Review of the Literature on the Current State of Periosteum-Mediated Craniofacial Bone Regeneration
Abstract
:1. Introduction
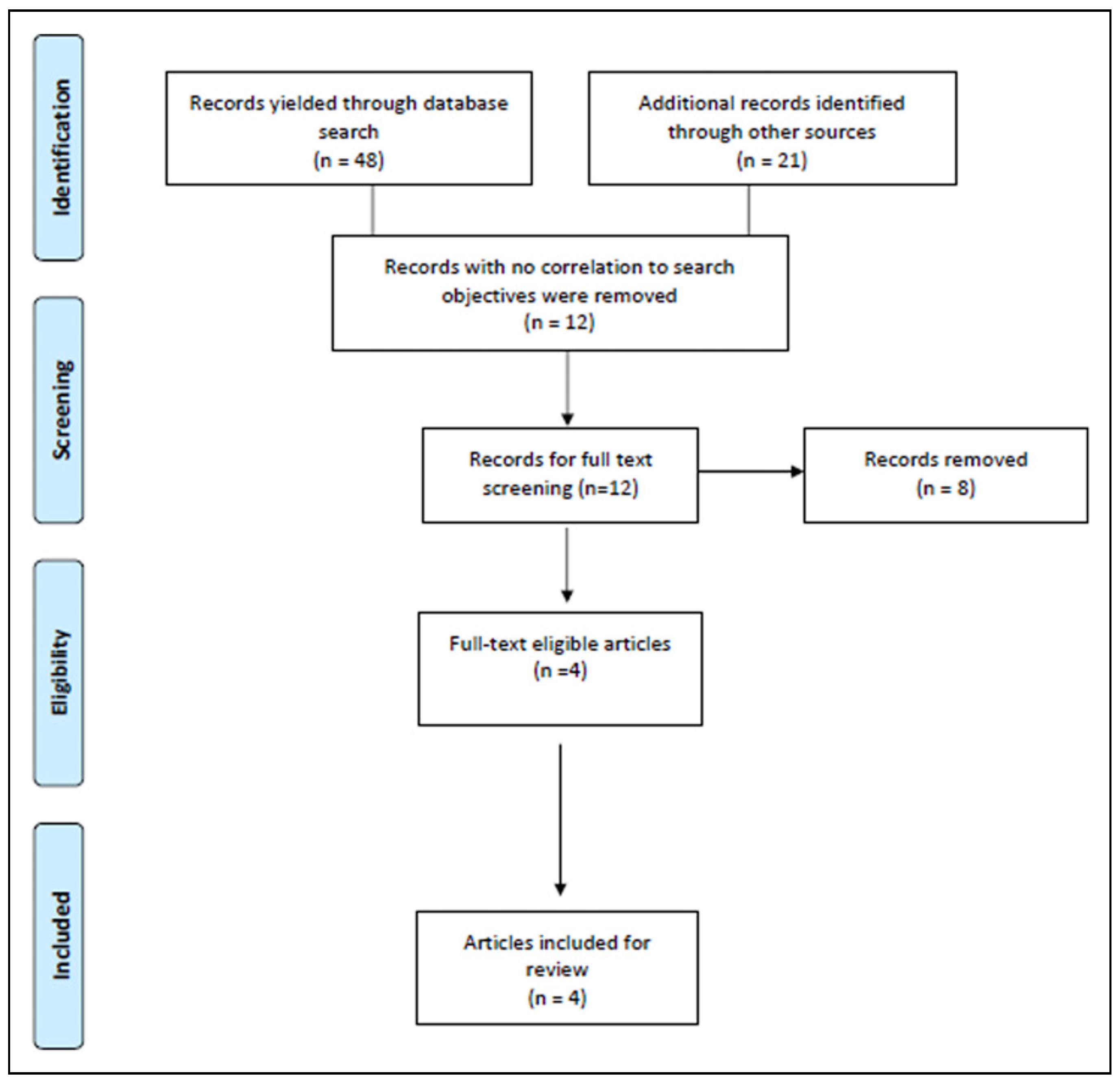
2. Methodology
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Quality Appraisal
2.4. Data Extraction and Analysis
3. Results
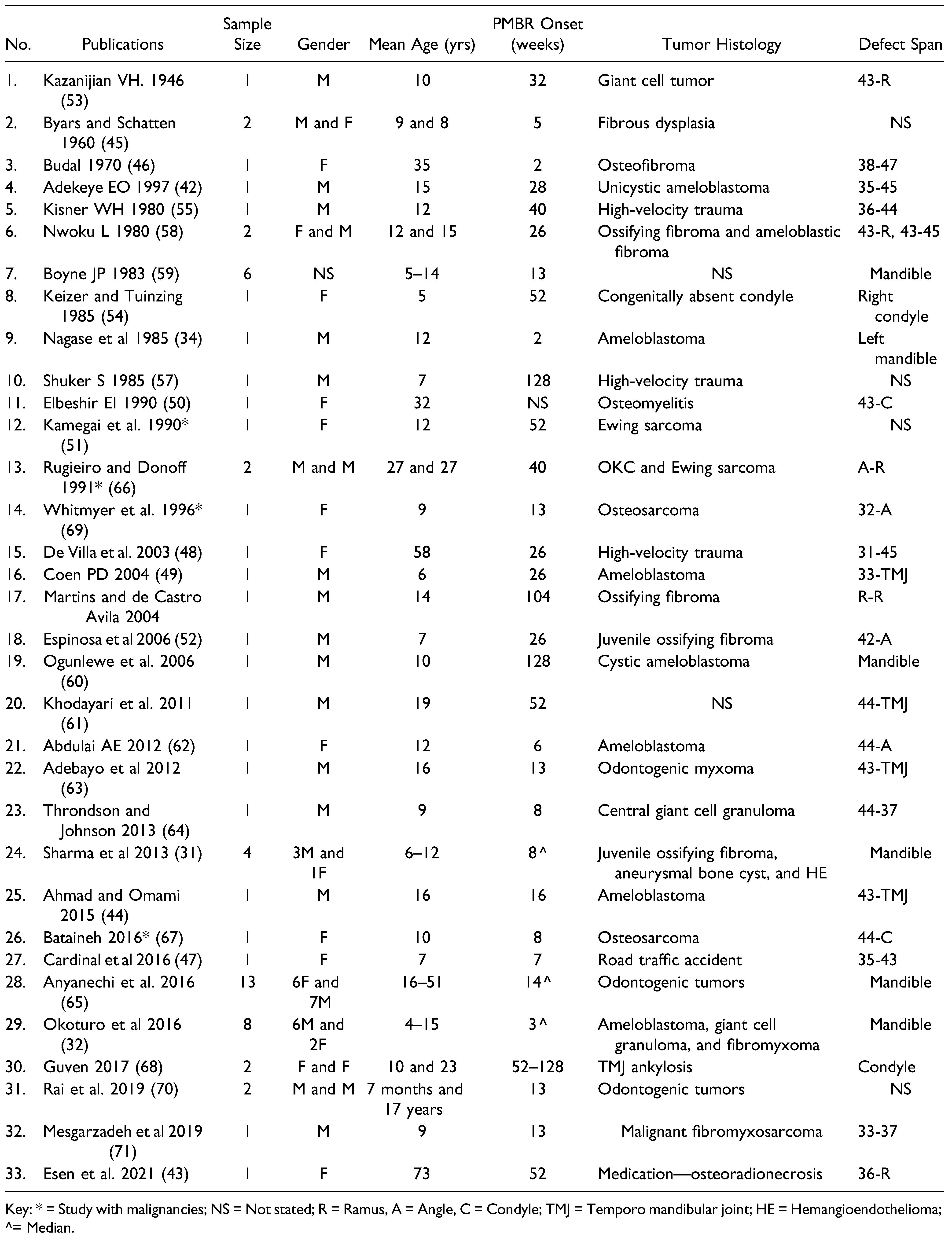
3.1. Search 1
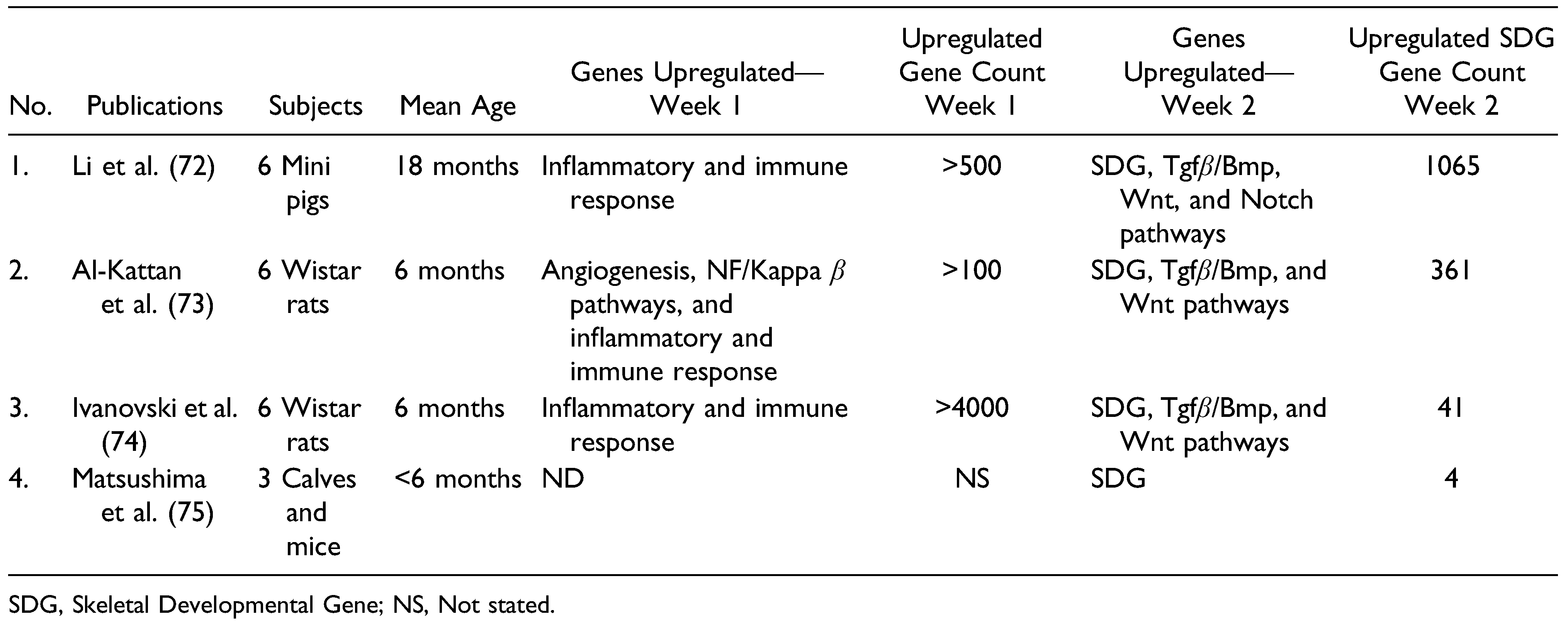
3.2. Search 2
4. Discussion
Funding
Conflicts of Interest
References
- Smith, A.; Petersen, D.; Samant, S.; Ver Halen, J.P. Pediatric mandibular reconstruction following resection of oral squamous cell carcinoma: A case report. Am J Otolaryngol. 2014, 35, 826–828. [Google Scholar] [PubMed]
- Posnick, J.C.; Wells, M.D.; Zuker, R.M. Use of the free fibular flap in the immediate reconstruction of pediatric mandibular tumors: Report of cases. J Oral Maxillofac Surg. 1993, 51, 189–196. [Google Scholar]
- Hildago, D.A.; Shenaq, S.M.; Larson, D.L. Mandibular reconstruction in the pediatric patient. Head Neck. 1996, 18, 359–365. [Google Scholar] [PubMed]
- Lydaki, E.; Bolonaki, I.; Stiakaki, E.; et al. Immediate free flap mandibular reconstruction in osteosarcoma of the mandible in childhood. Pediatr Hematol Oncol. 2000, 17, 335–340. [Google Scholar]
- Eckardt, A.; Swennen, G.; Teltzrow, T. Melanotic neuroectodermal tumor of infancy involving the mandible: 7-year follow-up after hemimandibulectomy and costochondral graft reconstruction. J Craniofac Surg. 2001, 12, 349–354. [Google Scholar]
- Nahabedian, M.Y.; Tufaro, A.; Manson, P.N. Improved mandible function after hemimandibulectomy, condylar head preservation, and vascularized fibular reconstruction. Ann Plast Surg. 2001, 46, 506–510. [Google Scholar] [PubMed]
- Phillips, J.H.; Rechner, B.; Tompson, B.D. Mandibular growth following reconstruction using a free fibula graft in the pediatric facial skeleton. Plast Reconstr Surg. 2005, 116, 419–424. [Google Scholar]
- Fenton, C.C.; Nish, I.A.; Carmichael, R.P.; Sandor, G.K. Metastatic mandibular retinoblastoma in a child reconstructed with soft tissue matrix expansion grafting: A preliminary report. J Oral Maxillofac Surg. 2007, 65, 2329–2335. [Google Scholar]
- Warren, S.M.; Borud, L.J.; Brecht, L.E.; Longaker, M.T.; Siebert, J.W. Microvascular reconstruction of the pediatric mandible. Plast Reconstr Surg. 2007, 119, 649–661. [Google Scholar]
