Sanguinarine Enhances the Integrity of the Blood–Milk Barrier and Inhibits Oxidative Stress in Lipopolysaccharide-Stimulated Mastitis
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Animal Treatment and Experimental Design
2.3. Histopathological Examination
2.4. Myeloperoxidase (MPO) Analysis
2.5. Cell Culture and Treatment
2.6. Cell Biological Detection and Viability Assay
2.7. Cytokine and Enzyme Activity Analyses
2.8. Western Blot Analysis
2.9. qRT-PCR Assay
2.10. Network Pharmacological Analysis
2.11. Immunofluorescence Analysis
2.12. ROS Analysis
2.13. Data Analysis
3. Results
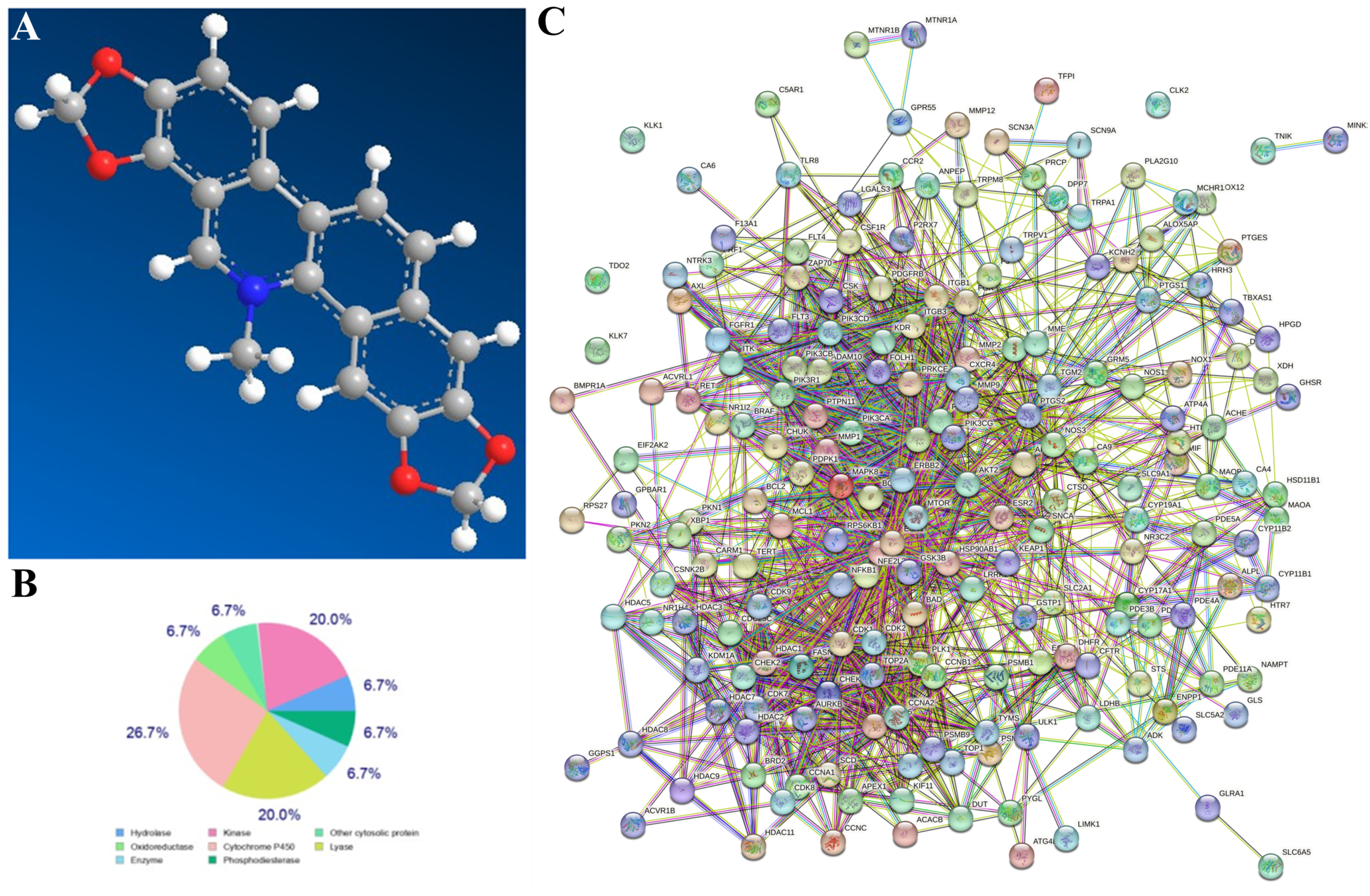
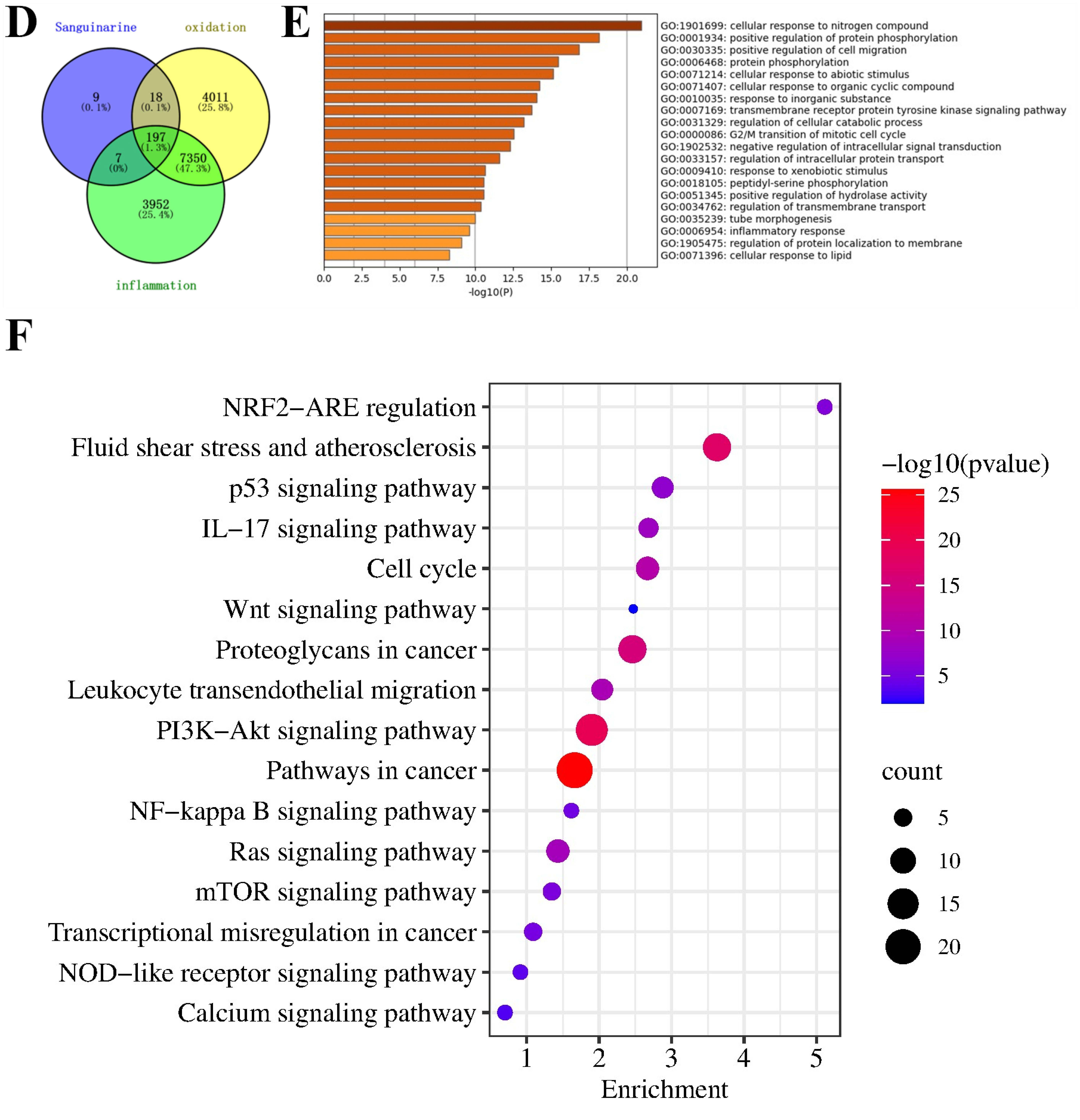
3.1. Network Pharmacological Analysis of SG
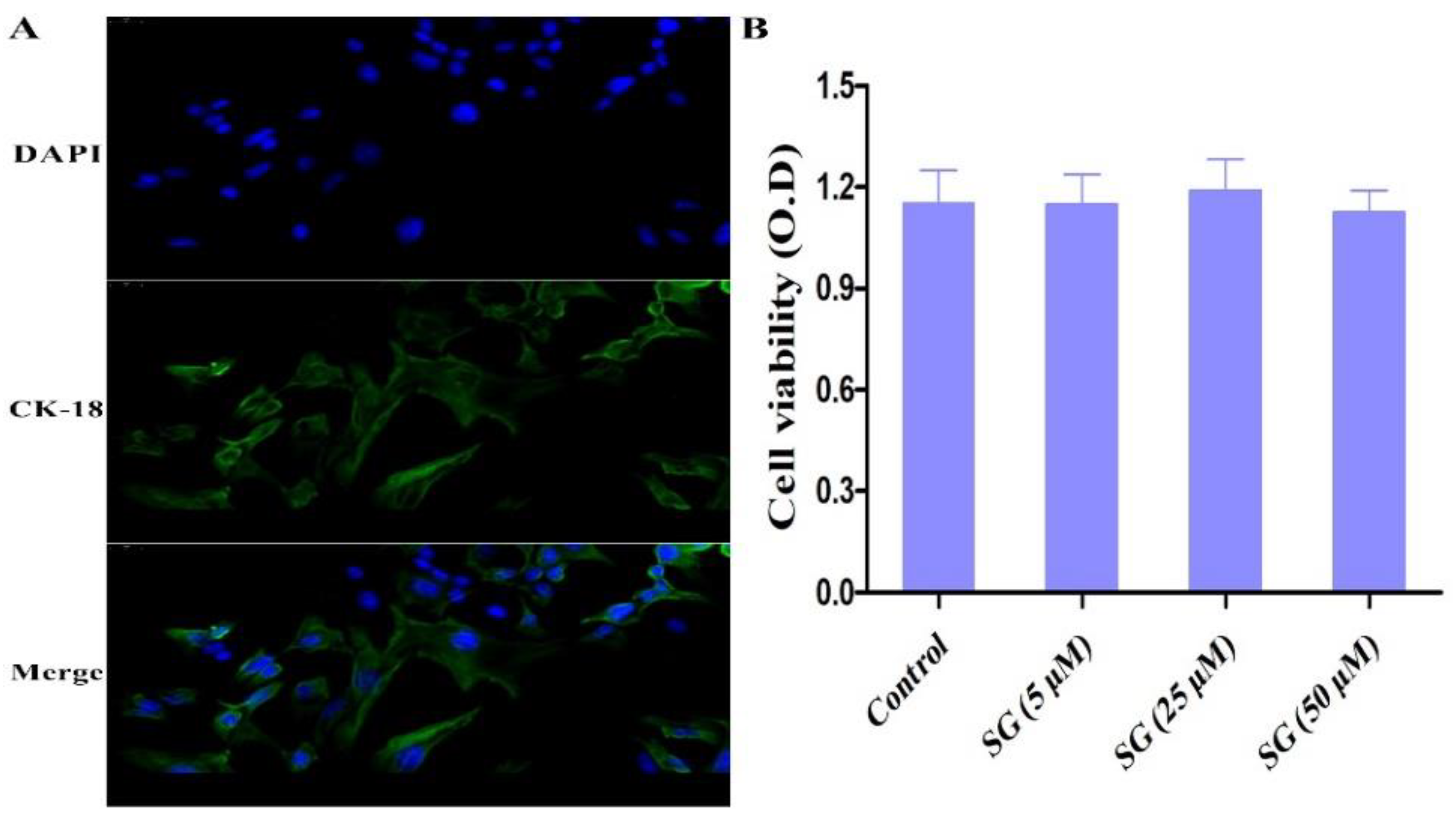
3.2. Cell Viability and Biological Assay
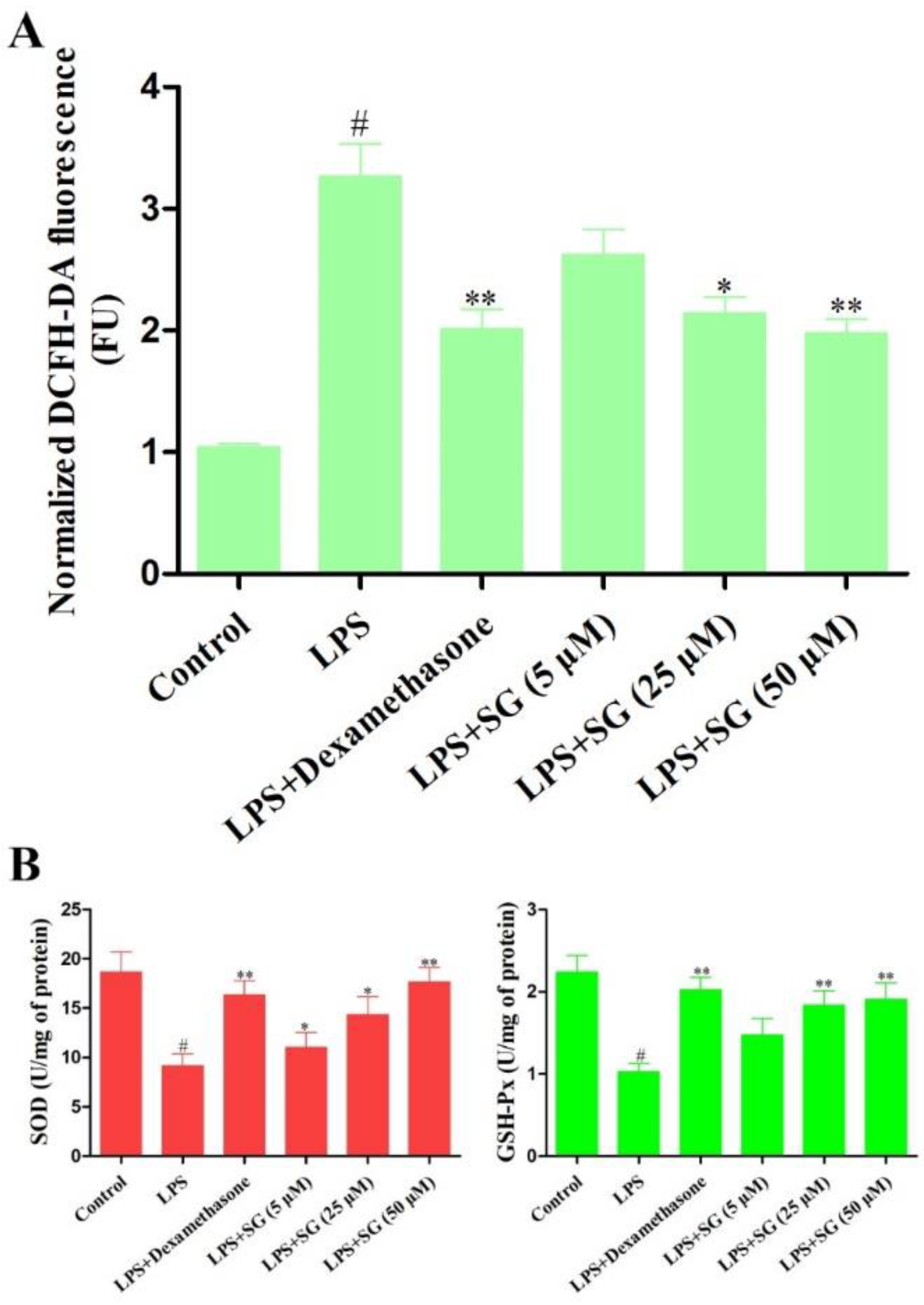
3.3. Effect of SG on LPS-Induced Oxidative Stress
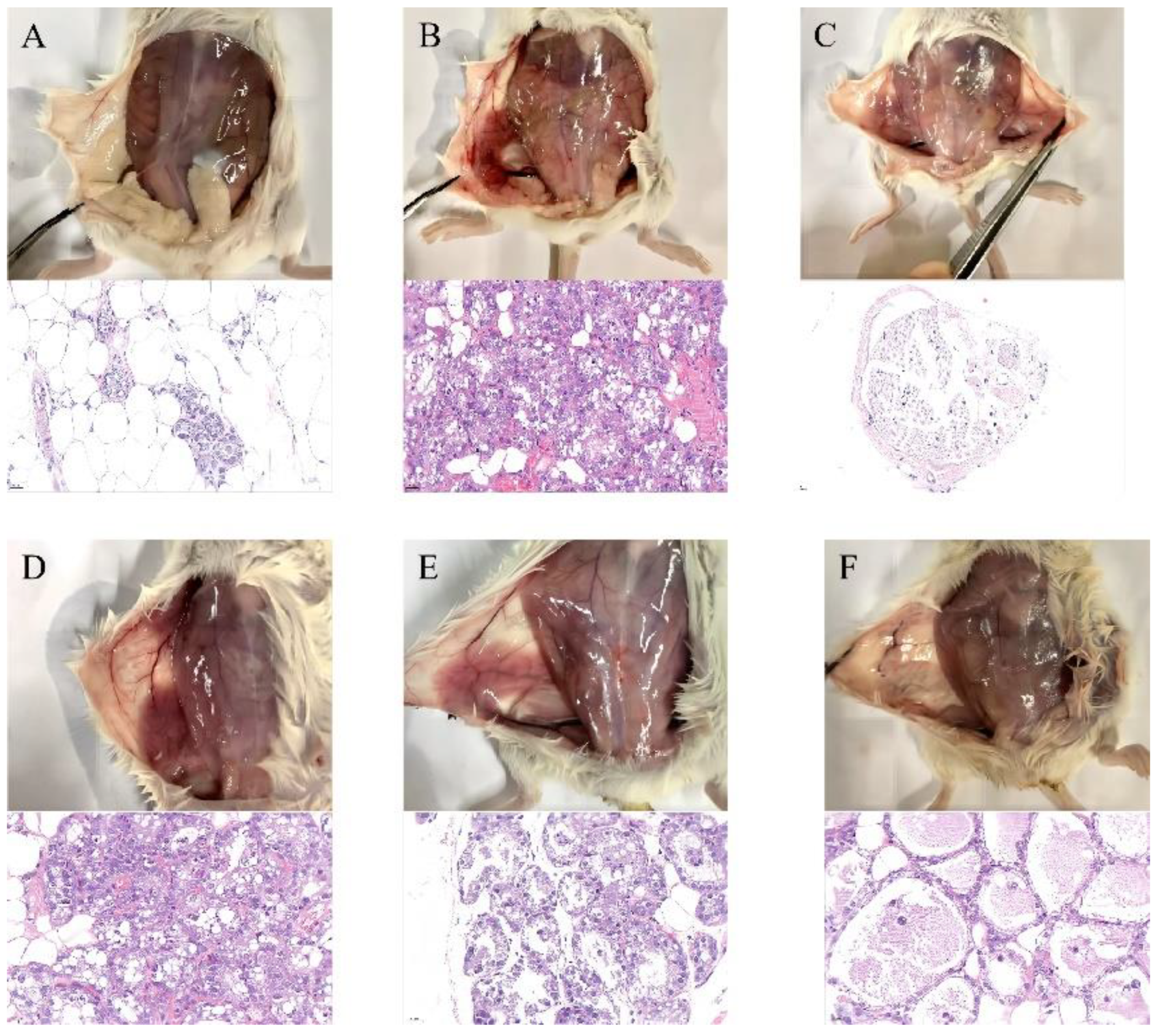
3.4. SG Alleviated LPS-Induced Mammary Gland Injury in Mice
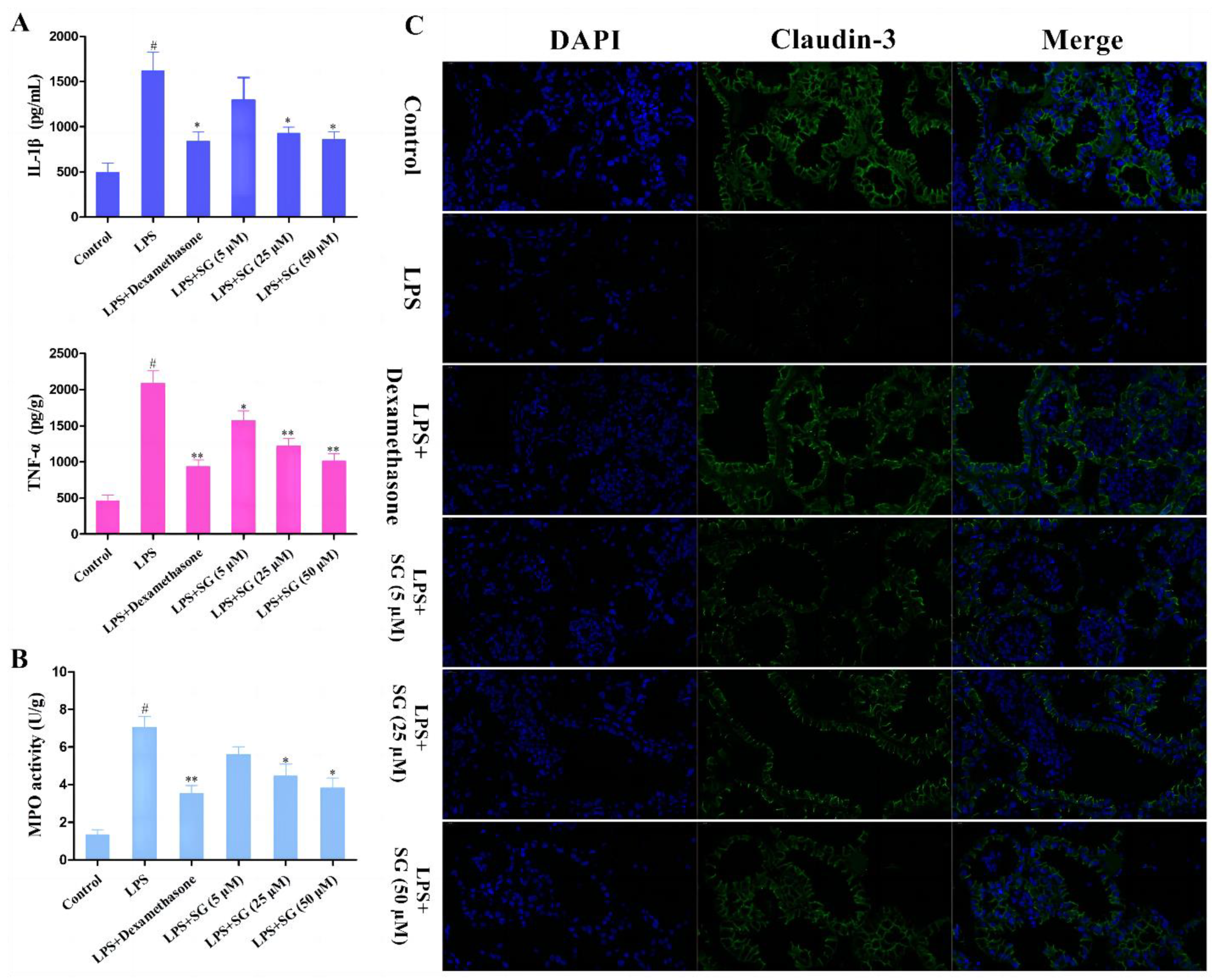
3.5. SG Reduced LPS-Induced Inflammatory Response and Improved the Integrity of the Blood–Milk Marrier
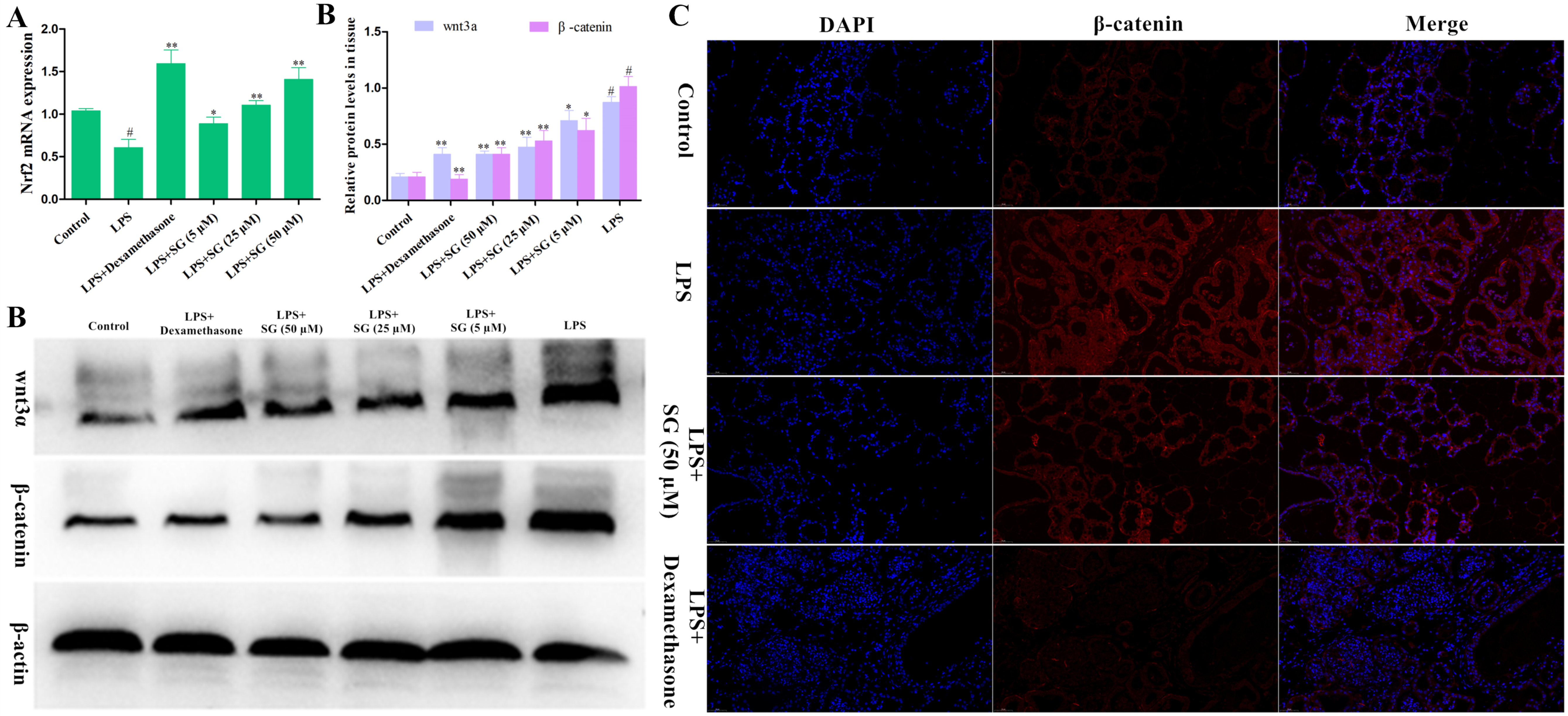
3.6. Effects of SG on the Activation of Nrf2 and the Wnt/β-Catenin Pathway
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gu, B.; Miao, J.; Fa, Y.; Lu, J.; Zou, S. Retinoic acid attenuates lipopolysaccharide-induced inflammatory responses by suppressing TLR4/NF-kappaB expression in rat mammary tissue. Int. Immunopharmacol. 