Titanium Mesh Exposure After Bone Grafting: Treatment Approaches—A Systematic Review
Abstract
Introduction
Materials and Methods
Protocol e Registration
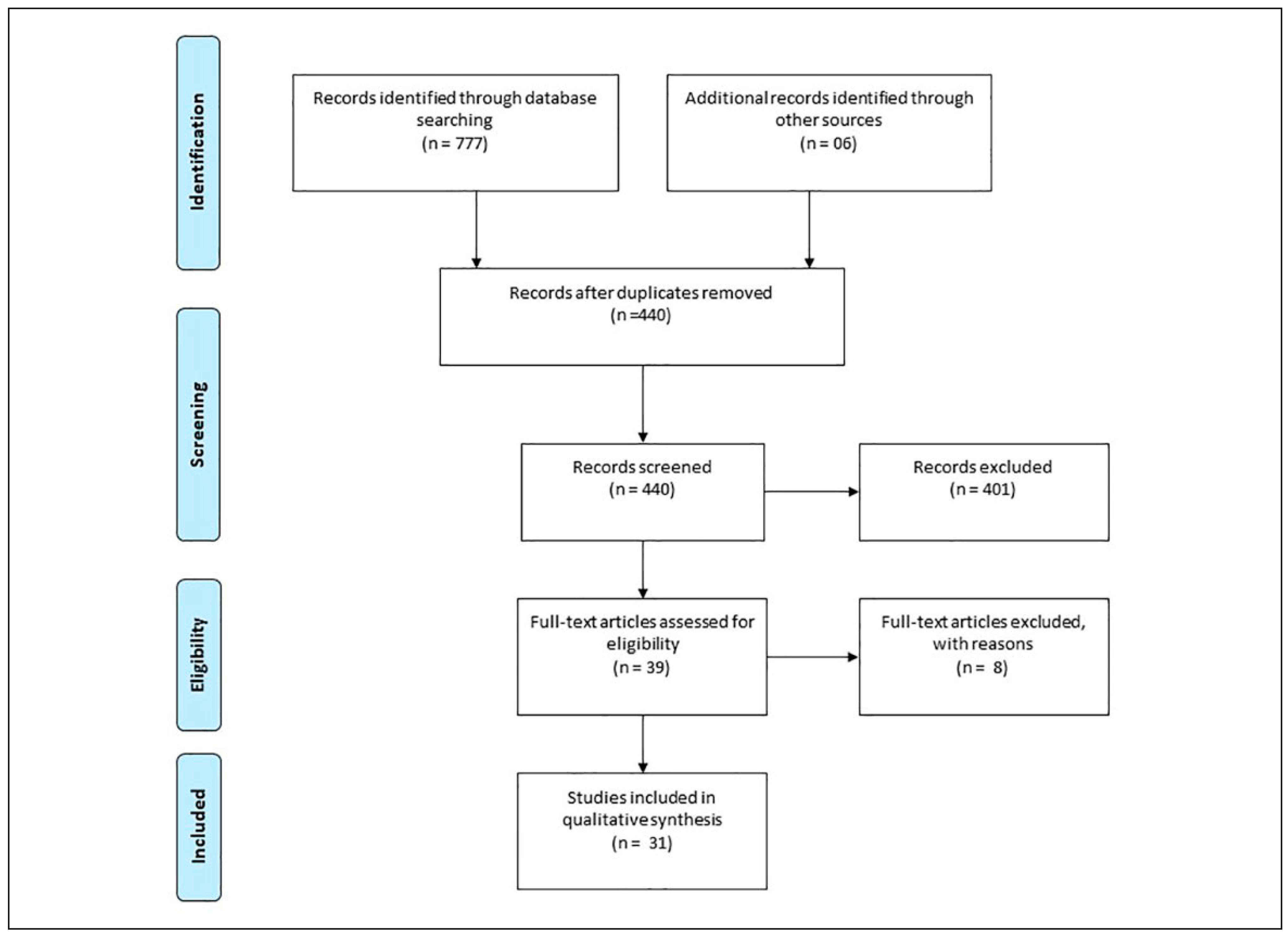
Study Selection
Eligibility Criteria
- -
- Studies which was described:
- -
- alveolar bone reconstruction/augmentation and subsequent exposure of the titanium mesh.
