In Vitro Enhanced Osteogenic Potential of Human Mesenchymal Stem Cells Seeded in a Poly (Lactic-co-Glycolic) Acid Scaffold: A Systematic Review
Abstract
:Introduction
Methods
Study Selection
Data Source and Search Strategy
Data Collection Process
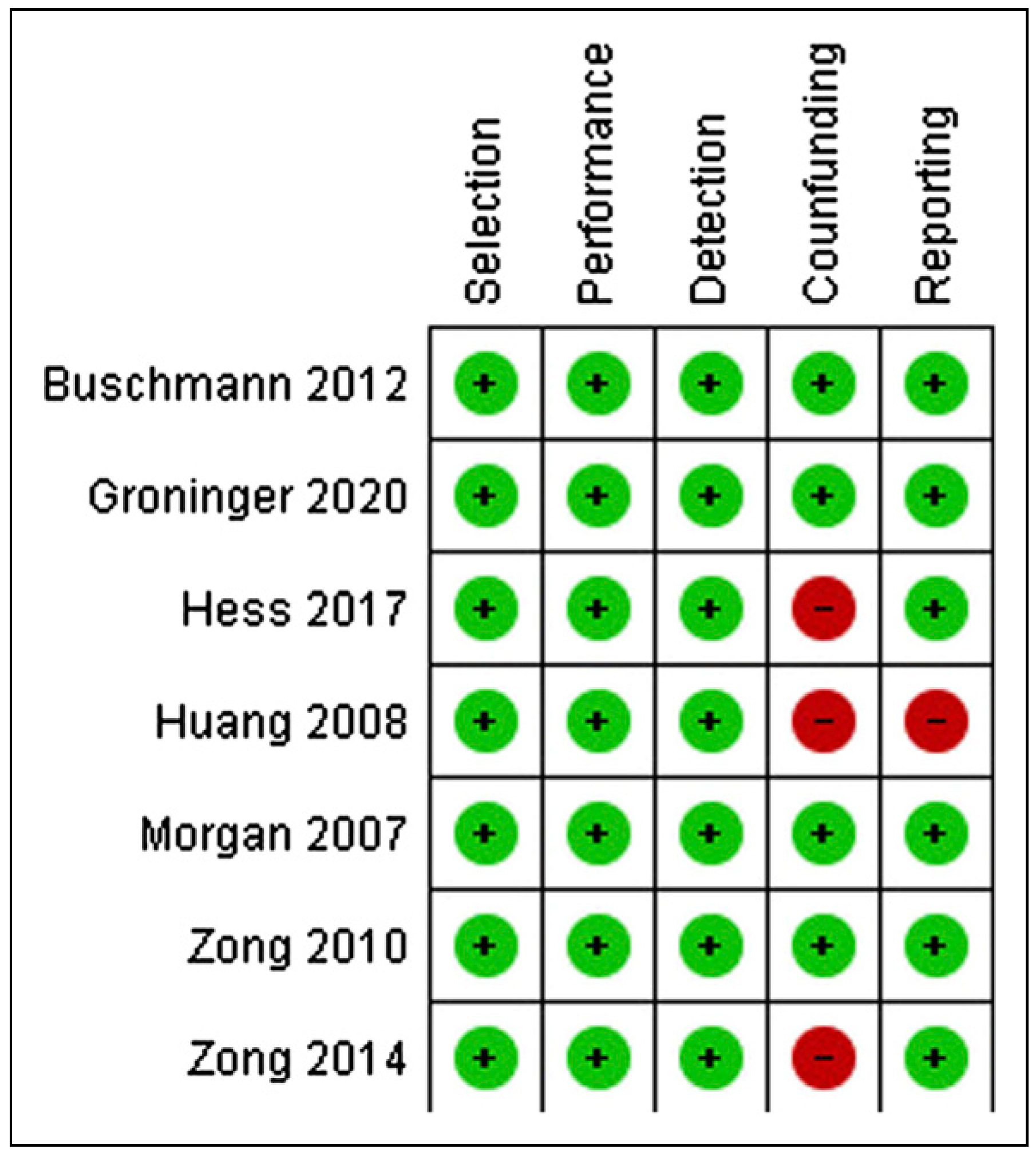
Risk of Bias
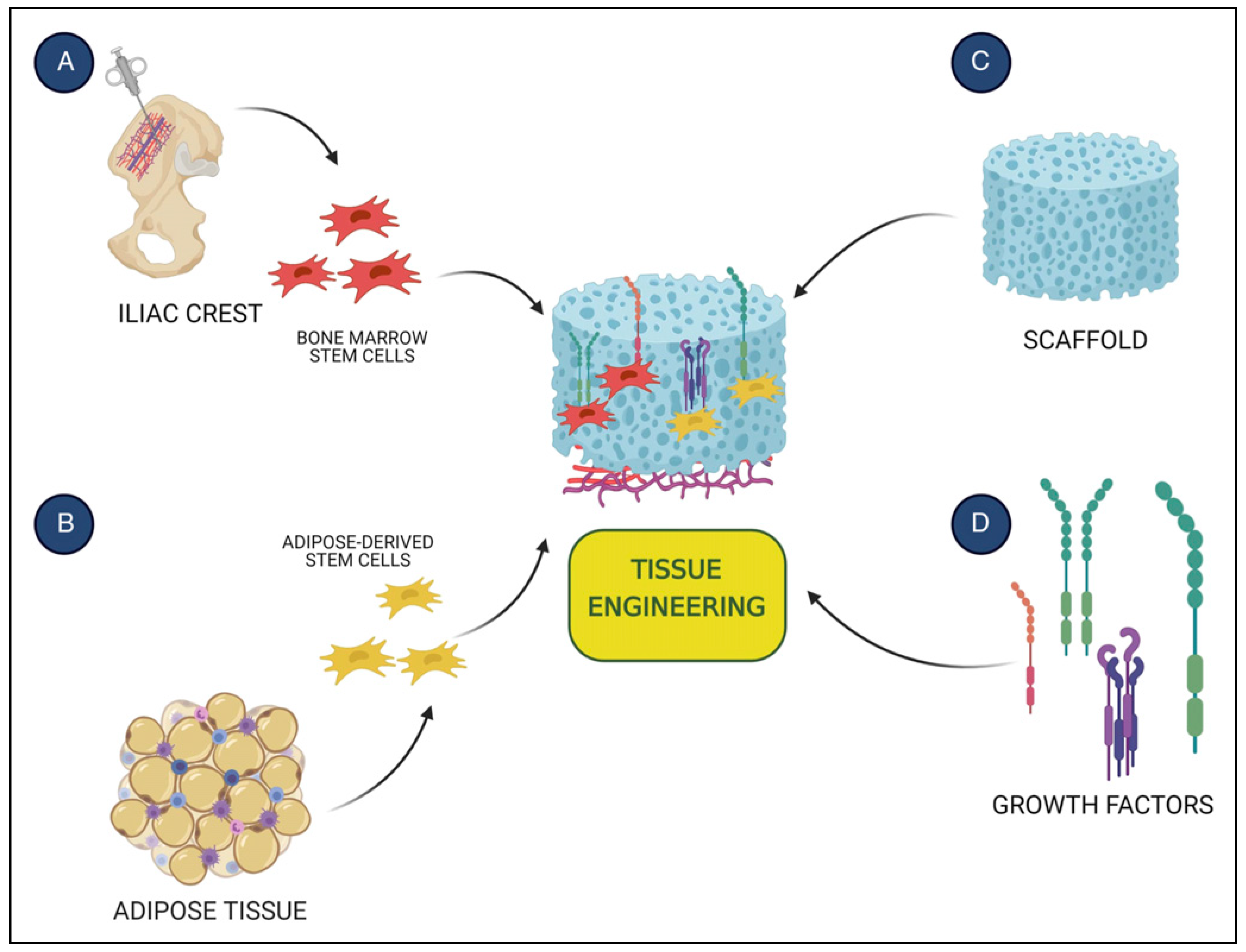
Results
Study Selection and Characteristics
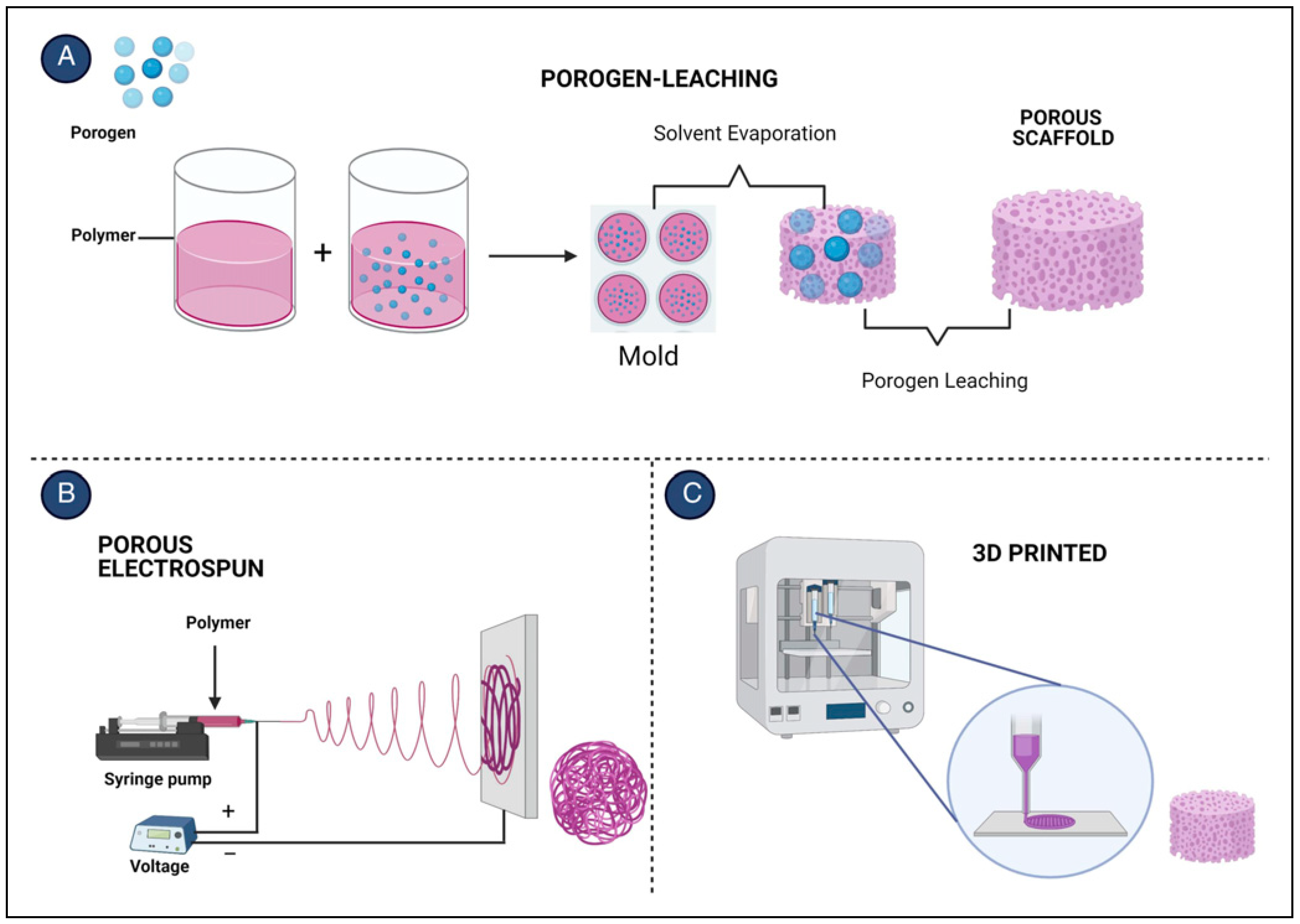
Poly (Lactic-co-Glycolic) Acid Scaffold Features
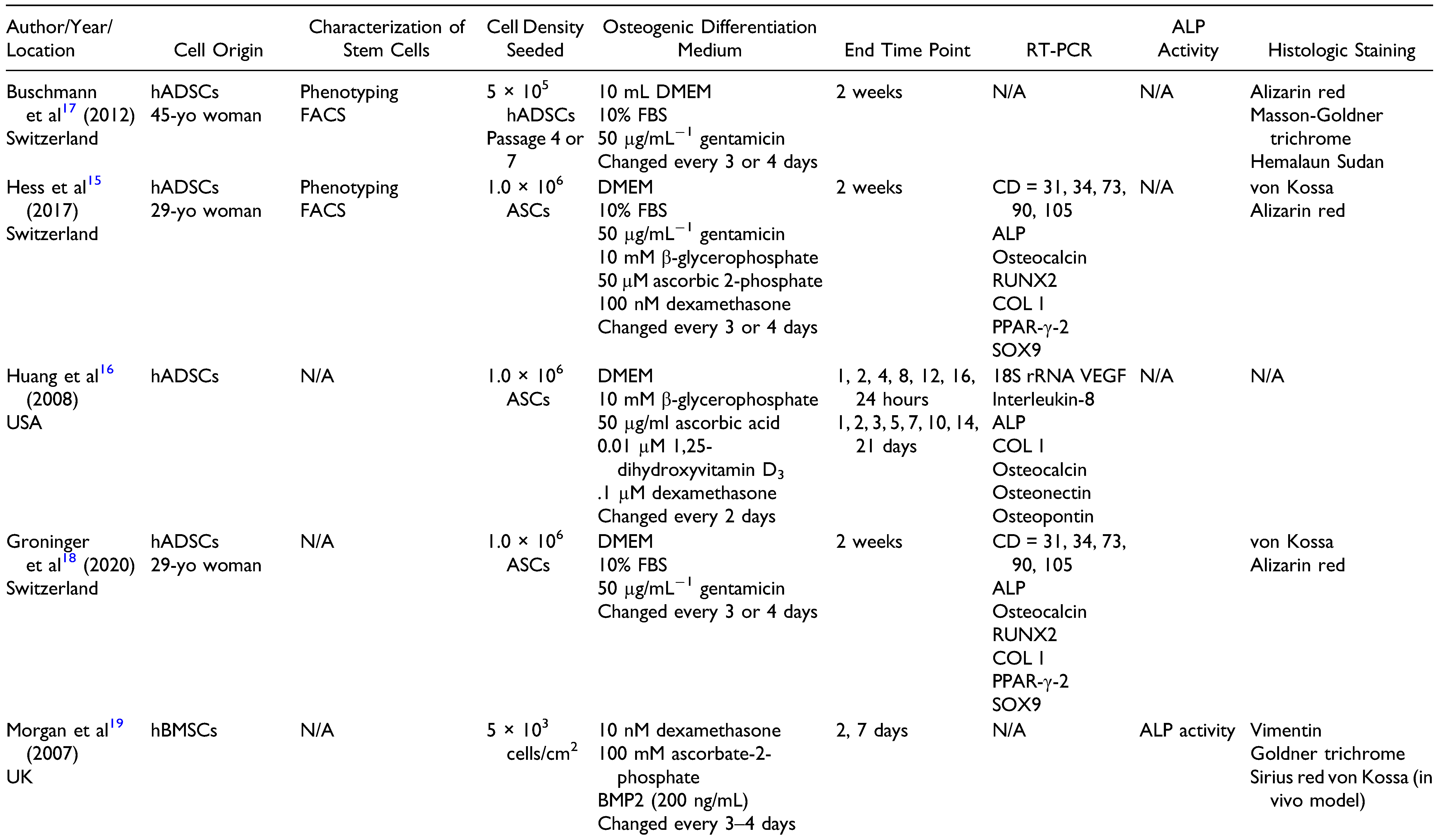
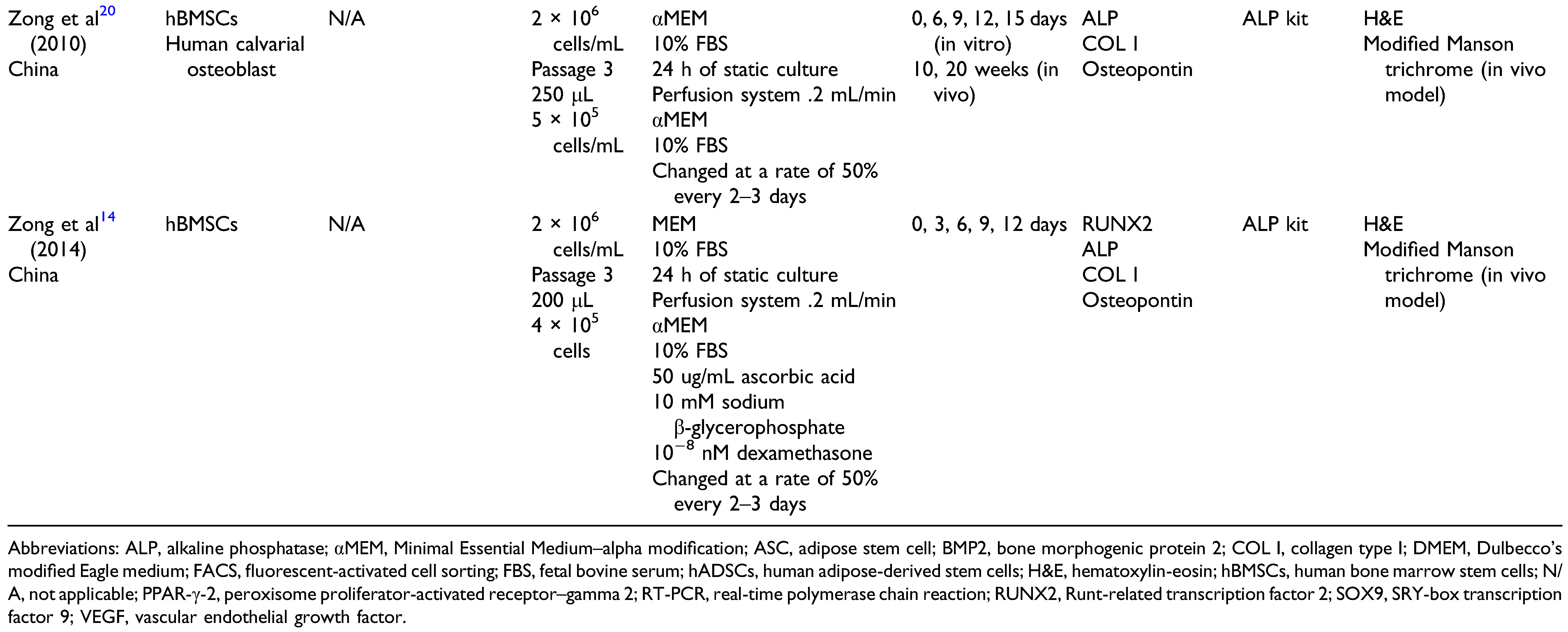
Characterization of hBMSCs in a PLGA Scaffold
Characterization of hADSCs in a PLGA Scaffold
Discussion
Future Clinical Implications
Conclusions
Funding
Declaration of Conflicting Interests
References
- Ho-Shui-Ling, A.; Bolander, J.; Rustom, L.E.; Johnson, A.W.; Luyten, F.P.; Picart, C. Bone regeneration strategies: Engineered scaffolds, bioactive molecules and stem cells current stage and future perspectives. Biomaterials. 2018, 180, 143–162. [Google Scholar]
- Kinaci, A.; Neuhaus, V.; Ring, D.C. Trends in bone graft use in the United States. Orthopedics. 2014, 37, e783–e788. [Google Scholar]
- Crist, T.E.; Mathew, P.J.; Plotsker, E.L.; Sevilla, A.C.; Thaller, S.R. biomaterials in craniomaxillofacial reconstruction: Past, present, and future. J Craniofac Surg. 2020, 32, 535–540. [Google Scholar]
