A Review of Literature on Ameloblastoma in Children and Adolescents and a Rare Case Report of Ameloblastoma in a 3-Year-Old Child
Abstract
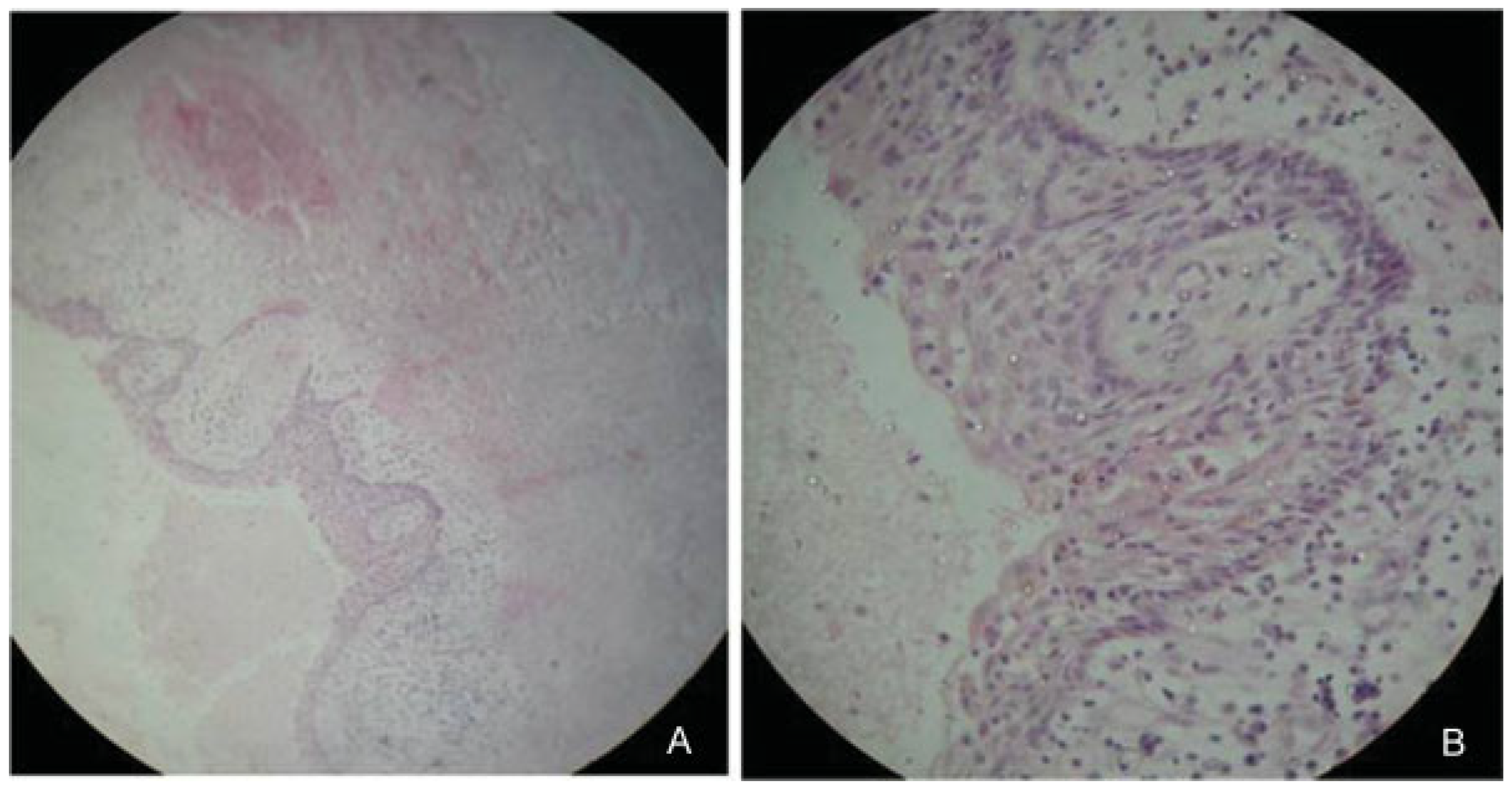
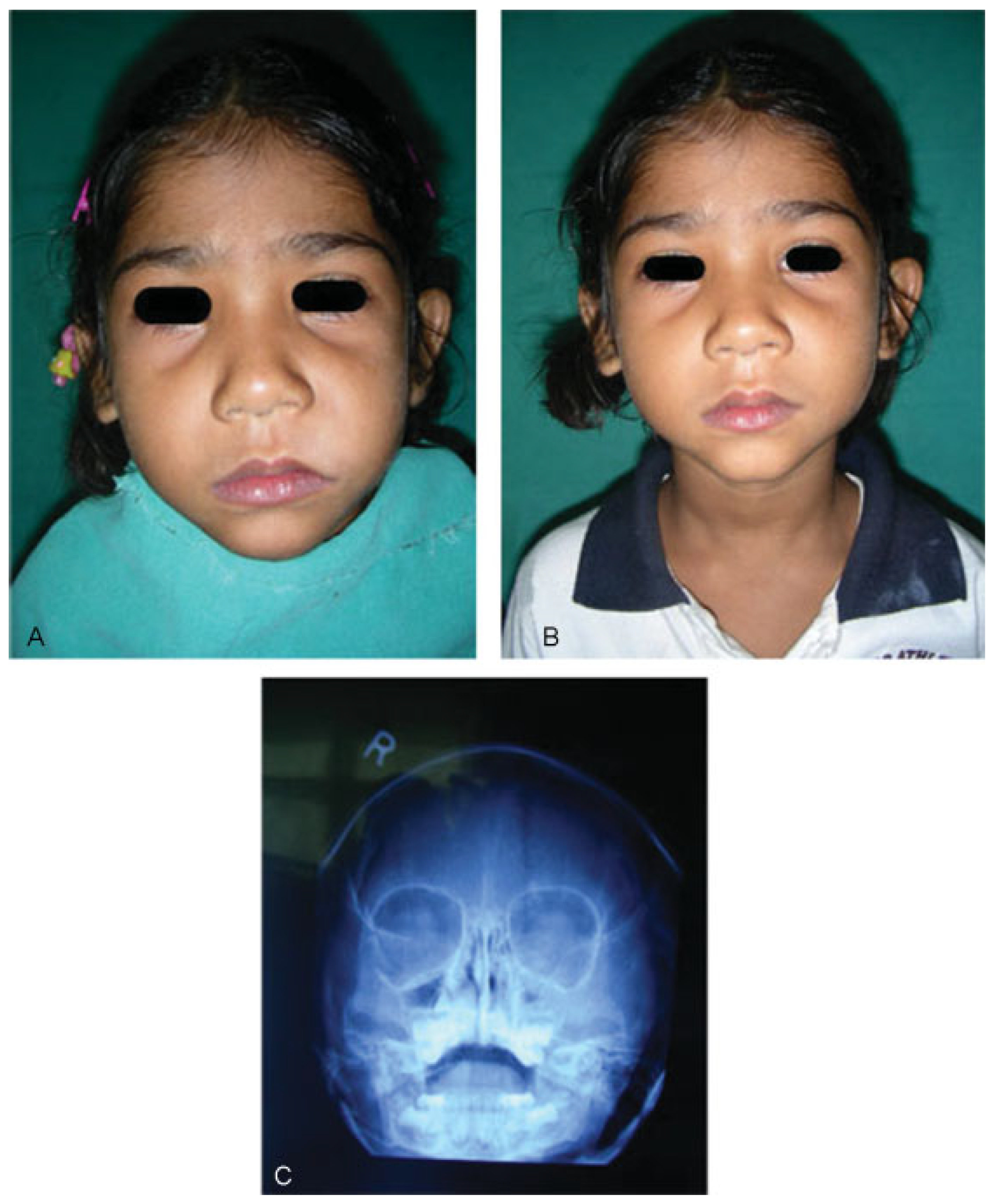
:Case Report
Materials and Methods
Results
Discussion
Age and Population
Age and Histopathological Correlation
Age and Treatment
Conclusion
References
- Blackwood, H.J.J. Odontogenic tumours in the child. Br Dent J 1965, 119, 431–438. [Google Scholar] [PubMed]
- Mehlisch, D.R.; Dahlin, D.C.; Masson, J.K. Ameloblastoma: A clinicopathologic report. J Oral Surg 1972, 30, 9–22. [Google Scholar] [PubMed]
- Small, I.A.; Waldron, C.A. Ameloblastoma of jaws. J Oral Surg 1955, 8, 281–297. [Google Scholar] [CrossRef]
- Al-Khateeb, T.; Ababneh, K.T. Ameloblastoma in young Jordanians: A review of the clinicopathologic features and treatment of 10 cases. J Oral Maxillofac Surg 2003, 61, 13–18. [Google Scholar] [CrossRef]
- Keszler, A.; Dominguez, F.V. Ameloblastoma in childhood. J Oral Maxillofac Surg 1986, 44, 609–613. [Google Scholar] [CrossRef]
- Ord, R.A.; Blanchaert, R.H., Jr.; Nikitakis, N.G.; Sauk, J.J. Ameloblastoma in children. J Oral Maxillofac Surg 2002, 60, 762–770; discussion 770–771. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Miyauchi, K.; Sato, K. Treatment of ameloblastoma in children. Br J Oral Maxillofac Surg 1998, 36, 453–456. [Google Scholar] [CrossRef]
- Zhang, J.; Gu, Z.; Jiang, L.; et al. Ameloblastoma in children and adolescents. Br J Oral Maxillofac Surg 2010, 48, 549–554. [Google Scholar] [CrossRef]
- Fulco, G.M.; Nonaka, C.F.W.; Souza, L.B.; Miguel, M.C.; Pinto, L.P. Solid ameloblastomas—Retrospective clinical and histopathologic study of 54 cases. Braz J Otorhinolaryngol 2010, 76, 172–177. [Google Scholar] [CrossRef]
- Kahn, M.A. Ameloblastoma in young persons: A clinicopathologic analysis and etiologic investigation. Oral Surg Oral Med Oral Pathol 1989, 67, 706–715. [Google Scholar] [CrossRef]
- Huang, I.Y.; Lai, S.T.; Chen, C.H.; Chen, C.M.; Wu, C.W.; Shen, Y.H. Surgical management of ameloblastoma in children. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2007, 104, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Adebiyi, K.E.; Ugboko, V.I.; Omoniyi-Esan, G.O.; Ndukwe, K.C.; Oginni, F.O. Clinicopathological analysis of histological variants of ameloblastoma in a suburban Nigerian population. Head Face Med 2006, 2, 42. [Google Scholar] [CrossRef]
