Introduction
Australian households subscribe to some of the highest rates of pet ownership in the world, rivalling that of the United States of America and the United Kingdom [
1]. Despite a comparatively small population of 25 million people, Australia is home to approximately 5 million dogs [
1,
2]. In practical terms, this equates to more than 40% of households having at least one dog and most significantly, rates of ownership highest amongst young adults and families with young children [
1]. The COVID-19 pandemic has further perpetuated this trend, with the Royal Society for the Prevention of Cruelty to Animals (RSPCA) in NSW recording a significant increase in adoptions since the initial wave of social distancing and lockdown restrictions [
3].
Over the past few generations, there has been a shift in attitude towards pet ownership, with dogs increasingly being considered as a family member [
1]. The evolution of the human-pet dynamic can be attributed in part to the ‘pet effect’, a well-established phenomenon describing the benefits of pet ownership on owner health [
4]. This includes improving cardiovascular health, physical fitness, immune system development, psychological health and child development [
4]. However, it becomes a salient public health issue when dog ownership begets dog bites [
4,
5].
The Australian Institute of Health and Welfare (AIHW) has reported an alarming trend in the incidence of hospitalisations for dog-related injury [
6]. In the 2001–2013 reporting period, an average of 2601 people were hospitalised annually, increasing to nearly 4000 in 2013–2014; a rate of 17 cases per 100 000 population [
5,
6]. From 2001 to 2014, there was a consistent proclivity towards injury in the male 0–9 age group. Most significantly, the body region predominantly injured in this younger age bracket was the head [
6]. A 10-year retrospective cohort analysis from Sydney Children’s Hospital of 628 patients similarly found that 64% of paediatric dog bites involved the facial, head and neck regions [
