Traumatic orbital apex syndrome is a well-known but rare complication of craniomaxillofacial trauma that combines features of the superior orbital fissure syndrome with traumatic optic neuropathy. The optimal treatment of traumatic orbital apex syndrome has not been established, because there have been so few cases. We report a case of traumatic orbital apex syndrome combined with the blow-in type of the orbital and zygomaticomaxillary complex (ZMC) fracture, which was successfully treated by emergency decompression of impinged nerves, and had complete recovery of visual and ocular function. We also discuss the indications for and timing of surgical intervention for cases of direct traumatic orbital apex syndrome with facial fracture.
Case
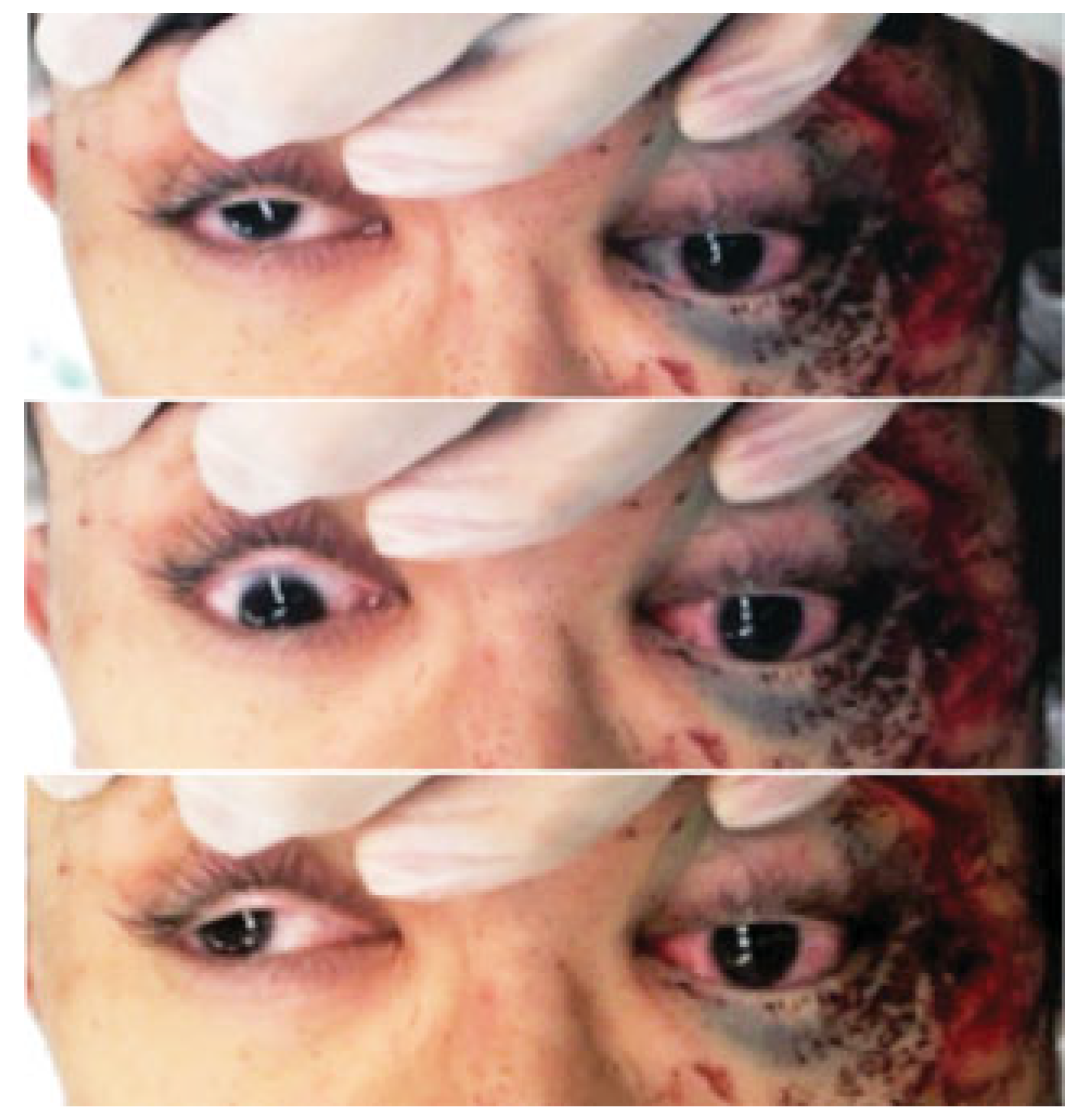
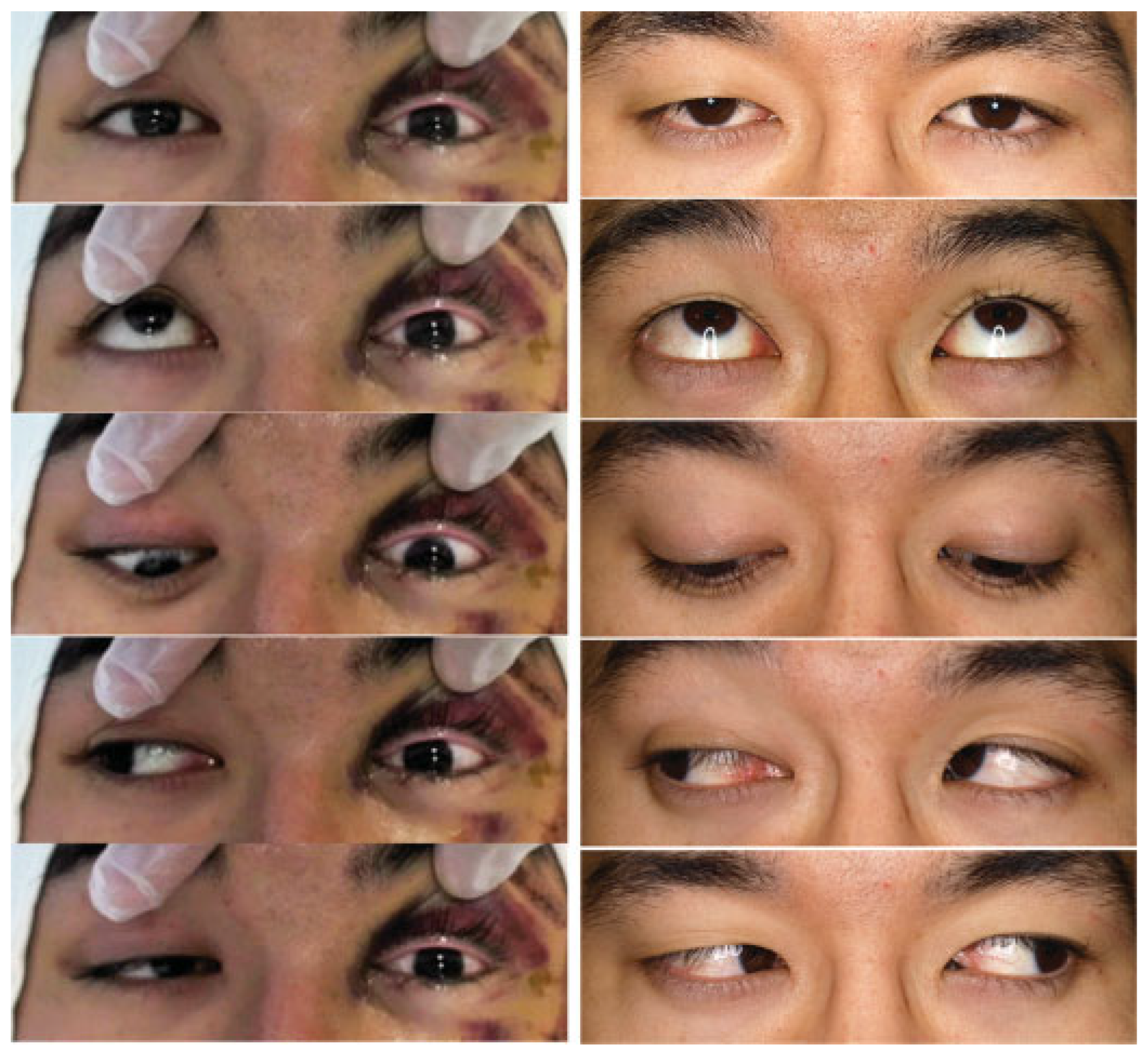
A 24-year-old male on a bicycle collided with a wall at the bottom of a hill due to brake failure. The main impact was sustained on the left side of his face. He was referred to our emergency center for urgent treatment of his facial injuries. On initial examination, tfwo hours after his accident, the patient presented with a Glasgow Coma Scale score of 12. He had left periorbital ecchymosis, left lid ptosis, and decreased left visual acuity. He had only the ability to count fingers at 50 cm, compared