- -
- Treatment approach and the treatment outcome as well (i.e., if it was possible to keep up the graft up to the implant phase, if the surgical site gets infected if the approach needed to be replaced).
- -
- Human studies.
- -
- Published in the English language from 1999 to 2019.
- -
- Reporting at least 6 months of follow-up from titanium mesh placement.
- -
- Informed consent document signed by the patients and registered at respective ethics committee (when applicable).
Exclusion Criteria
- -
- Absence of titanium mesh exposure.
- -
- Presence of infection at the time of bone grafting.
- -
- Less than 5 years after cancer treatment by head and neck irradiation and surgical resection.
- -
- Immediate bone reconstruction with titanium mesh in patients undergoing removal of malignant lesions of the jaws.
- -
- Patients with full or partial maxillectomy.
- -
- Patients with uncontrolled diabetes and/or other metabolic diseases.
- -
- Use of drugs that affect the bone quality (e.g., bisphosphonates or chemotherapy).
- -
- Pregnancy at the time of surgery or postoperative healing period.
Search Strategy
- ((((((Titanium cover) OR Titanium Shell) OR Titanium MEsh)) AND (((Graft Failure) OR dehiscence) OR Mesh exposure))) AND ((((treatment) OR approach) OR resolution) OR outcome)
- (Mesh OR titanium shell OR titanium mesh) AND (exposure OR dehiscence) AND (alveolar ridge augmentation OR alveolar ridge grafting OR alveolar ridge graft OR alveolar bone graft OR xenograft OR autogenous graft)
- “titanium” AND “mesh” AND “exposure” AND “bone” AND “augmentation.”
- “titanium” AND “mesh” AND “exposure” AND “bone” “reconstruction.”
- “titanium” AND “mesh” AND “exposure” AND “bone” AND “particulate.”
Data Extraction
Risk of Bias Assessment
Results
Discussion
Conclusions
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
References
- Brkovic, B.M.B.; Prasad, H.S.; Rohrer, M.D.; et al. Beta-tricalcium phosphate/type I collagen cones with or without a barrier membrane in human extraction socket healing: clinical, histologic, histomorphometric, and immunohistochemical evaluation. Clin Oral Investig. 2012, 16, 581–590. [Google Scholar] [PubMed]
- Marx, R.E.; Armentano, L.; Olavarria, A.; Samaniego, J. rhBMP2/ACS grafts versus autogenous cancellous marrow grafts in large vertical defects of the maxilla: an unsponsored randomized open-label clinical trial. Int J Oral Maxillofac Implants. 2013, 28, e243–e251. [Google Scholar] [PubMed]
- von Arx, T.; Kurt, B. Implant placement and simultaneous periimplant bone grafting using a micro titanium mesh for graft stabilization. Int J Periodontics Restorative Dent. 1998, 18, 117–127. [Google Scholar] [PubMed]
- von Arx, T.; Kurt, B. Implant placement and simultaneous ridge augmentation using autogenous bone and a micro titanium mesh: a prospective clinical study with 20 implants. Clin Oral Implants Res. 1999, 10, 24–33. [Google Scholar]
- Lizio, G.; Corinaldesi, G.; Marchetti, C. Alveolar ridge reconstruction with titanium mesh: a three-dimensional evaluation of factors affecting bone augmentation. Int J Oral Maxillofac Implants. 2014, 29, 1354–1363. [Google Scholar]
- Yamada, H.; Nakaoka, K.; Horiuchi, T.; et al. Mandibular reconstruction using custom-made titanium mesh tray and particulate cancellous bone and marrow harvested from bilateral posterior ilia. J Plast Surg Hand Surg. 2014, 48, 183–190. [Google Scholar]
