Abstract
Study Design: This was a retrospective study at Noma Children Hospital, Sokoto, Nigeria, from January 2018 to December 2021. Objective: The main objective of this appraisal was to present Braimah-Taiwo et al’s new classification system for mandibulo-maxillary synostosis secondary to noma and also to provide a guide to their treatment. Methods: Noma with mandibulo-maxillary synostosis was the main inclusion criteria. Excluded were cases of acute noma and noma without mandibulo-maxillary synostosis. Data retrieved include demographics of patients and extent of bony ankylosis and mandibulo-maxillary synostosis. Results: A total of 64 patients (30 (46.9%) males and 34 (53.1%) females) were managed. Ages ranged from 6 to 40 years with mean ± SD (18.2 ± 7.6) years. Regarding the new classification system of mandibulo-maxillary synostosis, 6 (9.4%) patients presented with Type 1 (Mild joint obliteration) ± Soft tissue scarring, 24 (37.5%) presented with Type II (Total joint obliteration) ± Soft tissue scarring, 21 (32.8%) presented with Type III (Coronoid, zygoma and maxilla) ± Soft tissue scarring, 4 (6.3%) presented with Type IV (Condyle, glenoid fossa, coronoid, sigmoid notch and zygoma) ± Soft tissue scarring, 7 (10.9%) presented with Type V (Condyle, glenoid fossa, coronoid, sigmoid notch, zygoma and pterygo-maxilla) ± Soft tissue scarring, while 2 (3.1%) patients presented with Type VI (condyle, glenoid fossa, coronoid, sigmoid notch, zygoma, pterygo-maxilla and the orbit) ± Soft tissue scarring. Conclusions: Pattern of tissue destruction in noma patients is complex involving both soft and hard tissues. This new classification will guide surgeons in the effective management of these patients.
Introduction
Noma also known as cancrum oris is a debilitating orofacial gangrenous process still seen in developing countries of the world.[] Historically, such conditions were reported during war whereby strenuous conditions and limitation to food have contributed to severe malnourishment and by extension inability to fight infections.[] Ironically, this condition is still prevalent in sub-Saharan Africa in which northern part of Nigeria fell into.[,]
The disease process involves 3 phases which include: acute, subacute and chronic phases. Most victims do not survive the acute phase and they succumb to the severe metabolic derangement.[,] Because of the close relationship of the Temporo-mandibular joint (TMJ) to the oral cavity, associated pain during the acute and sub-acute phases of the disease as well as the scarring phase process, prevents the child from opening the mouth and taking adequate food to improve nutrition. This vicious circle further contributes to poor recovery process.[]
The TMJ which is the joint were the mouth opens is a unique joint in the body whereby structures remote to the joint contributes significantly to its function. Specifically regarding noma, the gangrenous process which started from the teeth bearing region can easily spread to the joint causing joint immobility and mandibulo-maxillary synostosis (MMS). This immobility could be fibrous (false ankylosis) or bony (true ankylosis) depending on the stage of disease progress and history of previous surgical interventions. Bony ankylosis of the TMJ have been previously classified by Sawhney[] into 4 types (Types I, II, III and IV). However, Braimah et al[] in 2018 observed bony ankylosis pattern in noma patients and made a proposal for Sawhney’s modification by the addition of Type V (TMJ ankylosis with maxillary extension). Recently, the authors have further discovered involvement of the orbital bone in the ankylotic mass which was neither classified by Sawhney[] nor its modification by Braimah et al.[]
Generally, interpositional\gap arthroplasty is the surgical technique used in releasing Temporomandibular joint ankylosis (TMJA) which is a major component of MMS. In noma patients, pattern of bone union and extensive scarring requires modified management algorithm. Ma et al [] concluded that patients with TMJA could benefit more from Interpositional Arthroplasty (IA) than Gap Arthroplasty (GA), with a larger maximal interincisal opening (MIO), however with a similar incidence of re-ankylosis. IA shows to be an adequate option in the treatment of TMJA based on the results of MIO. However, in noma patients with late sequalae of scarring, achieving this MIO could be impossible if MMS is not released and the scaring not excised.
Based on the recent findings, the main aim of this appraisal was to present Braimah-Taiwo et al new classification system of MMS secondary to noma and also provide a guide to their management. In order to enhance clinical outcomes, disease classifications are to improve the patient selection process and to identify groups that will guide clinical decision-making. This new classification system can serve as a decision-making system that maybe used to allocate patients to one of the treatment categories regarding MMS in noma patients. (Table 1, Table 2 and Table 3).

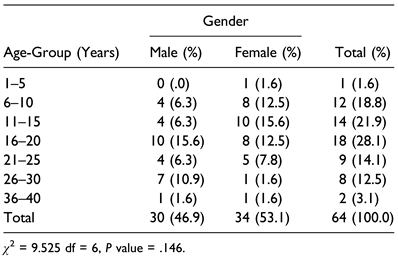
Table 1.
Age-Group of Patients With Mandibulo-Maxillary Synostosis According to Gender.

Table 2.
New Classification System of Mandibulo-Maxillary Synostosis According to Gender.