- Ebraheim, N.A.; Elgafy, H.; Xu, R. Bone-graft harvesting from iliac and fibular donor sites: Techniques and complications. J Am Acad Orthop Surg. 2001, 9, 210–218. [Google Scholar] [PubMed]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone tissue engineering: Recent advances and challenges. Crit Rev Biomed Eng. 2012, 40, 363–408. [Google Scholar]
- Liao, H.T.; Chen, C.T. Osteogenic potential: Comparison between bone marrow and adipose-derived mesenchymal stem cells. World J Stem Cells. 2014, 6, 288–295. [Google Scholar] [PubMed]
- Chen, G.; Kawazoe, N. Porous scaffolds for regeneration of cartilage, bone and osteochondral tissue. Adv Exp Med Biol. 2018, 1058, 171–191. [Google Scholar]
- Casagrande, S.; Tiribuzi, R.; Cassetti, E.; et al. Biodegradable composite porous poly(dl-lactide-co-glycolide) scaffold supports mesenchymal stem cell differentiation and calcium phosphate deposition. Artif Cells Nanomed Biotechnol. 2018, 46 (Suppl. 1), 219–229. [Google Scholar]
- Bharadwaz, A.; Jayasuriya, A.C. Recent trends in the application of widely used natural and synthetic polymer nanocomposites in bone tissue regeneration. Mater Sci Eng C Mater Biol Appl. 2020, 110, 110698. [Google Scholar]
- Friedenstein, A.J.; Petrakova, K.V.; Kurolesova, A.I.; Frolova, G.P. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968, 6, 230–247. [Google Scholar]
- Mizuno, H.; Tobita, M.; Uysal, A.C. Concise review: Adipose-derived stem cells as a novel tool for future regenerative medicine. Stem Cells. 2012, 30, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Pendleton, C.; Li, Q.; Chesler, D.A.; Yuan, K.; Guerrero-Cazares, H.; Quinones-Hinojosa, A. Mesenchymal stem cells derived from adipose tissue vs bone marrow: In vitro comparison of their tropism towards gliomas. PLoS One. 2013, 8, e58198. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016, 355, i4919. [Google Scholar] [CrossRef]
