Abstract
Reconstruction of cranial and maxillofacial defects is a challenging task. The standard reconstruction method has been bone grafting. In this review, we shall describe the biological principles of bone graft healing, as pertinent to craniofacial reconstruction. Different types and sources of bone grafts will be discussed, as well as new methods of bone defect reconstruction.
Bone defects in the craniomaxillofacial skeleton vary from the small (few millimeters) periodontal de- fects to the large segmental defects resulting from trauma, surgical excision, or cranioplasty. Such defects typically have complex three-dimensional structural needs, which are difficult to restore. In cranial vault defects, the underlying brain needs permanent protec- tion. Segmental jaw defects require restoration of me- chanical integrity, temporomandibular joint function, and intermaxillary dental occlusion. Maintaining ac- ceptable facial esthetics is another unique consideration in the treatment of facial defects, which cannot be underestimated. Bone grafts remain the gold standard for reconstructing segmental bone defects. We will overview the status of bone grafting techniques for craniofacial reconstruction, their biological foundation, as well as future directions.
The earliest report of a bone grafting procedure came in an 1682 book by Job Janszoo van Meekeren, a surgeon in Amsterdam [1]. In this account, the author reported a case in Russia, where the surgeon restored a cranial defect using a cranial bone graft from a dead dog [2]. In 1881, Sir William MacEwen of Rothesay, Scotland, published the first case report of successful interhuman transfer of bone grafts [3,4]. He used tibial bone wedges excised from three donors, during surgical correction of skeletal deformity, to reconstruct a humeral defect in a 3-year-old child. Subsequent clinical reports helped establish the efficacy of autogenous bone grafts in defect reconstruction [5,6,7].
Mechanism of Action of Bone Grafts
A bone graft is defined as any implanted material that promotes bone healing, whether alone or in combination with other material. Augmentation of bone healing at the recipient site occurs through one or more of the following mechanisms: osteoconduction, osteoinduc- tion, and osteogenesis. An osteoconductive material simply allows, or directs, new bone formation along its surfaces. Examples include bone graft matrix and syn- thetic osteoconductive polymers. An osteoinductive graft supplies recruitment and/or differentiation factors for bone-forming cells at the recipient site.
An osteogenic graft supplies induced, or inducible, bone-forming cells to the recipient site. Accordingly, an ideal bone graft is the one that functions through all three mechanisms by providing a template that directs three- dimensional bone growth (osteoconduction), recruits and induces differentiation of resident bone-forming cells, and supplies more bone-forming cells to the recipient site. Such grafts include cancellous and vascularized bone grafts [8].
Bone grafts can be employed for functions other than to stimulate bone formation within a defect. An onlay graft laid over facial bone surfaces could augment the cheek prominence or restore facial contour. In this case, more emphasis is directed toward the rate of graft resorption. Those grafts that are known for their slow resorption, such as calvarial and cortical bone, or nonresorption, such as synthetic materials, are pre- ferred. Such grafts might also be used for their me- chanical properties wherever mechanical support or immediate protection of vital structures is required, as in reconstructing orbital floor or calvarial defects.
Slow resorption is a disadvantage if the graft is used to augment bone formation at the recipient site. Graft incorporation is inversely proportional to how solid the graft is and how slow it resorbs [8]. Therefore, osteoconductive graft materials with interconnected in- ternal spaces that reach the outer surface are better scaffolds for directing three-dimensional bone invasion of the graft. This architecture provides more surface area along which native osteoclasts can attach themselves and start dissolving the graft, which is the first stage in graft incorporation.
Types of Bone Grafts
Bone grafts can be divided into the following subtypes: autografts, allografts, xenografts, synthetic materials, and any combination thereof. Autografting is the transfer of graft material obtained from one anatomic site to another within the same subject. It includes transferring cancellous, cortical, corticocancellous, or vascularized bone or aspirated bone marrow. Auto- grafts have the advantage of retaining at least some osteogenic cells and do not trigger an immune re- sponse. However, the total amount of bone that can be transferred is limited, and there can be high morbidity at the donor site [9].
Grafts that are transferred between two geneti- cally matched subjects, identical twins in humans, are called isografts. They would be expected to have the same advantages and disadvantages as autografts.
Grafts that are transferred between two geneti- cally unmatched subjects are called allografts. Bone allografts are unique in that the cellular component is typically removed to minimize their rejection. In addi- tion, they are thoroughly treated to eliminate any pos- sibility of disease transmission. Therefore, allografts can be subdivided according to their source, processing method, or available form [8].
With advancement in biomaterials technology, the use of animal-derived tissues for human tissue reconstruction is on the rise. These types of grafts are called xenografts. Several bone xenografts have been developed and are commercially available [10]. They are typically in the form of bovine or porcine collagen and can be used either alone or in combination with a synthetic carrier.
Synthetic bone substitutes and bone-augmenting preparations have been the focus of extensive research and have recently spawned a huge industry. Synthetic skeletal materials include osteoconductive polymers in the form of blocks, granules, or cements and osteoin- ductive proteins [8]. Synthetic osteoinductive proteins that have been extensively studied in bone reconstruction include differentiation factors, such as bone morpho- genic protein (BMP)-2 and -7 [11,12,13,14,15], and angiogenic factors, such as vascular endothelial growth factor (VEGF) [16,17,18].
Incorporation of Bone Grafts into the Recipient Site
It is true a bone graft may be used for its bulk or mechanical properties or to stimulate bone formation at the recipient site without necessarily being integrated into the newly formed bone. However, when bone grafts are used to bridge a critical-size bone defect, they are expected to become incorporated into the bed. Incorpo- ration of the bone graft in the recipient site involves two essential steps: first is the bony union between the edges of the graft to the edges of native bone segments, and second is graft remodeling, or gradual resorption of the graft material itself, concomitant with its replacement by new bone [19,20,21]. Graft remodeling can be of secondary importance in case of vascularized grafts, where the bone should be viable from the time of implantation. In this case, the remodeling process is expected be similar to that of normal bone.
Ideally, the whole bone graft should be incorpo- rated into the recipient site. In other words, the space that the graft originally occupies should ultimately become viable bone permanently accessible to the phys- iological remodeling mechanisms. That process is typi- cally very slow, and perfect outcome cannot always be achieved. Many factors determine how far the incorpo- ration process will proceed. These factors may be perti- nent to the graft itself, graft bed (recipient site), or the interface in between.
Factors related to the graft include the graft type, porosity, and mechanism of action. Autogenous cancel- lous and corticocancellous grafts are better incorporated due to their porous architecture, allowing easy cellular and vascular invasion. The graft trabeculae have a large surface area that is covered by osteoblasts, making it osteogenic as well as osteoconductive for three-dimen- sional bone growth. Additionally, due to the extensive vascular invasion, the bone matrix can readily be demin- eralized and its proteins exposed through the actions of osteoclasts. This leads to the release of osteoinductive matrix proteins.