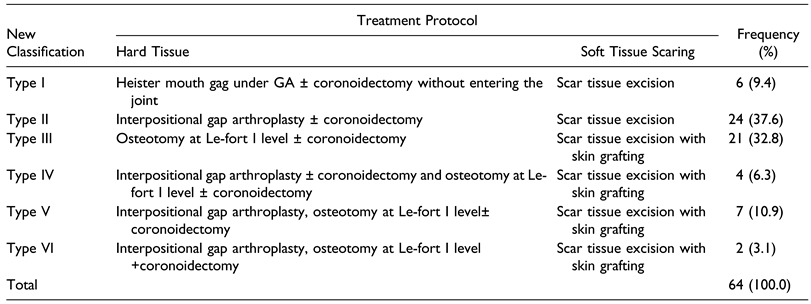
Table 3.
Management of Mandibulo-Maxillary Synostosis in Noma Patients Based on Current Classification System.
Materials and Methods
This was a retrospective study of patients with noma from January 2018 to December 2022 who presented for surgical correction of facial deformities at Noma Children Hospital Sokoto and Usmanu Danfodiyo University Teaching Hospital, Sokoto. Noma Children Hospital is the sole designated centre for the management of noma cases in Nigeria.
Noma with MMS was the main inclusion criteria. Excluded were cases of acute noma and noma without MMS. Data retrieved include demographics of patients and extent of bony ankylosis and MMS. Ethical clearance for the study was granted by the Ethics and Research Committee of the Sokoto State Ministry of Health with protocol number SKHREC/065/2022.
Data was stored and analyzed using IBM SPSS software version 25 for IOS (Armonk, NY: IBM Corp). Descriptive statistics was generated as part of the data analysis. Pearson chi-square was used to compare the relationship among the different variables (gender, age group and extent of TMJA). A P value of <.05 was considered significant.
Results
A total of 64 patients (30 (46.9%) males and 34 (53.1%) females) met the inclusion criteria. Ages ranged from 6 to 40 years with mean ± SD (18.2 ± 7.6) years (Table 1).
Regarding the new classification system of MMS in noma patients, 6 (9.4%) patients presented with Type 1 (Mild joint obliteration)±Soft tissue scarring (Figure 1A–C), 24 (37.5%) presented with Type II (Total joint obliteration) ±Soft tissue scarring (Figure 2A–E), 21 (32.8%) presented with Type III (Coronoid, zygoma and maxilla)±Soft tissue scarring (Figure 3A–E), 4 (6.3%%) presented with Type IV (Condyle, glenoid fossa, coronoid, sigmoid notch, and zygoma)±Soft tissue scarring (Figure 4A–C), 7 (10.9%) presented with Type V (Condyle, glenoid fossa, coronoid, sigmoid notch, zygoma and pterygo-maxilla)±Soft tissue scarring (Figure 5A–D), while 2 (3.1%) patients presented with Type VI (condyle, glenoid fossa, coronoid, sigmoid notch, zygoma, pterygo-maxilla and the orbit) ±Soft tissue scarring (Figure 6A–D) (Table 2).
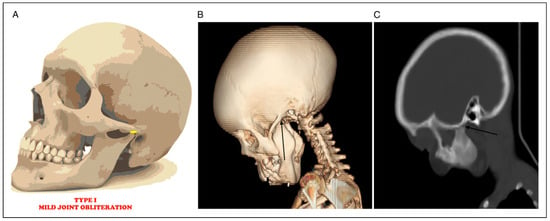
Figure 1.
(A) Mild joint space obliteration. (B) 3D CT showing mild joint obliteration (black arrow) (C) Sagittal CT radiograph showing mild joint obliteration (black arrow).
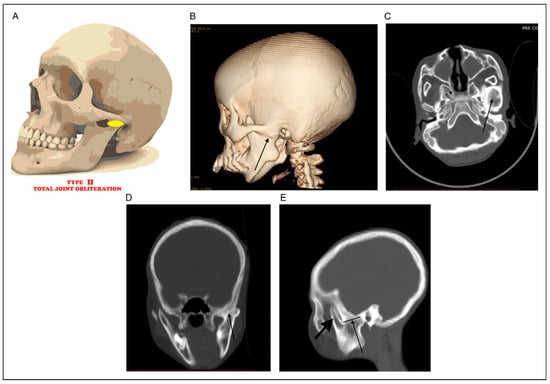
Figure 2.
(A) Total joint obliteration. (B) 3D CT showing total joint obliteration (black arrow). (C) Axial CT showing total joint obliteration (black arrow). (D) Coronal CT showing total joint obliteration (black arrow). (E) Sagittal CT showing total joint obliteration to the sigmoid notch and free coronoid (black arrow).
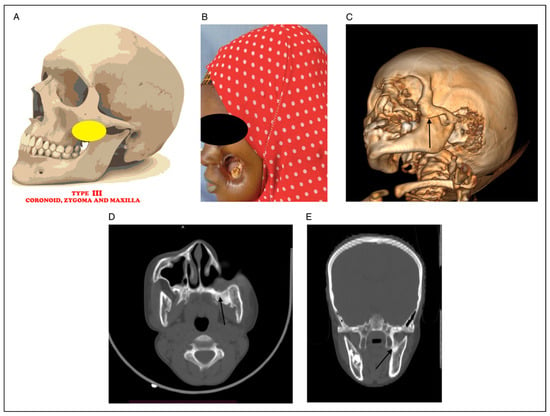
Figure 3.