- Bilkay, U.; Tiftikcioglu, Y.O.; Temiz, G.; Ozek, C.; Akin, Y. Freetissue transfers for reconstruction of oromandibular area in children. Microsurgery. 2008, 28, 91–98. [Google Scholar]
- Crosby, M.A.; Martin, J.W.; Robb, G.L.; Chang, D.W. Pediatric mandibular reconstruction using a vascularized fibula flap. Head Neck. 2008, 30, 311–319. [Google Scholar]
- Li, J.S.; Chen, W.L.; Huang, Z.Q.; Zhang, D.M. Pediatric mandibular reconstruction after benign tumor ablation using a vascularized fibular flap. J Craniofac Surg. 2009, 20, 431–434. [Google Scholar] [PubMed]
- Sinno, H.; Zadeh, T. Desmoid tumors of the pediatric mandible: Case report and review. Ann Plast Surg. 2009, 62, 213–219. [Google Scholar] [PubMed]
- Upton, J.; Guo, L.; Labow, B.I. Pediatric free-tissue transfer. Plast Reconstr Surg. 2009, 124 (Suppl. S6), e313–e326. [Google Scholar]
- Ducic, Y.; Young, L. Improving aesthetic outcomes in pediatric free tissue oromandibular reconstruction. Arch Facial Plast Surg. 2011, 13, 180–184. [Google Scholar]
- Pierse, J.; Ying-Peng Wun, E.; Pellecchia, R.; Wollenberg, J. Treatment of a rare ganglioneuroma with resection and reconstruction of the mandible: A case report and literature review. J Oral Maxillofac Surg. 2014, 72, 748.e1–784.e9. [Google Scholar]
- Zhang, W.B.; Liang, T.; Peng, X. Mandibular growth after paediatric mandibular reconstruction with the vascularized free fibula flap: A systematic review. Int J Oral Maxillofac Surg. 2016, 45, 440–447. [Google Scholar]
- Hu, L.; Yang, X.; Han, J.; et al. Secondary mandibular reconstruction for paediatric patients with long-term mandibular continuity defects: A retrospective study of six cases. Int J Oral Maxillofac Surg. 2017, 46, 447–452. [Google Scholar]
- Malloy, S.M.; Dronkers, W.J.; Firriolo, J.M.; et al. Outcomes following microvascular mandibular reconstruction in pediatric patients and young adults. Plast Reconstr Surg Glob Open. 2020, 8, e3243. [Google Scholar]
- Valentini, V.; Califano, L.; Cassoni, A.; et al. Maxillo-mandibular reconstruction in pediatric patients: How to do it? J Craniofac Surg. 2018, 29, 761–766. [Google Scholar] [PubMed]
- Nam, J.W.; Nam, W.; Cha, I.H.; Kim, H.J. Considerations for mandibular reconstruction in the pediatric patient following resection of malignant tumors. J Craniofac Surg. 2019, 30, e163–e168. [Google Scholar] [PubMed]