7].
In view of our anecdotal evidence of an increasing frequency of facial dog bite injures, the authors investigated their own institutional figures in order to accurately define the problem. There was a fourfold purpose to the study: (I) to estimate the frequency of facial dog bite injuries, (II) to investigate the epidemiology of facial dog bite injuries, (III), to stratify the range of injury types and (IV) to compare injury management with current guidelines and best practice.
The hypothesis that underpinned the study was that the paediatric population is at high risk, most likely as a result of provoked incidents. Given COVID-19 social distancing was associated with years 2020 and 2021, we further hypothesised that worsening rates of dog ownership coupled with the imposition of stay-at-home legislation (both lockdowns and self-isolation/quarantine) likely increased the frequency of these injuries.
The study had three specific aims: (I) to determine the most susceptible demographic within the study cohort, (II) to identify factors associated with an increased risk of severe injury and (III) to determine the morbidity associated with a facial dog bite injury.
Results
In total, 171 patients were managed by the Oral Maxillofacial Surgery Department for facial dog bite injuries in the designated period of study, from January 2017 to January 2022. Across the 5-year period, there was a demonstrable progressive increase in the total number of cases per year with 16 in 2017, 28 in 2018, 34 in 2019, 36 in 2020 and 57 in 2021.
In response to COVID-19, there were two strict lockdown periods of home confinement in Newcastle, Australia: (I) 31/03/2020 to 15/05/2020, (II) 05/08/2021 to 10/09/2021. During the first period, we recorded four cases, which was a reduction from the comparable dates of 2019 in which there were five cases. The second period reported five incidents, a minor increase from four cases in 2019.
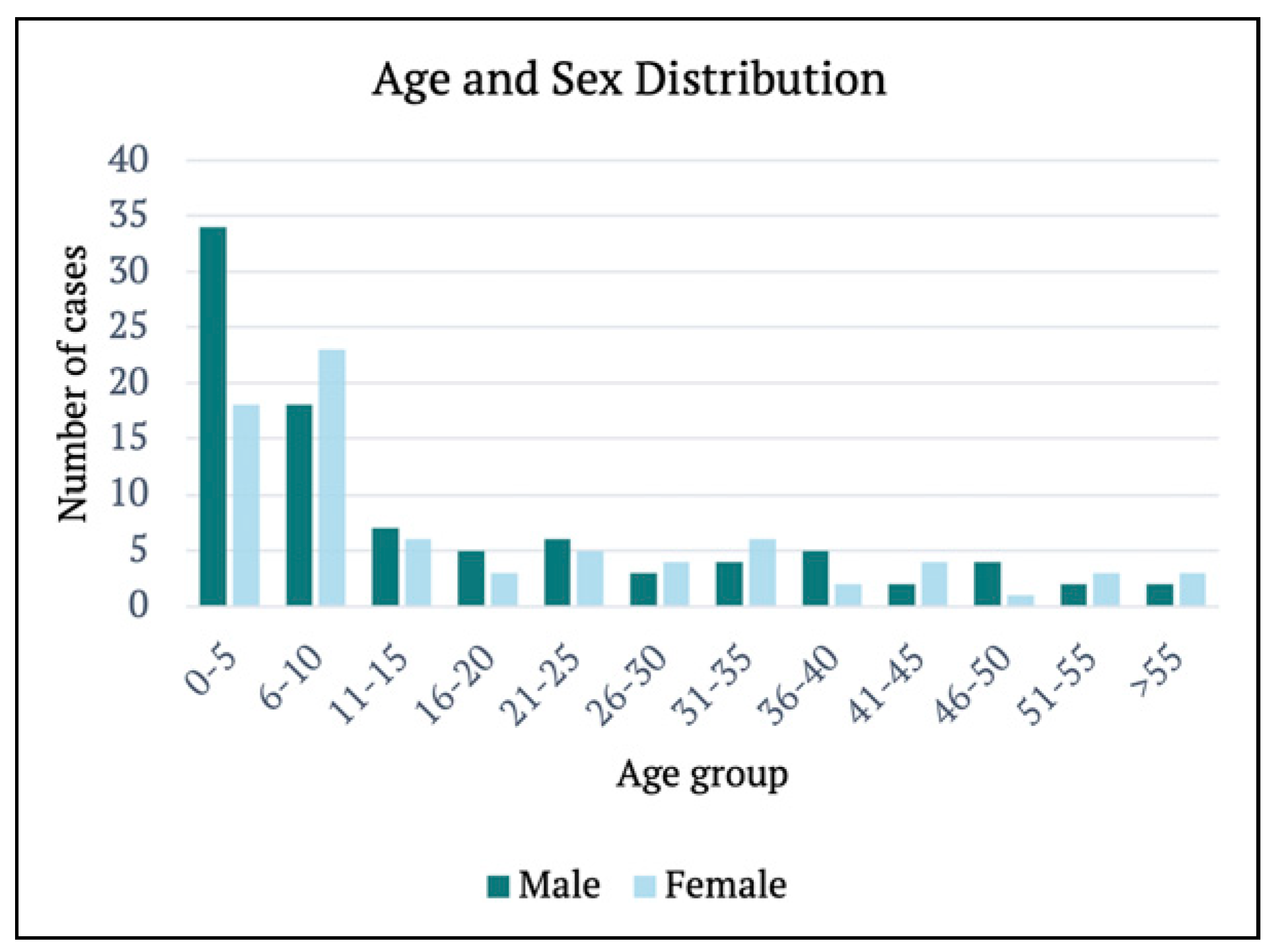
