Discussion
The role of salvage surgery is critical in head and neck cancers, and surgery is generally regarded as the best treatment option in recurrent resectable head and neck cancers. [
1] Surgeons face an increasingly challenging job of ablative and reconstructive procedures in patients with significant effects of failed previous surgical or non-surgical treatment. Reconstruction plays a pivotal role in salvage surgery for both functional rehabilitation and esthetic appearance. 55%–66% of patients need pedicle flaps or free flaps in salvage surgery to repair surgical defects. [
8,
9] Data from a meta-analysis by Goodwin et al [
10] showed that salvage surgery conveyed a fair chance of 5-year survival (39.4% in 1080 patients). Overall 2-year disease-free survival in the prospective observational study part of the same paper [
10] was comparable with the meta-analysis average (44% vs 51%). The lower mortality rate in the prospective observational study was attributed probably due to the more reliable reconstructive techniques than meta-analysis (1.8% vs 5.2%). Surgical complications were reported in 20% of patients in the prospective study and 39% of 388 patients of meta-analysis. Wound complications were manageable, even though 99% of patients in meta-analysis and 88% in the prospective study received prior full course radiotherapy. [
10] A recent systematic review and meta-analysis of pooled data reported complications rates as 33% fistulas, 24% wound infections, and 3% flap failures in salvage surgery for advanced-stage cancers who received prior radiation or chemoradiation. [
11]
It would be best to know the predictors of poor outcomes before embarking on flap reconstructive surgery. In the present study, none of the factors were found as statistically significant predictors for flap-related complications, especially previous treatments like radiotherapy, surgery, or chemotherapy. In the recurrent cases, salvage surgery itself would be the major determinant of flap-related complications, and the different subsets in it might not have significant variations within them. These findings are consistent with other studies in the literature. [
6,
12,
13,
14] But, Mücke et al [
15] study showed that previous neck dissections and radiotherapy were significant negative predictors for success in free flap transfer.
There are studies in literature comparing free flaps vs loco-regional flaps. A study by Chepeha et al comparing pectoralis flap vs free flap showed that minor complications rate (57% vs 21%,
P < .001) and hospital stay (14 days vs 12 days,
P < .006) was more in the pectoralis flap group. Gastric tube dependence was more in the pectoralis flap group than the free flap group (42% vs 17%,
P < .004) in the salvage setting. [
16] Quality of life analysis in oral cavity cancer patient study showed that free flap group had better speech, shoulder function and better mood status than pectoralis group. [