- Darshani Gunawardhana, K.S.; Jayasooriya, P.R.; Rambukewela, I.K.; Tilakaratne, W.M. A clinico-pathological comparison between mandibular and maxillary ameloblastomas in Sri Lanka. J Oral Pathol Med 2010, 39, 236–241. [Google Scholar] [CrossRef]
- Hong, J.; Yun, P.-Y.; Chung, I.H.; et al. Long-term follow up on recurrence of 305 ameloblastoma cases. Int J Oral Maxillofac Surg 2007, 36, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Feinberg, S.E.; Steinberg, B. Surgical management of ameloblastoma. Current status of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1996, 81, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Pogrel, M.A.; Montes, D.M. Is there a role for enucleation in the management of ameloblastoma? Int J Oral Maxillofac Surg 2009, 38, 807–812. [Google Scholar] [CrossRef]
- Ghandhi, D.; Ayoub, A.F.; Pogrel, M.A.; MacDonald, G.; Brocklebank, L.M.; Moos, K.F. Ameloblastoma: A surgeon’s dilemma. J Oral Maxillofac Surg 2006, 64, 1010–1014. [Google Scholar] [CrossRef]
- Daramola, J.O.; Ajagbe, A.; Oluwasanmi, J.O. Ameloblastoma of the jaws in Nigerian Children. A review of sixteen cases. Oral Surg 1975, 40, 458–463. [Google Scholar] [CrossRef]
- Chidzonga, M.M. Ameloblastoma in children. The Zimbabwean experience. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1996, 81, 168–170. [Google Scholar] [CrossRef]
- Adeline, V.L.; Dimba, E.A.; Wakoli, K.A. Clinicopathologic features of ameloblastoma in Kenya: A 10-year audit. J Craniofac Surg 2008, 19, 1589–1593. [Google Scholar] [CrossRef]
- Arotiba, G.T.; Ladeinde, A.L.; Arotiba, J.T.; Ajike, S.O.; Ugboko, V.I.; Ajayi, O.F. Ameloblastoma in Nigerian children and adolescents: A review of 79 cases. J Oral Maxillofac Surg 2005, 63, 747–751. [Google Scholar] [CrossRef] [PubMed]
- Fregnani, E.R.; da Cruz Perez, D.E.; de Almeida, O.P.; Kowalski, L.P.; Soares, F.A.; de Abreu Alves, F. Clinicopathological study and treatment outcomes of 121 cases of ameloblastomas. Int J Oral Maxillofac Surg 2010, 39, 145–149. [Google Scholar] [CrossRef]