The median age of patients was 9 years (range, 11 mth to 77 yr), with the highest incidence of a single age four years. The prevalence was also slightly greater amongst males (n = 93) than females (n = 78). The paediatric cohort (n = 110), particularly the 0–5 age group (n = 52), sustained more injuries than the adult population (n = 61). The age and sex distributions are summarised in
Figure 1.
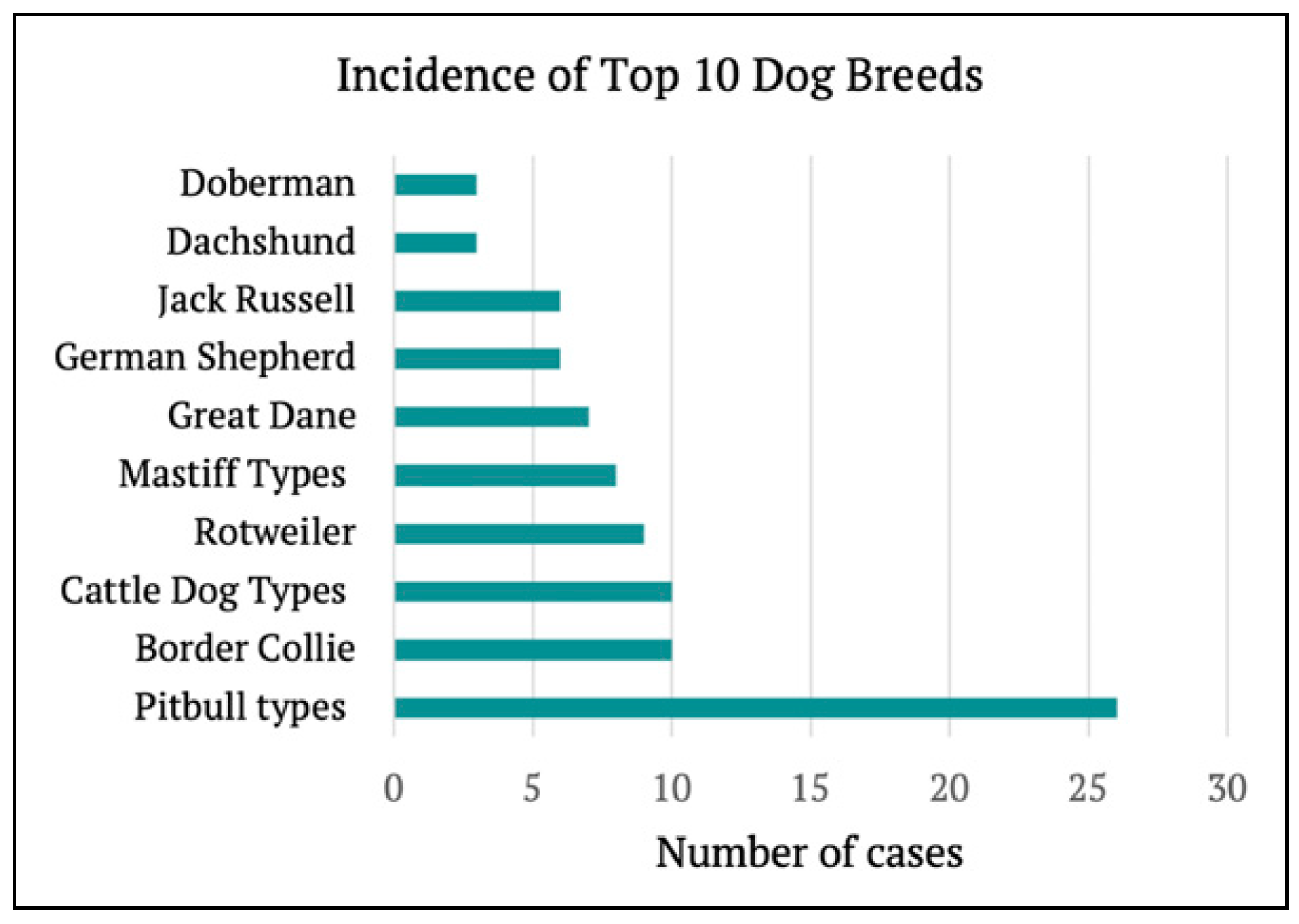
Dog breed was recorded in 108 (63%) cases. Pitbull types (n = 26), Border Collies (n = 10) and Cattle Dog types (n = 10) were the most frequent offenders. The most common breeds are represented in
Figure 2. Based on the average weight of the breed, dogs were further grouped by small (0–10 kg), medium (10–25 kg) or large (>25 kg). A similar incidence occurred between medium (n = 50) and large (n = 44) dogs, with fewer cases attributed to small dogs (n = 14).
In 87 (51%) presentations, the dog was identified as a family member. Most often, the incident transpired at their own home (n = 84), followed by a family member’s or friend’s house (n = 40), outside (n = 10), then neighbour’s house (n = 9). The preceding circumstances were documented in 130 (76%) cases. Of these, 64 incidents occurred whilst playing with the dog, 31 attacks were unprovoked, 16 occurred whilst showing affection towards the dog (i.e., cuddling, kissing or petting), 13 were provoked (i.e., removing food or toy, stepping on or pulling dog tail) and 6 whilst feeding or washing the dog.
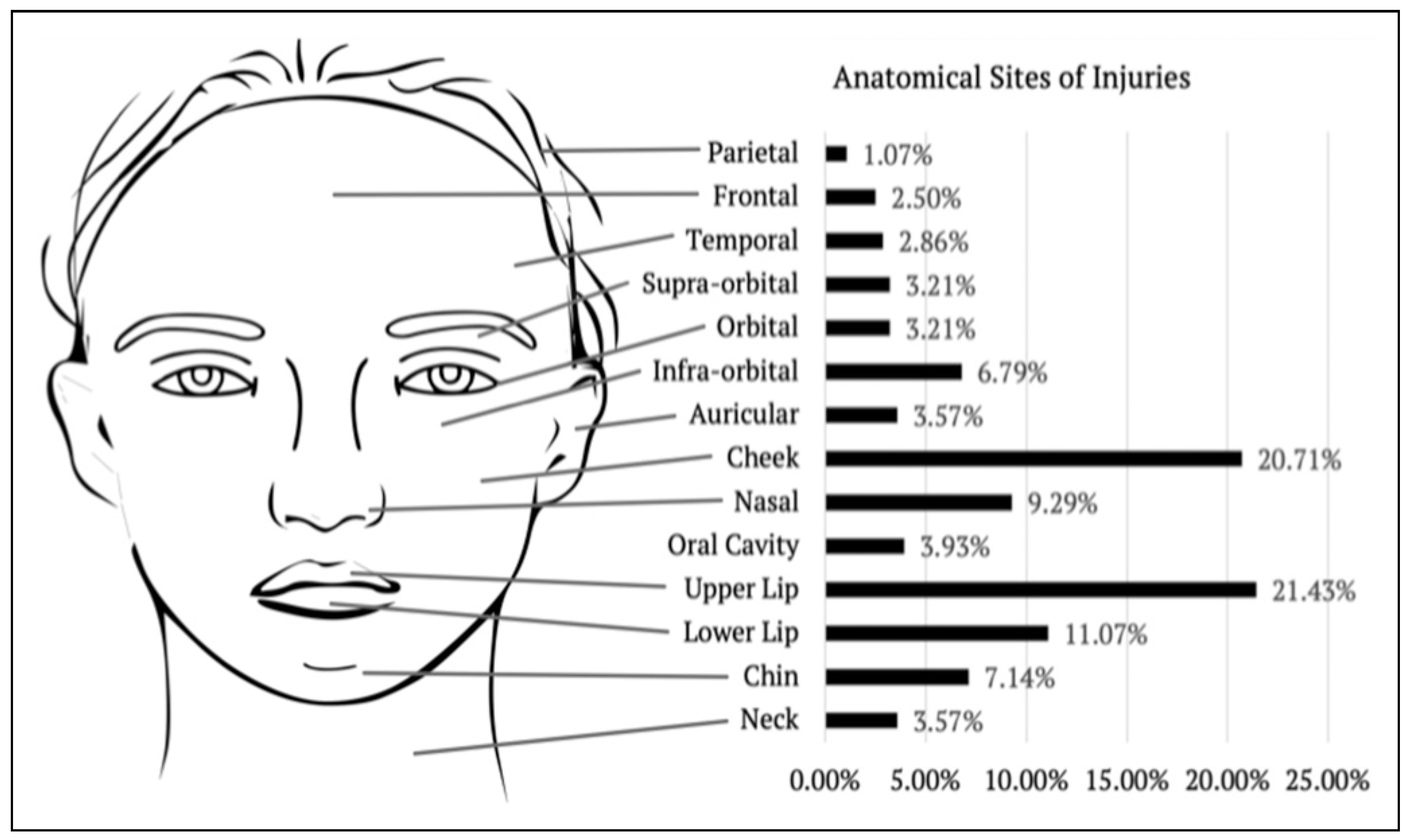
Injuries were reviewed systematically by primary anatomical site; specifically parietal (n = 3), frontal (n = 5), temporal (n = 1), nasal (n = 17), auricular (n = 10), supra-orbital (n = 7), orbital (n = 4), infra-orbital (n = 6), cheek (n = 33), oral cavity (n = 6), upper lip (n = 49), lower lip (n = 21), chin (n = 6) and neck (n = 3). Within our cohort, 120 cases (70%) occurred within the central ‘target area’, which is described by Palmer and Rees to encompass the lips, nose and cheek.