- Ciocca, L.; Lizio, G.; Baldissara, P.; Sambuco, A.; Scotti, R.; Corinaldesi, G. Prosthetically CAD-CAM-guided bone augmentation of atrophic jaws using customized titanium mesh: preliminary results of an open prospective study. J Oral Implantol. 2018, 44, 131–137. [Google Scholar]
- Malchiodi, L.; Scarano, A.; Quaranta, M.; Piattelli, A. Rigid fixation by means of titanium mesh in edentulous ridge expansion for horizontal ridge augmentation in the maxilla. Int J Oral Maxillofac Implants. 1998, 13, 701–705. [Google Scholar]
- Trento, G.S.; Carvalho, P.H.A.; Macedo, D.V.; Gabrielli, M.A.C.; Monnazzi, M.S.; Pereira-Filho, V.A. Titanium mesh associated with rhBMP-2 in alveolar ridge reconstruction. Int J Oral Maxillofac Surg. 2018, 48, 546–553. [Google Scholar]
- Torres, J.; Tamimi, F.; Alkhraisat, M.H.; et al. Platelet-rich plasma may prevent titanium-mesh exposure in alveolar ridge augmentation with anorganic bovine bone. J Clin Periodontol. 2010, 37, 943–951. [Google Scholar]
- Assenza, B.; Piattelli, M.; Scarano, A.; Iezzi, G.; Petrone, G.; Piattelli, A. Localized ridge augmentation using titanium micromesh. J Oral Implantol. 2001, 27, 287–292. [Google Scholar] [CrossRef]
- Khamees, J.; Darwiche, M.A.; Kochaji, N. Alveolar ridge augmentation using chin bone graft, bovine bone mineral, and titanium mesh: clinical, histological, and histomorphomtric study. J Indian Soc Periodontol. 2012, 16, 235–240. [Google Scholar] [CrossRef]
- Poli, P.P.; Beretta, M.; Cicciù, M.; Maiorana, C. Alveolar ridge augmentation with titanium mesh. A retrospective clinical study. Open Dent J. 2014, 8, 148–158. [Google Scholar] [CrossRef]
- Rasia dal Polo, M.; Poli, P.P.; Rancitelli, D.; Beretta, M.; Maiorana, C. Alveolar ridge reconstruction with titanium meshes: a systematic review of the literature. Med Oral Patol Oral Cir Bucal. 2014, 19, e639–e646. [Google Scholar] [CrossRef]
- Briguglio, F.; Falcomatà, D.; Marconcini, S.; Fiorillo, L.; Briguglio, R.; Farronato, D. The use of titanium mesh in guided bone regeneration: a systematic review. Int J Dent. 2019, 2019, 906542. [Google Scholar] [CrossRef]
- Her, S.; Kang, T.; Fien, M.J. Titanium mesh as an alternative to a membrane for ridge augmentation. J Oral Maxillofac Surg. 2012, 70, 803–810. [Google Scholar] [CrossRef]
- De Stavola, L.; Tunkel, J. The role played by a suspended external-internal suture in reducing marginal flap tension after bone reconstruction: a clinical prospective cohort study in the maxilla. Int J Oral Maxillofac Implants. 2014, 29, 921–926. [Google Scholar] [CrossRef]
- Sagheb, K.; Schiegnitz, E.; Moergel, M.; Walter, C.; Al-Nawas, B.; Wagner, W. Clinical outcome of alveolar ridge augmentation with individualized CAD-CAM-produced titanium mesh. Int J Implant Dent. 2017, 3, 36. [Google Scholar] [CrossRef]
- Abrahamsson, P.; Wälivaara, D.Å.; Isaksson, S.; Andersson, G. Periosteal expansion before local bone reconstruction using a new technique for measuring soft tissue profile stability: a clinical study. J Oral Maxillofac Surg. 2012, 70, e521–e530. [Google Scholar] [CrossRef]
- Mertens, C.; Thiele, O.; Engel, M.; Seeberger, R.; Hoffmann, J.; Freier, K. The use of self-inflating soft tissue expanders prior to bone augmentation of atrophied alveolar ridges. Clin Implant Dent Relat Res. 2015, 17, 44–51. [Google Scholar] [CrossRef]
