Abstract
Injectable gel is becoming increasingly popular for cosmetic reasons. The polyacrylamide gel (PAAG) is a permanent filler material used worldwide. In spite of the fact that the filler materials used today are considered quite safe, various complications have been reported in the literature. Hence PAAG use in the United States is not popular. As the area is very close to the dental field, a large complication potential is relatively considered following buccal dental injections. The aim of this article is to highlight a rare complication observed following a local anesthetic administration of a simple molar restoration in a healthy 33-year-old woman who had history of a filler augmentation in her cheek approximately 6 years ago.
The use of injectable filling agents for facial cosmetic affairs has had a recent dramatic rise in many countries [1]. Different types of filler agents are used these days to augment soft tissue in various parts of the face and body [2,3,4].
Polyacrylamide gel (PAAG) is one of the most frequently used products since 1997, which contain a hydrogel composed of polyacrylamide and water. This agent is used as a soft-tissue filler material for cosmetic augmentation [5,6]. PAAG is considered a permanent filler with its plasticity being similar to that of silicone. However, as PAAG is a hydrophilic content it is well tolerated by human tissue up to at least 9 years postoperatively [2,7].
In spite of the fact that the filler materials used today is quite safe it also has several unavoidable complications that include acute inflammation, persistent erythema, edema, discoloration, sensitivity, swelling, abscess formation, migration, asymmetry, induration, massive granuloma formation, necrosis, lymphadenopathy, bony erosion, and facial ulcer [6].
Acute inflammation with puncture of the implanted gel after supplement injections such as Botox, filler, and lipolysis even after long period has been reported [13]. Since there is no report on the complications caused following a dental injection of a gel-implanted site, this article is aimed at informing dental professionals for caution in patients with a history of gel implant for face augmentation by illustrating such difficulties in a case reported here.
Case Report
A 33-year-old woman with tenderness and swelling in the upper left malar and submalar area was referred to Oral and Maxillofacial Surgery Department at Shahid Beheshti University of Medical Sciences. She reported that the swelling had started following a dental injection for local anesthetic purpose before a restorative dental procedure in the left upper buccal mucosa. The dentist gave antibiotic and dexamethasone injections for a possible dental abscess when the patient presented at the clinic, but the swelling did not subside when she visited the dentist a day after. Since the symptoms did not subside after 1 week, she was referred to the department. In history taking no significant point was found in her medical history except an injection of PAAG filler received for cheek augmentation approximately 6 years ago.
Extraoral examination revealed the submalar swelling as being firm and tender with mild erythema without any fistula. Intraoral examination showed mild bulging in the left upper vestibular buccal region where the local anesthetic was administered. Oral hygiene was judged as fair and the laboratory tests were found to be within the normal range.
Diagnosis was put on the injection of local anesthetic solution into the gel implant site causing further expansion and discomfort. Treatment plan was given to remove the entire manipulated gel implant. This was performed through an intraoral incision under an intravenous sedation process (Figure 1). A pen-rose drain was placed at the incision site and patient was discharged after 2 days while being on an antibiotic, analgesic, and chlorhexidine mouthwash regimen for 10 days. Results of the follow-up of the case in 10, 20, and 30 days showed the reduced size of the swelling to normal gradually. Inflammation was subsided in a slow pattern, while the full recovery was achieved in 6 months with a normal facial appearance (Figure 2).
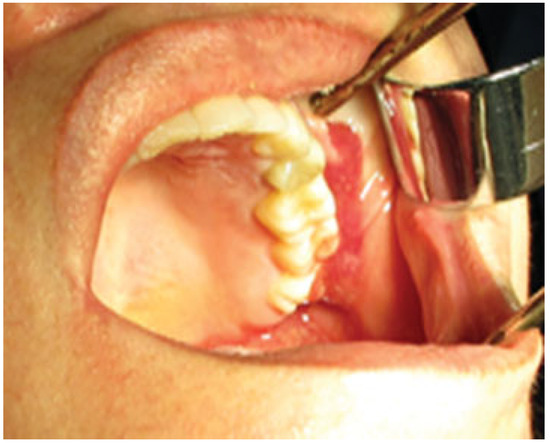
Figure 1.
Intraoperative feature showing easy evacuation of facial gel.
