Anesthetic Complication During Maxillofacial Trauma Surgery: A Case Report of Intraoperative Tension Pneumothorax
Abstract
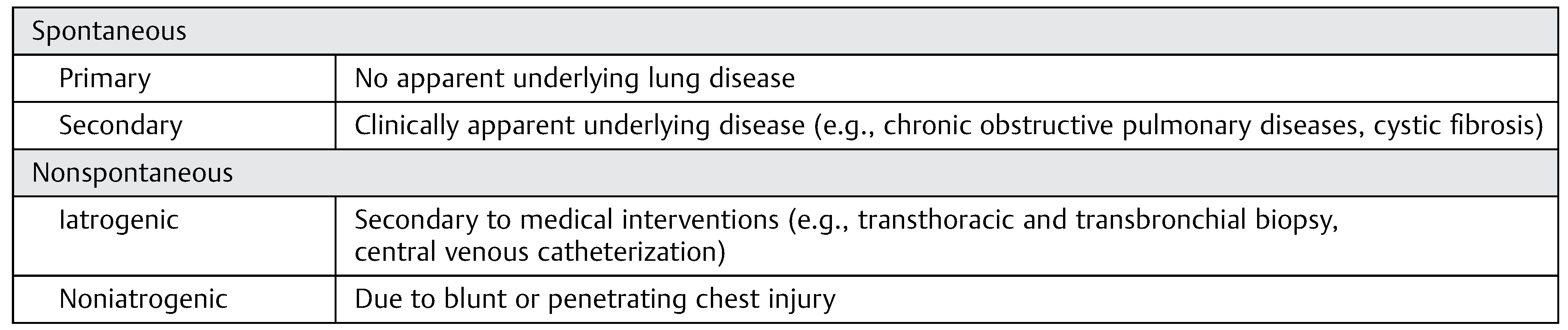
:Spontaneous
Nonspontaneous
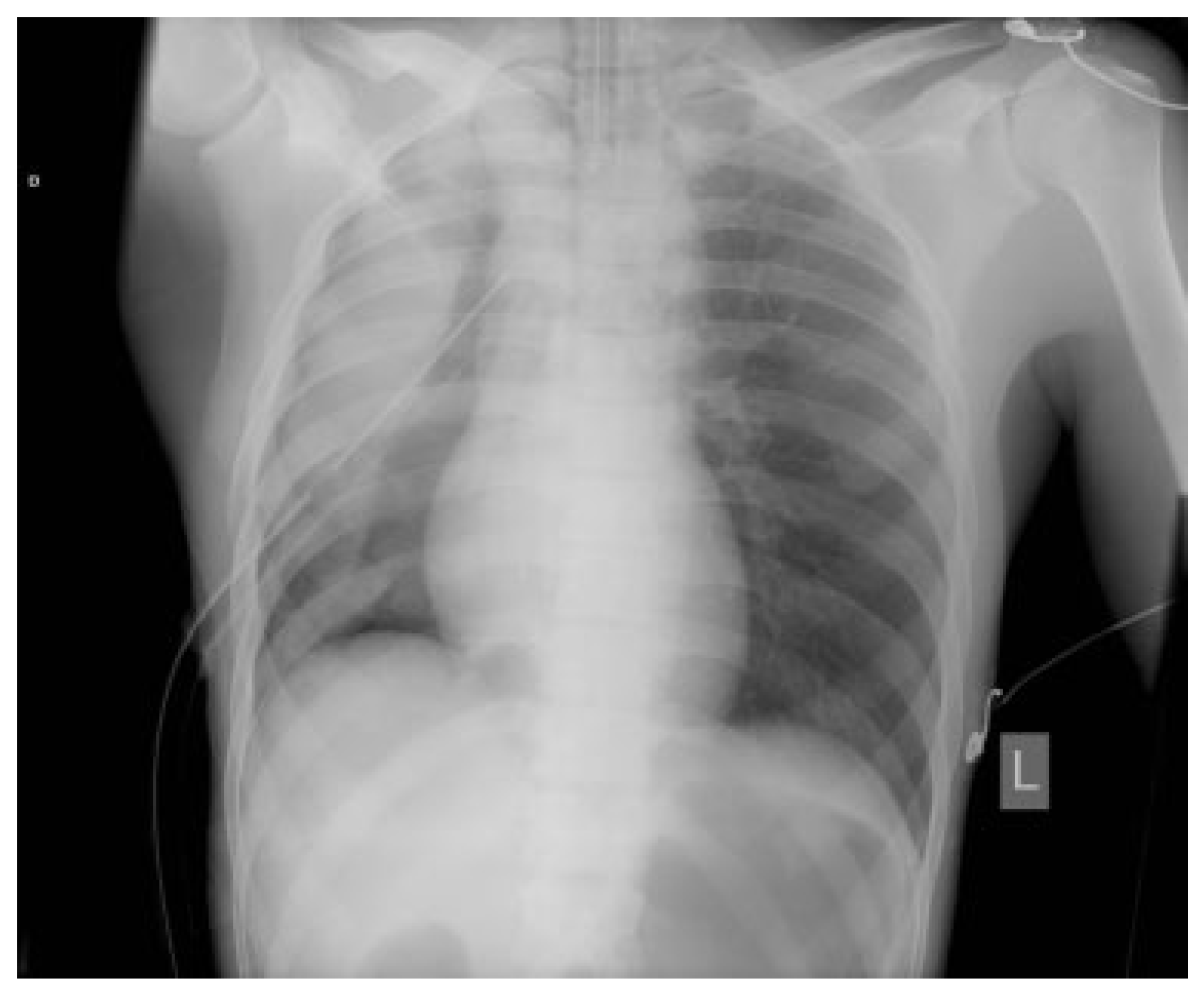
Case Report
Discussion
Conclusion
References
- Light, R.W.; Lee, Y.C.G. Pneumothorax, chylothorax, hemothorax, and fibrothorax. In Murray & Nadel’s Textbook of Respiratory Medicine, 6th ed.; Chapter 81; pp. 1439–1460.
- Noppen, M.; De Keukeleire, T. Pneumothorax. Respiration 2008, 76, 121–127. [Google Scholar] [PubMed]
- Sahn, S.A.; Heffner, J.E. Spontaneous pneumothorax. N Engl J Med 2000, 342, 868–874. [Google Scholar] [PubMed]
- Baumann, M.H.; Noppen, M. Pneumothorax. Respirology 2004, 9, 157–164. [Google Scholar]
- Chen, K.Y.; Jerng, J.S.; Liao, W.Y.; et al. Pneumothorax in the ICU: Patient outcomes and prognostic factors. Chest 2002, 122, 678–683. [Google Scholar]
- Leigh-Smith, S.; Harris, T. Tension pneumothorax—Time for a re-think? Emerg Med J 2005, 22, 8–16. [Google Scholar]
- Kortbeek, J.B.; Al Turki, S.A.; Ali, J.; et al. Advanced trauma life support, 8th edition, the evidence for change. J Trauma 2008, 64, 1638–1650. [Google Scholar]
- Piepho, T.; Thierbach, A.; Werner, C. Nasotracheal intubation: Look before you leap. Br J Anaesth 2005, 94, 859–860. [Google Scholar]
- Moore, D.C. Middle turbinectomy: A complication of IMPROPER nasal intubation? Anesthesiology 2000, 92, 1504–1505. [Google Scholar] [PubMed]
- Williams, A.R.; Burt, N.; Warren, T. Accidental middle turbinectomy: A complication of nasal intubation. Anesthesiology 1999, 90, 1782–1784. [Google Scholar]
- Watanabe, S.; Yaguchi, Y.; Suga, A.; Asakura, N. A “bubble-tip” (Air-guide) tracheal tube system: Its effects on incidence of epistaxis and ease of tube advancement in the subglottic region during nasotracheal intubation. Anesth Analg 1994, 78, 1140–1143. [Google Scholar]
- Kim, Y.C.; Lee, S.H.; Noh, G.J.; et al. Thermosoftening treatment of the nasotracheal tube before intubation can reduce epistaxis and nasal damage. Anesth Analg 2000, 91, 698–701. [Google Scholar] [CrossRef] [PubMed]