- Altiparmak, N.; Uckan, S.; Bayram, B.; Soydan, S. Comparison of tunnel and crestal incision techniques in reconstruction of localized alveolar defects. Int J Oral Maxillofac Implants. 2017, 32, 1103–1110. [Google Scholar] [PubMed]
- Lizio, G.; Mazzone, N.; Corinaldesi, G.; Marchetti, C. Reconstruction of extended and morphologically varied alveolar ridge defects with the titanium mesh technique: clinical and dental implants outcomes. Int J Periodontics Restorative Dent. 2016, 36, 689–697. [Google Scholar]
- Pellegrino, G.; Lizio, G.; Corinaldesi, G.; Marchetti, C. Titanium mesh technique in rehabilitation of totally edentulous atrophic maxillae: a retrospective case series. J Periodontol. 2016, 87, 519–528. [Google Scholar] [PubMed]
- Al-Ardah, A.J.; AlHelal, A.; Proussaefs, P.; AlBader, B.; Al Humaidan, A.A.; Lozada, J. Managing titanium mesh exposure with partial removal of the exposed site: a case series study. J Oral Implantol. 2017, 43, 482–490. [Google Scholar] [PubMed]
- Roccuzzo, M.; Ramieri, G.; Spada, M.C.; Bianchi, S.D.; Berrone, S. Vertical alveolar ridge augmentation by means of a titanium mesh and autogenous bone grafts. Clin Oral Implants Res. 2004, 15, 73–81. [Google Scholar]
- Roccuzzo, M.; Ramieri, G.; Bunino, M.; Berrone, S. Autogenous bone graft alone or associated with titanium mesh for vertical alveolar ridge augmentation: a controlled clinical trial. Clin Oral Implants Res. 2007, 18, 286–294. [Google Scholar] [PubMed]
- Cucchi, A.; Vignudelli, E.; Napolitano, A.; Marchetti, C.; Corinaldesi, G. Evaluation of complication rates and vertical bone gain after guided bone regeneration with non-resorbable membranes versus titanium meshes and resorbable membranes. A randomized clinical trial. Clin Implant Dent Relat Res. 2017, 19, 821–832. [Google Scholar] [CrossRef]
- Atef, M.; Tarek, A.; Shaheen, M.; Alarawi, R.M.; Askar, N. Horizontal ridge augmentation using native collagen membrane vs. titanium mesh in atrophic maxillary ridges: randomized clinical trial. Clin Implant Dent Relat Res. 2020, 22, 156–166. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar]
- Moura, L.B.; Carvalho, P.H.A.; Xavier, C.B.; et al. Autogenous non-vascularized bone graft in segmental mandibular reconstruction: a systematic review. Int J Oral Maxillofac Surg. 2016, 45, 1388–1394. [Google Scholar]
- La Torre, G.; Chiaradia, G.; Gianfagna, F.; De Laurentis, A.; Boccia, S.; Ricciardi, W. Quality assessment in meta-analysis. Ital J Public Heal. 2006, 3, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Clementini, M.; Morlupi, A.; Canullo, L.; Agrestini, C.; Barlattani, A. Success rate of dental implants inserted in horizontal and vertical guided bone regenerated areas: a systematic review. Int J Oral Maxillofac Surg. 2012, 41, 847–852. [Google Scholar]
- Naenni, N.; Schneider, D.; Jung, R.E.; Hüsler, J.; Hämmerle, C.H.F.; Thoma, D.S. Randomized clinical study assessing two membranes for guided bone regeneration of peri-implant bone defects: clinical and histological outcomes at 6 months. Clin Oral Implants Res. 2017, 28, 1309–1317. [Google Scholar]
- Merli, M.; Migani, M.; Esposito, M. Vertical ridge augmentation with autogenous bone grafts: resorbable barriers supported by ostheosynthesis plates versus titanium-reinforced barriers. A preliminary report of a blinded, randomized controlled clinical trial. Int J Oral Maxillofac Implants. 2007, 22, 373–382. [Google Scholar] [PubMed]