(A) Picture showing bony fusion between coronoid, zygoma and maxilla. (B) Clinical photograph of noma patient with fusion between coronoid, zygoma and maxilla. (C) 3D CT radiograph showing fusion between coronoid, zygoma and maxilla (black arrow). (D) Axial CT radiograph showing fusion between coronoid, zygoma and maxilla (black arrow). (E) Coronal CT showing coronoid, zygoma and maxilla (black arrow).
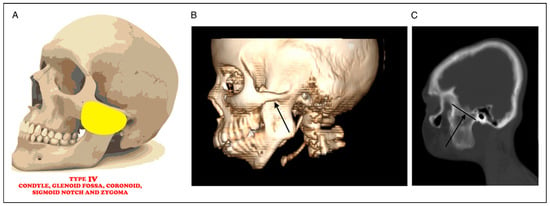
Figure 4.
(A) Picture showing bony fusion between condyle, glenoid fossa, coronoid, sigmoid notch, and zygoma. (B): 3D CT radiograph showing fusion between condyle, glenoid fossa, coronoid, sigmoid notch, and zygoma (black arrow). (C) Sagittal CT radiograph showing fusion between condyle, glenoid fossa, coronoid, sigmoid notch, and zygoma (black arrow).
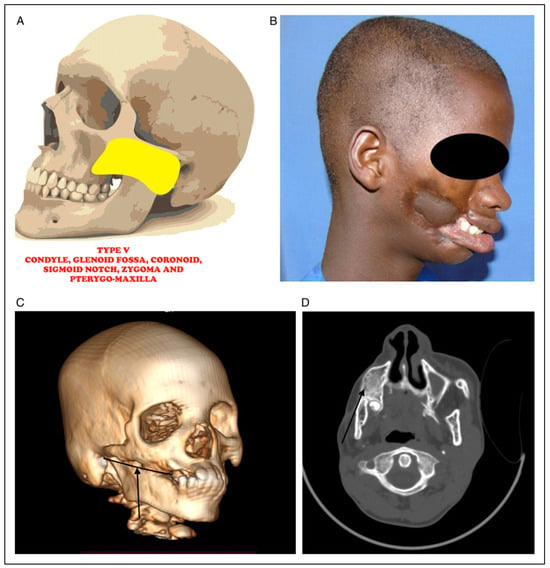
Figure 5.
(A) Picture showing bony fusion between condyle, glenoid fossa, coronoid, sigmoid notch, zygoma and pterygo-maxilla. (B) Clinical photograph of noma patient with fusion between condyle, glenoid fossa, coronoid, sigmoid notch, zygoma and pterygo-maxilla. (C) 3D CT radiograph showing fusion between condyle, glenoid fossa, coronoid, sigmoid notch, zygoma and pterygo-maxilla (black arrow). (D) Axial CT radiograph showing fusion between condyle, glenoid fossa, coronoid, sigmoid notch, zygoma and pterygo-maxilla (black arrow).
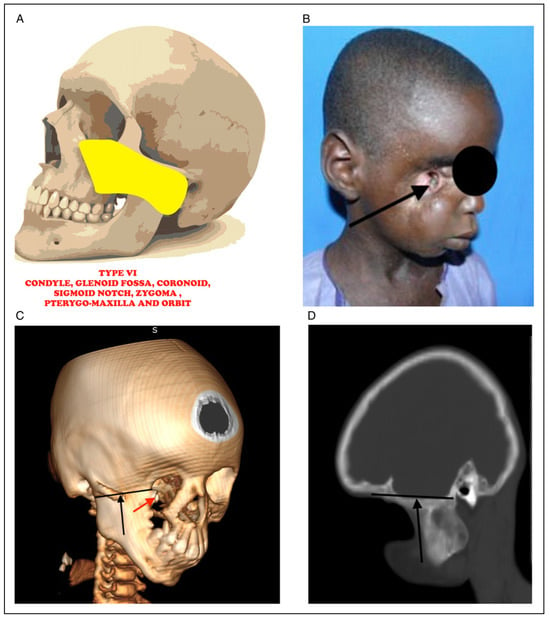
Figure 6.
(A) Picture showing bony fusion between condyle, glenoid fossa, coronoid, sigmoid notch, zygoma, pterygo-maxilla and the orbit. (B) Clinical photograph of noma patient with fusion between condyle, glenoid fossa, coronoid, sigmoid notch, zygoma, pterygomaxilla and the orbit with erupting tooth in the orbit (black arrow). (C) 3D CT radiograph showing fusion between condyle, glenoid fossa, coronoid, sigmoid notch, zygoma, pterygo-maxilla and the orbit (black arrow) with erupting tooth in the orbit (red arrrow). (D) Sagittal CT radiograph showing fusion between condyle, glenoid fossa, coronoid, sigmoid notch, zygoma, pterygo-maxilla and the orbit.
Regarding treatment of the specific classification, Type I patients were managed by forceful opening the mouth with Heister mouth gag under GA ± coronoidectomy without entering the joint space (Figure 7). Type II: Interpositional gap arthroplasty ± coronoidectomy (Figure 8), Type III: Osteotomy at Le-fort I level with scar excision and skin grafting (Figure 9), Type IV: Interpositional gap arthroplasty ± coronoidectomy with scar excision and skin grafting (Figure 10), Type V had Interpositional gap arthroplasty, Osteotomy at Le-fort I level ± coronoidectomy with scar excision and skin grafting (Figure 11) while Type VI had Interpositional gap arthroplasty, Osteotomy at Le-fort I level +coronoidectomy with scar excision and skin grafting (Figure 12). Twenty-one (33.3%) patients had scar tissue removal with split thickness skin graft (Table 3).