- Volk, A.S.; Riad, S.S.H.; Kania, K.E.; et al. Quantifying free fibula flap growth after pediatric mandibular reconstruction. J Craniofac Surg. 2020, 31, e710–e714. [Google Scholar] [PubMed]
- Iconomou, T.G.; Zuker, R.M.; Phillips, J.H. Mandibular reconstruction in children using the vascularized fibula. J Reconstr Microsurg. 1999, 15, 83–90. [Google Scholar] [PubMed]
- Castellon, L.; Jerez, D.; Mayorga, J.; Gallego, A.; Fuenzalida, C.; Laissle, G. Mandibular reconstruction for pediatric patients. J Craniofac Surg. 2018, 29, 1421–1425. [Google Scholar]
- Guo, L.; Ferraro, N.F.; Padwa, B.L.; Kaban, L.B.; Upton, J. Vascularized fibular graft for pediatric mandibular reconstruction. Plast Reconstr Surg. 2008, 121, 2095–2105. [Google Scholar]
- Genden, E.M.; Buchbinder, D.; Chaplin, J.M.; Lueg, E.; Funk, G.F.; Urken, M.L. Reconstruction of the pediatric maxilla and mandible. Arch Otolaryngol Head Neck Surg. 2000, 126, 293–300. [Google Scholar]
- Kolomvos, N.; Iatrou, I.; Theologie-Lygidakis, N.; Tzerbos, F.; Schoinohoriti, O. Iliac crest morbidity following maxillofacial bone grafting in children: A clinical and radiographic prospective study. J Cranio-Maxillo-Fac Surg. 2010, 38, 293–302. [Google Scholar]
- Rashid, M.; Tamimy, M.S.; Ehtesham Ul, H.; Sarwar, S.U.; Rizvi, S.T. Benign paediatric mandibular tumours: Experience in reconstruction using vascularised fibula. J Plast Reconstr Aesthet Surg. 2012, 65, e325–e331. [Google Scholar]
- Bianchi, B.; Ferri, A.; Ferrari, S.; et al. Microvascular reconstruction of mandibular defects in paediatric patients. J Cranio-Maxillo-Fac Surg. 2011, 39, 289–295. [Google Scholar]
- Chalmers, J.; Gray, D.H.; Rush, J. Observations on the induction of bone in soft tissues. J Bone Joint Surg Br. 1975, 57, 36–45. [Google Scholar]
- Sharma, P.; Williams, R.; Monaghan, A. Spontaneous mandibular regeneration: Another option for mandibular reconstruction in children? Br J Oral Maxillofac Surg. 2013, 51, e63–e66. [Google Scholar] [CrossRef]
- Okoturo, E.; Ogunbanjo, O.V.; Arotiba, G.T. Spontaneous regeneration of the mandible: An institutional audit of regenerated bone and osteocompetent periosteum. J Oral Maxillofac Surg. 2016, 74, 1660–1667. [Google Scholar] [CrossRef] [PubMed]
