Introduction of 3D Printing in a German Municipal Hospital—Practice Guide for CMF Surgery
Abstract
:1. Introduction
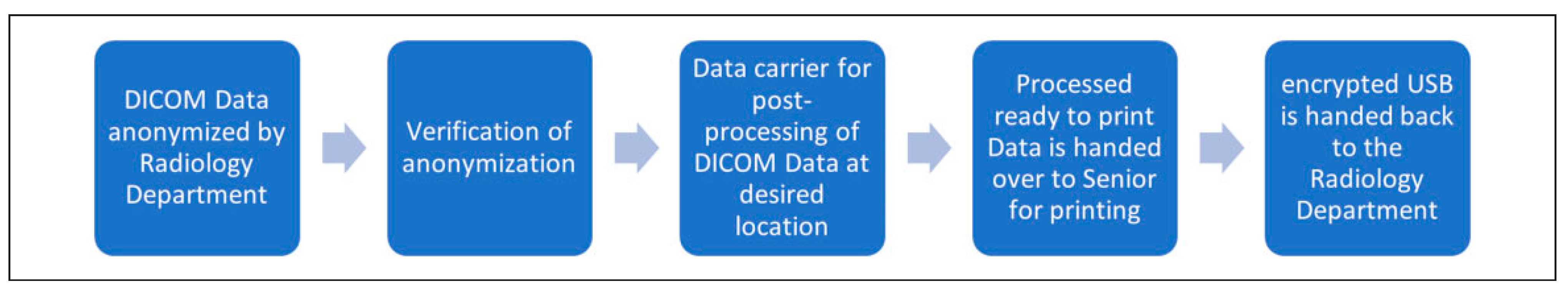
2. Requirements and Features