Of note, more than half of the cases (n = 95) involved multiple sites of injuries. It was the cheek (21%), upper lip (21%) and lower lip (11%) which recorded the highest involvement overall. The proportional incidence per facial region is shown in
Figure 3.
The extent of the laceration was recorded as less than one centimetre, one to five centimetres and greater than five centimetres. In the case of multiple sites of injury, patients were categorised based on the largest facial laceration. There were 125 cases in the 1–5 cm category, 33 cases less than 1 cm and 13 cases greater than 5 cm.
The presence of complex features was also analysed; this included avulsion injury, eye or nerve involvement and bony injury (such as teeth intrusion, dentoalveolar fracture or skull bone fracture). Avulsion injuries occurred in 42 (25%) cases. These ranged from skin defects of a few millimetres to more significant tissue loss involving the lips, nasal alar and pinna.
Bony injury was very uncommon with only two dentoalveolar fractures and three instances of tooth intrusion identified in the study cohort. There were no reports of damage to the eye itself, however, one case of a full thickness eyelid laceration and one episode of lacrimal canaliculi injury. Nerve injury was similarly uncommon with one instance of marginal mandibular nerve injury and one of mental nerve injury.
There were two cases complicated by cellulitis and a further two by abscess formation. In these instances, the patients had a delayed presentation to the OMFS department of greater than 48 hours from the initial injury.
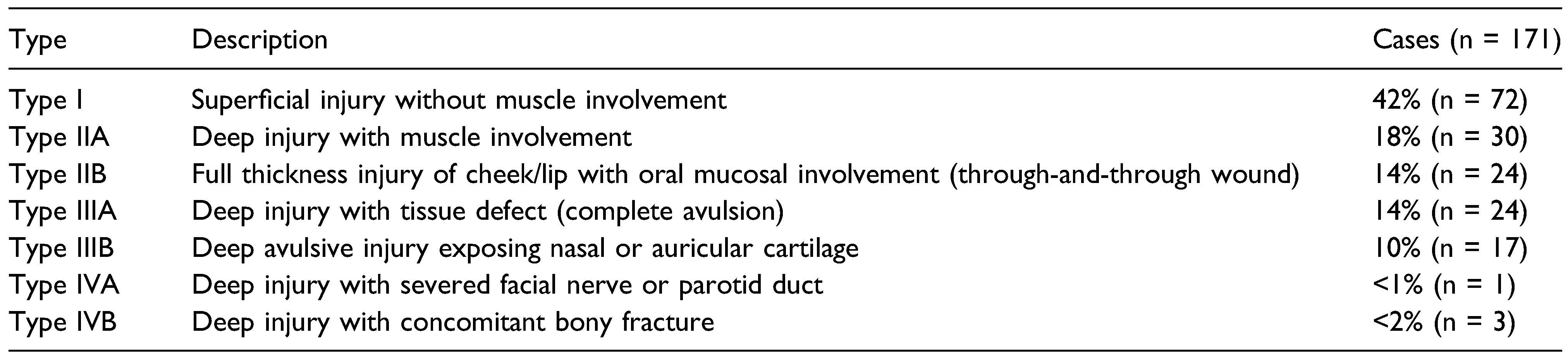
Modified Lackmann’s classification was employed to further stratify injuries. The number of cases per type are represented in
Table 1. Based on our criteria, 53 cases were considered to have sustained severe injuries. The median age was 14 years (range, 19 mth to 57 yr), again with a slight propensity of males (n = 29) to females (n = 24).