- Zong, C.; Qian, X.; Tang, Z.; et al. Biocompatibility and bone-repairing effects: Comparison between porous poly-lactic-co-glycolic acid and nano-hydroxyapatite/poly(lactic acid) scaffolds. J Biomed Nanotechnol. 2014, 10, 1091–1104. [Google Scholar] [PubMed]
- Hess, S.C.; Stark, W.J.; Mohn, D.; et al. Gene expression in human adipose-derived stem cells: Comparison of 2D films, 3D electrospun meshes or co-cultured scaffolds with two-way paracrine effects. Eur Cell Mater. 2017, 34, 232–248. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.K.; Huang, W.; Zuk, P.; et al. Genetic markers of osteogenesis and angiogenesis are altered in processed lipoaspirate cells when cultured on three-dimensional scaffolds. Plast Reconstr Surg. 2008, 121, 411–423. [Google Scholar] [CrossRef]
- Buschmann, J.; Harter, L.; Gao, S.; et al. Tissue engineered bone grafts based on biomimetic nanocomposite PLGA/amorphous calcium phosphate scaffold and human adipose-derived stem cells. Injury. 2012, 43, 1689–1697. [Google Scholar]
- Groninger, O.; Hess, S.; Mohn, D.; et al. Directing stem cell commitment by amorphous calcium phosphate nanoparticles incorporated in PLGA: Relevance of the free calcium ion concentration. Int J Mol Sci. 2020, 21, 2627. [Google Scholar]