- Colnot, C.; Zhang, X.; Knothe Tate, M.L. Current insights on the regenerative potential of the periosteum: Molecular, cellular, and endogenous engineering approaches. J Orthop Res. 2012, 30, 1869–1878. [Google Scholar] [CrossRef]
- Nagase, M.; Ueda, K.; Suzuki, I.; Nakajima, T. Spontaneous regeneration of the condyle following hemimandibulectomy by disarticulation. J Oral Maxillofac Surg. 1985, 43, 218–220. [Google Scholar] [CrossRef]
- Ma, J.L.; Pan, J.L.; Tan, B.S.; Cui, F.Z. Determination of critical size defect of minipig mandible. J Tissue Eng Regen Med. 2009, 3, 615–622. [Google Scholar] [CrossRef]
- Buccitelli, C.; Selbach, M. mRNAs, proteins and the emerging principles of gene expression control. Nat Rev Genet. 2020, 21, 630–644. [Google Scholar] [CrossRef]
- Ganguly, P.; Toghill, B.; Pathak, S. Aging, bone marrow and next-generation sequencing (NGS): Recent advances and future perspectives. Int J Mol Sci. 2021, 22, 12225. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ. 2021, 372, n160. [Google Scholar] [CrossRef]
- Shamliyan, T.; Kane, R.L.; Dickinson, S. A systematic review of tools used to assess the quality of observational studies that examine incidence or prevalence and risk factors for diseases. J Clin Epidemiol. 2010, 63, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- Little, J.; Higgins, J.P.; Ioannidis, J.P.; et al. STrengthening the REporting of genetic association studies (STREGA)--an extension of the STROBE statement. Genet Epidemiol. 2009, 33, 581–598. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.; Thomson, D.; Seto, I.; et al. Standard 6: Age groups for pediatric trials. Pediatrics. 2012, 129 (Suppl. S3), S153–S160. [Google Scholar] [PubMed]
- Adekeye, E.O. Rapid bone regeneration subsequent to subtotal mandibulectomy. Report of an unusual case. Oral Surg Oral Med Oral Pathol. 1977, 44, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Esen, A.; Gurses, G.; Akkulah, S. Spontaneous bone regeneration in resected non-continuous mandible due to medicationrelated osteonecrosis of the jaw. J Korean Assoc Oral Maxillofac Surg. 2021, 47, 465–470. [Google Scholar]
- Ahmad, O.; Omami, G. Self-regeneration of the mandible following hemimandibulectomy for ameloblastoma: A case report and review of literature. J Maxillofac Oral Surg. 2015, 14 (Suppl. S1), 245–250. [Google Scholar] [PubMed]