Further analysis with Chi-square tests was performed to determine if any primary predictor variables correlated with severe injury. There was no statistically significant association between gender, X
2 (1, n = 171) = .00,
P = .95, adult and paediatric population,
X2 (1, n = 171) = 3.1,
P = .08, home location,
X2 (1, n = 153) = 1.7,
P = .26, involvement of the family dog,
X2 (1, n = 154) = .4,
P = .53, or antecedent events,
X2 (4, n = 130) = 1.0,
P = .91. The details of these variables in reference to severe and non-severe cases are presented in
Table 2.
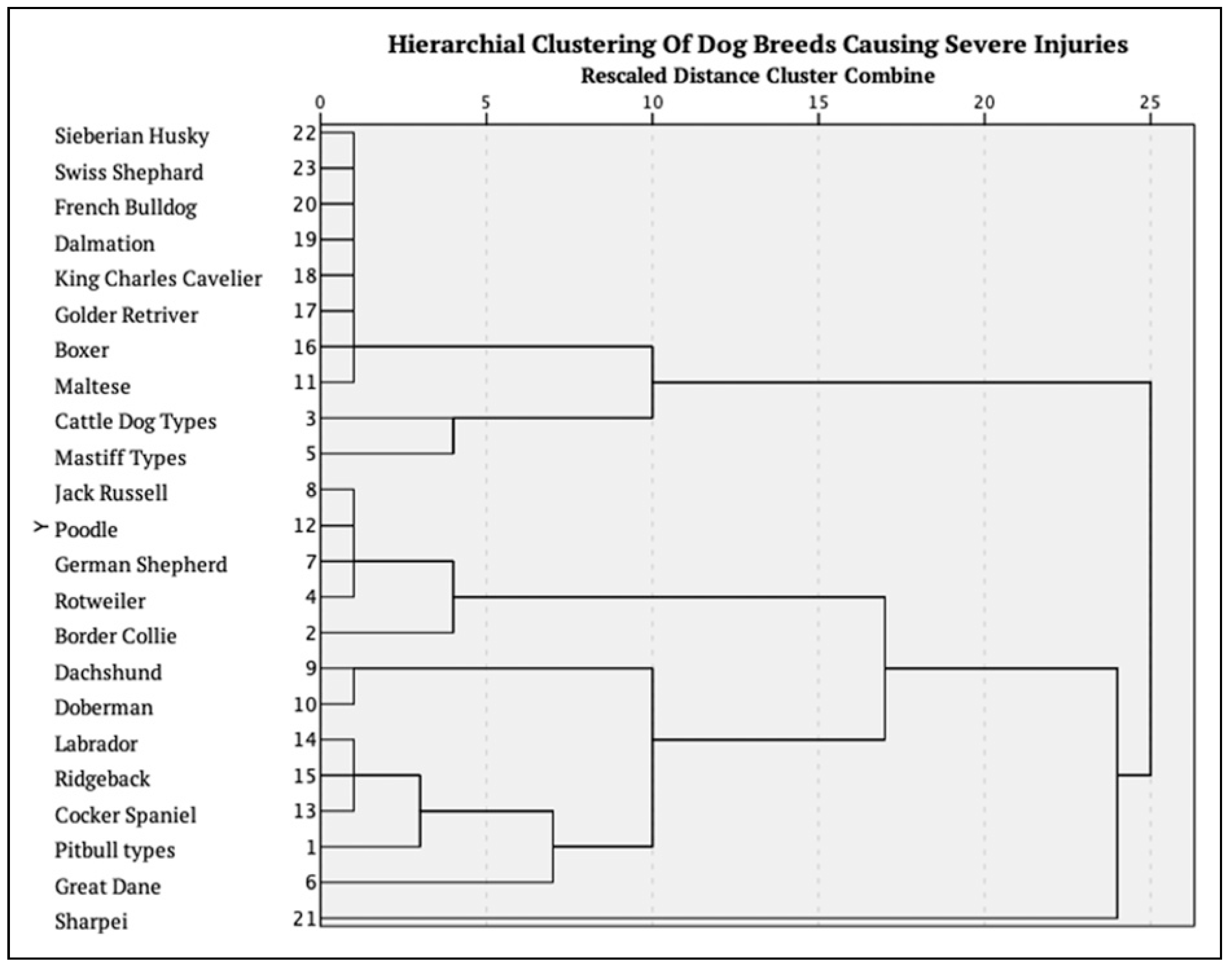
The dog breed was reported in 37 of the 53 severe cases. The Pitbull types (n = 12) remained the most common, followed by Great Dane (n = 4), Border Collie (n = 3) and Rottweiler (n = 3). Of note, it also included small dogs such as the Dachshund (n = 3), Jack Russell (n = 2), Poodle (n = 1) and Cocker Spaniel (n = 1). A hierarchical cluster analysis was performed to identify the groups of dog breeds that were associated with a higher incidence of severe injuries. This is presented in
Figure 4.