17] Pectoralis major myocutaneous flap patients had more complications than the free gracilis flap group (12 vs 8, statistically not significant) for buttressing the repair of salvage laryngectomy defect repair, but fistula rates were similar. [
18] A systematic review and meta-analysis by Jørgensen et al [
19] that compared submental flap vs free flap showed that complete flap loss, debulking revisions and oncological recurrences were comparable except operating time and length of hospital stay. Operating time (6.7 vs 8.1 hours,
P =.002) and hospital charges (32% lower,
P = .0001) were significantly lower in the supraclavicular island flap compared to the fascio-cutaneous free flaps for head and neck reconstruction, but wound infection rate, wound dehiscence rate and length of stay were comparable. [
20,
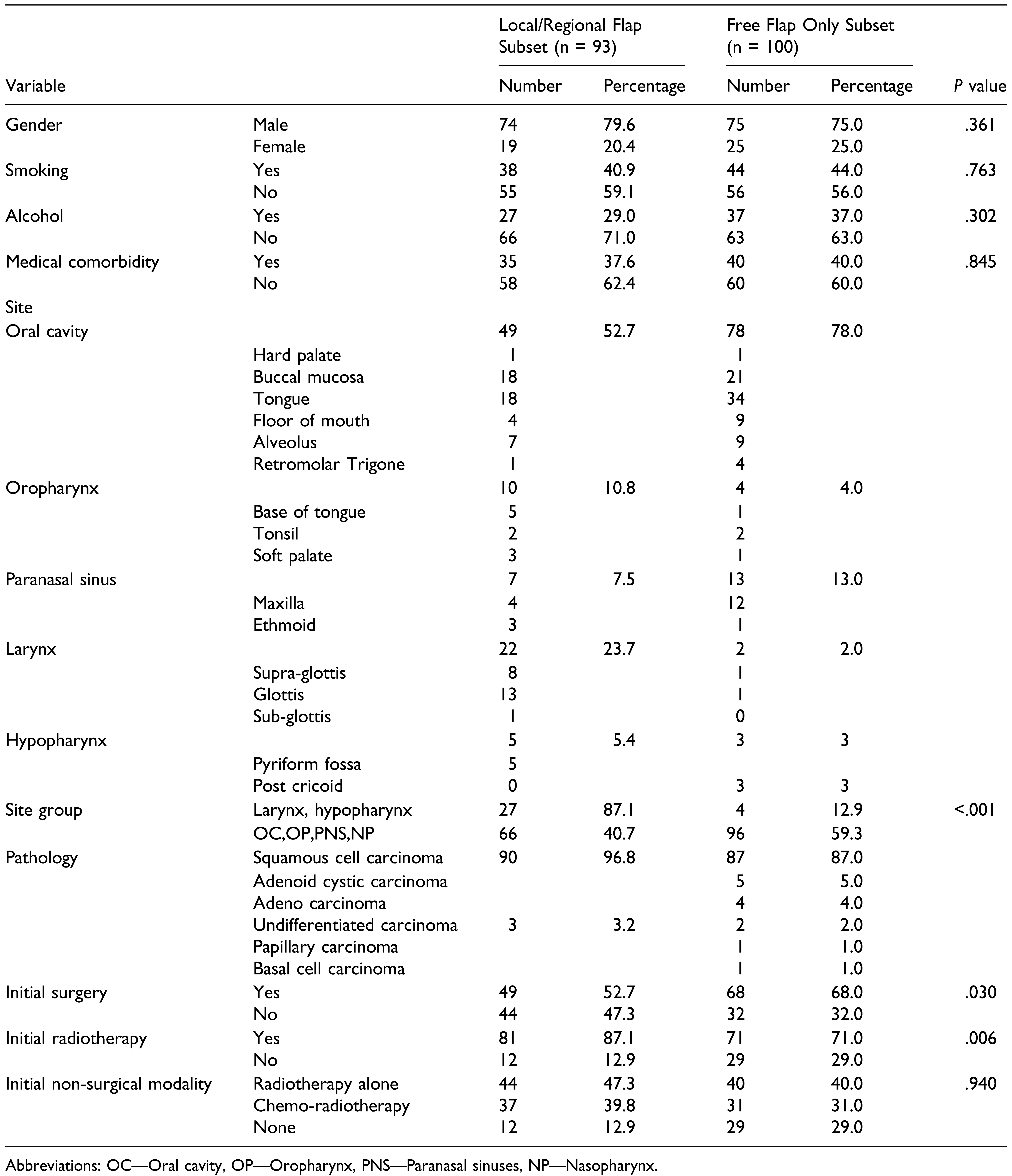
21] We have not made an attempt to statistically compare the outcomes of FF group and LRF group to prove the superiority of one over the other, in this setting. There is an obvious selection bias while selecting the reconstruction in salvage surgery. Free flaps are more done in the oral cavity and loco-regional flaps in the larynx. Patients who underwent prior surgical modality had more free flaps, and patients with previous radiotherapy had more loco-regional flaps. But, this preference is probably due to the appropriateness of the flap for the indications. Pectoralis major flap is preferred to and better in the larynx in laryngopharyngectomy defects. In salvage surgery, it is important that an appropriate flap is selected, suitable for the setting, according to the indications, neck and patient conditions. Comparison between free and non-free groups may be flawed in this situation due to the unavoidable selection bias and the choice is determined by the patient and defect characteristics.
Soft tissue flaps were preferred in salvage reconstruction, though the defects had a bony component. Bony reconstruction was done only in 23 cases (12%) in the present series. The reasons for this preference are multifactorial. Laryngopharyngeal defects do not have a bony component. Complex oromandibular defects in salvage surgery are usually large with an inner lining, outer cover, and bony components. But the preference is to give the lining and cover over the bone for immediate speech and swallowing rehabilitation. This is comfortably possible with ALT flap, radial forearm flap (RFFF) or pectoralis major flap, where bipaddling can be done to attain this goal. The soft tissue component of the defect is not satisfactorily covered by the skin paddle of the fibula flap or the musculocutaneous element of the deep circumflex iliac artery (DCIA) flap. Among the free flaps, vessel length is better for ALT and RFFF than bone flaps like free fibula or DCIA. Bony reconstruction is usually done only in absolute indications like central mandibular and maxillary defects to prevent gross functional and esthetic problems. The presence of trismus due to prior surgical and radiation-induced fibrosis is an added problem. Alleviation of trismus is usually better with a soft tissue alone reconstruction.