- Proussaefs, P.; Lozada, J.; Kleinman, A.; Rohrer, M.D.; McMillan, P.J. The use of titanium mesh in conjunction with autogenous bone graft and inorganic bovine bone mineral (bio-oss) for localized alveolar ridge augmentation: a human study. Int J Periodontics Restorative Dent. 2003, 23, 185–195. [Google Scholar]
- Maiorana, C.; Santoro, F.; Rabagliati, M.; Salina, S. Evaluation of the use of iliac cancellous bone and anorganic bovine bone in the reconstruction of the atrophic maxilla with titanium mesh: a clinical and histologic investigation. Int J Oral Maxillofac Implants. 2001, 16, 427–432. [Google Scholar] [PubMed]
- Louis, P.J.; Gutta, R.; Said-Al-Naief, N.; Bartolucci, A.A. Reconstruction of the maxilla and mandible with particulate bone graft and titanium mesh for implant placement. J Oral Maxillofac Surg. 2008, 66, 235–245. [Google Scholar]
- Zita Gomes, R.; Paraud Freixas, A.; Han, C.H.; Bechara, S.; Tawil, I. Alveolar ridge reconstruction with titanium meshes and simultaneous implant placement: a retrospective, multicenter clinical study. Biomed Res Int. 2016, 2016, 1–12. [Google Scholar]
- Pieri, F.; Corinaldesi, G.; Fini, M.; Aldini, N.N.; Giardino, R.; Marchetti, C. Alveolar ridge augmentation with titanium mesh and a combination of autogenous bone and anorganic bovine bone: a 2-year prospective study. J Periodontol. 2008, 79, 2093–2103. [Google Scholar]
- Khanna, R.; Khanna, R.; Pardhe, N.D.; Srivastava, N.; Bajpai, M.; Gupta, S. Pure titanium membrane (ultra-Ti®) in the treatment of periodontal osseous defects: a split-mouth comparative study. J Clin Diagn Res. 2016, 10, ZC47–ZC51. [Google Scholar]
- Hartmann, A.; Hildebrandt, H.; Schmohl, J.U.; Kämmerer, P.W. Evaluation of risk parameters in bone regeneration using a customized titanium mesh: results of a clinical study. Implant Dent. 2019, 28, 543–550. [Google Scholar] [PubMed]
- Tallarico, M.; Ceruso, F.M.; Muzzi, L.; et al. Effect of simultaneous immediate implant placement and guided bone reconstruction with ultra-fine titanium mesh membranes on radiographic and clinical parameters after 18 months of loading. Materials (Basel). 2019, 12, 1710. [Google Scholar] [CrossRef] [PubMed]
- Artzi, Z.; Dayan, D.; Alpern, Y.; Nemcovsky, C.E. Vertical ridge augmentation using xenogenic material supported by a configured titanium mesh: clinicohistopathologic and histochemical study. Int J Oral Maxillofac Implants. 2003, 18, 440–446. [Google Scholar]
- Proussaefs, P.; Lozada, J. Use of titanium mesh for staged localized alveolar ridge augmentation: clinical and histologic-histomorphometric evaluation. J Oral Implantol. 2006, 32, 237–247. [Google Scholar]
- Miyamoto, I.; Funaki, K.; Yamauchi, K.; Kodama, T.; Takahashi, T. Alveolar ridge reconstruction with titanium mesh and autogenous particulate bone graft: computed tomography-based evaluations of augmented bone quality and quantity. Clin Implant Dent Relat Res. 2012, 14, 304–311. [Google Scholar]
- Strietzel, F.P.; Reichart, P.A.; Graf, H.L. Lateral alveolar ridge augmentation using a synthetic nano-crystalline hydroxyapatite bone substitution material (Ostim): preliminary clinical and histological results. Clin Oral Implants Res. 2007, 18, 743–751. [Google Scholar]