By contrast, autogenous cortical bone grafts are more solid. The only available access for cellular and vascular invasion of such grafts is the junction with the adjacent bone segments, making the integration process slow and rarely complete [19]. This deficiency can be eliminated by using vascularized bone, which provides excellent long-term viability at the recipient site, even in large defects [8,19]. In fact, the only factor to worry about regarding integration of a viable vascularized graft is its mechanical stability [8].
As in fracture repair, rigid fixation of the graft to its bed is essential. Bone formation requires very low tissue strain levels. In addition, the ratio between the graft size and the contact area with circulation is a major determinant of how fast the graft can be incorporated, if at all. Large bone grafts with only minimal contact to bleeding, viable bone edges at the recipient site are expected to take a long time to become incorporated. One way to expose more of the graft core to the circulation is to mince the graft in a bone mill and pack it into the raw bed, given that it can be shielded from undue tissue strains.
Another important factor in determining graft incorporation is vascularity and viability of the graft bed. The bone graft typically needs to be attached to viable, bleeding bone edges. Too much reaming or excessive heat generation during saw cutting can cause necrosis of the bone edges and delay union to the graft [9]. Radiotherapy can jeopardize tissue vascularity, elimi- nating the option of reconstruction using a nonvascu- larized bone graft. In such cases, a vascularized bone graft should be used, given that reasonably viable bone edges can be found to connect to the graft, dependable vessels can be used for microvascular anas- tomosis, and absence of infection. Some reports suggest the use of hyperbaric oxygen therapy to promote tissue perfusion before reconstruction [22,23,24]. Finally, graft in- corporation depends also on the overall physiological healing capacity of the body.
The biological process leading to graft incorpo- ration is very similar to that of fracture repair. In brief, the cascade starts with the surgical hematoma, which involves the recruitment of platelets and white blood cells and the subsequent release of essential growth factors and cytokines. The recruited monocytes differ- entiate into osteoclasts and start removing the necrotic bone edges, with the demineralization of the matrix and release of bone augmenting factors. This leads to differ- entiation of osteoblasts and triggering the union between the graft and native bone edges. In the meantime, new blood vessels form within the granulation tissue and begin tunneling their way into the graft.
Since the early studies in bone transplantation immunity, it has been widely believed that at least some autograft-carried osteoblasts survive the transplantation process [25,26,27,28,29]. Cell survival is also believed to occur more often in vascularized autografts than in nonvascularized autografts and in cancellous more than in cortical auto- grafts [8,30]. These cells can play an essential role early during the incorporation process [8,31]. Graft incorporation has been summarized by Bauer and Muschler into five major steps [8]:
- Hematoma formation, release of bone inducing factors and cellular recruitment
- Inflammation and development of fibrovascular tissue, connecting the graft to the adjacent bone
- Vascular invasion of the graft
- Focal resorption of the graft by recruited osteoclasts
- New bone formation, union between the graft and the surrounding bone, and graft remodeling
Sources of Autogenous Bone Grafts for Craniofacial Reconstruction
Free Nonvascularized Bone Grafts
Iliac Crest
The iliac crest is one of the most common donor site for bone grafts, both vascularized and nonvascularized. Large segments of cortical, corticocancellous, or cancel- lous bone can be quickly obtained for different-sized defects. Furthermore, the location of the ilium allows harvesting by a separate surgical team to save operation time. A full-thickness iliac crest graft would have two thick cortices with ample amount of trabecular bone in between and can very closely resemble the thickness and height of mandibular bone. The graft shows reasonable long-term survival, and rehabilitation with osseointe- grated dental implants is possible [32,33,34]. Mandibular de- fects could be filled using nonvascularized iliac bone with a 70% success rate [35]. The graft could be implanted as corticocancellous blocks or particulate cancellous bone carried within either a titanium mesh tray or a crib of alloplastic rib bone. However, the rate of successful union drops sharply when the defect is longer than 6 cm.35,36
Posterior iliac crest graft can also be used for craniofacial reconstruction. However, the patient has to be tilted to the prone position, which eliminates the advantage of a simultaneous two-team approach. Donor site morbidity rate for anterior iliac crest grafts is around 23%, and much less for posterior iliac crest [37]. Compli- cations include postoperative pain, iliac or acetabular fractures or instability, persistent hematoma, herniation of abdominal contents, vascular injury, lateral femoral cutaneous nerve injury, and unsightly contour defects along the iliac crest [38,39,40,41].
Calvarial Graft
This is one of the most popular cortical bone grafts in craniofacial reconstruction, mainly for its mechanical properties and very slow resorption rate [8]. This makes it ideal for facial augmentation, orbital roof and floor reconstruction, and covering cranial defects. Typically, only the outer cortex is used, although a full-thickness graft could be taken and split into two grafts (Figure 1). Typically, the skull continues to grow until the age of 8, continues to thicken until the age of 20, and is thickest at the parietal region. This area can provide ~8 × 10 cm of bone and is considered the safest to harvest [42].
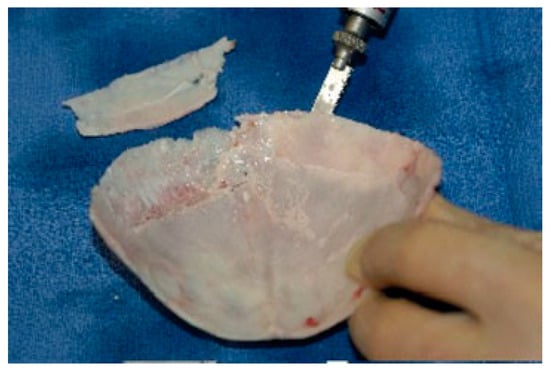
Figure 1.
Technique of splitting cranial bone using a reci- procating saw.
However, there are several key anatomic facts to consider before harvesting a calvarial bone graft:
- Thickness of the calvarium is highly variable to the point of being unpredictable, even within the parietal region [43]. Preoperative radiographic measurement of the bone thickness should give an idea of the area of bone that can safely be harvested.
- The dura is tightly adherent to the inner cortex and can easily be injured if the inner cortex is to be harvested with the graft.
- Various important vascular structures exist immedi- ately beneath the bone at various sites, including the superior sagittal sinus in the midline.
- The two cortices fuse together and the bone can become quite thin laterally and inferiorly to the temporal line, the attachment of the temporalis muscle, and at suture sites.
- Other anatomic variables, including transcortical emissary veins, subcortical vessels, and aberrant ara- chnoid plexuses (within the cortical calvarium), should also be considered [42].
The temporoparietal region provides more curved bone, which would be more suitable for orbital or malar reconstruction [44]. However, straight grafts can be harvested more posteriorly (i.e., from the occipitoparietal region). In any case, the bone is typically harvested as narrow strips (5 to 6 cm long × 1.5 to 2 cm wide) to avoid graft fracture during harvest. Then, several strips can be fixed together and used as one graft.