Soft tissue flaps were preferred in salvage reconstruction, though the defects had a bony component. Bony reconstruction was done only in 23 cases (12%) in the present series. The reasons for this preference are multifactorial. Laryngopharyngeal defects do not have a bony component. Complex oromandibular defects in salvage surgery are usually large with an inner lining, outer cover, and bony components. But the preference is to give the lining and cover over the bone for immediate speech and swallowing rehabilitation. This is comfortably possible with ALT flap, radial forearm flap (RFFF) or pectoralis major flap, where bipaddling can be done to attain this goal. The soft tissue component of the defect is not satisfactorily covered by the skin paddle of the fibula flap or the musculocutaneous el- ement of the deep circumflex iliac artery (DCIA) flap. Among the free flaps, vessel length is better for ALT and RFFF than bone flaps like free fibula or DCIA. Bony re- construction is usually done only in absolute indications like central mandibular and maxillary defects to prevent gross functional and esthetic problems. The presence of trismus due to prior surgical and radiation-induced fibrosis is an added problem. Alleviation of trismus is usually better with a soft tissue alone reconstruction.
Pectoralis major was the most commonly used flap in the present study. It has many advantages in the salvage setting. In the microvascular free flap reconstruction era, this flap has shifted its role as a “workhorse flap” to a “salvage flap.” It can be used for primary reconstruction, salvage, or emergency reconstruction due to free flap failure, oro/pharyngocutaneous fistula closure and protection to the carotid artery in emergency blowouts. It can also be used as a simultaneous reconstruction along with a free flap to fill the dead space and to cover the cervical skin defects and great vessels. [
22,
23,
24] It is available in the adjacent non-irradiated field, sturdy and better for logistic reasons in patients with multiple comorbidities. There is no need to depend on the status of the neck vessels. Also, its ability to accept skin grafts eliminates an additional necessity of a flap for skin cover. Sayles and Grant, in their systematic review and meta-analysis, have shown that prophylactic onlay flaps can prevent pharyngocutaneous fistula in total laryngectomy; the number needed to prevent one fistula was 6.05.
The authors concluded that prophylactic flaps should be offered in the salvage setting, especially in post chemoradiation patients. The most common flap used as an onlay in this study was the pectoralis major flap (151 out of 188 cases). [
25] A systematic review by Paleri et al showed the clear advantage of the vascularized flap outside the radiation field to repair the salvage laryngectomy defect. Pharyngocutaneous fistula formation is 50% higher in primary closure than in flap reconstruction, and to prevent one fistula, 11 patients need vascularized flaps for reconstruction of salvage laryngectomy defect. [
4]
There are reports showing the safety of free flaps in salvage surgery with a success rate of 91.3%–96.8%. [