Whilst the Pitbull types were the most common breed both overall and in severe injuries, there was no statistical significance between them and severe injuries, X2 (1, n = 108) = 2.2, P = .14. Furthermore, there was no association between injury severity and the size of the dog, X2 (2,n = 108) = .2, P = .90.
All 171 patients were managed by the OMFS department; 33 were treated solely within the Emergency Department, with the remaining 138 requiring hospital admission. The median duration of admission was 1 day (range, 0 to 16 days). Injuries were either managed non-surgically (n = 5), such as with antibiotics, or surgically under local anaesthetic (n = 36) or general anaesthetic (n = 130).
Of the 130 patients who required a general anaesthetic, the primary operation performed was a washout, debridement and repair (n = 112). The remaining cases (n = 18) required more complex intervention; specifically local flap (n = 4), full thickness skin graft (n = 3), buccal mucosa graft (n = 1), incision and drainage of abscess (n = 2), ear terminalisation (n = 1), closure of tongue (n = 1), neck exploration (n = 1), repair of canalicular injury (n = 1), repair of eyelid laceration (n = 3), reduction of dentoalveolar fracture (n = 1) and removal of teeth (n = 1).
Chi-square test was further used to determine if there was an association between severity of injury and management approach. There was not a statistically significant correlation between severe injuries and admission, X2 (1,n = 171) = .9, P = .35, or requirement for surgical management under general anaesthesia, X2 (1,n = 171) = 3.3, P = .068.
In total, 110 patients received at least one dose of intravenous antibiotics. The antibiotic selection was either amoxicillin + clavulanic acid (n = 86), pipercillin/tazobactam (n = 12), cefazolin (n = 8), clindamycin (n = 1) or another penicillin (n = 3). There was a statistically significant relationship between severe injuries and the use of intravenous therapy, X2 (1, n = 163) = 11.3, P = .00.
On discharge, 170 patients received oral antibiotics, with the selection documented in 160 cases. Penicillin-based antibiotics were the most commonly prescribed agent and included amoxicillin + clavulanic acid (known as Augmentin Duo Forte) (n = 148) and amoxicillin (n = 1). Less commonly prescribed were cephalosporins (n = 7), sulfonamides (n = 1), fluroquinolones (n = 1) or tetracyclines (n = 1). The median duration of oral therapy was 7 days (range 5 to 14). There was no statistically significant association between the severity of the injury and length of antibiotic course, X2 (1, n = 141) = .0, P = .94.
Within the study cohort, 152 patients had their tetanus vaccination status verified. Subsequently, 38 patients received the Adsorbed Diphtheria and Tetanus (ADT) Booster. There were no reported instances of patients developing tetanus secondary to the dog bite.
Whilst there were no intraoperative complications documented, there were two immediate post-operative adverse events; this included anaesthetic-related hypotension and an episode of acute upper airway obstruction post laryngeal mask airway removal. In addition, one patient required a return to theatre for a repeat washout due to persistent infection. Post the index admission, two patients re-presented to the Emergency Department with concern of infection at the site of injury. The cohort experienced no mortality from facial dog bites or their management.
In total, 105 patients attended follow-up appointments in the OMFS outpatient clinic. This occurred most frequently within one month of the procedure (n = 102). However, several were reviewed on two or more occasions (n = 19). At this time, 22 patients reported a concern or complication. There was no statistical correlation between severity of injury and presence of a complication at follow-up, X2 (1, n = 105) = 1.3, P = .26.