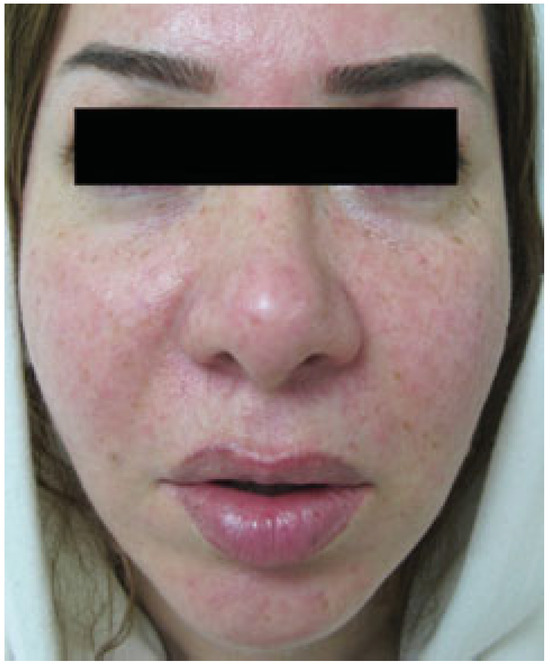
Figure 2.
Facial appearance was acceptable after 6 months follow-up.
Discussion
Acute inflammation has been reported associated with an accidental puncture of the implant after supplement injections such as Botox, filler, and lipolysis even after a long period from the implant insertion [13]. It appears that there is no evidence of any report on the cases originated from the administration of a dental local anesthetic injection during dental treatment. This was searched with different terms and phrases in Medline database from 1980 to 2010. PAAG is one of the cosmetic products being used within the past two decades in Iran on a large scale [8]. PAAG is a common permanent filler with a similar plasticity to silicone with a high biocompatibility [5]. Various complications of PAAG have been documented by earlier reports following its use [1,6,7,8,9,10,11,12,13]. Zielke et al. (2008) primarily reported pain, then formation of nodules, and hyperpigmentation has been found after the injection of PAAG [1]. On the other hand, Kalantar et al. [8] had reported several similar findings in an Iranian population. The complication rate reported was 7.7% which included swelling, abscess formation, lumpiness, changes in facial appearance, changes in gel location following injection, and sensitivity. This result was reported from a total population of 542 patients who received their treatment with PAAG in the facial regions [9].
This is consistent with the case series of 15 patients with adverse reactions to PAAG by Wang et al [11]. It was indicated that gel complications consist of nodule formation (80%), pain (60%), secondary deformity (20%), discomfort (13%), and long-lasting swelling (6.6%) [11].
An inflammatory reaction has been reported after injection of PAAG for the zygomatic facial augmentation [10]. ElShafey reported complications from repeated injection or puncture of old PAAG implant sites [13]. Notably, an acute inflammation of old PAAG has been occurred as a complication after a local anesthetic injection in a routine restorative dental process in the current case. She had a history of a malar and submalar augmentation undergone with PAAG approximately 6 years ago. She had not experienced any similar symptom of such sort following the facial gel insertion until the dental treatment was performed. She had reportedly received a single infiltration injection of lidocaine 2% (Darou Pakhsh, Iran). The injection has been administered in left upper vestibular region to perform a class II cavity preparation and its subsequent restoration on the first permanent molar. The patient had returned to the dentist a day later with swelling and tenderness in the region where she reported to have had a PAAG implant. Although the exact cause of this complication was not clear but it is assumed from the clinical and radiographic evaluations that possibly a needle contamination had occurred or puncture on the implant site caused the breakage of the implant capsule triggering inflammation. Similar story, although with different cause has earlier been reported by El-shafey [13].
After years of gel implantation, the thinned capsule may result in an increasing incidence of such complication [12]. In the present case, 6-year lapse is consistent with earlier reports. Dentists should be warned of the possibility of this side effect during dental injection.
El-Shafey stated that patients should be provided with their medical record of such surgical procedures in form of a filler card with details of the date, type, amount, and site of the filler injected. Patients should be warned of the possibility of inflammation with repeated injection or puncture of the implant site, even after long periods [13]. In this case, the dentist had not been warned about the patient’s facial gel and therefore no specific attention had been paid to the need for its clinical evaluation. No palpation of the vestibular aspect had been performed through which any gel migration could be detected. No compliant of gel displacement was reported by patient before dental treatment too. Based on the tissue reactions of this case, it is suggested that patients with a history of gel implant in face should follow steps to prevent any gel complication following a dental process. Following are also recommended to be observed in cases with a positive report of the gel injection history:
- Use of chlorhexidine mouthwashes or at least a mouth rinse with normal saline before any dental injection.
- Palpation of mucosa in the vestibular areas for detection of any gel migration mass before any attempt for dental injections.
- Avoidance of injection in vicinity of the gel implant site (Figure 3).