5,
6,
14,
15,
26,
27,
28,
29] The commonest free flaps used for salvage surgery in this study are ALT flap and RFFF. ALT flap can be harvested as thin fascio-cutaneous flap to chimeric flap with fascia lata, vastus lateralis and rectus femoris muscle and skin with good pedicle length, concealed donor site scar and simultaneous two-team approach for flap harvest and resection of head and neck cancers. [
30] Complex defects after salvage surgery can be reconstructed with good aesthetic, functional results and low complications by double island ALT flap. [
31] ALT flap can be used for total or partial circumferential pharyngo-esophageal defect reconstruction (71% had prior surgery, radiotherapy, or both) with comparable fistula and stricture rates, minimum donor site morbidity and quick recovery rates. [
32] RFFF is a thin fasciocutaneous flap with a long pedicle, able to reach the contralateral side of the neck for micro-anastomosis without any vein graft. RFFF can be used to cover the long mucosal salvage surgical defects in the oral cavity, tonsil, palate, larynx, and hypopharynx. [
12]
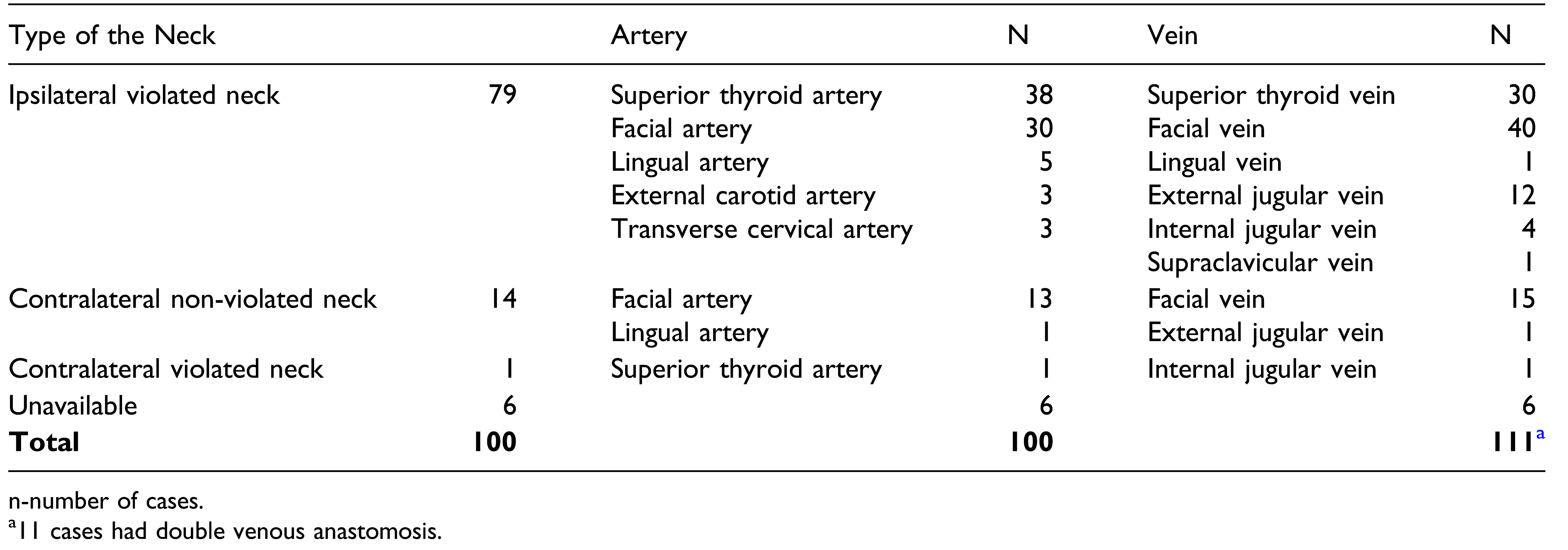
Vessel depleted neck is a challenge in salvage surgery. Ipsilateral neck vessels were available in 79% of our cases, though the neck was violated by previous surgery. Ipsilateral superior thyroid or facial vessels were available and used. Proper pre-operative assessment and multiple backup plans for each patient based on individual characteristics would be essential. [
33] Ipsilateral facial artery and vein, lingual artery and superior thyroid vessels are routinely used for microvascular reconstruction. In majority of the salvage surgical cases, these vessels would be either used in previous surgery or affected by the prior radiotherapy. Dual-phase Computed Tomography angiography was found helpful to find the recipient artery and vein. [
34] Options for arterial anastomosis in vessel depleted neck are superficial temporal artery, transverse cervical artery, external carotid artery, internal mammary artery, inferior thyroid artery, thoracoacromial artery, and common carotid artery. [
20,
35,
36,
37,
38] Veinous options are external jugular, superficial temporal, internal mammary, thoracoacromial veins, and cephalic vein transposition. [
33,
35,
38,
39] Corlett loop, that is, an extended length of the cephalic vein, can be used to achieve arterial and vein anastomosis when local artery and vessels are not available. [
40] In extreme situations, previous flap pedicle [
41,
42] and extracorporeal perfusion [
43] can also be used for microvascular reconstruction.
The strength of the study is the sample size. This is one of the largest studies which has looked into the morbidity related to flap reconstruction in the salvage surgery setting. The limitation from a methodological viewpoint would be the selection bias in the comparison between FF and LRF groups. But we aim to show that within the existing protocols of flap selection, either of these would be done with similar complication rates. Being a retrospective study, the inherent limitations might have also influenced the results.