- Byars, L.T.; Schatten, W.E. Subperiosteal segmental resection of the mandible. Plast Reconstr Surg Transplant Bull. 1960, 25, 142–145. [Google Scholar]
- Budal, J. The surgical removal of large osteofibromas. The postoperative osteogenic capacity of the periosteum. Oral Surg Oral Med Oral Pathol. 1970, 30, 303–308. [Google Scholar]
- Cardinal, L.; Dominguez, G.C.; Marodin, A.L.; Rau, L.H. Unusual spontaneous mandibular regeneration of a large defect followed by orthodontics, alveolar distraction, and dental implant rehabilitation: A 10-year follow-up. J Oral Maxillofac Surg. 2016, 74, 786–793. [Google Scholar]
- de Villa, G.H.; Chen, C.T.; Chen, Y.R. Spontaneous bone regeneration of the mandible in an elderly patient: A case report and review of the literature. Chang Gung Med J. 2003, 26, 363–369. [Google Scholar]
- Coen Pramono, D. Spontaneous bone regeneration after mandible resection in a case of ameloblastoma--a case report. Ann Acad Med Singap. 2004, 33, 59–62. [Google Scholar]
- Elbeshir, E.I. Spontaneous regeneration of the mandibular bone following hemimandibulectomy. Br J Oral Maxillofac Surg. 1990, 28, 128–130. [Google Scholar]
- Kamegai, A.; Mori, M.; Inoue, S. Mandibular reconstruction using electrically stimulated periosteum. J Cranio-Maxillo-Fac Surg. 1990, 18, 8–13. [Google Scholar]
- Espinosa, S.A.; Villanueva, J.; Hampel, H.; Reyes, D. Spontaneous regeneration after juvenile ossifying fibroma resection: A case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006, 102, e32–e35. [Google Scholar] [PubMed]
- Kazanjian, V.H. Spontaneous regeneration of bone following excision of section of the mandible. Am J Orthod Oral Surg 1946, 32, 242–248. [Google Scholar]
- Keizer, S.; Tuinzing, D.B. Spontaneous regeneration of a unilaterally absent mandibular condyle. J Oral Maxillofac Surg. 1985, 43, 130–132. [Google Scholar] [PubMed]
- Kisner, W.H. Spontaneous posttraumatic mandibular regeneration. Plast Reconstr Surg. 1980, 66, 442–447. [Google Scholar] [PubMed]
- Martins, W.D.; de Castro Avila, L.F. Partial spontaneous bone regeneration subsequent to mandibulectomy. J Contemp Dent Pract. 2004, 5, 108–120. [Google Scholar]
- Shuker, S. Spontaneous regeneration of the mandible in a child. A sequel to partial avulsion as a result of a war injury. J Maxillofac Surg. 1985, 13, 70–73. [Google Scholar]
