Abstract
Presurgical Nasoalveolar Molding (NAM) is an adjunctive treatment modality designed to reorient misaligned tissue structures and nasal cartilage in cleft lip and/or palate (CL/P) patients. Recent advances in NAM therapy focus on modifications to the intraoral molding plate or nasal stent intended to improve treatment outcomes, ease of use, compliance, and cost-effectiveness. Notably, 3D technological advancements have been employed to design NAM devices more efficiently and create objective, standardized means of measuring progressive morphological changes during therapy. These advances are designed to incorporate 3D technology in the treatment of cleft lip and/or palate to render it more precise, accurate, and time-efficient.
1. Introduction
Orofacial cleft (OFC) deformities constitute the most common congenital craniofacial anomaly in infants, occurring in approximately 1 in 700 live births worldwide. [1,2,3,4] Several studies have demonstrated that the etiology of OFC is both polygenic and multifactorial in nature. [5,6,7,8,9,10] The prevalence of cleft anomalies vary widely according to gender, [11,12] socioeconomic status, [13] ancestry, [4] maternal nutrition, [14] and environmental determinants such as prenatal exposure to tobacco and other teratogenic agents. [15,16,17]
Corrective treatment of cleft lip with or without palate involves extensive multispecialty longitudinal assessment and management. [18,19,20] Prior to cleft lip and/or palate surgical repair, multidisciplinary teams are responsible for monitoring the affected patient’s facial and oropharyngeal musculature arrangement and function, in addition to the individual’s orofacial bone structure, sinus partitioning, speech and sound production, and feeding efficacy. [19] The team’s armamentarium includes conducting feeding evaluations; conducting genetic tests to determine the cleft defect’s etiology and risks; assessing auditory function; performing corrective lip and tooth repairs and orthognathic procedures; conducting speech therapy; and providing emotional and psychological support for the affected individual and his/her family. [19,20,21]
Due to the considerable variation of nasolabial deformities seen in patients with orofacial cleft anomalies, presurgical manipulations are sometimes employed as corrective treatment to reduce the severity of the cleft prior to surgical repair. [22,23,24] In contrast to traditional presurgical orthopedic (PSO) treatment, nasoalveolar molding (NAM) primarily addresses the oronasal deformity in neonates by enabling repositioning of the maxillary alveolus and construction of the columella. [24] First described in the literature by Grayson et al., NAM utilizes an acrylic intraoral molding device and nasal stents to apply medially directed pressure on the neonate’s alveolar arch and nasal cartilage. [24,25,26,27] Over a 3to 4-month period, NAM has been demonstrated to reduce the alveolar gap, harmonize nasolabial symmetry in unilateral cases, and increase columellar length in bilateral cases. [28,29]
NAM therapy is predicated on the malleability of neonatal cartilaginous and soft tissue structures so that the molding device can guide the neonate’s alveolar development. While there is debate regarding NAM’s longitudinal clinical value, [29,30] its efficacy in redirecting soft tissue and cartilaginous growth has been shown to reduce scar formation, improve the aesthetic outcome of primary cleft repair, [31,32] and reduce the number of secondary follow-up visits, [33] thereby reducing overall health care costs associated with cleft care and management. [29,34] However, NAM has also been challenged for its burden of care, as the intraoral molding plate requires multiple impressions and adjustments in accordance with the infant’s alveolar development; [35] according to Sischo et al., unilateral cleft lip infants require 13 to 14 follow-up visits, while bilateral cleft lip patients require up to 20 to 22 visits. [36] NAM critics have also asserted that NAM compromises normal orofacial development [37] in affected infants and increases the emotional burden on families who already have to contend with their infant’s congenital defect. Notably, NAM has been challenged for the lack of standardization in study designs, outcome variables, and data reporting [29,38] and for its limited availability. Sischo et al. reported that one-third of specialized cleft care centers in the United States offer it as a therapeutic option for affected patients. [36]
Despite these challenges, NAM therapy has recently undergone substantive developments to render it more efficient, patient-specific, and cost-effective. With the advent of 3D photography or stereophotogrammetry, care providers have developed robust methods of objectively measuring progressive morphological changes during NAM therapy, thereby providing much needed evidence for its clinical utility. [39] Computer-aided design and computer-aided manufacturing (CAD/CAM) technologies can help care providers utilize a single CT scan of a patient to create dental casts and patient-specific intraoral plates in a cost-effective and efficient manner. Along the same vein, virtual workflows using semi-automated intraoral molding with a graphical user interface (GUI) have also been developed to create intraoral molding devices quickly and at a low cost. [35]
2. Traditional Presurgical Nasoalveolar Molding (NAM) Therapy
NAM’s overarching objective is to reduce the severity of the cleft deformity prior to surgical repair. In his seminal paper describing the NAM technique, Grayson et al. demonstrated that the deficient columella, misaligned alar cartilage, and deformed alveolar processes can be repositioned with the use of an intraoral molding plate and nasal stent. [24,25] An impression of the palate is taken followed by fabrication of a maxillary cast. This maxillary cast is used as a base upon which the molding plate is made through the use acrylic resin. [25] The initial impression of the cleft is taken 1 to 2 weeks after birth and when the neonate is fully awake in a prone position. After fabrication, the molding plate is examined and polished to remove any irregular rough areas and then inserted into the neonate’s oral cavity. Surgical adhesive tape is employed to secure the molding plate in position. The tape is placed externally on the neonate’s cheeks and to a protruding extension of the oral plate that is located between the cleft lip segments.