2010, 10, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Fusco, R.; Cordaro, M.; Siracusa, R.; Peritore, A.F.; D’Amico, R.; Licata, P.; Crupi, R.; Gugliandolo, E. Effects of Hydroxytyrosol against Lipopolysaccharide-Induced Inflammation and Oxidative Stress in Bovine Mammary Epithelial Cells: A Natural Therapeutic Tool for Bovine Mastitis. Antioxidants 2020, 9, 693. [Google Scholar] [CrossRef] [PubMed]
- Petersson-Wolfe, C.S.; Leslie, K.E.; Swartz, T.H. An Update on the Effect of Clinical Mastitis on the Welfare of Dairy Cows and Potential Therapies. Vet. Clin. N. Am. Food Anim. Pract. 2018, 34, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Fiore, E.; Arfuso, F.; Gianesella, M. Metabolic and hormonal adaptation in Bubalus bubalis around calving and early lactation. PLoS ONE 2018, 13, e0193803. [Google Scholar] [CrossRef]
- Bazzano, M.; Giannetto, C.; Fazio, F.; Arfuso, F.; Giudice, E.; Piccione, G. Metabolic profile of broodmares during late pregnancy and early post-partum. Reprod. Domest. Anim. Zuchthyg. 2014, 49, 947–953. [Google Scholar] [CrossRef]
- Mavangira, V.; Kuhn, M.J.; Abuelo, A.; Morisseau, C.; Hammock, B.D.; Sordillo, L.M. Activity of sEH and Oxidant Status during Systemic Bovine Coliform Mastitis. Antioxidants 2021, 10, 812. [Google Scholar] [CrossRef]
- Asaf, S.; Leitner, G.; Furman, O.; Lavon, Y.; Kalo, D.; Wolfenson, D.; Roth, Z. Effects of Escherichia coli- and Staphylococcus aureus-induced mastitis in lactating cows on oocyte developmental competence. Reproduction 2014, 147, 33–43. [Google Scholar] [CrossRef]