- Nwoku, A.L. Unusually rapid bone regeneration following mandibular resection. J Maxillofac Surg. 1980, 8, 309–315. [Google Scholar]
- Boyne, P.J. The restoration of resected mandibles in children without the use of bone grafts. Head Neck Surg. 1983, 6, 626–631. [Google Scholar]
- Ogunlewe, M.O.; Akinwande, J.A.; Ladeinde, A.L.; Adeyemo, W.L. Spontaneous regeneration of whole mandible after total mandibulectomy in a sickle cell patient. J Oral Maxillofac Surg. 2006, 64, 981–984. [Google Scholar]
- Khodayari, A.K.A.; Khojasteh, A.; Kiani, M.; Nayebi, A.; Mehrdad, L.; Vahdatinia, M. Spontaneous regeneration of the mandible after hemimandibulectomy: Report of a case. J Dent. 2011, 8, 152–156. [Google Scholar]
- Abdulai, A.E. Complete spontaneous bone regeneration following partial mandibulectomy. Ghana Med J. 2012, 46, 147–174. [Google Scholar]
- Adebayo, E.T.; Fomete, B.; Ajike, S.O. Spontaneous bone regeneration following mandibular resection for odontogenic myxoma. Ann Afr Med. 2012, 11, 182–185. [Google Scholar] [CrossRef] [PubMed]
- Throndson, R.R.; Johnson, J.M. Spontaneous regeneration of bone after resection of central giant cell lesion: A case report. Tex Dent J. 2013, 130, 1201–1209. [Google Scholar]
- Anyanechi, C.E.; Saheeb, B.D.; Bassey, G.O. Spontaneous bone regeneration after segmental mandibular resection: A retrospective study of 13 cases. Int J Oral Maxillofac Surg. 2016, 45, 1268–1272. [Google Scholar] [CrossRef]
- Ruggiero, S.L.; Donoff, R.B. Bone regeneration after mandibular resection: Report of two cases. J Oral Maxillofac Surg. 1991, 49, 647–652. [Google Scholar] [CrossRef]
- Bataineh, A. Spontaneous bone regeneration of the mandible: In a case of osteosarcoma and literature review. Acad Medustin J Dent. 2016, 3. [Google Scholar]
- Guven, O. Formation of condyle-like structure after treatment of temporomandibular joint ankylosis: Literature review and long-term follow-up of two patients. Case Rep Med. 2017, 2017, 9060174. [Google Scholar] [CrossRef]
- Whitmyer, C.C.; Esposito, S.J.; Smith, J.D.; Zins, J.E. Spontaneous regeneration of a resected mandible in a preadolescent: A clinical report. J Prosthet Dent. 1996, 75, 356–359. [Google Scholar] [CrossRef]