Calvarial bone can be harvested at three levels: partial-thickness outer cortex, full-thickness outer cor- tex, and bicortical [42]. Partial-thickness outer cortex can be harvested using a very sharp osteotome to curl off a sheet of cortical bone from the outer cortical plate. This technique can be used in children between the age of 4 and 8 years and can yield enough bone to fill a small defect.
In adults, full-thickness outer cortex can safely be harvested and is therefore the most commonly used calvarial graft. If a craniotomy has already been per- formed, the inner cortex can be harvested from the bone flap and used in the reconstruction, leaving the outer cortex to be placed back in its original position. This technique maintains the contour of the calvarium. If large quantities of bone are needed, bicortical grafts may be harvested, followed by splitting of the two cortices to double the surface of the graft. It is obvious that harvest- ing a bicortical calvarial graft would have the most complications hazard.
Complications of calvarial grafts include surface deformity at the donor and/or recipient site and graft fracture during harvest. Less commonly, dural exposure or tear can occur. If the dura is injured, the tear should be totally exposed, by expanding the bone defect with a rongeur, and patched with a temporalis fascia or, more recently, a synthetic graft. Intracranial hemorrhage after calvarial bone harvesting has been reported but is ex- tremely rare [42].
Chin Graft
Up to 3 cm of cortical and corticocancellous bone can be shaved off the chin bone through an intraoral approach. This can be sufficient for small defects, such as cleft palate and orthognathic osteotomy defects. Because of its slow resorption, it can be used as an onlay graft for facial augmentation.
Retromolar Graft
A small block of cortical or corticocancellous bone can be chiseled off the area behind the third molar [45]. This graft has the same indications as chin grafts; however, the amount of available bone is much smaller.
Tibial Graft
The anterior surface of the tibial plateau can be a good source of cortical or corticocancellous bone grafts. Me- chanical stiffness of the tibial cortex can be useful in augmentation of atrophic alveolar ridge for implant placement, facial bone augmentation, or bridging an osteotomy defect.
Rib Graft
Nonvascularized rib was the first autogenous bone graft used for reconstruction of mandibular segmental de- fects [45]. Osseous or osseochondral segments can be har- vested from ribs 5 to 7 and can either be used in full or split thickness (Figure 2). Costochondral grafts remain very popular in the treatment of ascending mandibular ramus and condylar defects [46,47,48,49]. Side effects and complications include postoperative chest wall pain, pleural injury leading to pleuritis or pneumothorax, and facial asym- metry due to overgrowth of the graft [47,50,51].
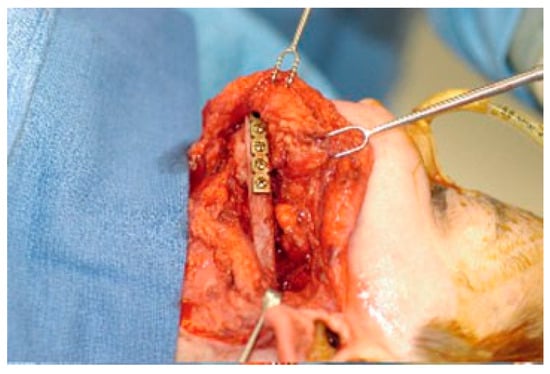
Figure 2.
Mandibular reconstruction with an osseocartila- ginous rib graft.
Although they were frequently used for facial bone augmentation, bridging osteotomy defects, and orbital floor reconstruction, osseous rib grafts are now rarely used in craniofacial reconstruction [45]. In addition to the problems mentioned previously, the amount and quality of bone obtained are inadequate for most recon- struction procedures. The availability of other sources of bone graft with better quality and quantity, as well as with safer approaches and synthetic bone substitute materials, has rendered rib grafting less popular.
Reimplantation of Resected Bone Segments
Limited studies have tested the possibility of ‘‘recycling’’ native bone segments that were removed as a part of tumor excision [52,53,54,55]. Intuitively, if tumor cells were successfully eradicated from the excised segments, they would be ideal for reconstructing the remaining defects. Resected mandibular segments could be reimplanted intact or hollowed out to remove trabecular bone, with use of the cortical bone shell as a tray for autogenous cancellous grafts. Larger long-term studies are needed to validate the safety and efficacy of this technique.
Regional Pedicled Bone Grafts
Pedicled Rib
In 1980, Cuono and Ariyan reported their successful use of the pectoralis major–attached rib as an osteomyocutaneous flap for oromandibular reconstruction [56]. How- ever, subsequent reports showed flap necrosis rates ranging from 21 to 75% [57,58]. The rib graft can also be carried along the latissimus dorsi or serratus anterior flaps [45]. In all the above-mentioned flaps, the pedicle only allows to rib graft to reach the lower third of the face, limiting its use to mandibular defects. As mentioned earlier, rib grafts are not suitable for such defects. Thus, these flaps are used only for soft tissue, and not bone, reconstruction [45].
Pedicled Clavicle
Sternocleidomastoid muscle (SCM) flaps have been extensively studied but not widely used. Several reports suggested the possibility of transferring clavicular peri- osteum[59] and bone segments of the clavicle itself [60]. The bone segment can either be partial or full thickness and can be utilized in reconstruction of small mandibular bone defects.
The technique preserves the neurovascular supply of the SCM muscle, thus allowing for its use in dynamic facial reconstruction [61]. This is particularly advantageous in cases where restoration of facial muscles, lower lip competence, mastication, or tongue movements is at- tempted. However, preserving the SCM muscle raises some concern in oncology cases due to the possibility of cervical lymph node involvement. In addition, the un- sightly contour defect at the donor site and in the lower neck is another disadvantage of this flap [61].
Pedicled Temporal Bone
The temporalis flap is one of the earliest described muscle flaps [62]. Over the years, it became one of the main techniques for reconstructing paralyzed facial muscles and midfacial full-thickness defects [63,64]. More relevant to our review, partial or full-thickness temporal bone can be raised with the muscle flap. It can be used to reconstruct maxillary, palatal, orbital rim, orbital floor, or ascending mandibular ramus defects. It can also be used as an onlay graft for facial augmentation [62].
However, significant donor site morbidity has been reported when calvarial bone is carried with the flap. These include limitation of mouth opening, which can be permanent, in addition to the mentioned com- plications of calvarial grafts [45].
Vascularized Bone Grafts
Although not widely used for midface, upper face, or cranial reconstruction, vascularized bone grafting is considered the gold standard for large mandibular defect reconstruction. Because the graft’s blood supply is coming through the anastomosis, it is independent of the condition of the recipient site. That makes it the most resistant to conditions like poor vascularity, ex- tensive scarring, and previous radiotherapy of the bed [45].