- Ibeagha-Awemu, E.M.; Lee, J.W.; Ibeagha, A.E.; Bannerman, D.D.; Paape, M.J.; Zhao, X. Bacterial lipopolysaccharide induces increased expression of toll-like receptor (TLR) 4 and downstream TLR signaling molecules in bovine mammary epithelial cells. Vet. Res. 2008, 39, 11. [Google Scholar] [CrossRef]
- Tang, X.; Liu, C.; Li, T.; Lin, C.; Hao, Z.; Zhang, H.; Zhao, G.; Chen, Y.; Guo, A.; Hu, C. Gambogic acid alleviates inflammation and apoptosis and protects the blood-milk barrier in mastitis induced by LPS. Int. Immunopharmacol. 2020, 86, 106697. [Google Scholar] [CrossRef]
- Yin, H.; Xue, G.; Dai, A.; Wu, H. Protective Effects of Lentinan Against Lipopolysaccharide-Induced Mastitis in Mice. Front. Pharmacol. 2021, 12, 755768. [Google Scholar] [CrossRef]
- Long, E.; Capuco, A.V.; Wood, D.L.; Sonstegard, T.; Tomita, G.; Paape, M.J.; Zhao, X. Escherichia coli induces apoptosis and proliferation of mammary cells. Cell Death Differ. 2001, 8, 808–816. [Google Scholar] [CrossRef] [PubMed]
- Tsugami, Y.; Matsunaga, K.; Suzuki, T.; Nishimura, T.; Kobayashi, K. Phytoestrogens Weaken the Blood-Milk Barrier in Lactating Mammary Epithelial Cells by Affecting Tight Junctions and Cell Viability. J. Agric. Food Chem. 2017, 65, 11118–11124. [Google Scholar] [CrossRef] [PubMed]
- Stelwagen, K.; Singh, K. The role of tight junctions in mammary gland function. J. Mammary Gland Biol. Neoplasia 2014, 19, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Lam, J.S.; Anderson, E.M.; Hao, Y. LPS quantitation procedures. Methods Mol. Biol. 2014, 1149, 375–402. [Google Scholar] [CrossRef]