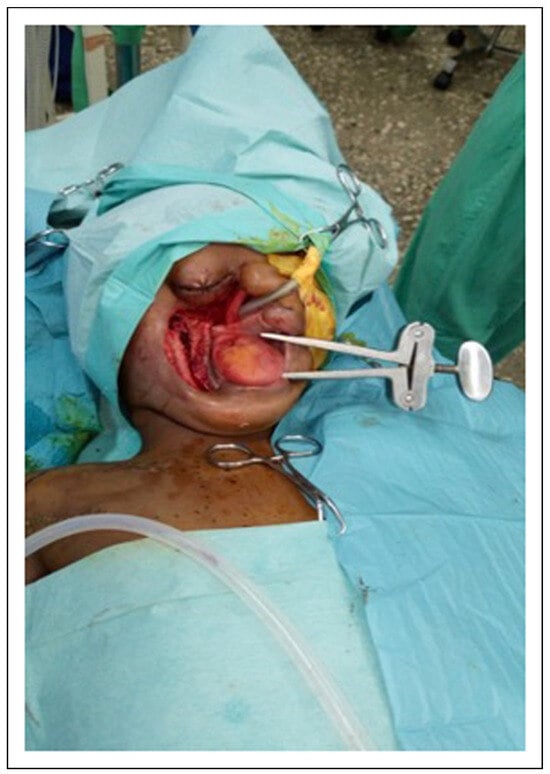
Figure 7.
Intraoperative photograph showing Heister mouth gag in opening of the mouth.
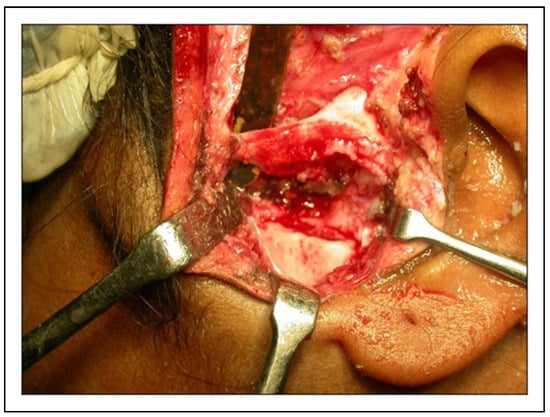
Figure 8.
Intraoperative photograph showing gap arthroplasty to be interposed with temporalis fascia.
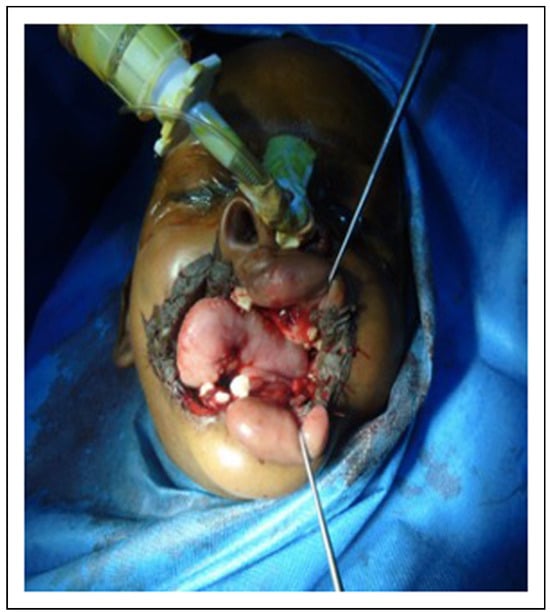
Figure 9.
Intraoperative photograph showing split thickness skin graft after scar tissue excision.
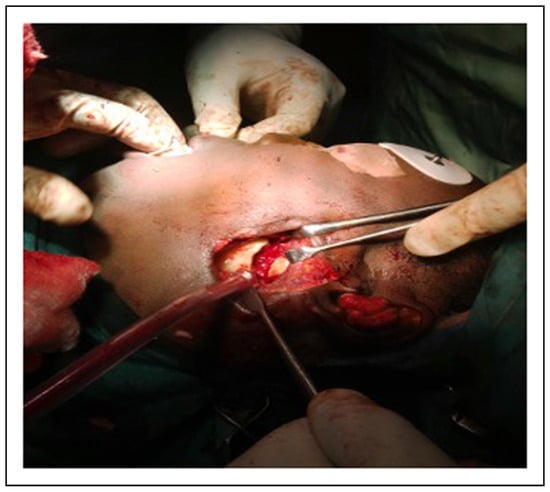
Figure 10.
Intraoperative photograph showing gap arthroplasty to be interposed with masseter muscle.