The molding plate’s position and acrylic composition is modified at various intervals in order to guide the neonate’s alveolar development. The care provider can add and remove the acrylic resin and the soft denture liner to the molding plate to shape the alveolar bone segments to approximate each other facilitating improved arch development. Adhesive tape is further employed to bring the lip segments closer together, gradually reducing the size of the cleft deformity, improving the symmetry between the nasal apertures, and bringing the columella to the midsagittal plane. [24]
According to Grayson et al., the nasal stent is added to the molding plate once the cleft deformity has been effectively reduced to 6 mm. [25] One nasal stent is used for unilateral cleft lip with or without palate cases and 2 stents are used for bilateral cleft cases. [40] Insertion of the nasal stent when the cleft is 6 mm wide is done to prevent unintended widening of the lateral alar wall’s circumference, which can unintentionally occur if the cleft deformity is greater than 6 mm in width. While the base of the nasal stent is located between the cleft lip segments, the nasal stent extends into the nose on the cleft side of the alar cartilage. The nasal stent is then modified at weekly intervals to shape the nasal cartilages and improve nasolabial symmetry until the structures begin to normalize. Force is applied to the nasal stent when the infant breastfeeds. Thus, this method differs from prior presurgical orthopedic therapies such as the Hotz plate [41] because of the NAM’s use of medially directed pressure to actively mold the alveolar arch and nasal cartilages into a preferred alignment. Due to alveolar growth or cleft reduction, the nasal stent must be exchanged at periodic intervals and placed onto a new molding plate. [42] After NAM therapy, primary lip-nose repair is performed in order to provide the patient with positive anatomic, aesthetic, and functional outcomes. [40] Thus, NAM has been credited with reducing the number of secondary surgical corrections associated with traditional cleft care management.
Despite the clinical benefits of NAM therapy, several studies have noted mild complications that are associated with this treatment modality. Grayson et al. discuss that the NAM intraoral molding plate can cause irritation of the gingival or oral mucosal tissue and that the surgical adhesive tape may cause skin irritation. [24] The pressure of the device against the hard and soft tissues may also result in ulcerations. [40] Levy-Bercowski et al. state that soft tissue complications additionally include fungal infections and bleeding. While hard tissue complications appear in reference to the asymmetrical configuration of the alveolar arches. They also stated that 39% of subjects reported lack of compliance. [43] Further, Grayson et al. acknowledge that parts of the NAM appliance itself may erode over time, thereby increasing its burden of care. [24]
3. Appliance Modifications to the Grayson NAM Technique
Many modifications of Grayson’s NAM technique have been introduced in order to improve its efficacy, reduce the occurrence of hard and soft tissue complications, and reduce the number of secondary visits. Certain techniques, like Bennun and Figueroa’s modified dynamic presurgical nasal remodeling (DPNR), [44] can be used for both bilateral and unilateral cases. In comparison to the Grayson technique, which initiates nasal molding when the alveolar gap is reduced to 6 mm, the Figueroa technique initiates nasal and alveolar molding at the same time. Further, the Figueroa appliance utilizes a larger nasal stent to decrease the risk of oral and mucosal ulcerations. Despite the lack of accuracy in the Figueroa method and its limited access to patients who live far from hospitals, it has been shown to increase comfort of the NAM appliance, increase efficiency (defined as the number of appliance adjustments), reduce the time needed for treatment for both bilateral and unilateral cases, and reduce the incidence of oral mucosal ulceration. [45]
4. NAM Treatment Modifications for Unilateral Cleft Lip and/or Palate Patients
There are several studies detailing recent NAM modifications that involve the nasal stent. In 2004, Mitsuyoshi et al. introduced use of a 1-mm wide cobalt-chrome nasal stent in order to leverage manual force of the stent more efficiently during treatment. [46] The modified stent also features a loop measuring 3-mm in diameter in the middle of the stent that enhances manual control of the stent’s force and direction. However, the authors note that this modification also has shortcomings, including causing instability of the molding plate. Following closely in 2005, Doruk and Kilic introduced the use of an extraoral nasal molding appliance (ENMA) that incorporates a headband to support the NAM device. [47] The nasal stent was a 0.8-mm stainless steel helical spring that was adjusted at 2-week intervals. This treatment modification proved to be efficacious in molding the oronasal cartilage and alveolar segments into a form that resembles normal structures. In 2015, Nagraj et al. described a double-loop technique using titanium molybdenum alloy wire nasal stents designed to simplify the modification process of the NAM appliance. [48]
Other studies describe modifications to the molding plate. Bajaj et al. describes the use of a 2to 3-mm-thick occlusal molding plate for fabrication of the NAM appliance. [49] The plate is designed with the addition of wax spacer to the cleft space of the molding plate, eliminating the need for weekly trimming of the appliance. The addition of wax spacer purportedly increases the time interval needed to modify the appliance, reducing the burden of care for both the care team and the patient’s primary care givers. Because of its time efficiency, the authors report that this modified appliance and technique can improve compliance with the treatment regimen. Similarly, Chen and Liao discuss a modified NAM technique that involves use of a “passive” palatal plate, which they describe as a “block-out” of alveolar and palate clefts prior to fabrication of the plate. [50] This plate is not attached to the nasal stent wire, reducing the amount of chair side time required to embed the nasal stent in traditional molding plates. Use of this passive palatal plate and a simplified taping technique (described as use of 1 long tape instead of 4 tapes) are reported to reduce caregivers’ stress about lip taping, medical costs, and the number of adjustments needed to achieve the desired aesthetic results.