- Tintinalli, J.E.; Claffey, J. Complications of nasotracheal intubation. Ann Emerg Med 1981, 10, 142–144. [Google Scholar] [CrossRef] [PubMed]
- Berry FAJr Blankenbaker, W.L.; Ball, C.G. Comparison of bacteremia occurring with nasotracheal and orotracheal intubation. Anesth Analg 1973, 52, 873–876. [Google Scholar]
- Gallagher, J.V.I.I.I.; Vance, M.V.; Beechler, C. Difficult nasotracheal intubation: A previously unreported anatomical cause. Ann Emerg Med 1985, 14, 258–260. [Google Scholar] [CrossRef]
- Gallagher, T.J.; Civetta, J.M. Acute maxillary sinusitis complicating nasotracheal intubation: A case report. Anesth Analg 1976, 55, 885–886. [Google Scholar] [CrossRef]
- Dauphinee, K. Nasotracheal intubation. Emerg Med Clin North Am 1988, 6, 715–723. [Google Scholar] [CrossRef]
- Chebel, N.A.; Ziade, D.; Achkouty, R. Bilateral pneumothorax and pneumomediastinum after treatment with continuous positive airway pressure after orthognathic surgery. Br J Oral Maxillofac Surg 2010, 48, e14–e15. [Google Scholar] [CrossRef]
- Goodson, M.L.; Manemi, R.; Paterson, A.W. Pneumothorax after orthognathic surgery. Br J Oral Maxillofac Surg 2010, 48, 180–181. [Google Scholar] [CrossRef]
- Kim, T.; Kim, J.Y.; Woo, Y.C.; Park, S.G.; Baek, C.W.; Kang, H. Pneumomediastinum and pneumothorax after orthognathic surgery—A case report-. Korean J Anesthesiol 2010, 59 (Suppl), S242–S245. [Google Scholar] [CrossRef]
- Edwards, D.B.; Scheffer, R.B.; Jackler, I. Postoperative pneumomediastinum and pneumothorax following orthognathic surgery. J Oral Maxillofac Surg 1986, 44, 137–141. [Google Scholar] [CrossRef]
- St-Hilaire, H.; Montazem, A.H.; Diamond, J. Pneumomediastinum after orthognathic surgery. J Oral Maxillofac Surg 2004, 62, 892–894. [Google Scholar] [CrossRef] [PubMed]
- Nannini, V.; Sachs, S.A. Mediastinal emphysema following Le Fort I osteotomy: Report of a case. Oral Surg Oral Med Oral Pathol 1986, 62, 508–509. [Google Scholar] [CrossRef]