- Burvenich, C.; Van Merris, V.; Mehrzad, J.; Diez-Fraile, A.; Duchateau, L. Severity of E. coli mastitis is mainly determined by cow factors. Vet. Res. 2003, 34, 521–564. [Google Scholar] [CrossRef]
- Khan, M.Z.; Ma, Y.; Xiao, J.; Chen, T.; Ma, J.; Liu, S.; Wang, Y.; Khan, A.; Alugongo, G.M.; Cao, Z. Role of Selenium and Vitamins E and B9 in the Alleviation of Bovine Mastitis during the Periparturient Period. Antioxidants 2022, 11, 657. [Google Scholar] [CrossRef]
- Huemer, M.; Mairpady Shambat, S.; Brugger, S.D.; Zinkernagel, A.S. Antibiotic resistance and persistence-Implications for human health and treatment perspectives. EMBO Rep. 2020, 21, e51034. [Google Scholar] [CrossRef]
- Green, M.; Bradley, A. The changing face of mastitis control. Vet. Rec. 2013, 173, 517–521. [Google Scholar] [CrossRef]
- Zhang, F.; Xie, J.; Wang, G.; Zhang, G.; Yang, H. Anti-osteoporosis activity of Sanguinarine in preosteoblast MC3T3-E1 cells and an ovariectomized rat model. J. Cell. Physiol. 2018, 233, 4626–4633. [Google Scholar] [CrossRef]
- Prabhu, K.S.; Bhat, A.A.; Siveen, K.S.; Kuttikrishnan, S.; Raza, S.S.; Raheed, T.; Jochebeth, A.; Khan, A.Q.; Chawdhery, M.Z.; Haris, M.; et al. Sanguinarine mediated apoptosis in Non-Small Cell Lung Cancer via generation of reactive oxygen species and suppression of JAK/STAT pathway. Biomed. Pharmacother. Biomed. Pharmacother. 2021, 144, 112358. [Google Scholar] [CrossRef]