5. NAM Treatment Modifications for Bilateral Cleft Lip and/or Palate
Bilateral cleft cases are more challenging to treat with NAM therapy because of the absence of soft tissue, malposition of the premaxilla, and severity of the cleft defect. Nevertheless, efforts have been made to increase lip and nasal symmetry, lengthen the columella, and increase nasal tip projection in bilateral cases. Patil et al. noted in their 2013 study that severe bilateral cleft lip and/or palate cases could be effectively addressed with minor modifications to the Grayson technique. [51] By adding a 1 mm layer of liner on the tissue surface of the NAM appliance, the authors were able to lengthen the columella by 6 mm. They were also able to reduce the cleft width of 12 mm on the right and 14 mm on the left. Ijaz et al. were able to utilize a modified plate with an acrylic ring to retract the premaxilla by 3.80 + 1.3 mm and elongate the columella by 2.24 mm. [52] Similarly, in 2018, Titiz and Aras introduced the use of a prefabricated retraction apparatus and retention arm to enable faster retraction of the premaxilla and reduction of the cleft width to less than 6 mm. [53]
6. Complications Associated with NAM Treatment Modifications
Despite their myriad benefits, the aforementioned treatment modifications still run the risk of causing soft tissue irritation and ulcerations. [40] The techniques are relatively time consuming and can be difficult to adjust to for the patients’ caregivers. Additionally, these modifications do not rule out the need for manual fabrication of NAM appliances and the frequent follow-on visits required to modify the appliance in accordance with the infant’s alveolar development. Finally, the modifications described are operator-dependent.
7. The Application of 3D Technologies to NAM Therapy
The advent of 3D technologies has spurred notable advances in NAM therapy. 3D photography, also known as stereophotogrammetry, has been utilized to quantitatively measure the progressive morphological changes that occur over the course of treatment. The adaptation of computer-aided design and computer-aided manufacturing (CAD/CAM) to NAM appliance fabrication has resulted in a production modality that is low-cost, efficient, and easier to use than other production techniques.
8. Application of 3D Printing to NAM Therapy
Since 2011, the application of 3D printing in the medical field has grown at an unprecedented rate. [54,55] Rapid prototyping (RP) and additive manufacturing have great potential for increasing technical refinement, precision, ease of use, and accessibility of medical devices compared to traditional manufacturing modalities. In contrast to subtractive manufacturing, additive manufacturing involves the fabrication of a 3D model via the successive deposition of material, layer by layer, from the ground-up. With subtractive manufacturing, the interior of the model is always solid, which can compromise the strength of the model. Because additive manufacturing allows the designer to have greater control over the interior of the object, it allows for greater versatility and precision. With regard to NAM therapy, various studies have documented the application of rapid prototyping and CAD/CAM additive manufacturing in efficiently producing versatile and structurally sound NAM appliances.
Selective laser sintering (SLS) utilizes a CO2 laser to fuse thermoplastic powder into solid layers 0.0004 to 0.006 mm thick. Direct metal laser sintering (DMLS) utilizes a laser to sinter different types of metal powder together into solid layer form of 0.0008 to 0.0012 mm in thickness. These metal powders can consist of bronze, steel, titanium, or aluminum. In contrast to SLS and DMLS techniques, stereolithography (SLA) additive manufacturing requires the use of an ultraviolet laser to draw cross-sections into a vat of epoxy resin. In a process called photopolymerization, the laser solidifies the resin to form layers 0.002 mm thick. The resin is then washed in isopropyl alcohol to remove debris, cured with ultraviolet light, and heated until it hardens. Fused deposition modeling (FDM), otherwise known as free form fabrication (FFF), utilizes an extruder to heat thermoplastic filaments until they form a malleable fluid. The fluid is then deposited in solidifying layers of 0.007 mm thickness to produce a complete 3D model. Finally, multi-jet and poly-jet printing techniques utilize an ink jet nozzle to spray liquid binding agents onto metallic or ceramic powders, thereby creating very fine layers (0.016 mm thick) of these powders. Once the layers are sintered in a furnace, they are covered with metal to yield the final 3D model.
The aforementioned 3D printing techniques utilize the same workflow to create a 3D NAM appliance. Imaging data is first collected often in the form of conventional orthodontic impressions which are then scanned 3-dimensionally. This data is then stored as Digital Imaging and Communications in Medicine (DICOM) files. [55] CAD software is used to construct a 3D model from the data contained in the DICOM files. The dataset is exported to Stereolithography (STL) format and can then be segmented. Finally, a 3D virtual model as illustrated in Figure 1 can be printed after it has been refined in a process called “geometric surface preparation.”
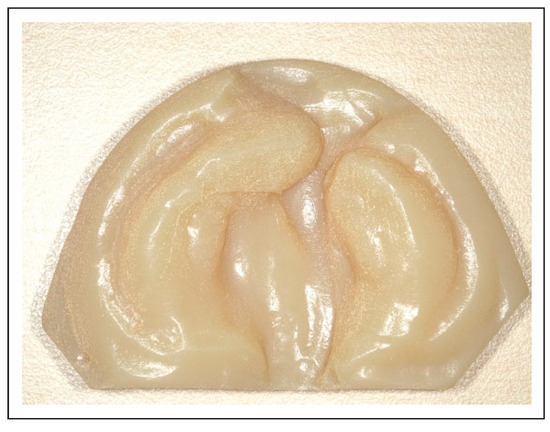
Figure 1.
3D printed maxillary cast.