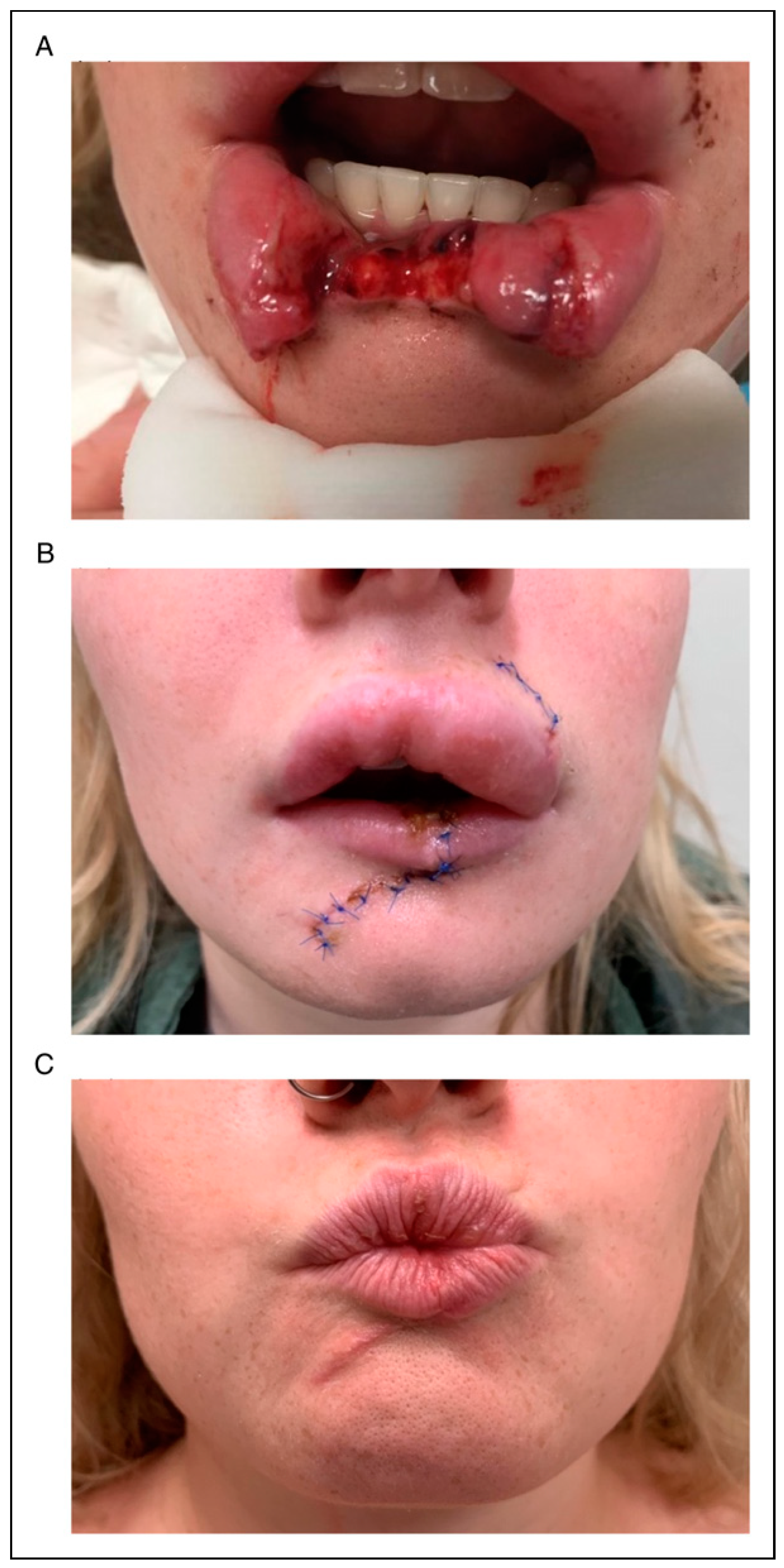
Figure 5 Depicts a clinical case from time of presentation, follow-up within the first month and at approximately 6 months.
Primarily, the concerns raised were aesthetic in nature (n = 14). This included hypertrophic scars, lip hyperplasia and asymmetric repair. An additional two patients reported residual nerve injury and one patient reported functional impairment from microstoma. Finally, there were two instances of persistent infection; one that required repeat debridement under local anaesthetic, with the other managed by a continuing course of oral antibiotics until clinical resolution of the sepsis.
Discussion
Facial dog bite injuries are a common and consistent presentation to our hospital. The rising incidence of these injuries is of particular concern as our evidence suggests the most susceptible demographic is that of young children. Khan et al. reported in their case series of 182 patients a similar predilection towards children and further correlated younger age with more extensive facial injury [
9]. Although our study was unable to associate specific demographic and aetiological factors with injury severity, the considerable number of patients subject to severe facial dog bites alone remains noteworthy.
The authors considered several methods for both describing and classifying facial dog bite injuries. Anatomically the face is composed of several unique features which pose individual challenges when considering repair. An initial description by upper, middle and lower face did not sufficiently encompass this and instead the authors recorded by specific facial subunit. Palmer and Rees did propose that there existed a ‘central target area’ which included the lips, nose and cheek [
10]. Within our cohort, 70% of cases occurred within this region, supporting that this is a high-risk area. Modified Lackmann’s classification was employed to stratify injury severity and ensure ease of comparison to other available studies [
8]. The authors propose one further change to the classification system, however, which would be the inclusion of Type Ib: superficial injury without muscle involvement, but with evidence of tissue defect. This was particularly relevant in injuries to the lip as often muscle or oral mucosa was not involved but avulsion of tissue occurred.