- Li, X.; Wu, X.; Wang, Q.; Xu, W.; Zhao, Q.; Xu, N.; Hu, X.; Ye, Z.; Yu, S.; Liu, J.; et al. Sanguinarine ameliorates DSS induced ulcerative colitis by inhibiting NLRP3 inflammasome activation and modulating intestinal microbiota in C57BL/6 mice. Phytomed. Int. J. Phytother. Phytopharm. 2022, 104, 154321. [Google Scholar] [CrossRef]
- Jiang, K.; Ma, X.; Guo, S.; Zhang, T.; Zhao, G.; Wu, H.; Wang, X.; Deng, G. Anti-inflammatory Effects of Rosmarinic Acid in Lipopolysaccharide-Induced Mastitis in Mice. Inflammation 2018, 41, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Buchan, K.D.; Prajsnar, T.K.; Ogryzko, N.V.; de Jong, N.W.M.; van Gent, M.; Kolata, J.; Foster, S.J.; van Strijp, J.A.G.; Renshaw, S.A. A transgenic zebrafish line for in vivo visualisation of neutrophil myeloperoxidase. PLoS ONE 2019, 14, e0215592. [Google Scholar] [CrossRef]
- Kan, X.; Liu, J.; Cai, X.; Huang, Y.; Xu, P.; Fu, S.; Guo, W.; Hu, G. Tartary buckwheat flavonoids relieve the tendency of mammary fibrosis induced by HFD during pregnancy and lactation. Aging 2021, 13, 25377–25392. [Google Scholar] [CrossRef]
- Ma, N.; Wei, G.; Zhang, H.; Dai, H.; Roy, A.C.; Shi, X.; Chang, G.; Shen, X. Cis-9, Trans-11 CLA Alleviates Lipopolysaccharide-Induced Depression of Fatty Acid Synthesis by Inhibiting Oxidative Stress and Autophagy in Bovine Mammary Epithelial Cells. Antioxidants 2021, 11, 55. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wang, J.; Chen, J.; Zhou, L.; Wang, H.; Chen, J.; Xu, Z.; Zhu, S.; Liu, W.; Yu, R.; et al. Morusin alleviates mycoplasma pneumonia via the inhibition of Wnt/β-catenin and NF-κB signaling. Biosci. Rep. 2019, 39. [Google Scholar] [CrossRef]