- Morgan, S.M.; Tilley, S.; Perera, S.; et al. Expansion of human bone marrow stromal cells on poly-(DL-lactide-coglycolide) (PDL LGA) hollow fibres designed for use in skeletal tissue engineering. Biomaterials. 2007, 28, 5332–5343. [Google Scholar] [CrossRef]
- Zong, C.; Xue, D.; Yuan, W.; et al. Reconstruction of rat calvarial defects with human mesenchymal stem cells and osteoblast-like cells in poly-lactic-co-glycolic acid scaffolds. Eur Cell Mater. 2010, 20, 109–120. [Google Scholar]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; et al. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef]
- Oliveira, É.R.; Nie, L.; Podstawczyk, D.; et al. Advances in growth factor delivery for bone tissue engineering. Int J Mol Sci. 2021, 22, 903. [Google Scholar] [CrossRef]
- Pereira, H.F.; Cengiz, I.F.; Silva, F.S.; Reis, R.L.; Oliveira, J.M. Scaffolds and coatings for bone regeneration. J Mater Sci Mater Med. 2020, 31, 27. [Google Scholar]
- Dominici, M.; Le Blanc, K.; Mueller, I.; et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International society for cellular therapy position statement. Cytotherapy. 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Satija, N.K.; Singh, V.K.; Verma, Y.K.; et al. Mesenchymal stem cell-based therapy: A new paradigm in regenerative medicine. J Cell Mol Med. 2009, 13, 4385–4402. [Google Scholar] [PubMed]
- Im, G.I.; Shin, Y.W.; Lee, K.B. Do adipose tissue-derived mesenchymal stem cells have the same osteogenic and chondrogenic potential as bone marrow-derived cells? Osteoarthritis Cartilage. 2005, 13, 845–853. [Google Scholar]