Moreover, they show less resorption than nonvascular- ized grafts and can immediately take endosteal implants for permanent dental restoration [65]. They are ideal for primary reconstruction, unlike free grafts that have very high failure rate in primary reconstruction. Another advantage is the possibility of simultaneous soft tissue and bone reconstruction with the same composite flap. Success rates of vascularized grafts is more than 90%.36,66,67
However, vascularized bone grafts are much more demanding and are technique sensitive as compared with nonvascularized grafts. Harvesting and an anastomosis require special surgical training and equipment. They add significantly to the operation time in cases of primary reconstruction, which can increase postoperative morbidity and mortality [66,68]. Microvascular reconstruc- tion is mostly limited to mandibular defects, with the most commonly used vascularized grafts being the fibula, iliac crest, scapula, and radius. Detailed description of these techniques is beyond the scope of this review.
Alloplastic Bone Grafts in Craniofacial Reconstruction
Demineralized bone matrix (DBM) allografts have been frequently used in craniofacial reconstruction [69,70,71,72,73]. Various DBM preparations are commercially available, varying from particles to blocks to sheets of different sizes. Generally, the smaller particles incorporate into the recipient bed faster than larger blocks or cortical sheets [8]. A recent study has shown that the bone aug- menting properties of DBM vary from one commercial preparation to another [74].
In addition to its osteoconductive and osteoin- ductive properties, DBM has some degree of mechanical stiffness, rendering it useful in reconstructing large cranial vault defects after cranioplasty procedures [73]. Sheets of cortical DBM could be molded into various shapes to match the three-dimensional configuration of the defect, providing a semirigid shield for the under- lying brain during the regeneration process.
Despite the overwhelming experimental evidence supporting the role of DBM as a bone augmenting material, incorporation of such allografts into recipient sites in human patients could be extremely slow. Re- placement of DBM with new calcified bone has been inconsistent, typically takes several months, especially in large defects [75,76]. During that period, mechanical stiff- ness of the DBM implants is not high enough to protect the underlying brain, necessitating the use of protective helmets. The process of graft incorporation and new bone formation can be markedly accelerated with the addition of bone augmenting factors, such as BMP-2 [75].
Synthetic Bone Substitutes and Bone Augmenting Factors
Advances in tissue engineering have provided a myriad of new tools for bone grafting. Growth factors, whether extracted or synthetic, adhesion molecules, and osteoconductive materials are becoming more available for bone reconstruction (Figure 3). These factors and materials vary widely in their osteoinductive, osteoconductive, and mechanical properties and there- fore in their applications.
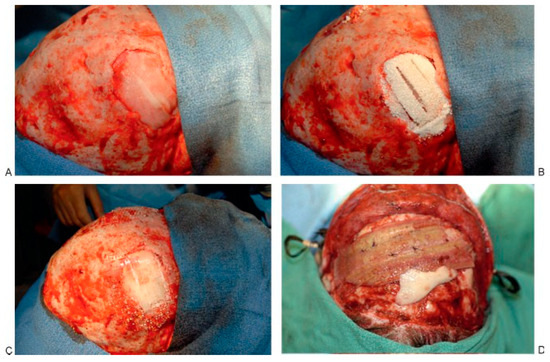
Figure 3.
Constructs for cranial defect reconstruction. (A) Acellular collagen sponge and bone morphogenic protein-2 in defect. (B) The addition of MastergraftTM (Medtronic Sofamor Danek, Memphis, TN), tricalcium phosphate, and hydroxyapatite collagen matrix. (C) Reconstruction using a resorbable plating cap. (D) Perforated demineralized bone matrix.
The general aim of using growth factors in augmentation of bone regeneration has been to stimulate the differentiation of bone-forming cells, angiogenic cells, or both. The transforming growth factor b (TGF-b) family is active in the periosteum in early stage of bone formation after fractures [77,78]. It stimulates the differentiation of cells of mesenchymal origin into os- teoblasts and chondrocytes[79,80] and inhibits cells of ectodermal origin [79]. Specifically, bone morphogenetic proteins, especially BMP-2, -3, -4, and -7, are potent inducers of osteogenesis [81,82,83]. Furthermore, hypoxia-in- ducing factor is expressed in high levels in fractures and is therefore considered as one of the major players in stimulation of angiogenic factors expression [84]. In frac- ture sites, hypoxia regulates osteoblast production of vascular modulators, such as VEGF and members of the TGF-b, insulin-like growth factor, and fibroblast growth factor families [85]. Recruited osteoclasts have been reported to produce heparinase, which releases VEGF from heparin in an active form, stimulating local angio- genesis and further osteoclast activity [86].
On the other hand, the vascular response during bone regeneration is extremely sensitive to the mechan- ical environment [87]. Endothelial cells subjected to me- chanical forces, hypoxia, or VEGF stimulation could start producing BMP-2 [88,89]. Other products of endo- thelial cells, including endothelin-1 and endothelial- derived angiotensin II, can also stimulate osteoblasts during bone healing [90]. Of these factors, BMP-2, BMP-7, and VEGF have shown the most potential for successful clinical use [75,91,92]. It has been reported that platelet-rich plasma (PRP) promotes angiogenesis and osteogenesis via the presence of growth factors, which include platelet-derived growth factor, platelet-derived endothelial cell growth factor, and TGF-b [93,94,95].
Kim and coworkers reported that demineralized bone and PRP produced a significantly higher percent- age of bone regeneration as compared with the use of demineralized bone alone [96]. However, Marden and coworkers found that platelet-derived growth factor inhibited bone regeneration induced by osteogenin, a bone morphogenetic protein, in rat craniotomy de- fects [97]. In our experience, we found no evidence that PRP either promotes or interferes with osteogenesis occurring in the presence of exogenous recombinant human bone morphogenetic protein-2 (rhBMP2) [75].
Two types of bone substitute materials have been used in craniofacial reconstruction: calcium phosphate cements and calcium sulfate (plaster of paris) [98]. Several preparations of calcium phosphates are commercially available for bone defect reconstruction. They have been successfully used to block cerebrospinal fluid leaks [99], obliterate the frontal sinus [100], and reconstruct contour defects in the cranium [101]. Calcium sulfate hemi- hydrate, in combination with porous ceramic hydroxya- patite granules, has also been successfully used for cranial defect reconstruction [102].
One major problem with cranial reconstruction has been how to maintain mechanical stability and protection for the underlying brain until sufficient bone regenerates to give permanent protection. Tempo- rary stability can be provided with either resorbable or nonresorbable fixation materials. During growth, non- resorbable metal fixation should be removed after recon- struction so as not to interfere with subsequent cranial remodeling. Nonresorbable fixation materials include titanium and cobalt chrome, the latter being easier to remove due to lack of osseointegration.