- Yang, Z.; Yin, R.; Cong, Y.; Yang, Z.; Zhou, E.; Wei, Z.; Liu, Z.; Cao, Y.; Zhang, N. Oxymatrine lightened the inflammatory response of LPS-induced mastitis in mice through affecting NF-κB and MAPKs signaling pathways. Inflammation 2014, 37, 2047–2055. [Google Scholar] [CrossRef]
- Li, H.; Sun, P. Insight of Melatonin: The Potential of Melatonin to Treat Bacteria-Induced Mastitis. Antioxidants 2022, 11, 1107. [Google Scholar] [CrossRef]
- Krömker, V.; Leimbach, S. Mastitis treatment-Reduction in antibiotic usage in dairy cows. Reprod. Domest. Anim. Zuchthyg. 2017, 52 (Suppl. S3), 21–29. [Google Scholar] [CrossRef]
- Mackraj, I.; Govender, T.; Gathiram, P. Sanguinarine. Cardiovasc. Ther. 2008, 26, 75–83. [Google Scholar] [CrossRef]
- Bruckmaier, R.M.; Wellnitz, O. Triennial Lactation Symposium/Bolfa: Pathogen-specific immune response and changes in the blood-milk barrier of the bovine mammary gland. J. Anim. Sci. 2017, 95, 5720–5728. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.; Martinson, H.; Durand-Rougely, C.; Schedin, P. Macrophages are crucial for epithelial cell death and adipocyte repopulation during mammary gland involution. Development 2012, 139, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.A.; Bobe, G.; Vorachek, W.R.; Kasper, K.; Traber, M.G.; Mosher, W.D.; Pirelli, G.J.; Gamroth, M. Effect of supranutritional organic selenium supplementation on postpartum blood micronutrients, antioxidants, metabolites, and inflammation biomarkers in selenium-replete dairy cows. Biol. Trace Elem. Res. 2014, 161, 272–287. [Google Scholar] [CrossRef]
