Lee, S.; Lee, J.D.; Lee, G.W.; Seo, Y. Separation of SF6 from gas mixtures using gas hydrate formation. Environ. Sci. Technol. 2010, 44, 6117–6122. [Google Scholar] [CrossRef]
- Dyadin, Y.A.; Larionov, E.G.; Manakov, A.Y.; Zhurko, F.V.; Aladko, E.Y.; Mikina, T.V.; Komarov, V.Y. Clathrate hydrates of hydrogen and neon, Mendeleev Communications. R. Soc. Chem. 1999, 9, 209–210. [Google Scholar]
- Smirnov, G.S.; Stegailov, V.V. Toward determination of the new hydrogen hydrate clathrate structures. J. Phys. Chem. Lett. 2013, 4, 3560–3564. [Google Scholar] [CrossRef]
- Du, J.; Wang, L.; Liang, D.; Li, D. Phase equilibria and dissociation enthalpies of hydrogen semi-clathrate hydrate with tetrabutyl ammonium nitrate. J. Chem. Eng. Data 2012, 57, 603–609. [Google Scholar] [CrossRef]
- Hashimoto, S.; Sugahara, T.; Moritoki, M.; Sato, H.; Ohgaki, K. Thermodynamic stability of hydrogen + tetra-n-butyl ammonium bromide mixed gas hydrate in nonstoichiometric aqueous solutions. Chem. Eng. Sci. 2008, 63, 1092–1097. [Google Scholar] [CrossRef]
- Gambelli, A.M.; Rossi, F. Experimental investigation on the possibility of defining the feasibility of CO2/CH4 exchange into a natural gas hydrate marine reservoir via fast analysis of sediment properties. Chem. Eng. Res. Des. 2021, 171, 327–339. [Google Scholar] [CrossRef]
- Wang, P.; Li, K.; Yang, J.; Zhu, J.; Zhao, Y.; Teng, Y. Experimental and theoretical study on dissociation thermodynamics and kinetics of hydrogen-propane hydrate. Chem. Eng. J. 2021, 426, 131279. [Google Scholar] [CrossRef]
- Zhang, Y.; Bhattacharjee, G.; Kumar, R.; Linga, P. Solidified hydrogen storage (Solid-HyStore) via clathrate hydrates. Chem. Eng. J. 2022, 431, 133702. [Google Scholar] [CrossRef]
- Mao, W.L.; Mao, H.K.; Goncharov, A.F.; Struzhkin, V.V.; Guo, Q.; Hu, J.; Shu, J.; Hemley, R.J.; Somayazulu, M.; Zhao, Y. Hydrogen clusters in clathrate hydrate. Science 2022, 297, 2247–2249. [Google Scholar] [CrossRef]
- Strobel, T.A.; Hester, K.C.; Sloan, E.D.; Koh, C.A. A hydrogen clathrate hydrate with cyclohexane: Structure and stability. J. Am. Chem. Soc. 2007, 129, 9544–9545. [Google Scholar] [CrossRef]
- Katsumasa, K.; Koga, K.; Tanaka, H. On the thermodynamic stability of hydrogen clathrate hydrates. J. Chem. Phys. 2007, 127, 044509. [Google Scholar] [CrossRef] [Green Version]
- Sundramoorthy, J.D.; Hammonds, P.; Lal, B.; Philips, G. Gas hydrate equilibrium measurement and observation of gas hydrate dissociation with/without a KHI. Procedia Eng. 2016, 148, 870–877. [Google Scholar] [CrossRef] [Green Version]
- Buleiko, V.M.; Grigoriev, B.A.; Mendoza, J. Calorimetric investigation of hydrates of pure isobutane and iso- and normal butane mixtures. Fluid Phase Equilibr. 2018, 462, 14–24. [Google Scholar] [CrossRef]
- Mooijer-van den Heuvel, M.M.; Peters, C.J.; Arons, J.S. Gas hydrate phase equilibria for propane in the presence of additive components. Fluid Phase Equilibr. 2002, 193, 245–249. [Google Scholar] [CrossRef]
- Nagashima, H.D.; Fukushima, N.; Ohmura, R. Phase equilibria condition measurements in carbon dioxide clathrate hydrate forming system from 199.1 K to 247.1 K. Fluid Phase Equilibr. 2016, 413, 53–56. [Google Scholar] [CrossRef]
- Nema, Y.; Ohmura, R.; Senaha, I.; Yasuda, K. Quadruple point determination in carbon dioxide hydrate forming system. Fluid Phase Equilibr. 2017, 441, 49–53. [Google Scholar] [CrossRef]
- Babu, P.; Yang, T.; Veluswamy, H.P.; Kumar, R.; Linga, P. Hydrate phase equilibrium of ternary gas mixture containing carbon dioxide, hydrogen and propane. J. Chem. Thermodyn. 2013, 61, 58–63. [Google Scholar] [CrossRef]
- Zhang, Z.; Kusalik, P.G.; Guo, G.J. Molecular insight into the growth of hydrogen and methane binary hydrates. J. Phys. Chem. C 2018, 122, 7771–7778. [Google Scholar] [CrossRef]
- Zhong, J.R.; Chen, L.T.; Liu, T.C.; Zeng, X.Y.; Sun, Y.F.; Sun, C.Y.; Liu, B.; Chen, G.J.; Ripmeester, J.A. Serving of hydrogen-containing gas mixtures with tetrahydrofuran hydrate. J. Phys. Chem. C 2017, 121, 27822–27829. [Google Scholar] [CrossRef]
- Ghaani, M.R.; Takeya, S.; English, N.J. Hydrogen storage in propane-hydrate: Theoretical and experimental study. Appl. Sci. 2020, 10, 8962. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gambelli, A.M.; Rossi, F.; Cotana, F. Gas Hydrates as High-Efficiency Storage System: Perspectives and Potentialities. Energies 2022, 15, 8728. https://doi.org/10.3390/en15228728
Gambelli AM, Rossi F, Cotana F. Gas Hydrates as High-Efficiency Storage System: Perspectives and Potentialities. Energies. 2022; 15(22):8728. https://doi.org/10.3390/en15228728
Chicago/Turabian StyleGambelli, Alberto Maria, Federico Rossi, and Franco Cotana. 2022. "Gas Hydrates as High-Efficiency Storage System: Perspectives and Potentialities" Energies 15, no. 22: 8728. https://doi.org/10.3390/en15228728
APA StyleGambelli, A. M., Rossi, F., & Cotana, F. (2022). Gas Hydrates as High-Efficiency Storage System: Perspectives and Potentialities. Energies, 15(22), 8728. https://doi.org/10.3390/en15228728