In the past several years, evidence has shown that 3D-printed NAM appliances can potentially minimize appointment length, improve precision, and reduce costs associated with 3D fabrication modalities. [42] It has also been shown that the use of CAD-generated NAM appliances can achieve similar clinical outcomes and similar risk of hard and soft tissue complications compared to traditional NAM devices. [40] For instance, in 2012, Yu et al. demonstrated that 3D images of the maxillary casts of unilateral cleft lip and palate patients could be generated using a laser surface scanner. [56] The digital geometrical data could then be used to print a 3D scale model of the original cast via rapid prototyping. The authors state that the rapid prototyping technique could significantly simplify the NAM appliance fabrication workflow by allowing the care provider to manufacture an entire set of NAM appliances for 1 patient at once with quantitative precision. This technique can effectively reduce the number of follow-on visits required for treatment. Moreover, Yu et al. provide compelling evidence that CAD-generated NAM appliances improve nasal symmetry, increase columellar length, and reduce the distance between the alveolar segments, similar to more traditional NAM techniques.
In 2013, Gong and Yu demonstrated that the same reverse engineering and rapid prototyping approach could be applied to fabricate NAM devices for patients with bilateral cleft lip and palate. [57] Using a 3D image of the patients’ cleft anatomy, the study authors constructed digital models of the NAM appliances all at once using Rapidform 2006 software. Similar to the findings reported by Yu et al., the alveolar segments were aligned and the columellar length increased after therapy.
In a 2013 randomized control trial, Yu et al. demonstrated that CAD-NAM therapy was effective in reducing the alveolar gap and correcting the maxilla’s sagittal length. [58] They utilized CAD technology to assess the anteroposterior, vertical, and transverse alveolar morphology of neonatal patients with unilateral cleft lip and palate. A maxillary cast was created for each infant during the first impression taking. The cast data was uploaded to a reverse engineering software system (Rapidform XOR3, Inus Technology, Inc., Seoul, South Korea) using a 3D laser scanner. The software was then employed to print 3D models of the casts based on several predicted adjustments of the intraoral casts. Overall, the results reported by Yu et al. indicated that CAD-NAM could be effectively employed to reduce the alveolar gap and arch length and normalize the maxillary alveolar contours. However, the authors duly noted a complication in the form of stunted upper alveolar vertical growth.
In a prospective study conducted in 2015, Shen et al. demonstrated that computer-aided manufacturing (CAM) could indeed be utilized to create a NAM device. [59] In their study, they describe a technique that first requires an intraoral impression during the first visit. This impression would then be utilized to design a maxillary cast. During each follow-on visit, the shape of the maxillary cast would be modified based on a simulated “molding course” using a computer-generated algorithm. The molding course would project changes to the alveolar segments in order to minimize the alveolar gap by 1 mm at weekly intervals. Each corresponding maxillary cast would then be fabricated using 3D printing. In the study, the alveolar gap decreased significantly (P < 0.01) and the alveolar arch became more symmetrical (P < 0.01). Several complications were noted, including bleeding, mucosal irritation, and ulceration. Despite these complications, the study demonstrated that linear and angular freeform manipulations of the cleft palate plaster model can precisely represent anatomic structures in a neonatal cleft case. Importantly, the study also demonstrated that 3D technology can significantly reduce the number of follow-on visits and appliance modifications, thereby minimizing the burden of care and the costs associated with NAM treatment.
More recent studies have investigated the integration of graphical user interfaces with CAD-CAM technology to automate the design of several intraoral molding plates from a single impression taking. For example, Bauer et al. reported in 2017 the RapidNAM system, which utilizes a “growth prediction factor” to project the patient’s cleft growth and generate a series of molding plates based on these projections. [60] In their study, the computer-generated algorithm generated plates that were able to reduce the cleft gap by nearly 50%. The NAM appliances are produced with a PolyJet 3D printer using a biocompatible resin. Three appliances were utilized for the entire treatment course. The study authors note that no cases of mucosal ulcerations or skin irritation were found and that the NAM appliances significantly increased ease of use and cut costs for the patients’ families. An example of digitally produced sequential study models and molding plates is shown in Figure 2.
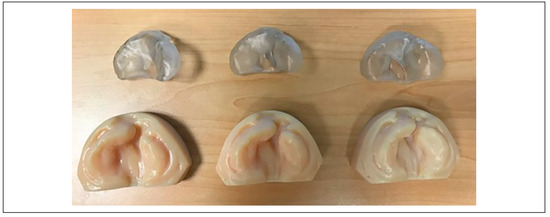
Figure 2.
Sequential maxillary casts and NAMs demonstrating projected movements.
In 2018, Grill et al. applied the “RapidNAM” system to generate a virtual series of molding plates at once using a single impression. [35] Compared to the manual CAD/CAM method, which takes up to 1.5 h per plate, the RapidNAM system reported by Grill et al. created each plate in 10 to 15 min. In terms of cost, the RapidNAM plates cost 155 USD compared to 223 USD for conventional intraoral molding plates. The study authors note, however, that certain elements of the workflow must be done manually, such as selecting the alveolar segments, due to the considerable variation present in cleft cases. The results from the study demonstrate that the RapidNAM technique produces clinical outcomes similar to those of manual intraoral molding plate production; it reduced the alveolar gap, improved nasal symmetry, and unlike the findings from Yu et al., allowed for vertical maxillary growth. Sequential maxillary cast design using free form manipulation software (Autodesk Fusion 360) is shown in Figure 3.