- Ni, H.; Martínez, Y.; Guan, G.; Rodríguez, R.; Más, D.; Peng, H.; Valdivié Navarro, M.; Liu, G. Analysis of the Impact of Isoquinoline Alkaloids, Derived from Macleaya cordata Extract, on the Development and Innate Immune Response in Swine and Poultry. BioMed Res. Int. 2016, 2016, 1352146. [Google Scholar] [CrossRef]
- Glynn, D.J.; Hutchinson, M.R.; Ingman, W.V. Toll-like receptor 4 regulates lipopolysaccharide-induced inflammation and lactation insufficiency in a mouse model of mastitis. Biol. Reprod. 2014, 90, 91. [Google Scholar] [CrossRef] [PubMed]
- Galijasevic, S. The development of myeloperoxidase inhibitors. Bioorg. Med. Chem. Lett. 2019, 29, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Liu, Q.; Wen, Z.; Feng, H.; Deng, X.; Ci, X. Xanthohumol ameliorates lipopolysaccharide (LPS)-induced acute lung injury via induction of AMPK/GSK3β-Nrf2 signal axis. Redox Biol. 2017, 12, 311–324. [Google Scholar] [CrossRef]
- Lietman, C.; Wu, B.; Lechner, S.; Shinar, A.; Sehgal, M.; Rossomacha, E.; Datta, P.; Sharma, A.; Gandhi, R.; Kapoor, M.; et al. Inhibition of Wnt/β-catenin signaling ameliorates osteoarthritis in a murine model of experimental osteoarthritis. JCI Insight 2018, 3, e96308. [Google Scholar] [CrossRef]