- Piecuch, J.F.; West, R.A. Spontaneous pneumomediastinum associated with orthognathic surgery. A case report. Oral Surg Oral Med Oral Pathol 1979, 48, 506–508. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, F.; Ward-Booth, R.P.; Banks, J.M. Pneumomediastinum after facial trauma. Oral Surg Oral Med Oral Pathol 1988, 66, 540–542. [Google Scholar] [CrossRef]
- Haberkamp, T.J.; Levine, H.L.; O’Brien, G. Pneumomediastinum secondary to a mandible fracture. Otolaryngol Head Neck Surg 1989, 101, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Chuong, R.; Boland, T.J.; Piper, M.A. Pneumomediastinum and subcutaneous emphysema associated with temporomandibular joint surgery. Oral Surg Oral Med Oral Pathol 1992, 74, 2–6. [Google Scholar] [CrossRef]
- Horowitz, I.; Hirshberg, A.; Freedman, A. Pneumomediastinum and subcutaneous emphysema following surgical extraction of mandibular third molars: Three case reports. Oral Surg Oral Med Oral Pathol 1987, 63, 25–28. [Google Scholar] [CrossRef]
- Nahlieli, O.; Neder, A. Iatrogenic pneumomediastinum after endodontic therapy. Oral Surg Oral Med Oral Pathol 1991, 71, 618–619. [Google Scholar] [CrossRef]


 |
© 2016 by the author. The Author(s) 2016.
Share and Cite
Al Shetawi, A.H.; Golden, L.; Turner, M. Anesthetic Complication During Maxillofacial Trauma Surgery: A Case Report of Intraoperative Tension Pneumothorax. Craniomaxillofac. Trauma Reconstr. 2016, 9, 251-254. https://doi.org/10.1055/s-0036-1572504
Al Shetawi AH, Golden L, Turner M. Anesthetic Complication During Maxillofacial Trauma Surgery: A Case Report of Intraoperative Tension Pneumothorax. Craniomaxillofacial Trauma & Reconstruction. 2016; 9(3):251-254. https://doi.org/10.1055/s-0036-1572504
Chicago/Turabian StyleAl Shetawi, Al Haitham, Leonard Golden, and Michael Turner. 2016. "Anesthetic Complication During Maxillofacial Trauma Surgery: A Case Report of Intraoperative Tension Pneumothorax" Craniomaxillofacial Trauma & Reconstruction 9, no. 3: 251-254. https://doi.org/10.1055/s-0036-1572504
APA StyleAl Shetawi, A. H., Golden, L., & Turner, M. (2016). Anesthetic Complication During Maxillofacial Trauma Surgery: A Case Report of Intraoperative Tension Pneumothorax. Craniomaxillofacial Trauma & Reconstruction, 9(3), 251-254. https://doi.org/10.1055/s-0036-1572504