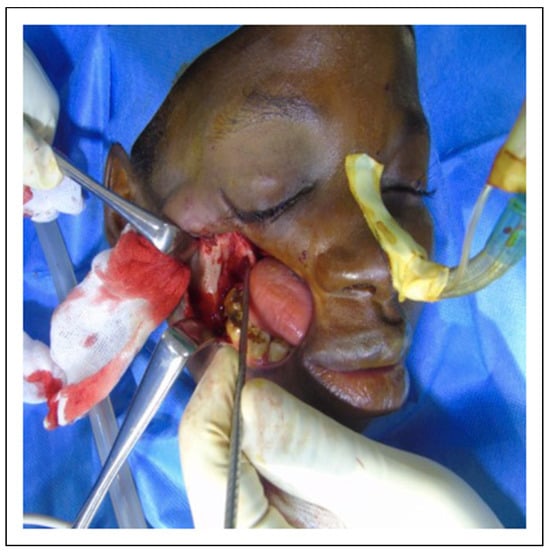
Figure 11.
Intraoperative photograph showing osteotomy at Le-fort I level to separate the coronoid from the zygoma, maxilla and pterygoid bones with scar excision.
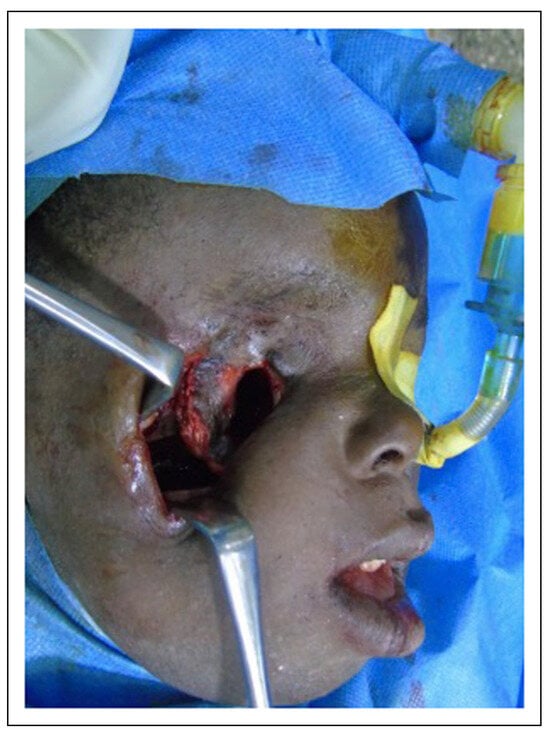
Figure 12.
Intraoperative photograph showing osteotomy at Le-fort I to separate the coronoid from the zygoma, maxilla, pterygoid and orbital bones with scar excision.
No intraoperative nor immediate post-operative complications was observed. After 10 months review we observed only 1 (1.6%) case of limited mouth opening (Figure 13A–D).
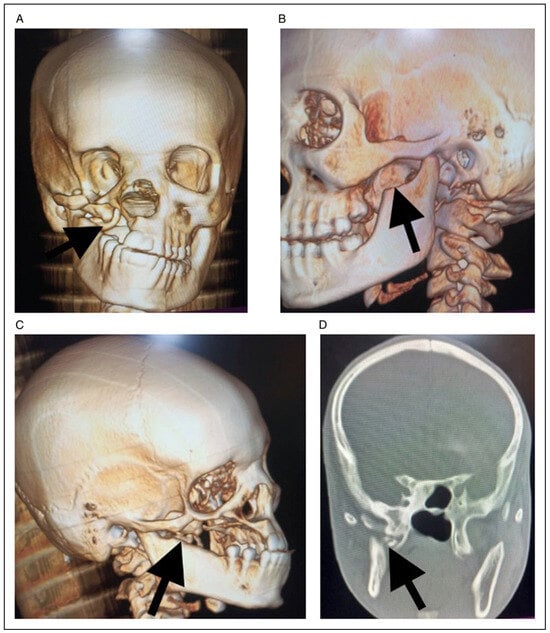
Figure 13.
(A) Frontal 3D reconstruction CT showing calcified tissue (black arrow) extending from skull base to the lingula of the mandible at 10 months post-operative review. (B). Left lateral 3D reconstruction CT showing coronoidectomy (black arrow) and free joint space at 10 months post-operative review. (C). Right lateral 3D reconstruction CT showing coronoidectomy (black arrow) and free joint space at 10 months post-operative review.(D) Coronal view showing calcified tissue (black arrow) extending from skull base to the lingula of the mandible at 10 months post-operative review.
Discussions
One of the most important sequelae of noma is prominent facial deformities, MMS, soft tissue scarring (trismus) and ankylosis of the temporomandibular joint.[] All these sequelae further results in difficulty with mouth opening, difficulty with mastication and swallowing, oral incontinence and speech impairment.[] Several classification systems have been developed by several authors to describe the sequelae of noma.[] The two most widely used classifications were the NOITULP, introduced by Marck et al[] and Montandon/WHO, first described by Montandon et al.[] The Montandon/WHO system was based on two publications by Raynaud et al[] and Cariou[] were they suggested rating the defect based on its severity and surgical strategy needed to successfully reconstruct the face. Montandon et al[] Type I represents a localised lip, commissure or cheek defect that normally can be lined by local tissues and covered with a single flap, Type II includes an upper lip and nose amputation, Type III involves a lower lip and mandible amputation, and Type IV marks large defects involving the lips, cheek, palate, maxillary bone, orbital floor and other entities.
Despite the validation of Montandon et al[] classification of noma sequelae by WHO in 1994, it does not fully address the disease severity by omitting the most important aspect of the sequelae which is ‘difficulty in mouth opening’.