- Funato, A.; Ishikawa, T.; Kitajima, H.; Yamada, M.; Moroi, H. A novel combined surgical approach to vertical alveolar ridge augmentation with titanium mesh, resorbable membrane, and rhPDGF-BB: a retrospective consecutive case series. Int J Periodontics Restorative Dent. 2013, 33, 437–445. [Google Scholar] [PubMed]
- Uehara, S.; Kurita, H.; Shimane, T.; et al. Predictability of staged localized alveolar ridge augmentation using a micro titanium mesh. Oral Maxillofac Surg. 2015, 19, 411–416. [Google Scholar]
- Kim, Y.; Kim, T.K.; Leem, D.H. Clinical study of a flap advancement technique without vertical incision for guided bone regeneration. Int J Oral Maxillofac Implants. 2015, 30, 1113–1118. [Google Scholar] [CrossRef]
- Cucchi, A.; Sartori, M.; Parrilli, A.; Aldini, N.N.; Vignudelli, E.; Corinaldesi, G. Histological and histomorphometric analysis of bone tissue after guided bone regeneration with nonresorbable membranes vs. resorbable membranes and titanium mesh. Clin Implant Dent Relat Res. 2019, 21, 693–701. [Google Scholar] [CrossRef]
- Giannelli, M.; Chellini, F.; Margheri, M.; Tonelli, P.; Tani, A. Effect of chlorhexidine digluconate on different cell types: a molecular and ultrastructural investigation. Toxicol Vitr. 2008, 22, 308–317. [Google Scholar]
- Marx, R.E.; Armentano, L.; Olavarria, A.; Samaniego, J. rhBMP-2/ACS grafts versus autogenous cancellous marrow grafts in large vertical defects of the maxilla: an unsponsored randomized open-label clinical trial. Int J Oral Maxillofac Implants. 2013, 28, e243–e251. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Celletti, R.; Davarpanah, M.; Etienne, D.; et al. Guided tissue regeneration around dental implants in immediate extraction sockets: comparison of e-PTFE and a new titanium membrane. Int J Periodontics Restorative Dent. 1994, 14, 242–253. [Google Scholar] [PubMed]
- Kan, J.Y.K.; Rungcharassaeng, K.; Lozada, J.L.; Zimmerman, G. Facial gingival tissue stability following immediate placement and provisionalization of maxillary anterior single implants: a 2- to 8-year follow-up. Int J Oral Maxillofac Implants. 2011, 26, 179–187. [Google Scholar]
© 2021 by the author. The Author(s) 2021.
Share and Cite
Cunha, G.; Carvalho, P.H.d.A.; Quirino, L.C.; Torres, L.H.S.; Filho, V.A.P.; Gabrielli, M.F.R.; Gabrielli, M.A.C. Titanium Mesh Exposure After Bone Grafting: Treatment Approaches—A Systematic Review. Craniomaxillofac. Trauma Reconstr. 2022, 15, 397-405. https://doi.org/10.1177/19433875211046114
Cunha G, Carvalho PHdA, Quirino LC, Torres LHS, Filho VAP, Gabrielli MFR, Gabrielli MAC. Titanium Mesh Exposure After Bone Grafting: Treatment Approaches—A Systematic Review. Craniomaxillofacial Trauma & Reconstruction. 2022; 15(4):397-405. https://doi.org/10.1177/19433875211046114
Chicago/Turabian StyleCunha, Giovanni, Pedro Henrique de Azambuja Carvalho, Lílian Caldas Quirino, Luiz Henrique Soares Torres, Valfrido Antônio Pereira Filho, Mario Francisco Real Gabrielli, and Marisa Aparecida Cabrini Gabrielli. 2022. "Titanium Mesh Exposure After Bone Grafting: Treatment Approaches—A Systematic Review" Craniomaxillofacial Trauma & Reconstruction 15, no. 4: 397-405. https://doi.org/10.1177/19433875211046114
APA StyleCunha, G., Carvalho, P. H. d. A., Quirino, L. C., Torres, L. H. S., Filho, V. A. P., Gabrielli, M. F. R., & Gabrielli, M. A. C. (2022). Titanium Mesh Exposure After Bone Grafting: Treatment Approaches—A Systematic Review. Craniomaxillofacial Trauma & Reconstruction, 15(4), 397-405. https://doi.org/10.1177/19433875211046114