with the ability of the nonaffected right eye to read figures without his glasses, which were lost at the trauma scene. The pupils were anisocoric: 5 mm on the left versus 3.5 mm on the right. Direct pupillary reflex was sluggish on the left eye and intact on the right. Indirect pupillary reflex of both eyes was maintained. He also had lost the left corneal reflex, and had paresthesias over the left frontal region, ocular motility disorder of the left eye in all directions of gaze, and elevated left ocular pressure on palpation (
Figure 1). Opthalmologic evaluation performed in the emergency room was limited due to head trauma and generalized convulsion. The relative afferent pupillary defect (RAPD) was positive for the affected left eye but negative for the right eye. Although the lower eyelid was edematous, the optic media, such as the iris, lens, and vitreum was found intact. The left optic disc was found to be slightly erythematous.
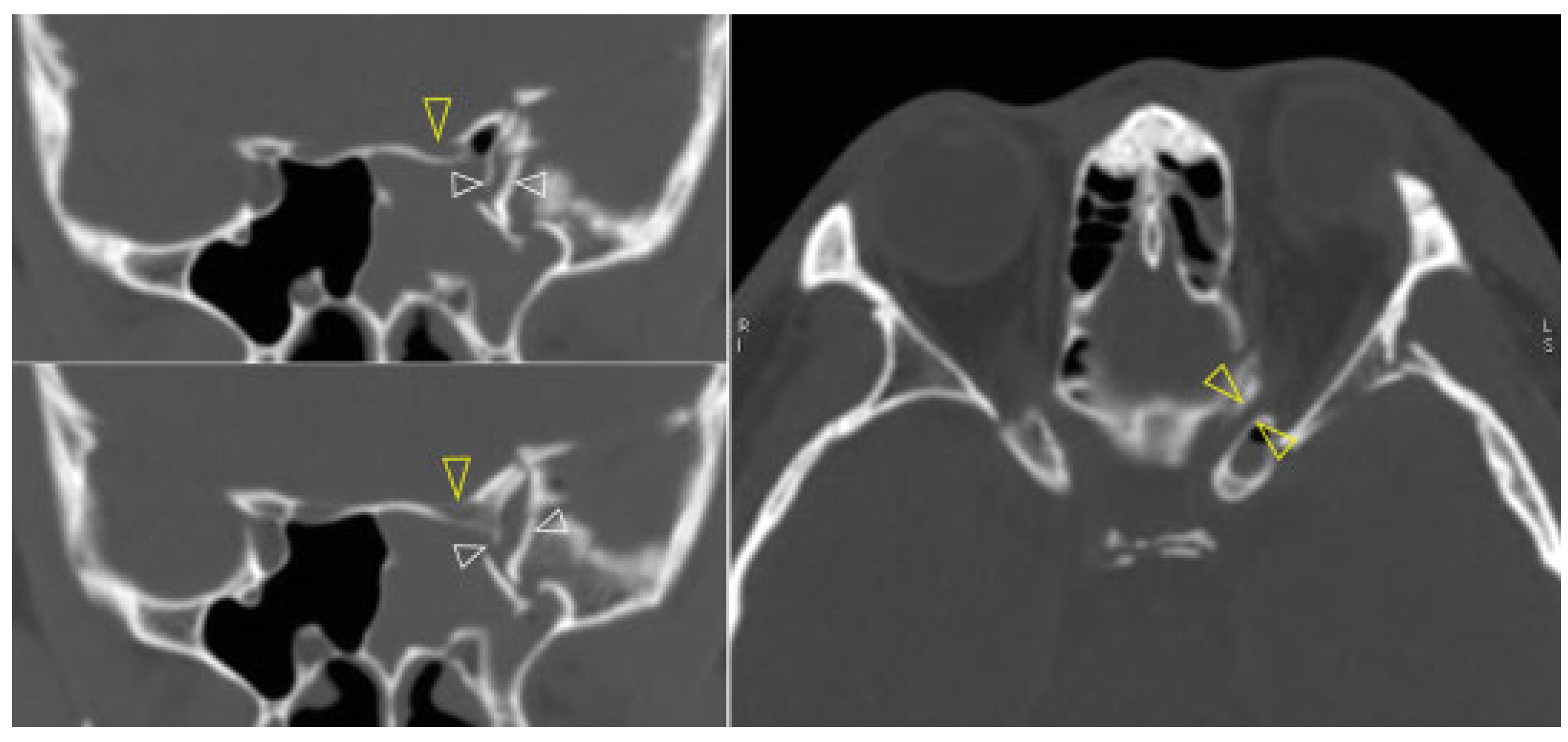
Computed tomography (CT) revealed a left sided orbital and ZMC fracture of the blow-in type, in which the inward displacement of the fractured segments results in decreased orbital volume; there was resultant proptosis of the left eye globe (
Figure 2). The superior orbital fissure was compressed by the medially displacement of the fractured segment of the greater wing of the left sphenoid (