- Liu, T.M.; Martina, M.; Hutmacher, D.W.; Hui, J.H.P.; Lee, E.H.; Lim, B. Identification of common pathways mediating differentiation of bone marrow- and adipose tissue-derived human mesenchymal stem cells into three mesenchymal lineages. Stem Cells. 2007, 25, 750–760. [Google Scholar]
- Vishnubalaji, R.; Al-Nbaheen, M.; Kadalmani, B.; Aldahmash, A.; Ramesh, T. Comparative investigation of the differentiation capability of bone-marrow- and adipose-derived mesenchymal stem cells by qualitative and quantitative analysis. Cell Tissue Res. 2012, 347, 419–427. [Google Scholar] [CrossRef]
- Park, S.H.; Sim, W.Y.; Min, B.H.; Yang, S.S.; Khademhosseini, A.; Kaplan, D.L. Chip-based comparison of the osteogenesis of human bone marrow- and adipose tissue-derived mesenchymal stem cells under mechanical stimulation. PLoS One. 2012, 7, e46689. [Google Scholar] [CrossRef]
- Shafiee, A.; Seyedjafari, E.; Soleimani, M.; Ahmadbeigi, N.; Dinarvand, P.; Ghaemi, N. A comparison between osteogenic differentiation of human unrestricted somatic stem cells and mesenchymal stem cells from bone marrow and adipose tissue. Biotechnol Lett. 2011, 33, 1257–1264. [Google Scholar]
- Kern, S.; Eichler, H.; Stoeve, J.; Klüter, H.; Bieback, K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006, 24, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.T.; Lee, M.J.; Chen, C.H.; et al. Proliferation and differentiation potential of human adipose-derived mesenchymal stem cells isolated from elderly patients with osteoporotic fractures. J Cell Mol Med. 2012, 16, 582–593. [Google Scholar] [CrossRef]