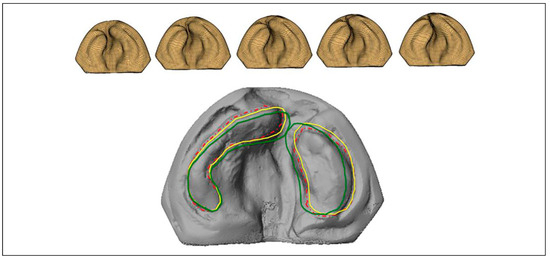
Figure 3.
Computer-aided design of sequential maxillary casts.
An additional study in 2018 published by Grill et al. incorporates the use of RapidNAM to automate nasal stent transfers between the different intraoral molding plates. [42] This technique involves the use of a click-in nasal stent that can be automatically detached and inserted onto a new intraoral molding plate, bypassing the need for creating a new nasal stent for every new molding plate. Like the traditional technique, the nasal stent is composed of stainless steel and a nasal bulb. Alternatively, nasal stents have been used in conjunction with an extraoral attachment, typically using the forehead of the patient. These can be produced using conventional laboratory or CAD/CAM technology, as demonstrated in Figure 4.
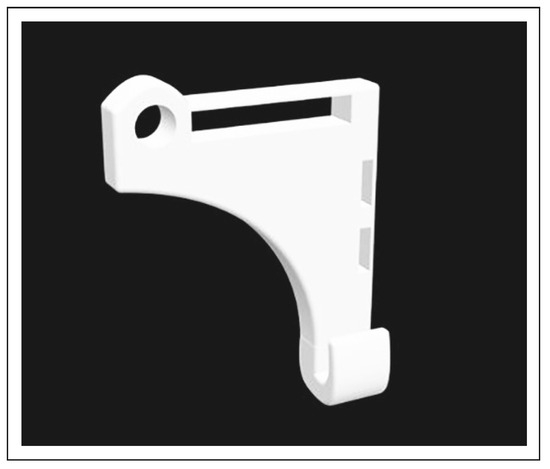
Figure 4.
3D rendering of nasal stent with extraoral attachment.
9. Applications of 3D Stereophotogrammetry in NAM Therapy
In addition to rapid prototyping technology, computeraided design (CAD) enables highly precise 3D measurements of nasal and alveolar development over the course of NAM treatment. [61] In a prospective study conducted by
Baek and Son, 3D stereophotogrammetry was used to measure the cleft gap width during NAM treatment. [62] The authors found that the posterior alveolar segments experienced the greatest growth in area and distance compared to the anterior alveolar segments but that the anterior alveolar segments were primarily affected by NAM therapy. Similarly, in 2007, Singh et al. utilized digital stereophotogrammetry to collect 3D facial images of patients with unilateral cleft lip and/or palate. [63] In their study, they were able to find that the 3D facial morphology of the cleft patients after NAM therapy was “virtually indistinguishable” from patients in the control group.
Most recently, Staderini et al. published a study in 2019 describing use of the 3dMD system to standardize the capture of 3D photographs of patients with unilateral cleft lip and/or palate before and after NAM treatment. [64] The authors used Geomagic Studio 2014 software to evaluate morphological changes. It selects the eyes and chin to calculate the asymmetry index and the symmetry plane. The authors conclude that such software can more accurately detect changes in facial symmetry in comparison to human or photometric techniques. Notably, this study highlights the need for standardized protocols and use of consistent operators to make more generalizable conclusions about the efficacy of this treatment modality. Figure 5 illustrates the progressive nasolabial changes expected from NAM therapy.
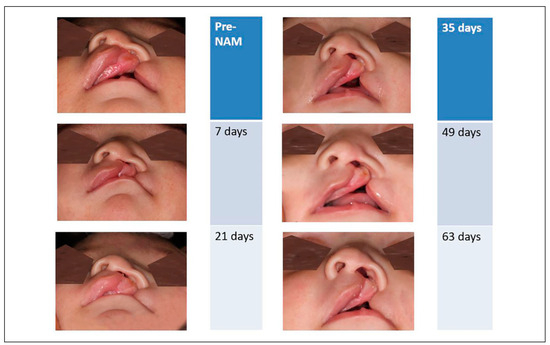
Figure 5.
Progressive evaluation of nasolabial aesthetics during presurgical NAM therapy.
10. Conclusions
The craniofacial community has widely adopted the application of computer-guided technologies for 3D image analysis of infants with cleft lip and/or palate. Three-dimensional stereophotogrammetry and CAD/CAM NAM treatment have been utilized to quantify the morphological linear and angular dimensional changes of the neonate’s face in an accurate, practical, and convenient way. Studies have concluded that CAD/CAM NAM treatment is effective in the reduction of total arch length, premaxillary protrusion, nasal projection, columella length, nasal symmetry and nasal width. Additional benefits of such a workflow are to reduce chairside adjustment time and provide the clinician with a quantitatively detailed visual feedback mechanism of treatment progress. More rigorous investigations are needed to determine whether these computer-guided technologies should be adopted en masse by the craniofacial community. There are a variety of software and hardware options for these technologies, each requiring different workflows, none of which have demonstrated superiority over another.