Figure 3, left). This medially displaced fragment pushed the left anterior clinoid process further medially and caused it also to fracture, which subsequently resulted in optic canal fracture and optic nerve compression (
Figure 3, right). A slight left temporal epidural hematoma was also noted. The diagnosis was made of the left blow-in type of orbital and ZMC fracture with associated orbital apex syndrome, due to direct compression of the optic canal and the superior orbital fissure from the displaced fracture fragments.
Urgent surgery was pursued, in conjunction with neurosurgery, for decompression of the optic canal and the superior orbital fissure via a transcranial approach. Mega-dose corticosteroid therapy was administered, beginning with a loading dose of 30 mg/kg of intravenous methylprednisolone followed by continuous intravenous infusion of 5.4 mg/kg/ hour for 24 h. While waiting for availability of an operating room, four hours after his trauma, the direct light reflex of his left pupil disappeared.
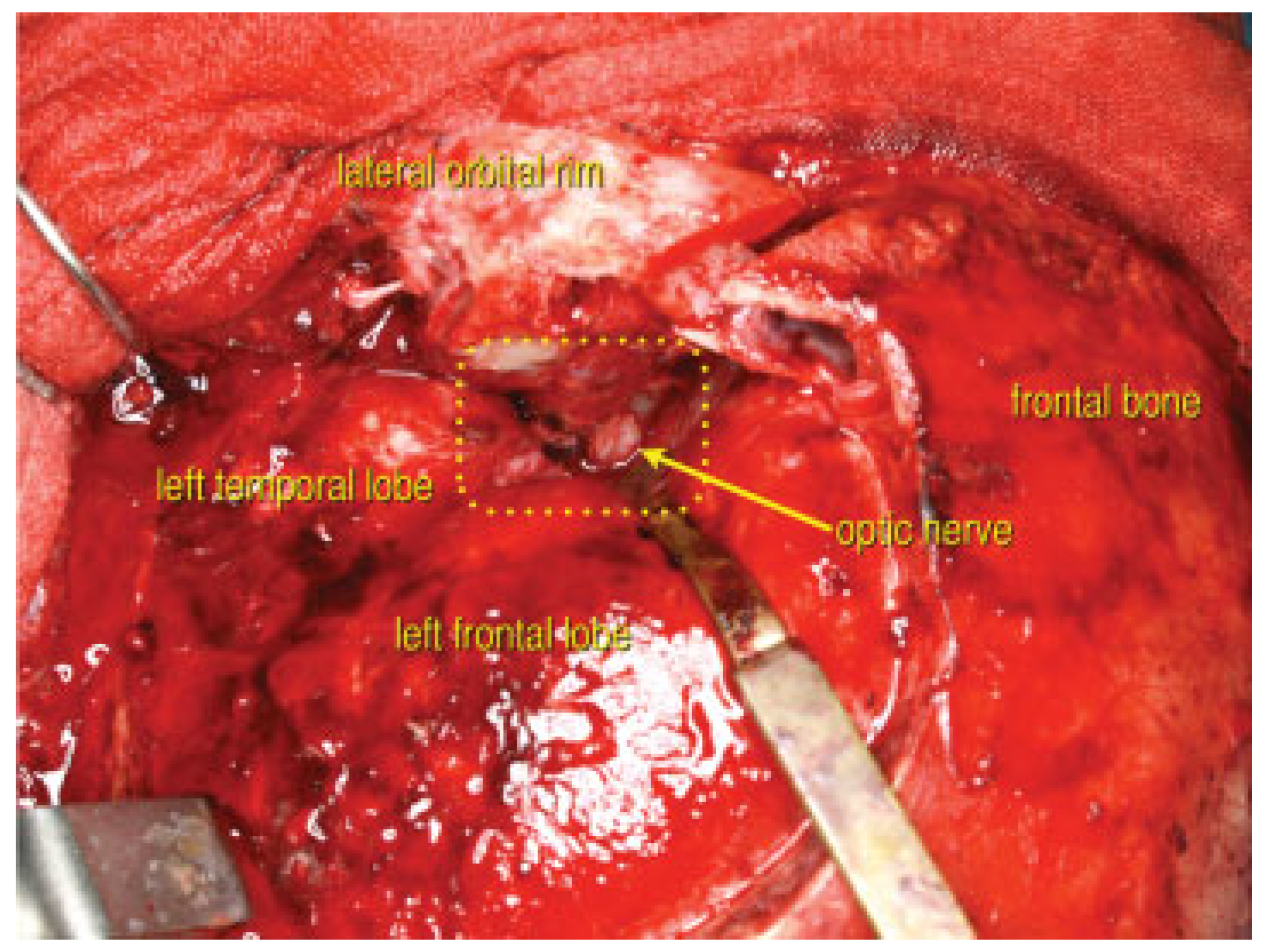
Surgery began six hours after his accident. First, using a coronal incision, the medially displaced left zygoma and inferiorly displaced superior orbital rim fractures were reduced to decrease the elevated orbital pressure and