- Olaitan, A.A.; Adeola, D.S.; Adekeye, E.O. Ameloblastoma: Clinical features and management of 315 cases from Kaduna, Nigeria. J Craniomaxillofac Surg 1993, 21, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Chapelle, K.A.; Stoelinga, P.J.; de Wilde, P.C.; Brouns, J.J.; Voorsmit, R.A. Rational approach to diagnosis and treatment of ameloblastomas and odontogenic keratocysts. Br J Oral Maxillofac Surg 2004, 42, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Meer, S.; Galpin, J.S.; Altini, M.; Coleman, H.; Ali, H. Proliferating cell nuclear antigen and Ki67 immunoreactivity in ameloblastomas. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2003, 95, 213–221. [Google Scholar] [CrossRef]
- Robinson, L.; Martinez, M.G. Unicystic ameloblastoma: A prognostically distinct entity. Cancer 1977, 40, 2278–2285. [Google Scholar] [CrossRef]
- Gardner, D.G. Some current concepts on the pathology of ameloblastomas. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1996, 82, 660–669. [Google Scholar] [CrossRef]
- Lau, S.L.; Samman, N. Recurrence related to treatment modalities of unicystic ameloblastoma: A systematic review. Int J Oral Maxillofac Surg 2006, 35, 681–690. [Google Scholar] [CrossRef]

| Literature Data | Daramola et al. [18] | Keszler et al. [5] | Khan et al. [10] | Chidzonga et al. [19] | Takahasi et al. [7] | Ord et al. [6] | |||||||
| Published year | 1975 | 1986 | 1989 | 1996 | 1998 | 2002 | |||||||
| Case Numbers | 16 | 8 | 38 | 20 | 6 | 11 | |||||||
| Incidence | 16/70 | 8/92 | 38/311 | 20/117 | 6/27 | 11/38 | |||||||
| Age | |||||||||||||
| Range | 5–17 | 4–15 | 7–19 | 11–18 | 8–15 | 12–20 | |||||||
| <10 | 3 | 3 | 1 | 0 | 1 | 0 | |||||||
| 10–20 | 13 | 5 | 37 | 20 | 5 | 11 | |||||||
| Mean | 13.4 | 10.8 | 14.8 | 15.5 | 12.3 | 15.5 | |||||||
| Sex | |||||||||||||
| Male | 10 | 4 | 18 | 10 | 3 | 4 | |||||||
| Female | 6 | 4 | 20 | 10 | 3 | 7 | |||||||
| M/F ratio | 1.7:1 | 1:1 | 1:1.1 | 1:1 | 1:1 | 0.56:1 | |||||||
| Site | |||||||||||||
| Maxilla | 1 | 0 | 0 | 1 | 0 | 1 | |||||||
| Mandible | 15 | 8 | 38 | 19 | 6 | 10 | |||||||
| Center/Country | Ibadeb, Nigeria | B A*, Argentina | TN*, USA | Harare, Zimbabwe | Chiba, Japan | BL*, USA | |||||||