Several forms of resorbable fixation materials are available, which are mostly different forms of poly- lactate and polyglycolate polymers [103,104]. When using these materials, however, it should be noted that the time needed to lose mechanical stiffness is much shorter than the resorption time. Additionally, there might be some interaction between certain bone graft materials, such as DBM or hydroxyapatite cements and some resorbable materials, such as Lactosorb [1] (Walter Lorenz Surgical, Inc., Jacksonville, FL) [75,105].
Future Directions
Autogenous bone grafts remain the gold standard for surgical reconstruction of bone defects. However, ad- vances in tissue engineering and biomaterials technology will provide more tools for these procedures. Several problems remain that limit the wide utilization of such options, including regulatory requirements, high costs, lack of randomized controlled human studies, uncertain long-term results, as well as method-specific limitations.
References
- Meekeren, J.J. Observationes Medico-Chirugicae; Ex Officina Henrici & Vidnae Theodori Boom: Amsterdam, The Netherlands, 1682. [Google Scholar]
- Sanan, A.; Haines, S.J. Repairing holes in the head: a history of cranioplasty. Neurosurgery 1997, 40, 588–603. [Google Scholar] [PubMed]
- Macewen, W. Observations concerning transplantation of bone illustrated by a case of inter-human osseous trans- plantation, whereby over two-thirds of the shaft of a humerus was restored. Proc Roy Soc Lond 1881, 32, 232–247. [Google Scholar]
- Meikle, M.C. On the transplantation, regeneration and induction of bone: the path to bone morphogenetic proteins and other skeletal growth factors. Surgeon 2007, 5, 232–243. [Google Scholar] [CrossRef]
- Albee, F.H. Fundamentals in bone transplantation: experi- ences in three thousand bone graft operations. JAMA 1923, 81, 1429–1432. [Google Scholar] [CrossRef]
- Phemister, D.B. The fate of transplanted bone and regenerative power of its various constituents. Surg Gynecol Obstet 1914, 19, 303–333. [Google Scholar]
- Phemester, D. Treatment of ununited fractures by onlay bone grafts without screw or tie fixation and without breaking down of fibrous union. J Bone Joint Surg Am 1947, 29, 946–960. [Google Scholar]
- Bauer, T.W.; Muschler, G.F. Bone graft materials. An overview of the basic science. Clin Orthop Relat Res 2000, 371, 10–27. [Google Scholar] [CrossRef]
- Khan, S.N.; Cammisa, F.P., Jr.; Sandhu, H.S.; Diwan, A.D.; Girardi, F.P.; Lane, J.M. The biology of bone grafting. J Am Acad Orthop Surg 2005, 13, 77–86. [Google Scholar] [CrossRef]
- Laurencin, C.T.; El-Amin, S.F. Xenotransplantation in orthopaedic surgery. J Am Acad Orthop Surg 2008, 16, 4–8. [Google Scholar] [CrossRef]
- Boden, S.D. The ABCs of BMPs. Orthop Nurs 2005, 24, 49–52quiz. [Google Scholar] [CrossRef]
- Boyne, P.J.; Salina, S.; Nakamura, A.; Audia, F.; Shabahang, S. Bone regeneration using rhBMP-2 induction in hemi- mandibulectomy type defects of elderly sub-human pri- mates. Cell Tissue Bank 2006, 7, 1–10. [Google Scholar] [CrossRef]
- Seto, I.; Marukawa, E.; Asahina, I. Mandibular reconstruction using a combination graft of rhBMP-2 with bone marrow cells expanded in vitro. Plast Reconstr Surg 2006, 117, 902–908. [Google Scholar] [CrossRef] [PubMed]
- Urist, M.R.; Sato, K.; Brownell, A.G.; et al. Human bone morphogenetic protein (hBMP). Proc Soc Exp Biol Med 1983, 173, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Wikesjo¨, U.M.; Qahash, M.; Thomson, R.C.; et al. rhBMP-2 significantly enhances guided bone regeneration. Clin Oral Implants Res 2004, 15, 194–204. [Google Scholar] [CrossRef]
- Geiger, F.; Lorenz, H.; Xu, W.; et al. VEGF producing bone marrow stromal cells (BMSC) enhance vascularization and resorption of a natural coral bone substitute. Bone 2007, 41, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Koefoed, M.; Tiyapatanaputi, P.; et al. Remodeling of cortical bone allografts mediated by adherent rAAV- RANKL and VEGF gene therapy. Nat Med 2005, 11, 291–297. [Google Scholar] [CrossRef]
- Peng, H.; Usas, A.; Olshanski, A.; et al. VEGF improves, whereas sFlt1 inhibits, BMP2-induced bone formation and bone healing through modulation of angiogenesis. J Bone Miner Res 2005, 20, 2017–2027. [Google Scholar] [CrossRef]
- Dell, P.C.; Burchardt, H.; Glowczewskie, F.P., Jr. A roentgeno- graphic, biomechanical, and histological evaluation of vascularized and non-vascularized segmental fibular canine autografts. J Bone Joint Surg Am 1985, 67, 105–112. [Google Scholar] [CrossRef]
- Goldberg, V.M.; Stevenson, S. Natural history of autografts and allografts. Clin Orthop Relat Res 1987, 225, 7–16. [Google Scholar] [CrossRef]
- Stevenson, S.; Li, X.Q.; Davy, D.T.; Klein, L.; Goldberg, V.M. Critical biological determinants of incorporation of non- vascularized cortical bone grafts. Quantification of a complex process and structure. J Bone Joint Surg Am 1997, 79, 1–16. [Google Scholar] [CrossRef]
- Myers, R.A.; Marx, R.E. Use of hyperbaric oxygen in postradiation head and neck surgery. NCI Monogr 1990, 9, 151–157. [Google Scholar]
- Vudiniabola, S.; Pirone, C.; Williamson, J.; Goss, A.N. Hyper- baric oxygen in the therapeutic management of osteoradio- necrosis of the facial bones. Int J Oral Maxillofac Surg 2000, 29, 435–438. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, S.; Cimsit, M.; Ilgezdi, S.; et al. Hyperbaric oxygen therapy used to treat radiation injury: two case reports. Ostomy Wound Manage 2006, 52, 14–16. [Google Scholar]
- Burwell, R.G. Studies in the transplantation of bone. V. The capacity of fresh and treated homografts of bone to evoke transplantation immunity. J Bone Joint Surg Br 1963, 45-B, 386–401. [Google Scholar] [CrossRef]
- Chalmers, J. Transplantation immunity in bone homografting. J Bone Joint Surg Br 1959, 41-B, 160–179. [Google Scholar] [CrossRef]