- Rai, S.; Rattan, V.; Jolly, S.S.; Sharma, V.K.; Mubashir, M.M. Spontaneous regeneration of bone in segmental mandibular defect. J Maxillofac Oral Surg. 2019, 18, 224–228. [Google Scholar] [CrossRef]
- Mesgarzadeh, A.H.; Abadi, A.; Keshani, F. Seven-year follow-up of spontaneous bone regeneration following segmental mandibulectomy: Alternative option for mandibular reconstruction. Dent Res J. 2019, 16, 435–440. [Google Scholar]
- Li, Z.; Pan, J.; Ma, J.; Zhang, Z.; Bai, Y. Microarray gene expression of periosteum in spontaneous bone regeneration of mandibular segmental defects. Sci Rep. 2017, 7, 13535. [Google Scholar] [CrossRef]
- Al-Kattan, R.; Retzepi, M.; Calciolari, E.; Donos, N. Microarray gene expression during early healing of GBR-treated calvarial critical size defects. Clin Oral Implants Res. 2017, 28, 1248–1257. [Google Scholar] [CrossRef] [PubMed]
- Ivanovski, S.; Hamlet, S.; Retzepi, M.; Wall, I.; Donos, N. Transcriptional profiling of “guided bone regeneration” in a critical-size calvarial defect. Clin Oral Implants Res. 2011, 22, 382–389. [Google Scholar] [CrossRef]
- Matsushima, S.; Isogai, N.; Jacquet, R.; Lowder, E.; Tokui, T.; Landis, W.J. The nature and role of periosteum in bone and cartilage regeneration. Cells Tissues Organs. 2011, 194, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Stricker, S.; Mundlos, S. FGF and ROR2 receptor tyrosine kinase signaling in human skeletal development. Curr Top Dev Biol. 2011, 97, 179–206. [Google Scholar]
- Berendsen, A.D.; Olsen, B.R. Bone development. Bone development. Bone. 2015, 80, 14–18. [Google Scholar] [CrossRef]
- Ripamonti, U.; Duarte, R.; Ferretti, C.; Reddi, A.H. Osteogenic competence and potency of the bone induction principle: inductive substrates that initiate bone: Formation by autoinduction. J Craniofac Surg. 2022, 33, 971–984. [Google Scholar] [CrossRef]
- Verdugo, F.; Simonian, K.; D’Addona, A.; Ponton, J.; Nowzari, H. Human bone repair after mandibular symphysis block harvesting: A clinical and tomographic study. J Periodontol. 2010, 81, 702–709. [Google Scholar] [CrossRef]
- Wang, X.; Yu, Y.Y.; Lieu, S.; et al. MMP9 regulates the cellular response to inflammation after skeletal injury. Bone. 2013, 52, 111–119. [Google Scholar] [CrossRef]
- Kolar, P.; Gaber, T.; Perka, C.; Duda, G.N.; Buttgereit, F. Human early fracture hematoma is characterized by inflammation and hypoxia. Clin Orthop Relat Res. 2011, 469, 3118–3126. [Google Scholar] [PubMed]