These injuries present both a burden on the public healthcare system and the patient due to the potential for a myriad of long-term physical and psychosocial sequalae.
Dog bites are complex injuries that are associated with relatively high risk of infection, and as identified in the Australian Prescriber, this may be as high as 25% [
11]. An additional consideration in these injuries is the risk of tetanus transmission, a life-threatening bacterial infection with a mortality rate around 1 in 10 [
12]. Whilst animals in Australia are not carriers of the rabies virus, it remains a concern in returning overseas travellers from endemic countries, which is outside the scope of this study [
13].
Consequently, particularly in the craniofacial region, dog bite injuries can result in poor clinical and cosmetic outcomes. The psychosocial impacts are variable between patients and ages. Particularly within the paediatric population, dog attacks predispose to behaviour changes such as fear and avoidance of dogs, nightmares and difficulty sleeping, emotional dysregulation and in some cases may develop into post-traumatic stress disorder (PTSD) [
14,
15].
In 2001, Bennet and Righetti reported the cost of managing dog bite injuries in Australian public hospital Emergency Departments to be more than
$7 million per annum [
16]. Depending on whether the patient is subsequently admitted, their length of stay and need for operative intervention, this cost is likely even greater. According to the AIHW, the average cost of services delivered per public hospital admission lies between
$4500 and
$4800 [
17]. Specifically for dog bite injuries, a cohort analysis from Sydney Children’s hospital identified the mean clinical cost per patient to be
$2968 [
7].
Given the morbidity that is associated with dog bites, preventable factors must be addressed. This leads to the question as to whether it is the inherent nature of a dog or rather the human element that is the primary precipitant. Dog temperament and their propensity towards aggressive behaviour has largely been determined by statistics of breed involvement in dog attacks [
18]. Within New South Wales, between July 2018 and 30 June 2021, the most common breeds involved in dog attack incidents requiring medical intervention were American Staffordshire Terrier, Bull Terrier types and Australian Cattle Dogs [
19]. This breed pattern was similarly mirrored by our study findings.
Whilst these larger dog breeds have a greater tendency to cause serious injury, several authors associate this with their size and power rather than an inherent aggressive nature [
18]. In fact, Duffy et al. performed a behavioural study of 9813 dogs from over 30 different breeds which identified the highest rates of human-directed aggression (bites or bite attempts) amongst small dogs; Dachshunds, Chihuahuas and Jack Russel Terriers [
20]. The Australian Veterinary Association (AVA) further determined that breed-specific legislation against certain ‘aggressive breeds’ had no impact to reduce the frequency of dog bites in Australia [
18].
The AVA has instead proposed that a coalescence of genetic, social and environmental factors is responsible for the tendency of a dog to bite [
18]. They identified that male and undesexed dogs were more than twice as likely to be involved than their respective counterparts [
18]. The concept of ‘dogs at large’ being the primary antecedent was refuted with approximately 80% of attacks in Australia, occurring in the domestic environment with the dog known to the victim [
18,
19]. In a case study performed by Kahn et al., 56 out of the 65 dog bites that occurred at home could be attributed to the child or adults’ behaviour preceding the incident [
10]. The majority of historical and emergent studies that have been published in relation to facial dog bites have similarly either quantified or qualified the nature and extent of the injuries that was sustained in their respective study cohort [
9,
21,
22]. Some published studies have taken this one step further and have recommended a range of preventative strategies that can be instituted in order to try to reduce the frequency of dog bite injuries [
23,
24,
25]. In the main, the best recommendations have concluded that young children should never be left alone in the company of a dog and, that where practical, dogs should be socialised to accept children, with owners encouraged to discourage dog biting behaviours.
We believe that, whilst these are undoubtedly important directives, the public health ramifications of facial dog bite injuries are largely overlooked and reasonably need to specifically target high-risk groups. This will form the basis of our ongoing research.
The strength of this study is that our hospital subserves a large and diverse residential population. It is based on a major urban-metropolitan hub, with several large regional centres and an array of small rural towns and remote communities. Dog ownership in such instances therefore covers a diverse demographic with persons and families from a variety of societal roles.
The weakness of this study is a reflection of the weakness of the majority of comparable studies, and that is, that there is generally no attempt to introduce real effective change as a research outcome. Our study further possessed the inherent flaws associated with a retrospective study; this included inaccurate and/or absent data available for collection, potential for collection bias as the author was not blinded to the study hypothesis and lack of a comparison or control group. In addition, the relatively small sample size may have impacted our ability to associate statistical significance between injury severity and the primary predictor variables.
In conclusion, this study has provided a snapshot of the authors experience in understanding and dealing with a facial dog bite epidemic that in all likelihood has become a pandemic. It highlights the need for further investigation of the human-dog relationship and the need to develop a sustainable and robust preventive program targeting the most vulnerable victims.