- El-Badawy, A.; Amer, M.; Abdelbaset, R.; et al. Adipose stem cells display higher regenerative capacities and more adaptable electro-kinetic properties compared to bone marrow-derived mesenchymal stromal cells. Sci Rep. 2016, 6, 37801. [Google Scholar]
- Gosain, A.K.; Riordan, P.A.; Song, L.; et al. A 1-year study of osteoinduction in hydroxyapatite-derived biomaterials in an adult sheep model: Part, I.I. Bioengineering implants to optimize bone replacement in reconstruction of cranial defects. Plast Reconstr Surg. 2004, 114, 1155–1163; discussion 1164–1155. [Google Scholar]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Préat, V. PLGA-based nanoparticles: An overview of biomedical applications. J Control Release. 2012, 161, 505–522. [Google Scholar] [PubMed]
- Ferrara, N.; Bunting, S. Vascular endothelial growth factor, a specific regulator of angiogenesis. Curr Opin Nephrol Hypertens. 1996, 5, 35–44. [Google Scholar] [PubMed]
- Kim, Y.; Kim, H.; Cho, H.; Bae, Y.; Suh, K.; Jung, J. Direct comparison of human mesenchymal stem cells derived from adipose tissues and bone marrow in mediating neovascularization in response to vascular ischemia. Cell Physiol Biochem. 2007, 20, 867–876. [Google Scholar]
- Mokhtari-Jafari, F.; Amoabediny, G.; Dehghan, M.M. Role of biomechanics in vascularization of tissue-engineered bones. J Biomech. 2020, 110, 109920. [Google Scholar]
- Bhuiyan, D.B.; Middleton, J.C.; Tannenbaum, R.; Wick, T.M. Mechanical properties and osteogenic potential of hydroxyapatite-PLGA-collagen biomaterial for bone regeneration. J Biomater Sci Polym Ed. 2016, 27, 1139–1154. [Google Scholar]