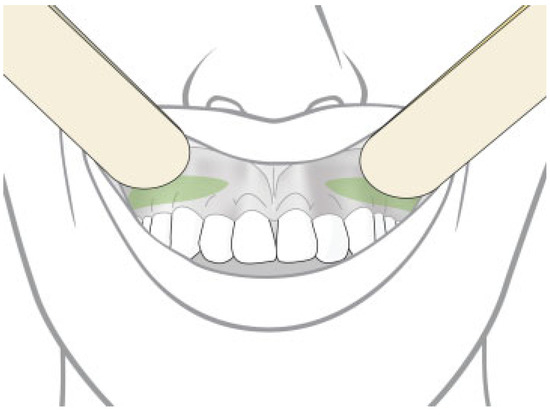 Figure 3. In cases with malar and submalar gel augmentation, the dentist could be more conscious when dental injection was needed in the marked area.
Figure 3. In cases with malar and submalar gel augmentation, the dentist could be more conscious when dental injection was needed in the marked area. - Taking more care about sterilization process.
- Changing the needle between each attempt for anesthesia.
- Informed consent should be obtained before any injections in orofacial region (medico legal consequences).
Conclusion
The use of gel implants in facial region has increased in recent years for cosmetic reasons. The dentists who work very closely to the site of gel injection need to ask their patients about any gel implants in their medical history so that care should be taken while performing a dental surgery.
References
- Zielke, H.; Wölber, L.; Wiest, L.; Rzany, B. Risk profiles of different injectable fillers: Results from the Injectable Filler Safety Study (IFS Study). Dermatol Surg 2008, 34, 326–335. [Google Scholar] [CrossRef]
- Breiting, V.; Aasted, A.; Jørgensen, A.; Opitz, P.; Rosetzsky, A. A study on patients treated with polyacrylamide hydrogel injection for facial corrections. Aesthetic Plast Surg 2004, 28, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Homicz, M.R.; Watson, D. Review of injectable materials for soft tissue augmentation. Facial Plast Surg 2004, 20, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Beer, K. Dermal fillers and combinations of fillers for facial rejuvenation. Dermatol Clin 2009, 27, 427–432. [Google Scholar] [CrossRef]
- von Buelow, S.; Pallua, N. Efficacy and safety of polyacrylamide hydrogel for facial soft-tissue augmentation in a 2-year follow-up: A prospective multicenter study for evaluation of safety and aesthetic results in 101 patients. Plast Reconstr Surg 2006, 118 (Suppl. S3), 85S–91S. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.X.; Xu, S.L.; Deng, H.; et al. Migration of implants: A problem with injectable polyacrylamide gel in aesthetic plastic surgery. Aesthetic Plast Surg 2006, 30, 215–225. [Google Scholar] [CrossRef]
- Christensen, L.H.; Breiting, V.B.; Aasted, A.; Jørgensen, A.; Kebuladze, I. Long-term effects of polyacrylamide hydrogel on human breast tissue. Plast Reconstr Surg 2003, 111, 1883–1890. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Hormozi, A.; Mozafari, N.; Rasti, M. Adverse effects after use of polyacrylamide gel as a facial soft tissue filler. Aesthet Surg J 2008, 28, 139–142. [Google Scholar] [CrossRef]
- Liu, H.L.; Cheung, W.Y. Complications of polyacrylamide hydrogel (PAAG) injection in facial augmentation. J Plast Reconstr Aesthet Surg 2010, 63, e9–e12. [Google Scholar] [CrossRef]
- Amin, S.P.; Marmur, E.S.; Goldberg, D.J. Complications from injectable polyacrylamide gel, a new nonbiodegradable soft tissue filler. Dermatol Surg 2004, 30 Pt 2, 1507–1509. [Google Scholar]
- Wang, Y.B.; Huang, J.J.; Qiao, Q.; et al. Clinically analyzing the possible side-effects after injecting hydrophilic polyacrylamide gel as a soft-tissue filler. Chinese Journal of Plastic Surgery 2003, 19, 328–330. [Google Scholar]
- Christensen, L.; Breiting, V.; Janssen, M.; Vuust, J.; Hogdall, E. Adverse reactions to injectable soft tissue permanent fillers. Aesthetic Plast Surg 2005, 29, 34–48. [Google Scholar] [CrossRef] [PubMed]
- El-Shafey, S.I. Complications from repeated injection or puncture of old polyacrylamide gel implant sites: Case reports. Aesthetic Plast Surg 2008, 32, 162–165. [Google Scholar] [CrossRef] [PubMed]
© 2013 by the author. The Author(s) 2013.