| Histology & Age | |||||||||||||
| Follicular | — | — | — | — | — | — | |||||||
| Plexiform | — | — | — | — | 66% | — | |||||||
| Desmoplastic | — | — | — | — | — | — | |||||||
| Achanthomatous | — | — | — | — | — | — | |||||||
| Unicystic | — | — | — | — | — | 81% | |||||||
| Literature Data | Al Khateeb et al. [4] | Arotiba et al. [21] | Adebiyi et al. [12] | Huang et al. [11] | Adeline et al. [20] | ||||||||
| Published year | 2003 | 2005 | 2006 | 2007 | 2008 | ||||||||
| Case Numbers | 10 | 79 | 14 | 15 | 40 | ||||||||
| Incidence | 10/26 | 79/360 | 14/77 | 15/223 | 40/184 | ||||||||
| Age | |||||||||||||
| Range | 9–20 | 6–19 | 11–20 | 9–17 | 10–19 | ||||||||
| <10 | 1 | 9 | 0 | 1 | 0 | ||||||||
| 10–20 | 9 | 70 | 14 | 14 | 40 | ||||||||
| Mean | 16 | 14.7 | NS | 13.7 | NS | ||||||||
| Sex | |||||||||||||
| Male | 4 | 45 | NS | 9 | NS | ||||||||
| Female | 6 | 34 | NS | 6 | NS | ||||||||
| M/F ratio | 1:1.5 | 1.3:1 | NS | 1.5:1 | NS | ||||||||
| Site | |||||||||||||
| Maxilla | 0 | 4 | NS | 1 | NS | ||||||||
| Mandible | 10 | 75 | NS | 14 | NS | ||||||||
| Center/Country | Irbid, Jordan | Lagos, Nigeria | Ife Ife, Nigeria | Kaohsiung, Taiwan | Nairobi, Kenya | ||||||||
| Histology & Age | |||||||||||||
| Follicular | — | — | — | — | — | ||||||||
| Plexiform | — | — | — | — | — | ||||||||
| Desmoplastic | — | — | — | — | — | ||||||||
| Achanthomatous | — | — | — | — | — | ||||||||
| Unicystic | 60% | — | — | — | — | ||||||||
| Literature Data | Gunawardana et al. [13] | Fregnani et al. [22] | Zhang et al. [8] | Total | |||||||||
| Published year | 2010 | 2010 | 2010 | ||||||||||
| Case Numbers | 62 | 16 | 37 | 372 | |||||||||
| Incidence | 62/286 | 16/121 | 37/267 | 372/2199 | |||||||||
| Age | |||||||||||||
| Range | 5–19 | 2–18 | 5–18 | ||||||||||
| <10 | 7 | NS | 3 | 29/356 | |||||||||
| 10–20 | 55 | NS | 34 | 327/356 | |||||||||
| Mean | NS | NS | 14.4 | ||||||||||
| Sex | |||||||||||||
| Male | NS | NS | 23 | 130/240 | |||||||||
| Female | NS | NS | 14 | 110/240 | |||||||||
| M/F ratio | NS | NS | 1.6:1 | 1.18:1 | |||||||||
| Site | |||||||||||||
| Maxilla | NS | NS | 0 | 8/240 | |||||||||
| Mandible | NS | NS | 37 | 232/240 | |||||||||