- Bhuiyan, D.B.; Middleton, J.C.; Tannenbaum, R.; Wick, T.M. Bone regeneration from human mesenchymal stem cells on porous hydroxyapatite-PLGA-collagen bioactive polymer scaffolds. Biomed Mater Eng. 2017, 28, 671–685. [Google Scholar]
- Qi, H.; Ye, Z.; Ren, H.; et al. Bioactivity assessment of PLLA/PCL/HAP electrospun nanofibrous scaffolds for bone tissue engineering. Life Sci. 2016, 148, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Xu, H.; Gao, Y.; et al. Promoting osteoblast proliferation on polymer bone substitutes with bone-like structure by combining hydroxyapatite and bioactive glass. Mater Sci Eng C Mater Biol Appl. 2019, 96, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Shuai, C.; Yu, L.; Feng, P.; Gao, C.; Peng, S. Interfacial reinforcement in bioceramic/biopolymer composite bone scaffold: The role of coupling agent. Colloids Surf B Biointerfaces. 2020, 193, 111083. [Google Scholar] [CrossRef] [PubMed]
- Boyan, B.D.; Hummert, T.W.; Dean, D.D.; Schwartz, Z. Role of material surfaces in regulating bone and cartilage cell response. Biomaterials. 1996, 17, 137–146. [Google Scholar] [CrossRef]
- Du, B.; Yin, H.; Chen, Y.; et al. A waterborne polyurethane 3D scaffold containing PLGAwith a controllable degradation rate and an anti-inflammatory effect for potential applications in neural tissue repair. J Mater Chem B. 2020, 8, 4434–4446. [Google Scholar] [CrossRef]


  |
 |
© 2023 by the author. The Author(s) 2023.