Previously, difficulty in mouth opening in noma patients have been generally regarded to as trismus and have been classified by Marck et al[] in their experience in the management of noma patients in Sokoto, Northwest Nigeria. They noted that previous classification system lacked brevity hence the introduction of NOITULP system that covers anatomical subunits and severity of defect, not previously taken into account by any system.[] The NOITULP approach uses the letters N-‘nose’, O-‘outer cheek’, I-‘inner cheek’, T-‘trismus’, U-‘upper lip’, L-‘lower lip’ and P-‘particularities’ to describe the anatomical subunits affected and gives them a rating from 0 to 4, depending on the severity of the defect.[]
The ‘T’ component which grades the degree of mouth opening (Trismus) was further graded as: Grade-0 implies no mouth opening difficulty, Grade-1 (imply mouth opening of >40 mm), Grade-2 (imply mouth opening of 20-40 mm), Grade-3 (imply mouth opening of 1-20 mm), Grade-4 (imply complete locked jaw). As robust and widely acceptable this classification was, unfortunately, did not address the components and peculiarities of trismus in this group of patients. Trismus, which is only a soft tissue phenomenon did not address the bony component (the temporomandibular joints) of the mouth opening impairment (MMS) with its unique presentations in noma patients.
Based on this knowledge lacunae, the present authors have comprehensively evaluated the soft tissue and hard tissue components of ‘inability to open mouth’ in noma patients and have developed a holistic classification system that guides treatment options to be adopted in the management of ‘inability to open mouth’ in noma patients.
Proposed New Classification System
This new classification system of MMS secondary to noma is centred on Braimah et al classifications of TMJA in noma patients of 2018[] because TMJA is a major component of MMS. The new classification have taken into consideration both the soft and hard tissue components of the ‘inability to open the mouth’ in noma patients. The new classification include:
Type 1 MSS (Mild TMJ obliteration) ± Soft tissue scarring.
Type II MSS (Total TMJ obliteration) ± Soft tissue scarring.
Type III MSS (Coronoid, zygoma and maxilla) ± Soft tissue scarring.
Type IV MSS (Condyle, glenoid fossa, coronoid, sigmoid notch, and zygoma)±Soft tissue scarring.
Type V MSS (Condyle, glenoid fossa, coronoid, sigmoid notch, zygoma and pterygo-maxilla) ± Soft tissue scarring.
Type VI MSS (condyle, glenoid fossa, coronoid, sigmoid notch, zygoma, pterygo-maxilla and the orbit) ± Soft tissue scarring.
This new classification system is simple and comprehensive as it has addressed both the soft (soft tissue scarring) and hard (TMJA) tissue components of ‘inability to open mouth/MMS’ in noma patients. Furthermore, the classification will guide surgeons on the choice of surgical treatment protocol to be adopted while managing these groups of patients.
Proposed Treatment Algorithm Based On New Classification
Inter-positional or gap arthroplasty is the surgical technique used in releasing temporomandibular joint ankylosis (TMJA) which is a major component of MMS.[,] In noma patients, pattern of bone union and extensive scarring and tissue loss requires modified management algorithm because of the MMS outside the joint space. Ma et al[] concluded that patients with TMJA could benefit more from Interpositional arthroplasty (IA) than Gap arthroplasty (GA), with a larger maximal interincisal opening (MIO), however with a similar incidence of re-ankylosis. IA shows to be an adequate option in the treatment of TMJA based on the results of MIO. Proposed management algorithm of MMS related to noma based on the current classification is as follows:
Type 1 Mandibulo-Maxillary Synostosis: Forceful opening under GA ± coronoidectomy using Heister mouth gag only. The advantage of this mouth gag is that it can be inserted even with 2 mm MIO. This has been very helpful in forcing the mouth to open without entering the joint space. When MIO of less than 3.5 cm is achieved intra-operatively, then an ipsilateral coronoidectomy should be performed. If 4.0 cm intraoperative MIO is still not adequate, then contralateral coronoidectomy should be made. Scar tissue excision if present should be excised.
Type II Mandibulo-Maxillary Synostosis: Interpositional gap arthroplasty ± coronoidectomy. It is essential to make osteotomy cut below the ankylotic mass to prevent accidental sigmoid sinus breach that can lead to severe haemorrhage. This manoeuvre has been reported previously in the literature.[] Interpositional materials used in our series include: temporalis myofascial flap and masseter muscle flap. These interpositional tissues have been established in previous literatures as viable materials for interpositional gap arthroplasty of TMJA.[,] Protocol for coronoidectomy as described in Type I should be applied. Scar tissue excision if present should be excised.
Type III Mandibulo-Maxillary Synostosis: Osteotomy at Le-fort I level with scar excision and skin grafting. In this classification, there is no bony involvement of the joint architecture, therefore osteotomy at Le-fort I level to separate the coronoid from the zygoma and maxilla should be performed. Mouth gag should thereafter be introduced to open the mouth. There is no need to enter the joint space surgically. Because of raw surface intraorally, split thickness skin graft should be placed to prevent scaring that may further limit MIO. Protocol for coronoidectomy as described in Type I should be applied.