Author’s Note: Leslie Slowikowski is now affiliated with Department of Dentistry, Children’s Hospital New Orleans, New Orleans, LA, USA.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflicts of Interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Mossey, P.A.; Modell, B. Epidemiology of oral clefts 2012: An international perspective. Front Oral Biol. 2012, 16, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.K.; Bui, A.H.; Taioli, E. Epidemiology of Cleft Lip and Palate. Des. Strateg. Cleft Lip Palate Care 2017, 3–22. [Google Scholar] [CrossRef]
- World Health Organization. Addressing the Global Challenges of Craniofacial Anomalies Report of a WHO Meeting on International Collaborative Research on Craniofacial Anomalies; WHO: Geneva, Switzerland, 2006. [Google Scholar]
- Leslie, E.J.; Marazita, M.L. Genetics of cleft lip and cleft palate. Am. J. Med. Genet. C Semin. Med. Genet. 2013, 163, 246–258. [Google Scholar] [CrossRef]
- Wilson-Nagrani, C.; Richmond, S.; Paternoster, L. Nonsyndromic cleft lip and palate polymorphisms affect normal lip morphology. Front. Genet. 2018, 9, 413. [Google Scholar] [CrossRef]
- Dixon, M.J.; Marazita, M.L.; Beaty, T.H.; Murray, J.C. Cleft lip and palate: Understanding genetic and environmental influences. Nat. Rev. Genet. 2011, 12, 167–178. [Google Scholar] [CrossRef]
- Marazita, M.L.; Lidral, A.C.; Murray, J.C.; et al. Genome scan, finemapping, and candidate gene analysis of non-syndromic cleft lip with or without cleft palate reveals phenotype-specific differences in linkage and association results. Hum. Hered. 2009, 68, 151–170. [Google Scholar] [CrossRef]
- Birnbaum, S.L.K.; Reutter, H.; Herms, S.; et al. Key susceptibility locus for nonsyndromic cleft lip with or without cleft palate on chromosome 8q24. Nat. Genet. 2009, 41, 473–477. [Google Scholar] [PubMed]
- Mangold, E.; Ludwig, K.U.; Birnbaum, S.; et al. Genome-wide association study identifies two susceptibility loci for nonsyndromic cleft lip with or without cleft palate. Nat. Genet. 2010, 42, 24–26. [Google Scholar]
- Auslander, A.; McKean-Cowdin, R.; Brindopke, F.; Magee, K.; DiBona, M.; Magee, W. Environmental risk factors for cleft lip and palate in low-resource settings: A case-control study. Lancet Glob. Health 2019, 7, 19. [Google Scholar] [CrossRef]
- Nagase, Y.; Natsume, N.; Kato, T.; Hayakawa, T. Epidemiological analysis of cleft lip and/or palate by cleft pattern. J. Maxillofac. Oral. Surg. 2010, 9, 389–395. [Google Scholar] [CrossRef]
- Hlongwa, P.; Levin, J.; Rispel, L.C. Epidemiology and clinical profile of individuals with cleft lip and palate utilising specialised academic treatment centres in South Africa. PLoS ONE 2019, 14, e0215931. [Google Scholar] [CrossRef] [PubMed]
- Acun˜a-Gonza’lez, G.; Medina-Sol’ıs, C.E.; Maupome, G.; et al. Family history and socioeconomic risk factors for non-syndromic cleft lip and palate: A matched case-control study in a less developed country. Biomedica 2011, 31, 381–391. [Google Scholar]
- Shaw, G.M.; Carmichael, S.L.; Laurent, C.; Rasmussen, S.A. Maternal nutrient intakes and risk of orofacial clefts. Epidemiology 2006, 17, 285–291. [Google Scholar] [CrossRef]
- Little, J.; Cardy, A.; Munger, R.G. Tobacco smoking and oral clefts: A meta-analysis. Bull. World Health Organ. 2002, 82, 213–218. [Google Scholar]
- Shi, M.; Christensen, K.; Weinberg, C.R.; et al. Orofacial cleft risk is increased with maternal smoking and specific detoxification-gene variants. Am. J. Hum. Genet. 2007, 80, 76–90. [Google Scholar] [CrossRef]
- Lie, R.T.; Wilcox, A.J.; Taylor, J.; et al. Maternal smoking and oral clefts: The role of detoxification pathway genes. [Published correction appears in Epidemiology 2010 May;21(3):432]. Epidemiology. 2008, 19, 606–615. [Google Scholar] [CrossRef]
- Stuppia, L.; Cagogreco, M.; Marzo, G.; et al. Genetics of syndromic and nonsyndromic cleft lip and palate. J. Craniofac. Surg. 2011, 22, 1722–1726. [Google Scholar] [CrossRef]
- Nahai, F.R.; Williams, J.K.; Burstein, F.D.; Martin, J.; Thomas, J. The management of cleft lip and palate: Pathways for treatment and longitudinal assessment. Semin. Plast. Surg. 2005, 19, 275–285. [Google Scholar] [CrossRef]
- Robin, N.H.; Baty, H.; Franklin, J.; et al. The multidisciplinary evaluation and management of cleft lip and palate. South. Med. J. 2006, 99, 1111–1120. [Google Scholar] [CrossRef]
- Strauss, R.P.; Broder, H. Interdisciplinary team care of cleft lip and palate: Social and psychological aspects. Clin. Plast. Surg. 1985, 12, 543–551. [Google Scholar] [CrossRef]
- Rau, A.; Ritschl, L.M.; Mu¨cke, T.; Wolff, K.-D.; Loeffelbein, D.J. Nasoalveolar molding in cleft care—Experience in 40 patients from a single centre in Germany. PLoS ONE 2015, 10, e0118103. [Google Scholar]