Share and Cite
Maita, K.C.; Avila, F.R.; Torres-Guzman, R.A.; Sarabia-Estrada, R.; Zubair, A.C.; Quinones-Hinojosa, A.; Forte, A.J. In Vitro Enhanced Osteogenic Potential of Human Mesenchymal Stem Cells Seeded in a Poly (Lactic-co-Glycolic) Acid Scaffold: A Systematic Review. Craniomaxillofac. Trauma Reconstr. 2024, 17, 61-73. https://doi.org/10.1177/19433875231157454
Maita KC, Avila FR, Torres-Guzman RA, Sarabia-Estrada R, Zubair AC, Quinones-Hinojosa A, Forte AJ. In Vitro Enhanced Osteogenic Potential of Human Mesenchymal Stem Cells Seeded in a Poly (Lactic-co-Glycolic) Acid Scaffold: A Systematic Review. Craniomaxillofacial Trauma & Reconstruction. 2024; 17(1):61-73. https://doi.org/10.1177/19433875231157454
Chicago/Turabian StyleMaita, Karla C., Francisco R. Avila, Ricardo A. Torres-Guzman, Rachel Sarabia-Estrada, Abba C. Zubair, Alfredo Quinones-Hinojosa, and Antonio J. Forte. 2024. "In Vitro Enhanced Osteogenic Potential of Human Mesenchymal Stem Cells Seeded in a Poly (Lactic-co-Glycolic) Acid Scaffold: A Systematic Review" Craniomaxillofacial Trauma & Reconstruction 17, no. 1: 61-73. https://doi.org/10.1177/19433875231157454
APA StyleMaita, K. C., Avila, F. R., Torres-Guzman, R. A., Sarabia-Estrada, R., Zubair, A. C., Quinones-Hinojosa, A., & Forte, A. J. (2024). In Vitro Enhanced Osteogenic Potential of Human Mesenchymal Stem Cells Seeded in a Poly (Lactic-co-Glycolic) Acid Scaffold: A Systematic Review. Craniomaxillofacial Trauma & Reconstruction, 17(1), 61-73. https://doi.org/10.1177/19433875231157454