Type IV Mandibulo-Maxillary Synostosis: Interpositional gap arthroplasty ± coronoidectomy and Osteotomy at Le-fort I level with scar excision and skin grafting. Same protocol of Types III and IV should be applied. Protocol for coronoidectomy as described in Type I should also be applied.
Type V Mandibulo-Maxillary Synostosis: Interpositional gap arthroplasty ± coronoidectomy and Osteotomy at Le-fort I level to separate the coronoid from the zygoma, maxilla and pterygoid bones with scar excision and skin grafting. Same protocol of Types III and IV should be applied. Protocol for coronoidectomy as described in Type I should also be applied.
Type VI Mandibulo-Maxillary Synostosis: Interpositional gap arthroplasty ± coronoidectomy and Osteotomy at Le-fort I level to separate the coronoid from the zygoma, maxilla, pterygoid and orbital bones with scar excision and skin grafting. Protocol for coronoidectomy as described in Type I should also be applied.
In noma patients with late sequalae of scarring, achieving this MIO could be impossible if the scaring is not excised and its recurrence prevented. In the current classification, the problem of scarring have been managed by scar tissue total excision and lining with split thickness skin graft as explained above. However, were scar tissue is wide enough, partial excision and turning in of scar tissue to reconstruct the inner/ mucosa lining was adopted. This utilization of scar tissue as mucosa repair in noma patients has not been reported in the literature. The soft tissue defects were managed with local, regional or distant flaps based on the NOITULP classification of soft tissue defects which uses the letters N-‘nose’, O-‘outer cheek’, I-‘inner cheek’, T-‘trismus’, U-‘upper lip’, L-‘lower lip’ and P-‘particularities’ to describe the anatomical subunits affected.[]
Although soft tissue defect was not the main focus of the current study, it is important to mention that various flaps were utilized in this series to include: Local flaps (Abbe’s flap, Abbe’s Eslander flap, karapanzic flaps). For regional flaps, cervicofacial and visor flaps were used while radial forearm flap, delto-pectoral flap and pectoralis major flaps were used as distant flaps. All these flaps have been documented for oral and maxillofacial soft tissue defects reconstruction.[,] Additionally, soft tissue repairs including skin grafts and flaps should be carried out at MIO. This will prevent overstretching and eventual vascular compromise that may lead to soft tissue necrosis if done when mouth is closed. In our series no intraoperative nor immediate post-operative complications was observed. Most common intraoperative and immediate post-operative complication observed in our previous series was haemorrhage from sigmoid sinus injury.[,] Based on this experience, approach to the ankylosed TMJ in the current series was post-rami with great ability to directly visualize the mesial aspect of the ankylotic mass. Osteotomy cut was placed in the sub-ankylotic region thereby preventing injury to the sinus and the great vessels. Metwalli[] have reported that the distances between the internal carotid artery, the internal jugular vein, the maxillary artery and the medial pole of the mandibular condyle were reduced in TMJA patients when compared to patients without ankylosis.
After 10 months review, we observed one (1.6%) case of limited mouth opening (0.5 cm) that we opined was due to the calcification of spheno-mandibular ligament. This was because CT scan did not show any bony union but a calcified tissue was seen extending from the base of the skull to the lingula region of the ramus as shown in Figure 13A–D. This calcified tissue was removed and patients mouth opening improved to 4.5 cm.
Prevention of late sequelae of scarring and inability to open the mouth (MMS) should be further strengthened in noma patients. In our practice, this was achieved by the early introduction of aggressive physiotherapy during the acute and subacute stages of the disease. Although this was quite challenging during this phase of the disease process, however, long term benefit supersedes those challenges. Additionally, because these patients were still within the hospital care, adequate analgesic and sedation could be an added arsenal in ensuring regular and adequate physiotherapy to prevent TMJA and scaring as practiced in our facility. Early and long term aggressive physiotherapy should also be ensued after surgical intervention to prevent the incidence of re-ankylosis.[]
Limitation
This study was retrospective in design, however, data generated and experience gathered has given an insight into both hard and soft tissue components of ‘inability to open the mouth’ in Noma patients. Also, long term follow-up of these patients was also difficult as the majority of them travel from very far distances to receive care.
Conclusion
Pattern of tissue destruction in noma patients is complex involving both soft and hard tissues. Survivors of this neglected tropical disease suffer late sequelae of noma which include facial soft tissue defects, scarring, mandibulo-maxillary synostosis and inability to open the mouth. Reconstruction of these defects needs to address both esthetics and functional concerns which is one of the greatest challenges confronting the surgical team. The majority of noma patients wants to feed satisfactorily, have acceptable faces and to be accepted in their local communities where they live. Goal setting must be realistic, practicable and acceptable to patients, relatives and the surgical team while accommodating these expectations. The Braimah-Taiwo et al new classification system of mandibulo-maxillary synostosis related to noma will provide a guide to surgeons in the effective management of these patients in order to address these concerns.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Institutional Review Board Statement
Ethical clearance for the study was granted by the Ethics and Research Committee of the Sokoto State Ministry of Health with protocol number SKHREC/065/2022.