- De Bruyn, P.P.; Kabisch, W.T. Bone formation by fresh and frozen, autogenous and homogenous transplants of bone, bone marrow and periosteum. Am J Anat 1955, 96, 375–417. [Google Scholar] [CrossRef]
- Heiple, K.G.; Chase, S.W.; Herndon, C.H. A comparative study of the healing process following different types of bone transplantation. J Bone Joint Surg Am 1963, 45, 1593–1616. [Google Scholar] [CrossRef]
- Kruyt, M.C.; Dhert, W.J.; Oner, C.; van Blitterswijk, C.A.; Verbout, A.J.; de Bruijn, J.D. Osteogenicity of autologous bone transplants in the goat. Transplantation 2004, 77, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Doi, K.; Tominaga, S.; Shibata, T. Bone grafts with micro- vascular anastomoses of vascular pedicles: an experimental study in dogs. J Bone Joint Surg Am 1977, 59, 809–815. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, C.; Lin, A.S.; et al. Periosteal progenitor cell fate in segmental cortical bone graft transplantations: implications for functional tissue engineering. J Bone Miner Res 2005, 20, 2124–2137. [Google Scholar] [CrossRef]
- Gu¨ ven, O. Rehabilitation of severely atrophied mandible using free iliac crest bone grafts and dental implants: report of two cases. J Oral Implantol 2007, 33, 122–126. [Google Scholar] [CrossRef]
- Laine, J.; Va¨ha¨talo, K.; Peltola, J.; Tammisalo, T.; Happonen, R.P. Rehabilitation of patients with congenital unrepaired cleft palate defects using free iliac crest bone grafts and dental implants. Int J Oral Maxillofac Implants 2002, 17, 573–580. [Google Scholar] [PubMed]
- Sekine, J.; Sano, K.; Ikeda, H.; Inokuchi, T. Rehabilitation by means of osseointegrated implants in oral cancer patients with about four to six years follow-up. J Oral Rehabil 2006, 33, 170–174. [Google Scholar] [CrossRef]
- Pogrel, M.A.; Podlesh, S.; Anthony, J.P.; Alexander, J. A comparison of vascularized and nonvascularized bone grafts for reconstruction of mandibular continuity defects. J Oral Maxillofac Surg 1997, 55, 1200–1206. [Google Scholar] [CrossRef]
- Foster, R.D.; Anthony, J.P.; Sharma, A.; Pogrel, M.A. Vascular- ized bone flaps versus nonvascularized bone grafts for mandibular reconstruction: an outcome analysis of primary bony union and endosseous implant success. Head Neck 1999, 21, 66–71. [Google Scholar] [CrossRef]
- Ahlmann, E.; Patzakis, M.; Roidis, N.; Shepherd, L.; Holtom, P. Comparison of anterior and posterior iliac crest bone grafts in terms of harvest-site morbidity and functional outcomes. J Bone Joint Surg Am 2002, 84-A, 716–720. [Google Scholar] [CrossRef]
- Boone, D.W. Complications of iliac crest graft and bone grafting alternatives in foot and ankle surgery. Foot Ankle Clin 2003, 8, 1–14. [Google Scholar] [CrossRef]
- Nocini, P.F.; Bedogni, A.; Valsecchi, S.; et al. Fractures of the iliac crest following anterior and posterior bone graft harvesting. Review of the literature and case presentation. Minerva Stomatol 2003, 52, 441–448. [Google Scholar] [PubMed]
- Velchuru, V.R.; Satish, S.G.; Petri, G.J.; Sturzaker, H.G. Hernia through an iliac crest bone graft site: report of a case and review of the literature. Bull Hosp Jt Dis 2006, 63, 166–168. [Google Scholar]
- Zijderveld, S.A.; ten Bruggenkate, C.M.; van Den Bergh, J.P.; Schulten, E.A. Fractures of the iliac crest after split-thickness bone grafting for preprosthetic surgery: report of 3 cases and review of the literature. J Oral Maxillofac Surg 2004, 62, 781–786. [Google Scholar] [CrossRef]
- Frodel, J.L. Calvarial bone graft harvesting techniques: considerations for their use with rigid fixation techniques in the craniomaxillofacial region. In Craniomaxillofacial Reconstructive and Corrective Bone Surgery; Greenberg, A., Prein, J., Eds.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Pensler, J.; McCarthy, J.G. The calvarial donor site: an anatomic study in cadavers. Plast Reconstr Surg 1985, 75, 648–651. [Google Scholar] [CrossRef] [PubMed]
- Powell, N.B.; Riley, R.W. Cranial bone grafting in facial aesthetic and reconstructive contouring. Arch Otolaryngol Head Neck Surg 1987, 113, 713–719. [Google Scholar] [CrossRef]
- Ehrenfeld, M.; Hagenmaier, C. Autogenous bone grafts in maxillofacial reconstruction. In Craniomaxillofacial Reconstructive and Corrective Bone Surgery; Greenberg, A., Prein, J., Eds.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Gu¨ zel, M.Z.; Arslan, H.; Sarac¸, M. Mandibular condyle reconstruction with inlay application of autogenous costo- chondral graft after condylectomy: Cerrahpaa’s technique. J Oral Maxillofac Surg 2007, 65, 615–620. [Google Scholar] [CrossRef]
- Medra, A.M. Follow up of mandibular costochondral grafts after release of ankylosis of the temporomandibular joints. Br J Oral Maxillofac Surg 2005, 43, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Poswillo, D.E. Biological reconstruction of the mandibular condyle. Br J Oral Maxillofac Surg 1987, 25, 100–104. [Google Scholar] [CrossRef]
- Troulis, M.J.; Tayebaty, F.T.; Papadaki, M.; Williams, W.B.; Kaban, L.B. Condylectomy and costochondral graft recon- struction for treatment of active idiopathic condylar resorption. J Oral Maxillofac Surg 2008, 66, 65–72. [Google Scholar] [CrossRef]
- Peltoma¨ki, T.; Isotupa, K. The costochondral graft: a solution or a source of facial asymmetry in growing children. A case report. Proc Finn Dent Soc 1991, 87, 167–176. [Google Scholar]
- Siavosh, S.; Ali, M. Overgrowth of a costochondral graft in a case of temporomandibular joint ankylosis. J Craniofac Surg 2007, 18, 1488–1491. [Google Scholar] [CrossRef]
- Bradley, P.F. A two-stage procedure for reimplantation of autogenous freeze-treated mandibular bone. J Oral Max- illofac Surg 1982, 40, 278–284. [Google Scholar] [CrossRef]