- Katoh, M. Multi-layered prevention and treatment of chronic inflammation, organ fibrosis and cancer associated with canonical WNT/β-catenin signaling activation (Review). Int. J. Mol. Med. 2018, 42, 713–725. [Google Scholar] [CrossRef]

| Name | Sequence (5′→3′): Forward and Reverse | GenBank Accession No. | Product Size (bp) |
|---|---|---|---|
| Nrf2 | GACCTAAAGCACAGCCAACACAT CTTCAATCGGCTTGAATGTTTGTC | NM_010902.5 | 182 |
| GAPDH | CAATGTGTCCGTCGTGGATCT GTCCTCAGTGTAGCCCAAGATG | NM_001289726.1 | 124 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Z.; Zheng, Y.; Liang, X.; Xue, G.; Wu, H. Sanguinarine Enhances the Integrity of the Blood–Milk Barrier and Inhibits Oxidative Stress in Lipopolysaccharide-Stimulated Mastitis. Cells 2022, 11, 3658. https://doi.org/10.3390/cells11223658
Zheng Z, Zheng Y, Liang X, Xue G, Wu H. Sanguinarine Enhances the Integrity of the Blood–Milk Barrier and Inhibits Oxidative Stress in Lipopolysaccharide-Stimulated Mastitis. Cells. 2022; 11(22):3658. https://doi.org/10.3390/cells11223658
Chicago/Turabian StyleZheng, Zhijie, Yonghui Zheng, Xiaoben Liang, Guanhong Xue, and Haichong Wu. 2022. "Sanguinarine Enhances the Integrity of the Blood–Milk Barrier and Inhibits Oxidative Stress in Lipopolysaccharide-Stimulated Mastitis" Cells 11, no. 22: 3658. https://doi.org/10.3390/cells11223658
APA StyleZheng, Z., Zheng, Y., Liang, X., Xue, G., & Wu, H. (2022). Sanguinarine Enhances the Integrity of the Blood–Milk Barrier and Inhibits Oxidative Stress in Lipopolysaccharide-Stimulated Mastitis. Cells, 11(22), 3658. https://doi.org/10.3390/cells11223658