Conflicts of Interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Barmes, D.E.; Enwonwu, C.O.; Leclercq, M.H.; Bourgeois, D.; Falkler, W.A. The need for action against oro-facial gangrene (noma). Trop Med Int Health 1997, 2, 1111–1114. [Google Scholar] [CrossRef] [PubMed]
- Dawson, J. Cancrum oris. Br Dent J. 1945, 79, 151–157. [Google Scholar]
- Braimah, R.O.; Adeniyi, A.S.; Taiwo, A.O.; et al. Risk factors and mortality rate of acute cancrum oris (noma) in Sokoto north west Nigeria: a 13 year survey. J Pediatr Dent. 2017, 5, 1–5. [Google Scholar] [CrossRef]
- Ibikunle, A.A.; Adeniyi, S.A.; Taiwo, A.O.; et al. Management of 159 cases of acute cancrum oris: our experience at the Noma children hospital. Sokoto. Arch Med Health Sci. 2017, 5, 172–176. [Google Scholar]
- Myburgh, N.G.; Hobdell, M.H.; Lalloo, R. African countries propose a regional oral health strategy: the Dakar Report from 1998. Oral Dis. 2004, 10, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Adeniyi, S.A.; Taiwo, A.O.; Ibikunle, A.A.; et al. Pattern of tissue destruction among patients diagnosed with cancrum oris (Noma) at a Northwestern Nigerian Hospital, Sokoto. Saudi J Oral Sci. 2017, 4, 101–115. [Google Scholar]
- Sawhney, C.P. Bony ankylosis of the temporomandibular joint: follow up of 70 patients treated with Arthroplasty and acrylic spacer interposition. Plast Reconstr Surg. 1986, 77, 29–38. [Google Scholar] [PubMed]
- Braimah, R.O.; Taiwo, O.A.; Ibikunle, A.A.; et al. Temporomandibular Joint Ankylosis with maxillary extension: proposal for modification of Sawhney’s classification. Craniomaxillofac Trauma Reconstr Open. 2018, 2, 15–21. [Google Scholar] [CrossRef]
- Ma, J.; Liang, L.; Jiang, H.; Gu, B. Gap arthroplasty versus interpositional arthroplasty for temporomandibular joint ankylosis: a meta- analysis. PLoS One. 2015, 10, e0127652. [Google Scholar]
- Enwonwu, C.O.; Falkler, J.R.W.A.; Idigbe, E.O.; et al. Pathogenesis of cancrum oris (NOMA): confounding interactions of malnutrition with infection. Am J Trop Med Hyg. 1999, 60, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Speiser, S.; Langridge, B.; Birkl, M.M.; Kubiena, H.; Rodgers, W. Update on Noma: systematic review on classification, outcomes and follow-up of patients undergoing reconstructive surgery after Noma disease. BMJ Open 2021, 11, e046303. [Google Scholar] [CrossRef] [PubMed]
- Marck, K.W.; DeBruikin, H.P.; Schmid, F.; et al. Noma: the Sokoto approach. Eur J Plast Surg. 1998, 21, 277–281. [Google Scholar] [CrossRef]
- Montandon, D.; Lehmann, C.; Chami, N. The surgical treatment of noma. Plast Reconstr Surg. 1991, 87, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Reynaud, J.; Garand, G.; Ployet, M.J. Du noma Au syndrome de Silvermann: une pathogEnie ‡ discuter. Ann Chir Plast. 1978, 23, 227–230. [Google Scholar] [PubMed]
- Cariou, J. Le noma dans La corne de l’Afrique: approche the´rapeutique. Ann Chir Plast Esthet. 1986, 31, 374–380. [Google Scholar] [PubMed]
- Liu, X.; Shen, P.; Zhang, S.; Yang, C.; Wang, Y. Effectiveness of different surgical modalities in the management of temporomandibular joint ankylosis: a meta-analysis. Int J Clin Exp Med. 2015, 8, 19831–19839. [Google Scholar] [PubMed]
- Braimah, R.O.; Taiwo, A.O.; Ibikunle, A.A.; et al. Clinical experience in the management of temporo-mandibular joint ankylosis: five year appraisal in a Nigerian sub population. Journal of Korean Association of Oral and Maxillofacial Surgery. 2018, 44, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Braimah, R.O.; Oladejo, T.; Olarinoye, T.O.; Adetoye, A.O.; Osho, P.O. A multidisciplinary approach to the management of temporomandibular joint ankylosis in a sickle cell anemia patient in a resource limited setting. Ann Maxillofac Surg. 2016, 6, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Urner, B.; Collin, J.; Fernandes, R. Soft tissue reconstruction of the maxillofacial region. In Oral and Maxillofacial Surgery for the Clinician; Bonanthaya, K., Panneerselvam, E., Manuel, S., Kumar, V.V., Rai, A., Eds.; Springer: Singapore, 2021; pp. 1941–1967. [Google Scholar]
- Nthumba, P.; Carter, L. Visor flap for total upper and lower lip reconstruction: a case report. J Med Case Rep. 2009, 3, 7312–7316. [Google Scholar] [CrossRef] [PubMed]
- Metwalli, S. Computerized Tomography of the Temporal Bone in TMJ Ankylosis. Master’s Thesis, Cairo University, Giza, Egypt, 1993; pp. 20–36. [Google Scholar]
- Kauser, M.S.; Mohammad, A. Importance of physiotherapy in postoperative TMJ ankylosis patients. Arch CranOroFac Sc. 2014, 1, 61–62. [Google Scholar]
© 2023 by the authors. The Author(s) 2023.