- Marciani, R.D.; Giansanti, J.S.; Massey, G.B. Reimplantation of freeze-treated and saline-treated mandibular bone. J Oral Surg 1976, 34, 314–319. [Google Scholar]
- Plezia, R.A.; Weaver, A.W.; Pietruk, T.; Gilbert, H.D. Evaluation of osteogenesis following immediate and delayed reimplan- tation of frozen autogenous mandibular bone. Oral Surg Oral Med Oral Pathol 1983, 56, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.; Arrigoni, G. Reimplantation of the mandibular condyle in cases of intraoral resection and reconstruction of the mandible. J Maxillofac Surg 1979, 7, 1–5. [Google Scholar] [CrossRef]
- Cuono, C.B.; Ariyan, S. Immediate reconstruction of a composite mandibular defect with a regional osteomuscu- locutaneous flap. Plast Reconstr Surg 1980, 65, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Biller, H.F.; Krespi, Y.P.; Lawson, W.; Baek, S.M. A one-stage flap reconstruction following resection for stomal recur- rence. Otolaryngol Head Neck Surg 1980, 88, 357–360. [Google Scholar] [CrossRef]
- Lam, K.H.; Wei, W.I.; Siu, K.F. The pectoralis major costomyocutaneous flap for mandibular reconstruction. Plast Reconstr Surg 1984, 73, 904–910. [Google Scholar] [CrossRef]
- Tovi, F.; Gittot, A. Sternocleidomastoid myoperiosteal flap for the repair of laryngeal and tracheal wall defects. Head Neck Surg 1983, 5, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Siemssen, S.O.; Kirkby, B.; O’Connor, T.P. Immediate reconstruction of a resected segment of the lower jaw, using a compound flap of clavicle and sternomastoid muscle. Plast Reconstr Surg 1978, 61, 724–735. [Google Scholar] [CrossRef]
- Urken, M.L.; Biller, H.F. Muscle and musculocutaneous flaps: sternocleidomastoid. In Atlas of Regional and Free Flaps for Head and Neck Reconstruction; Urken, M.L., Cheney, M.L., Sullivan, M.J., Eds.; Raven Press: New York, NY, USA, 1995. [Google Scholar]
- Cheney, M.L. Muscle and musculocutaneous flaps: tempo- ralis. In Atlas of Regional and Free Flaps for Head and Neck Reconstruction; Urken, M.L., Cheney, M.L., Sullivan, M.J., Eds.; Raven Press: New York, NY, USA, 1995. [Google Scholar]
- Cheney, M.L.; McKenna, M.J.; Megerian, C.A.; Ojemann, R.G. Early temporalis muscle transposition for the management of facial paralysis. Laryngoscope 1995, 105 Pt 1, 993–1000. [Google Scholar] [CrossRef]
- Rubin, L.R.; Mishriki, Y.; Lee, G. Anatomy of the nasolabial fold: the keystone of the smiling mechanism. Plast Reconstr Surg 1989, 83, 1–10. [Google Scholar] [CrossRef]
- Chana, J.S.; Chang, Y.M.; Wei, F.C.; et al. Segmental mandibulectomy and immediate free fibula osteoseptocuta- neous flap reconstruction with endosteal implants: an ideal treatment method for mandibular ameloblastoma. Plast Reconstr Surg 2004, 113, 80–87. [Google Scholar] [CrossRef]
- Haughey, B.H.; Wilson, E.; Kluwe, L.; et al. Free flap reconstruction of the head and neck: analysis of 241 cases. Otolaryngol Head Neck Surg 2001, 125, 10–17. [Google Scholar] [CrossRef]
- Keller, E.E.; Tolman, D.E.; Eckert, S. Endosseous implant and autogenous bone graft reconstruction of mandibular dis- continuity: a 12-year longitudinal study of 31 patients. Int J Oral Maxillofac Implants 1998, 13, 767–780. [Google Scholar] [PubMed]
- Komisar, A. The functional result of mandibular recon- struction. Laryngoscope 1990, 100, 364–374. [Google Scholar] [CrossRef]
- Chen, T.M.; Wang, H.J. Cranioplasty using allogeneic perforated demineralized bone matrix with autogenous bone paste. Ann Plast Surg 2002, 49, 272–277. [Google Scholar] [CrossRef]
- Moss, S.D.; Joganic, E.; Manwaring, K.H.; Beals, S.P. Trans- planted demineralized bone graft in cranial reconstructive surgery. Pediatr Neurosurg 1995, 23, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Salyer, K.E.; Bardach, J.; Squier, C.A.; Gendler, E.; Kelly, K.M. Cranioplasty in the growing canine skull using demineral- ized perforated bone. Plast Reconstr Surg 1995, 96, 770–779. [Google Scholar] [CrossRef]
- Salyer, K.E.; Gendler, E.; Menendez, J.L.; Simon, T.R.; Kelly, K.M.; Bardach, J. Demineralized perforated bone implants in craniofacial surgery. J Craniofac Surg 1992, 3, 55–62. [Google Scholar] [CrossRef]
- Salyer, K.E.; Gendler, E.; Squier, C.A. Long-term outcome of extensive skull reconstruction using demineralized perfo- rated bone in Siamese twins joined at the skull vertex. Plast Reconstr Surg 1997, 99, 1721–1726. [Google Scholar] [CrossRef]
- Bae, H.W.; Zhao, L.; Kanim, L.E.; Wong, P.; Delamarter, R.B.; Dawson, E.G. Intervariability and intravariability of bone morphogenetic proteins in commercially available deminer- alized bone matrix products. Spine 2006, 31, 1299–1306dis. [Google Scholar] [CrossRef] [PubMed]
- Elsalanty, M.E.; Por, Y.C.; Genecov, D.G.; et al. Recombinant human BMP-2 enhances the effects of materials used for reconstruction of large cranial defects. J Oral Maxillofac Surg 2008, 66, 277–285. [Google Scholar] [CrossRef]
- Por, Y.C.; Barcelo’, C.R.; Salyer, K.E.; et al. Bone generation in the reconstruction of a critical size calvarial defect in an experimental model. Ann Acad Med Singapore 2007, 36, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Andrew, J.G.; Hoyland, J.; Andrew, S.M.; Freemont, A.J.; Marsh, D. Demonstration of TGF-beta 1 mRNA by in situ hybridization in normal human fracture healing. Calcif Tissue Int 1993, 52, 74–78. [Google Scholar] [CrossRef]
- Bourque, W.T.; Gross, M.; Hall, B.K. Expression of four growth factors during fracture repair. Int J Dev Biol 1993, 37, 573–579. [Google Scholar]
- Lind, M. Growth factors: possible new clinical tools. A review. Acta Orthop Scand 1996, 67, 407–417. [Google Scholar] [CrossRef]
- Massague’, J. The transforming growth factor-beta family. Annu Rev Cell Biol 1990, 6, 597–641. [Google Scholar] [CrossRef] [PubMed]