to relieve the compression of the superior orbital fissure. A frontotemporal craniotomy was made. The lesser wing of the sphenoid and the base of the left anterior clinoid process were widely resected sequentially by the epidural approach (
Figure 4). Sufficient decompression of the superior orbital fissure and the optic canal was accomplished successively (
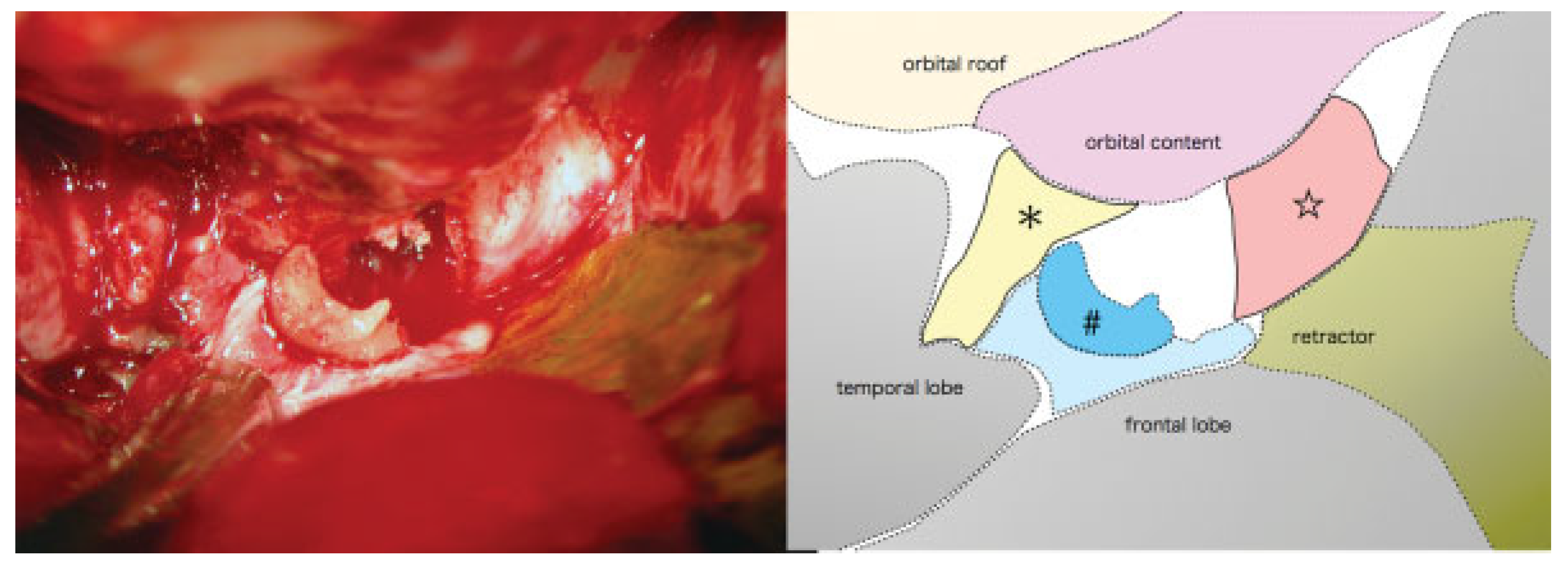
Figure 5 and
Figure 6). Finally all the fractures were reduced and fixed with titanium plates. The left orbital floor was reconstructed with the calvarial bone graft. After surgery, the patient had a dexamethasone taper over six days to reduce cerebral edema, beginning with a dose of 4 mg/day, on the advice of our neurosurgical consultant.
Immediately after surgery, his left eye regained direct pupillary light reflex. A few hours later, his left visual acuity recovered enough to enable him to count fingers accurately, and he recovered a prompt pupillary light reflex. After postsurgical day one, his left eye globe started to demonstrate adduction. By two days after surgery, his left eye globe moved in most directions but with decreased range of motion for supraduction and abduction (