- Matsuo, K.; Hirose, T. Preoperative non-surgical overcorrection of cleft lip nasal deformity. Br. J. Plast. Surg. 1991, 44, 5–11. [Google Scholar] [CrossRef]
- Grayson, B.H.; Maull, D. Nasoalveolar molding for infants born with clefts of the lip, alveolus, and palate. Semin. Plast. Surg. 2005, 19, 294–301. [Google Scholar] [CrossRef]
- Grayson, B.H.; Santiago, P.E.; Brecht, L.E.; Cutting, C.B. Presurgical nasoalveolar molding in infants with cleft lip and palate. Cleft Palate Craniofac. J. 1999, 36, 486–498. [Google Scholar] [PubMed]
- Attiguppe, P.R.; Karuna, Y.M.; Yavagal, C.; et al. Presurgical nasoalveolar molding: A boon to facilitate the surgical repair in infants with cleft lip and palate. Contemp. Clin. Dent. 2016, 7, 569–573. [Google Scholar]
- Rubin, M.S.; Clouston, S.; Ahmed, M.M.; et al. Assessment of presurgical clefts and predicted surgical outcome in patients treated with and without nasoalveolar molding. J. Craniofac. Surg. 2015, 26, 71–75. [Google Scholar] [CrossRef]
- Shetye, P.R. Presurgical infant orthopedics. J. Craniofac. Surg. 2012, 23, 210–211. [Google Scholar] [PubMed]
- Ahmed, M.K.; Bui, A.H.; Barnett, R.; Rousso, J.J. Quantitative evaluation of nasolabial alterations following nasoalveolar molding (NAM) therapy in patients with unilateral cleft lip. Facial Plast. Surg. 2019, 35, 73–77. [Google Scholar]
- Alzain, I.; Batwa, W.; Cash, A.; Murshid, Z.A. Presurgical cleft lip and palate orthopedics: An overview. Clin. Cosmet. Investig. Dent. 2017, 9, 53–59. [Google Scholar] [CrossRef]
- Broder, H.L.; Flores, R.L.; Clouston, S.; et al. Surgeon’s and caregivers’ appraisals of primary cleft lip treatment with and without nasoalveolar molding: A prospective multicenter pilot study. Plast. Reconstr. Surg. 2016, 137, 938–945. [Google Scholar] [CrossRef]
- Abbott, M.M.; Meara, J.G. Nasoalveolar Molding in Cleft Care: Is It Efficacious? Plast. Reconstr. Surg. 2012, 130, 659–666. [Google Scholar]
- Patel, P.A.; Rubin, M.S.; Clouston, S.; et al. Comparative study of early secondary nasal revisions and costs in patients with clefts treated with and without nasoalveolar molding. J. Craniofac. Surg. 2015, 26, 1229–1233. [Google Scholar] [PubMed]
- Pfeifer, T.M.; Grayson, B.H.; Cutting, C.B. Nasoalveolar molding and gingivoperiosteoplasty versus alveolar bone graft: An outcome analysis of costs in the treatment of unilateral cleft alveolus. Cleft Palate Craniofac. J. 2002, 39, 26–29. [Google Scholar]
- Grill, F.D.; Ritschl, L.M.; Bauer, F.X.; et al. A semi-automated virtual workflow solution for the design and production of intraoral molding plates using additive manufacturing: The first clinical results of a pilot study. Sci. Rep. 2018, 8, 11845. [Google Scholar]
- Sischo, L.; Chan, J.W.; Stein, M.; Smith, C.; van Aalst, J.; Broder, H.L. Nasoalveolar molding: Prevalence of cleft centers offering Nam and who seeks it. Cleft Palate Craniofac. J. 2012, 49, 270–275. [Google Scholar] [PubMed]
- Berkowitz, S. Gingivoperiosteoplasty as well as early palatal cleft closure is unproductive. J. Craniofac. Surg. 2009, 20, 1747–1758. [Google Scholar] [PubMed]
- van der Heijden, P.; Dijkstra, P.U.; Stellingsma, C.; van der Laan, B.F.; Korsten-Meijer, A.G.W.; Goorhuis-Brouwer, S.M. Limited evidence for the effect of presurgical nasoalveolar molding in unilateral cleft on nasal symmetry: A call for unified research. Plast. Reconstr. Surg. 2013, 131, 62e–71e. [Google Scholar]
- Chou, P.Y.; Hallac, R.R.; Ajiwe, T.; et al. The role of nasoalveolar molding: A 3D prospective analysis. Nature Sci. Rep. 2017, 7, 9901. [Google Scholar]
- Maillard, S.; Retrouvey, J.M.; Ahmed, M.K.; Taub, P.J. Correlation between nasoalveolar molding and surgical, aesthetic, functional and socioeconomic outcomes following primary repair surgery: A systematic review. J. Oral. Maxillofac. Res. 2017, 8, e2. [Google Scholar] [CrossRef]
- Isogawa, N.; Ochiai, S.; Mito, T.; et al. Three-dimensional comparison in palatal forms between modified presurgical nasoalveolar molding plate and Hotz’s plate applied to the infants with unilateral cleft lip and palate. Singapore Dent. J. 2010, 31, 36–42. [Google Scholar]
- Grill, F.D.; Ritschl, L.M.; Dikel, H.; et al. Facilitating CAD/CAM nasoalveolar molding therapy with a novel click-in system for nasal stents ensuring a quick and user-friendly chairside nasal stent exchange. Nature Sci. Rep. 2018, 8, 12084. [Google Scholar]
- Levy-Bercowski, D.; Abreu, A.; DeLeon, E.; et al. Complications and solutions in presurgical nasoalveolar molding therapy. Cleft Palate Craniofac. J. 2009, 46, 521–528. [Google Scholar] [PubMed]
- Bennun, R.D.; Figueroa, A.A. Dynamic presurgical nasal remodeling in patients with unilateral and bilateral cleft lip and palate: Modification to the original technique. Cleft Palate Craniofac. J. 2006, 43, 639–648. [Google Scholar]