- Kolar, P.; Schmidt-Bleek, K.; Schell, H.; et al. The early fracture hematoma and its potential role in fracture healing. Tissue Eng Part B Rev. 2010, 16, 427–434. [Google Scholar] [CrossRef]
- Ruocco, M.G.; Karin, M. Control of osteoclast activity and bone loss by IKK subunits: New targets for therapy. Adv Exp Med Biol. 2007, 602, 125–134. [Google Scholar] [PubMed]
- Hashimoto, K.; Kaito, T.; Furuya, M.; et al. In vivo dynamic analysis of BMP-2-induced ectopic bone formation. Sci Rep. 2020, 10, 4751. [Google Scholar]
- Reyes, R.; Rodriguez, J.A.; Orbe, J.; Arnau, M.R.; Evora, C.; Delgado, A. Combined sustained release of BMP2 and MMP10 accelerates bone formation and mineralization of calvaria critical size defect in mice. Drug Deliv. 2018, 25, 750–756. [Google Scholar] [PubMed]
- Minear, S.; Leucht, P.; Jiang, J.; et al. Wnt proteins promote bone regeneration. Sci Transl Med. 2010, 2, 29ra30. [Google Scholar]
- Minear, S.; Leucht, P.; Miller, S.; Helms, J.A. rBMP represses Wnt signaling and influences skeletal progenitor cell fate specification during bone repair. J Bone Miner Res. 2010, 25, 1196–1207. [Google Scholar]

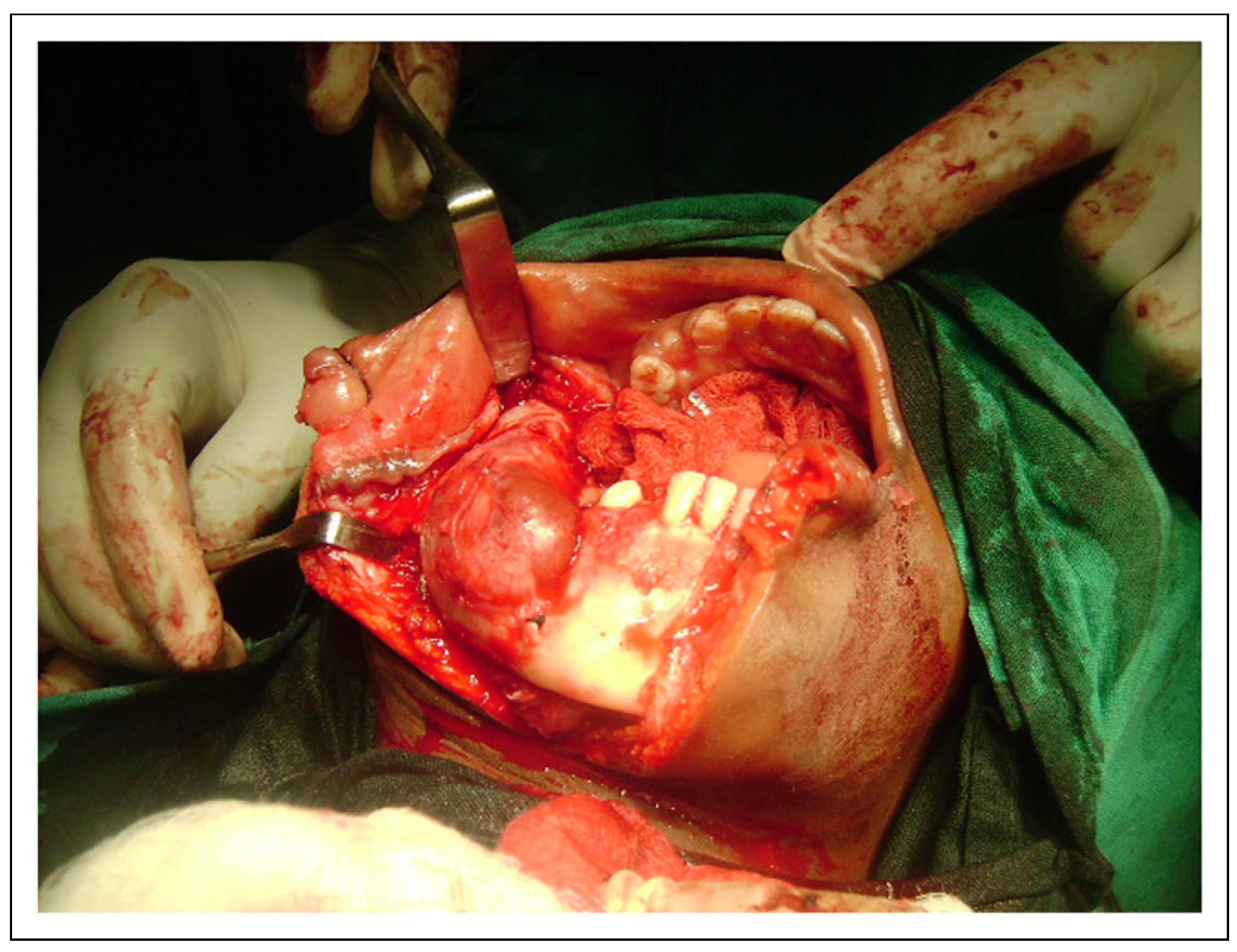
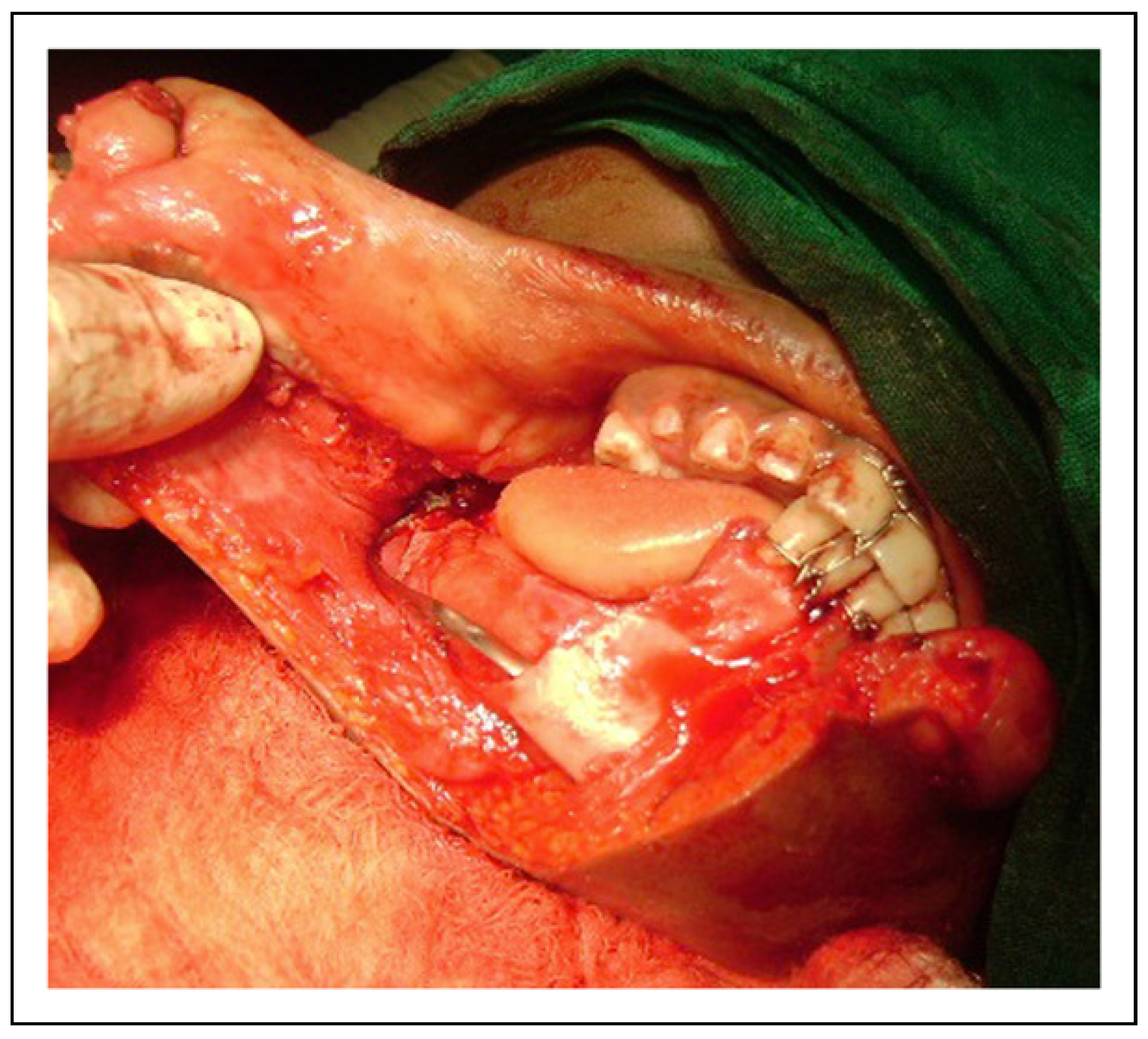
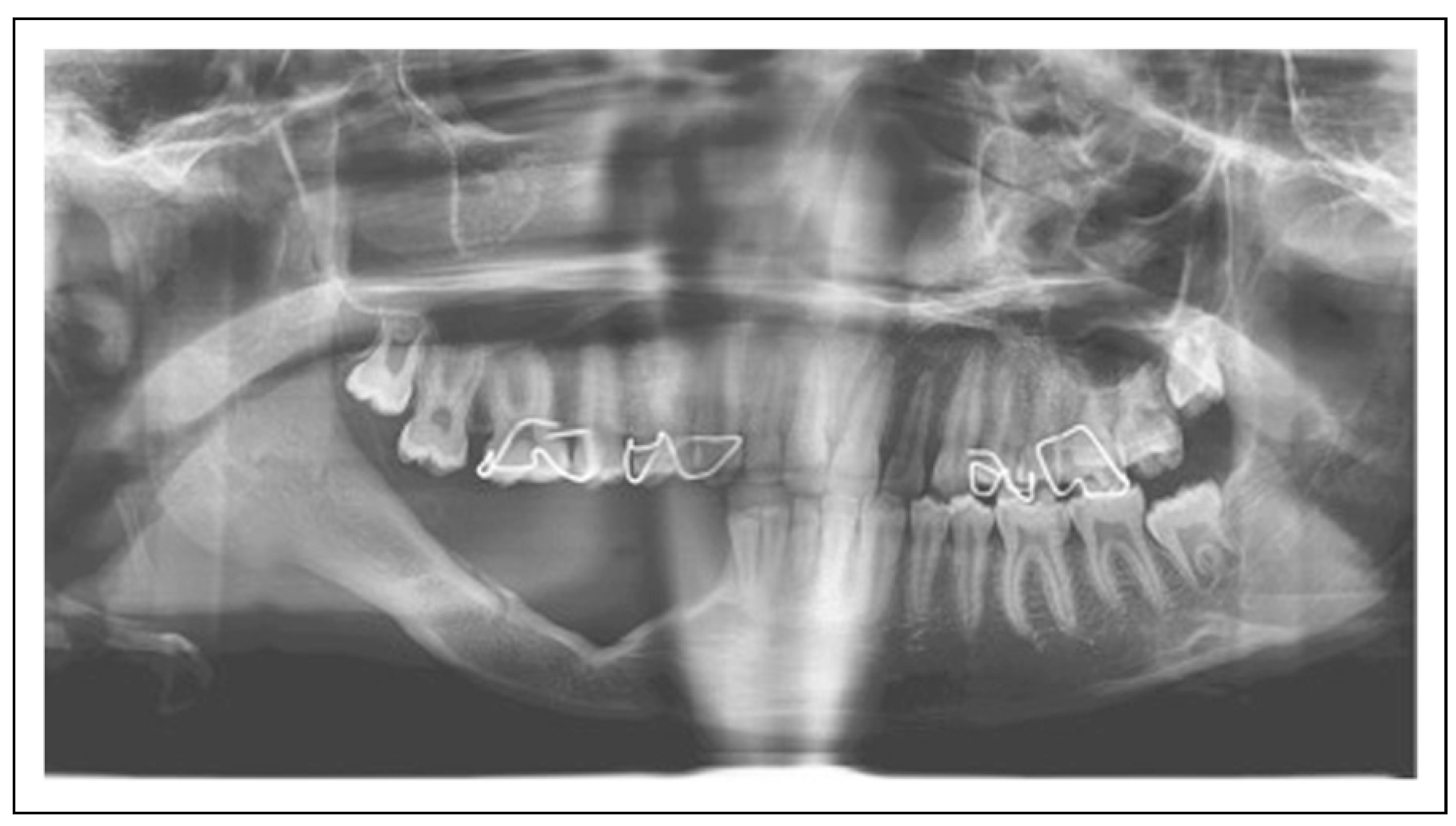
© 2023 by the author. The Author(s) 2008.
Share and Cite
Okoturo, E. Review of the Literature on the Current State of Periosteum-Mediated Craniofacial Bone Regeneration. Craniomaxillofac. Trauma Reconstr. 2024, 17, 253-262. https://doi.org/10.1177/19433875231214068
Okoturo E. Review of the Literature on the Current State of Periosteum-Mediated Craniofacial Bone Regeneration. Craniomaxillofacial Trauma & Reconstruction. 2024; 17(3):253-262. https://doi.org/10.1177/19433875231214068
Chicago/Turabian StyleOkoturo, Eyituoyo. 2024. "Review of the Literature on the Current State of Periosteum-Mediated Craniofacial Bone Regeneration" Craniomaxillofacial Trauma & Reconstruction 17, no. 3: 253-262. https://doi.org/10.1177/19433875231214068
APA StyleOkoturo, E. (2024). Review of the Literature on the Current State of Periosteum-Mediated Craniofacial Bone Regeneration. Craniomaxillofacial Trauma & Reconstruction, 17(3), 253-262. https://doi.org/10.1177/19433875231214068