- Barnes, G.L.; Kostenuik, P.J.; Gerstenfeld, L.C.; Einhorn, T.A. Growth factor regulation of fracture repair. J Bone Miner Res 1999, 14, 1805–1815. [Google Scholar] [CrossRef]
- Eingartner, C.; Coerper, S.; Fritz, J.; Gaissmaier, C.; Koveker, G.; Weise, K. Growth factors in distraction osteogenesis. Immuno-histological pattern of TGF-beta1 and IGF-I in human callus induced by distraction osteogenesis. Int Orthop 1999, 23, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Ishidou, Y.; Kitajima, I.; Obama, H.; et al. Enhanced expression of type I receptors for bone morphogenetic proteins during bone formation. J Bone Miner Res 1995, 10, 1651–1659. [Google Scholar] [CrossRef]
- Pacicca, D.M.; Patel, N.; Lee, C.; et al. Expression of angiogenic factors during distraction osteogenesis. Bone 2003, 33, 889–898. [Google Scholar] [CrossRef]
- Steinbrech, D.S.; Mehrara, B.J.; Saadeh, P.B.; et al. Hypoxia increases insulinlike growth factor gene expression in rat osteoblasts. Ann Plast Surg 2000, 44, 529–534. [Google Scholar] [CrossRef]
- Saijo, M.; Kitazawa, R.; Nakajima, M.; Kurosaka, M.; Maeda, S.; Kitazawa, S. Heparanase mRNA expression during fracture repair in mice. Histochem Cell Biol 2003, 120, 493–503. [Google Scholar] [CrossRef]
- Wallace, A.L.; Draper, E.R.; Strachan, R.K.; McCarthy, I.D.; Hughes, S.P. The vascular response to fracture micromove- ment. Clin Orthop Relat Res 1994, 301, 281–290. [Google Scholar] [CrossRef]
- Bouletreau, P.J.; Warren, S.M.; Spector, J.A.; et al. Hypoxia and VEGF up-regulate BMP-2 mRNA and protein expression in microvascular endothelial cells: implications for fracture healing. Plast Reconstr Surg 2002, 109, 2384–2397. [Google Scholar] [CrossRef] [PubMed]
- Sorescu, G.P.; Sykes, M.; Weiss, D.; et al. Bone morphogenic protein 4 produced in endothelial cells by oscillatory shear stress stimulates an inflammatory response. J Biol Chem 2003, 278, 31128–31135. [Google Scholar] [CrossRef] [PubMed]
- von Schroeder, H.P.; Veillette, C.J.; Payandeh, J.; Qureshi, A.; Heersche, J.N. Endothelin-1 promotes osteoprogenitor pro- liferation and differentiation in fetal rat calvarial cell cultures. Bone 2003, 33, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Kaigler, D.; Wang, Z.; Horger, K.; Mooney, D.J.; Krebsbach, P.H. VEGF scaffolds enhance angiogenesis and bone regener- ation in irradiated osseous defects. J Bone Miner Res 2006, 21, 735–744. [Google Scholar] [CrossRef]
- Ripamonti, U.; Ma, S.S.; Cunningham, N.S.; Yeates, L.; Reddi, A.H. Reconstruction of the bone—bone marrow organ by osteogenin, a bone morphogenetic protein, and demineralized bone matrix in calvarial defects of adult primates. Plast Reconstr Surg 1993, 91, 27–36. [Google Scholar] [CrossRef]
- Kawase, T.; Okuda, K.; Saito, Y.; Amizuka, N.; Suzuki, H.; Yoshie, H. Platelet-rich plasma provides nucleus for mineralization in cultures of partially differentiated periodontal ligament cells. In Vitro Cell Dev Biol Anim 2005, 41, 171–176. [Google Scholar] [CrossRef]
- Kawase, T.; Okuda, K.; Wolff, L.F.; Yoshie, H. Platelet-rich plasma-derived fibrin clot formation stimulates collagen synthesis in periodontal ligament and osteoblastic cells in vitro. J Periodontol 2003, 74, 858–864. [Google Scholar] [CrossRef]
- Okuda, K.; Kawase, T.; Momose, M.; et al. Platelet-rich plasma contains high levels of platelet-derived growth factor and transforming growth factor-beta and modulates the pro- liferation of periodontally related cells in vitro. J Periodontol 2003, 74, 849–857. [Google Scholar] [CrossRef]
- Kim, S.G.; Kim, W.K.; Park, J.C.; Kim, H.J. A comparative study of osseointegration of Avana implants in a demineralized freeze-dried bone alone or with platelet-rich plasma. J Oral Maxillofac Surg 2002, 60, 1018–1025. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Marden, L.J.; Fan, R.S.; Pierce, G.F.; Reddi, A.H.; Hollinger, J.O. Platelet-derived growth factor inhibits bone regeneration induced by osteogenin, a bone morphogenetic protein, in rat craniotomy defects. J Clin Invest 1993, 92, 2897–2905. [Google Scholar] [CrossRef] [PubMed]
- Pou, A.M. Update on new biomaterials and their use in reconstructive surgery. Curr Opin Otolaryngol Head Neck Surg 2003, 11, 240–244. [Google Scholar] [CrossRef]
- Costantino, P.D.; Hiltzik, D.H.; Sen, C.; et al. Sphenoethmoid cerebrospinal fluid leak repair with hydroxyapatite cement. Arch Otolaryngol Head Neck Surg 2001, 127, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Petruzzelli, G.J.; Stankiewicz, J.A. Frontal sinus obliteration with hydroxyapatite cement. Laryngoscope 2002, 112, 32–36. [Google Scholar] [CrossRef]
- Baker, S.B.; Weinzweig, J.; Kirschner, R.E.; Bartlett, S.P. Applications of a new carbonated calcium phosphate bone cement: early experience in pediatric and adult craniofa- cial reconstruction. Plast Reconstr Surg 2002, 109, 1789–1796. [Google Scholar] [CrossRef]
- Costantino, P.D.; Hiltzik, D.; Govindaraj, S.; Moche, J. Bone healing and bone substitutes. Facial Plast Surg 2002, 18, 13–26. [Google Scholar] [CrossRef]
- Tiainen, J.; Leinonen, S.; Iloma¨ki, J.; et al. Comparison of the pull-out forces of bioabsorbable polylactide/glycolide screws (Biosorb and Lactosorb) and tacks: a study on the stability of fixation in human cadaver parietal bones. J Craniofac Surg 2002, 13, 538–543. [Google Scholar] [CrossRef]
- Wiltfang, J.; Merten, H.A.; Schultze-Mosgau, S.; Schrell, U.; We’nzel, D.; Kessler, P. Biodegradable miniplates (LactoSorb): long-term results in infant minipigs and clinical results. J Craniofac Surg 2000, 11, 239–243. [Google Scholar] [CrossRef]
- Genecov, D.G.; Kremer, M.; Agarwal, R.; et al. Norian craniofacial repair system: compatibility with resorbable and nonresorbable plating materials. Plast Reconstr Surg 2007, 120, 1487–1495. [Google Scholar] [CrossRef]
© 2008 by the author. The Author(s) 2008.