- Liao, Y.F.; Wang, Y.C.; Chen, I.J.; Pai, C.J.; Ko, W.C.; Wang, Y.C. Comparative outcomes of two nasoalveolar molding techniques for bilateral cleft nose deformity. Plast. Reconstr. Surg. 2014, 133, 103–110. [Google Scholar] [PubMed]
- Mitsuyoshi, I.; Masahiko, W.; Masayuki, F. Simple modified preoperative nasoalveolar moulding in infants with unilateral cleft lip and palate. Br. J. Oral. Maxillofac. Surg. 2004, 42, 578–580. [Google Scholar]
- Doruk, C.; Kilic, B. Extraoral nasal molding in a newborn with unilateral cleft lip and palate: A case report. Cleft PalateCraniofac. J. 2005, 42, 699–702. [Google Scholar]
- Nagraj, N.; Nagarjuna, M.; Desai, A.K.; Gandedkar, N.; Jayade, B.; Gopalkrishnan, K. Double-loop technique using titanium molybdenum alloy wire for fabrication of nasal stents in nasoalveolar molding therapy for cleft lip and palate patients. Cleft Palate Craniofac. J. 2015, 52, 246–249. [Google Scholar]
- Bajaj, A.; Rao, K.S.; Sharma, S.M.; Shetty, V. Modified presurgical nasoalveolar molding in the infants with complete unilateral cleft lip and palate: A stepwise approach. J. Maxillofac. Oral. Surg. 2011, 10, 275–280. [Google Scholar] [CrossRef]
- Chen, Y.F.; Liao, Y.F. A modified nasoalveolar molding technique for correction of unilateral cleft nose deformity. J. Craniomaxillofac Surg. 2015, 43, 2100–2105. [Google Scholar]
- Patil, P.G.; Patil, S.P.; Sarin, S. Nasoalveolar molding with active columellar lengthening in severe bilateral cleft lip/palate: A clinical report. J. Prosthodont. 2013, 22, 137–142. [Google Scholar]
- Ijaz, A.; Raffat, A.; Israr, J. Nasoalveolar molding of bilateral cleft of the lip and palate infants with orthopaedic ring plate. J. Pak. Med. Assoc. 2010, 60, 527–531. [Google Scholar] [PubMed]
- Titiz, S.; Aras, I. Modifications in presurgical nasoalveolar molding treatment of bilateral cleft lip and palate patients with severely malpositioned premaxillae. Cleft Palate Craniofac. J. 2018, 55, 1316–1320. [Google Scholar] [PubMed]
- Hoang, D.; Perrault, D.; Stevanovic, M.; Ghiassi, A. Surgical applications of three-dimensional printing: A review of the current literature & how to get started. Ann. Transl. Med. 2016, 4, 456. [Google Scholar] [PubMed]
- Garcia, J.; Yang, Z.; Mongrain, R.; Leask, R.L.; Lachapelle, K. 3D printing materials and their use in medical education: A review of current technology and trends for the future. BMJ Simul. Technol. Enhanc. Learn. 2018, 4, 27–40. [Google Scholar]
- Zgong, X.; Yu, Q.; Yu, Z.Y.; Wang, G.M.; Qian, Y.F. Presurgical alveolar molding using computer aided design in infants with unilateral complete cleft lip and palate. Shanghai Kou Qiang Yi Xue. 2012, 21, 180–184. [Google Scholar]
- Gong, X.; Yu, Q. Correction of maxillary deformity in infants with bilateral cleft lip and palate using computer-assisted design. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. 2012, 114, S74–S78. [Google Scholar]
- Yu, Q.; Gong, X.; Shen, G. CAD presurgical nasoalveolar molding effects on the maxillary morphology in infants with UCLP. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. 2013, 116, 418–426. [Google Scholar]
- Shen, C.; Yao, C.A.; Magee, W., 3rd; Chai, G.; Zhang, Y. Presurgical nasoalveolar molding for cleft lip and palate: The application of digitally designed molds. Plast. Reconstr. Surg. 2015, 135, 1007e–1015e. [Google Scholar]
- Bauer, F.X.; Scho¨nberger, M.; Gattinger, J.; et al. RapidNAM: Generative manufacturing approach of nasoalveolar molding devices for presurgical cleft lip and palate treatment. Biomed Tech. 2017, 62, 407–414. [Google Scholar] [CrossRef]
- Ritschl, L.M.; Rau, A.; Gull, F.D.; et al. Pitfalls and solutions in virtual design of nasoalveolar molding plates by using CAD/CAM technology—A preliminary clinical study. J. Craniomaxillofac Surg. 2016, 44, 453–459. [Google Scholar]
- Baek, S.H.; Son, W.S. Difference in alveolar molding effect and growth in the cleft segments: 3-dimensional analysis of unilateral cleft lip and palate patients. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 2006, 102, 160–168. [Google Scholar] [PubMed]
- Singh, G.D.; Levy-Bercowski, D.; Ya’n˜ez, M.A.; Santiago, P.E. Three-dimensional facial morphology following surgical repair of unilateral cleft lip and palate in patients after nasoalveolar molding. Orthod. Craniofac. Res. 2007, 10, 161–166. [Google Scholar] [PubMed]
- Staderini, E.; Patini, R.; Camodeca, A.; Guglielmi, F.; Gallenzi, P. Three-dimensional assessment of morphological changes following nasoalveolar molding therapy in cleft lip and palate patients: A case report. Dent. J. 2019, 7, 27. [Google Scholar] [CrossRef] [PubMed]
© 2021 by the author. The Author(s) 2021.