Figure 7, left). Three days after surgery, a left corneal reflex was elicitable. His visual acuity recovered to read figures with a distinction of colors. Bestcorrected visual acuity of the left eye improved from 20/63 on sixth day to 20/32 by the third week after surgery. His left eyelid ptosis and the loss of sensation over his left forehead both completely recovered by the sixth post-operative week. Critical flicker fusion frequency of the affected left eye recovered from 28 Hz (40Hz on the right) on seven days after surgery to 39 Hz (40 Hz on the right) by the eight months after surgery. Although the left optic disc found to be pale and the visual field was slightly restricted, he ultimately regained best-corrected visual acuity of 20/20 with full eye movement and negative RAPD for the left eye, and then returned to his previous college studies without any residual visual impairment (
Figure 7, right).
Discussion
This case illustrates all of the hallmarks of an orbital apex syndrome, which include: visual loss from optic neuropathy, ophthalmoplegia resulting from multiple cranial nerves (oculomotor, trochlear, and abducens), and numbness in the territory of the ophthalmic branch of the trigeminal nerve [
1]. Craniomaxillofacial trauma is the one of major causes of orbital apex syndrome, although the overall incidence is very low. Considering a reported incidence of traumatic superior orbital fissure syndrome of only 0.3–0.8% [
2,
3,
4,
5], and of traumatic optic neuropathy of 0.8–6.0% [
6,
7,
8,
9], then the incidence of traumatic orbital apex syndrome (the coexistence of both conditions) would be expected to be much lower than either alone. Only a few such cases have been reported in the literature [
2,
10].
The rarity of traumatic orbital apex syndrome, as well as traumatic superior orbital fissure syndrome and traumatic optic neuropathy, has made it difficult to define treatment guidelines for this condition [
9,
11]. Gossman et al. proposed classification of traumatic optic neuropathy into direct and indirect categories [
12]. They defined direct traumatic optic neuropathy as optic neuropathy which arises from radiologically evident compression along the course of the optic nerve and optic canal fracture, in which nerve transection was excluded. Indirect type traumatic optic neuropathy was defined as optic neuropathy without fracture or radiographic evidence of another abnormality contiguous to the orbital, intracanalicular, or intracranial optic nerve. The same classification has been applied to the superior orbital fissure syndrome [
4]. For example, when the CT scan confirms that there is significant narrowing of the superior orbital fissure from the displaced fracture fragment, this constitutes direct-type traumatic superior orbital fissure syndrome [
5,
13].