| Center/Country | Peradeniya, SriLanka | Sao Paulo, Brazil | Xi’an, China | ||||||||||
| Histology & Age | |||||||||||||
| Follicular | — | — | 48.7% | ||||||||||
| Plexiform | — | — | — | ||||||||||
| Desmoplastic | — | — | — | ||||||||||
| Achanthomatous | — | — | — | ||||||||||
| Unicystic | — | — | 24.3% | ||||||||||
| Histological Pattern | Age Range (Years) | Country |
|---|---|---|
| Follicular | 21–30 | Ife Ife, Nigeria [12] |
| 21–30 | Peradeniya, Sri Lanka [13] | |
| 12–92 | Rio Grande, Brazil [9] | |
| Plexiform | 21–30 | Ife Ife, Nigeria [12] |
| 21–30 | Peradeniya, Sri Lanka [13] | |
| 12–92 | Rio Grande, Brazil [9] | |
| Desmoplastic | 31–40 | Ife Ife, Nigeria [12] |
| 21–50 | Peradeniya, Sri Lanka [13] | |
| 20–51 | Rio Grande, Brazil [9] | |
| Acanthomatous | 61–70 | Ife Ife, Nigeria [12] |
| 31–50 | Peradeniya, Sri Lanka [13] | |
| Unicystic | 31–40 | Ife Ife, Nigeria [12] |
| 11–20 | Peradeniya, Sri Lanka [13] |
© 2012 by the author. The Author(s) 2012.
Share and Cite
Chaudhary, Z.; Krishnan, S.; Sharma, P.; Sharma, R.; Kumar, P. A Review of Literature on Ameloblastoma in Children and Adolescents and a Rare Case Report of Ameloblastoma in a 3-Year-Old Child. Craniomaxillofac. Trauma Reconstr. 2012, 5, 161-168. https://doi.org/10.1055/s-0032-1313358
Chaudhary Z, Krishnan S, Sharma P, Sharma R, Kumar P. A Review of Literature on Ameloblastoma in Children and Adolescents and a Rare Case Report of Ameloblastoma in a 3-Year-Old Child. Craniomaxillofacial Trauma & Reconstruction. 2012; 5(3):161-168. https://doi.org/10.1055/s-0032-1313358
Chicago/Turabian StyleChaudhary, Zainab, Sriram Krishnan, Pankaj Sharma, Rakesh Sharma, and Priya Kumar. 2012. "A Review of Literature on Ameloblastoma in Children and Adolescents and a Rare Case Report of Ameloblastoma in a 3-Year-Old Child" Craniomaxillofacial Trauma & Reconstruction 5, no. 3: 161-168. https://doi.org/10.1055/s-0032-1313358
APA StyleChaudhary, Z., Krishnan, S., Sharma, P., Sharma, R., & Kumar, P. (2012). A Review of Literature on Ameloblastoma in Children and Adolescents and a Rare Case Report of Ameloblastoma in a 3-Year-Old Child. Craniomaxillofacial Trauma & Reconstruction, 5(3), 161-168. https://doi.org/10.1055/s-0032-1313358