This same distinction may be helpful when applied to the management traumatic orbital apex syndrome, as the treatment strategy is quite different between direct and indirect injuries, in terms of the indications for, and urgency of, surgery.
Surgical intervention is recommended for the direct type traumatic optic neuropathy [
5,
9,
14] and direct traumatic superior orbital fissure syndrome [
4,
13]. Although these recommendations merely constitute expert opinion informed by a limited number of cases, the underlying principle of reducing impinging bone fragments would reasonably be expected to reduce the pathological changes of impinged ischemic nerve tissue [
15] and reduce the morbidity of the retina, which is extremely sensitive to pressure and hypoxia [
16,
17,
18,
19]. We additionally recommend that once impingement is clinically or radiologically evident, surgical intervention should be performed as soon as possible. While the timing of irreversible ischemic damage to the optic nerve is controversial, it has been reported to occur as quickly as 60 min after injury, and is highly probable by 2 h [
20].
Patients with an initial visual acuity of more than just light perception, such as our case, have been reported to have better chances of visual recovery compared with cases presenting without light perception [
21,
22,
23]. Although our patient experienced a rapid decrease in visual acuity, from counting fingers to no light perception, timely surgical intervention may have halted the process of optic nerve ischemia before damage became irreversible.
Various surgical approaches have been described for the orbital apex region, from endoscopic transnasal to transcranial approach [
9]. However, potential approaches are limited in the setting of traumatic orbital apex syndrome with craniomaxillofacial fracture. Most cases with traumatic orbital apex syndrome or traumatic superior orbital fissure syndrome sustain fractures of the ipsilateral sphenoid or the superior orbital rim, especially blow-in fractures [
3,
4], which necessitate a combined extra- and trans-cranial approach to adequately expose the deep aspect of the orbital cavity and accomplish sufficient decompression of the involved nerves. In the early stage of the process of transcranial approach in our case, we could safely reduce the impinged blow-in fractured segments and the sphenoid fragments, relieving some of the elevated intraorbital pressure. Complete neurosurgical decompression of the superior orbital fissure and the optic nerve allowed the impinged nerves and surrounding tissue to expand without rigid bony restriction. This decompression procedure via transcranial approach is a common neurosurgical practice in the treatment of the aneurysms of the internal carotid artery [
24].
Indirect neuropathy must surely be present, even in cases of direct traumatic optic neuropathy or traumatic superior orbital fissure syndrome with craniomaxillofacial trauma. Treatment of indirect traumatic optic neuropathy is still controversial [
25] and its discussion is beyond the scope of this report. We used mega-dose steroids as adjunctive therapy in this case. In a series of cases of traumatic superior orbital fissure syndrome, Chen et al. reported a higher percentage of complete recovery of impinged cranial nerves in patients who were treated with corticosteroids (40%) than those who did not receive steroids (21%) [
4,
5], although they concluded that the role of steroid therapy was unclear. We opted to use a mega-dose steroid protocol to reduce swelling of the impinged nerves, but regard appropriate reduction of impinging bone segments and sufficient decompression as the most effective and important part of treatment.
In cases of traumatic orbital apex syndrome, traumatic optic neuropathy or traumatic superior orbital fissure syndrome, concern might arise about the potential for early surgical reduction of the facial fracture to increase tissue swelling and ocular pressure, thereby worsening optic and cranial nerve palsies [
23,
26]. We propose that the direct type traumatic orbital apex syndrome is a trauma that requires immediate surgery for two reasons. First, the time-window of reversible ischemic damage of the optic nerve is short. Second, the combined blow-in type fracture frequently impairs venous drainage of the orbital contents and the already impinged optic and other cranial nerves, which can worsen tissue swelling further [
3]. Prior reports of case series of traumatic optic neuropathy and traumatic superior orbital fissure syndrome have shown that early reduction of facial fracture does not negatively affect the overall visual outcome [
4,
21]. Additionally, a comminuted ZMC fracture such as this case usually requires a coronal approach. Therefore, we regard simultaneous reduction and fixation of facial fractures with decompression of the superior orbital apex and the optic canal to be a very reasonable approach.