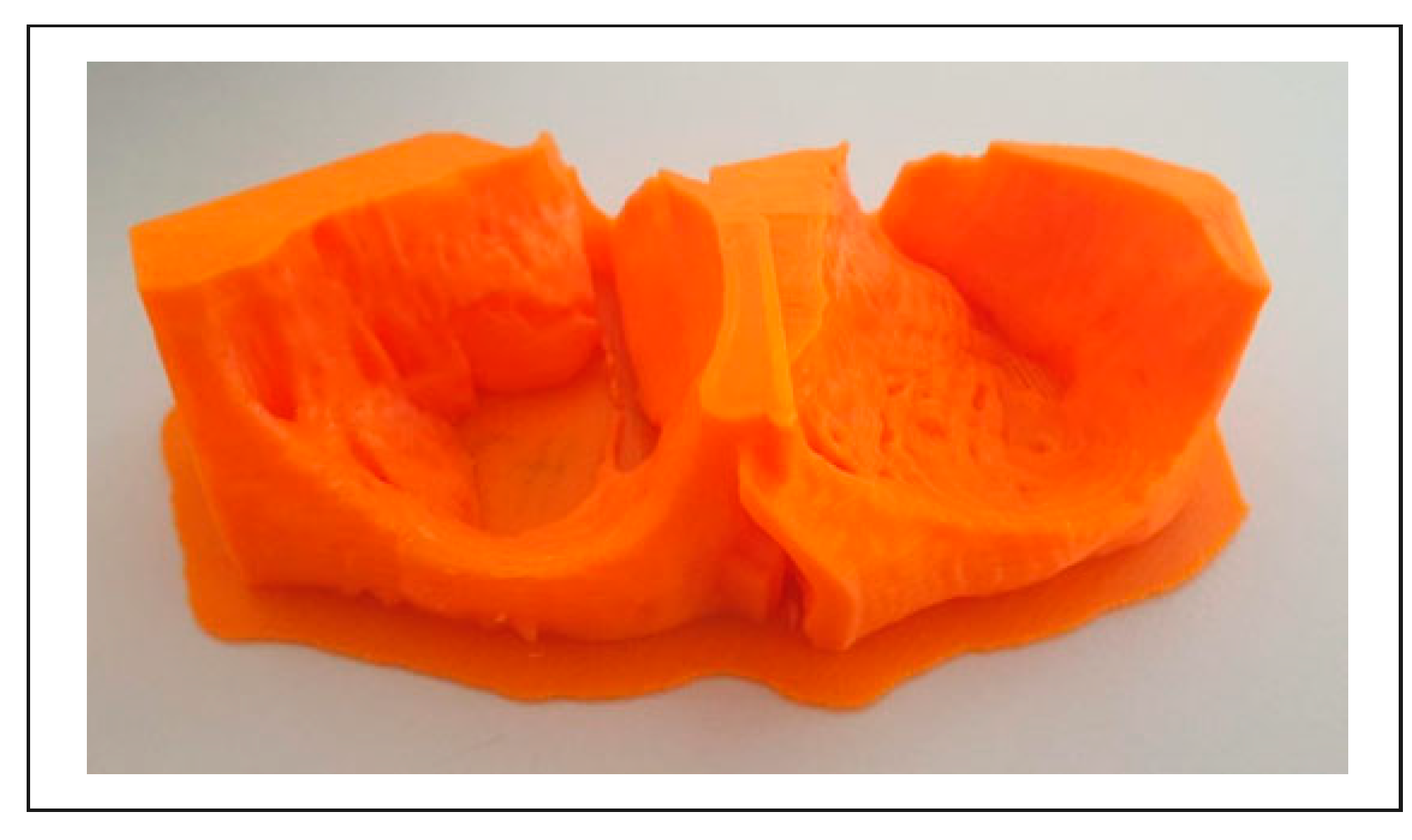
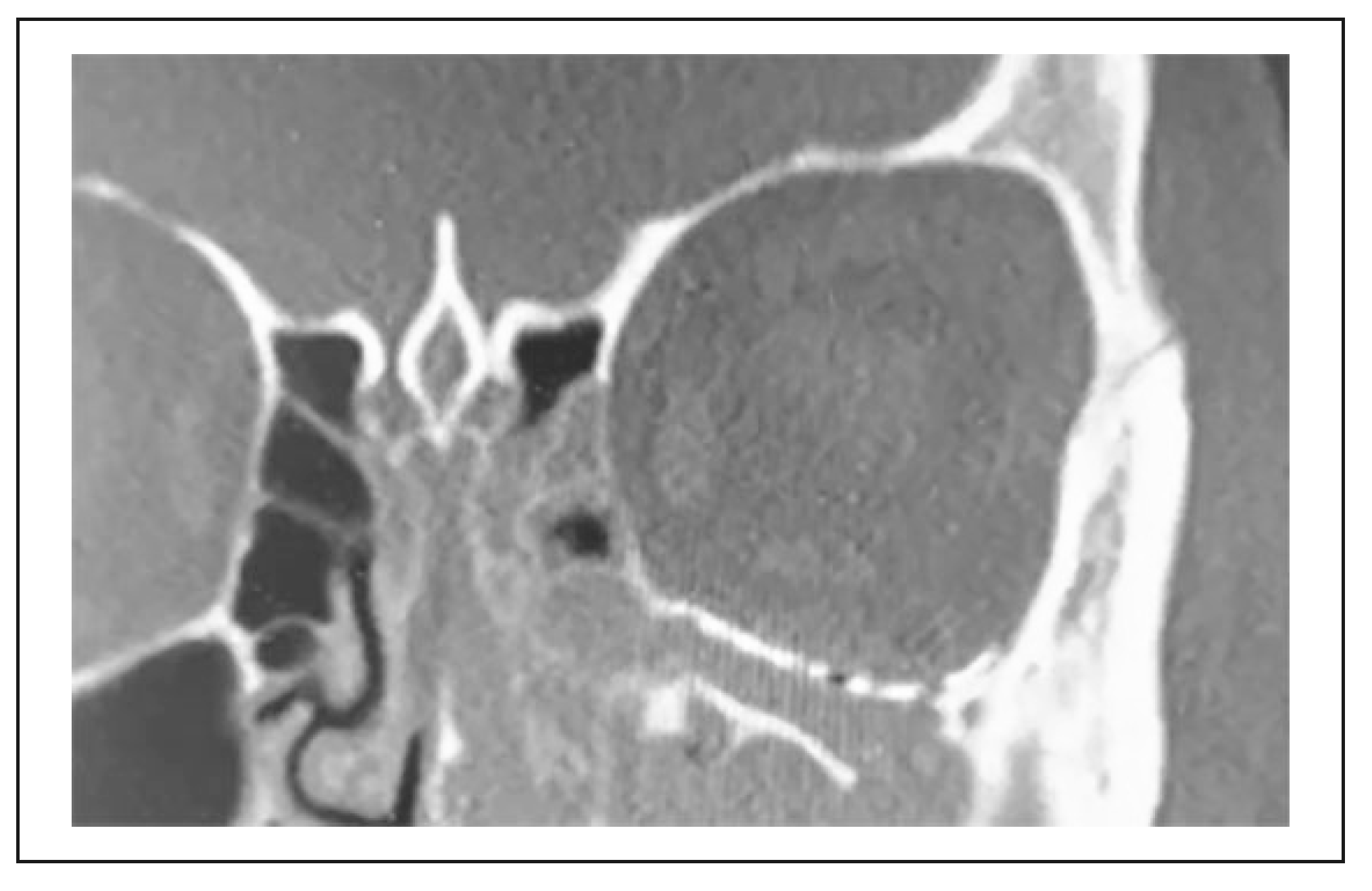
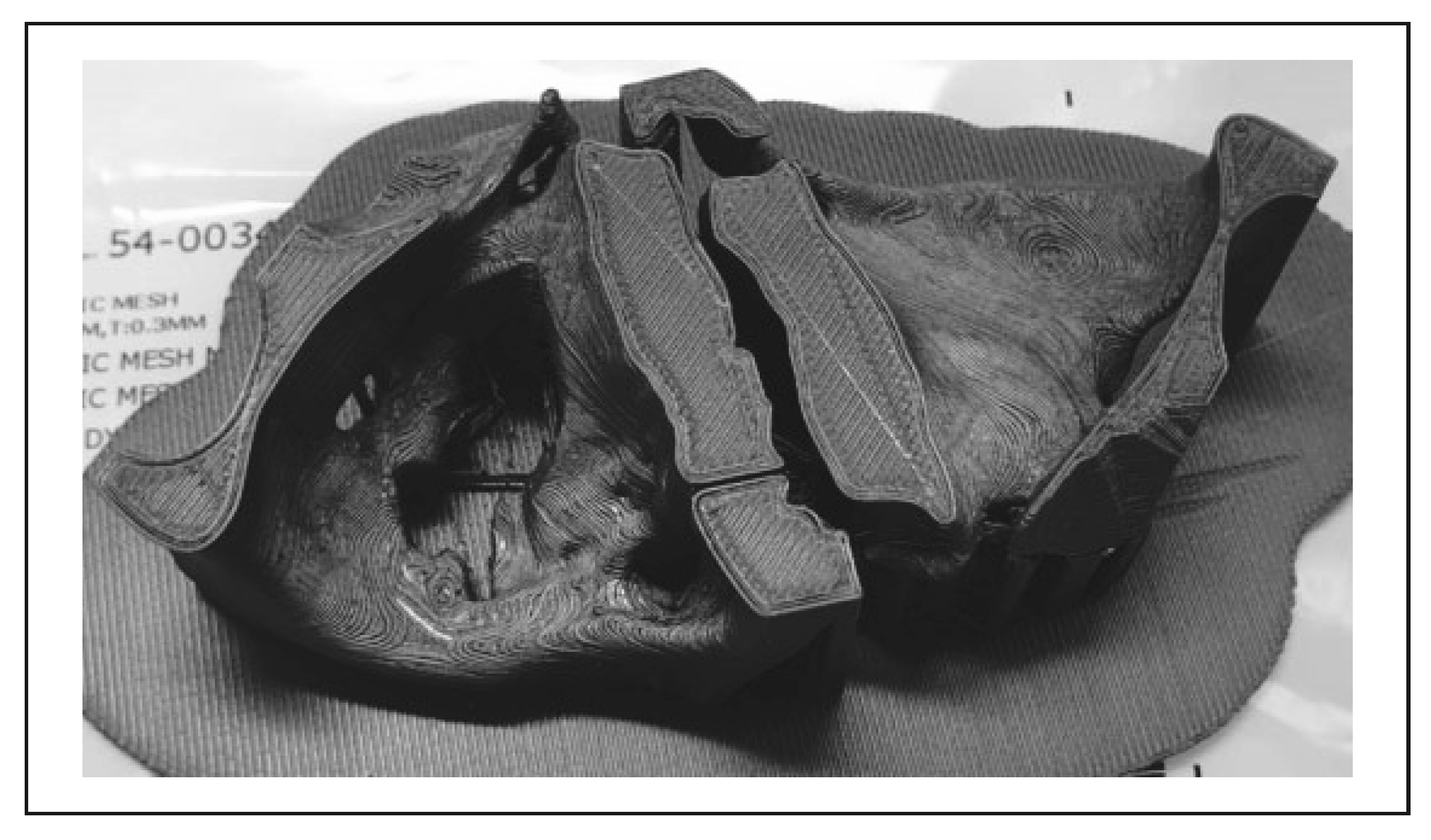
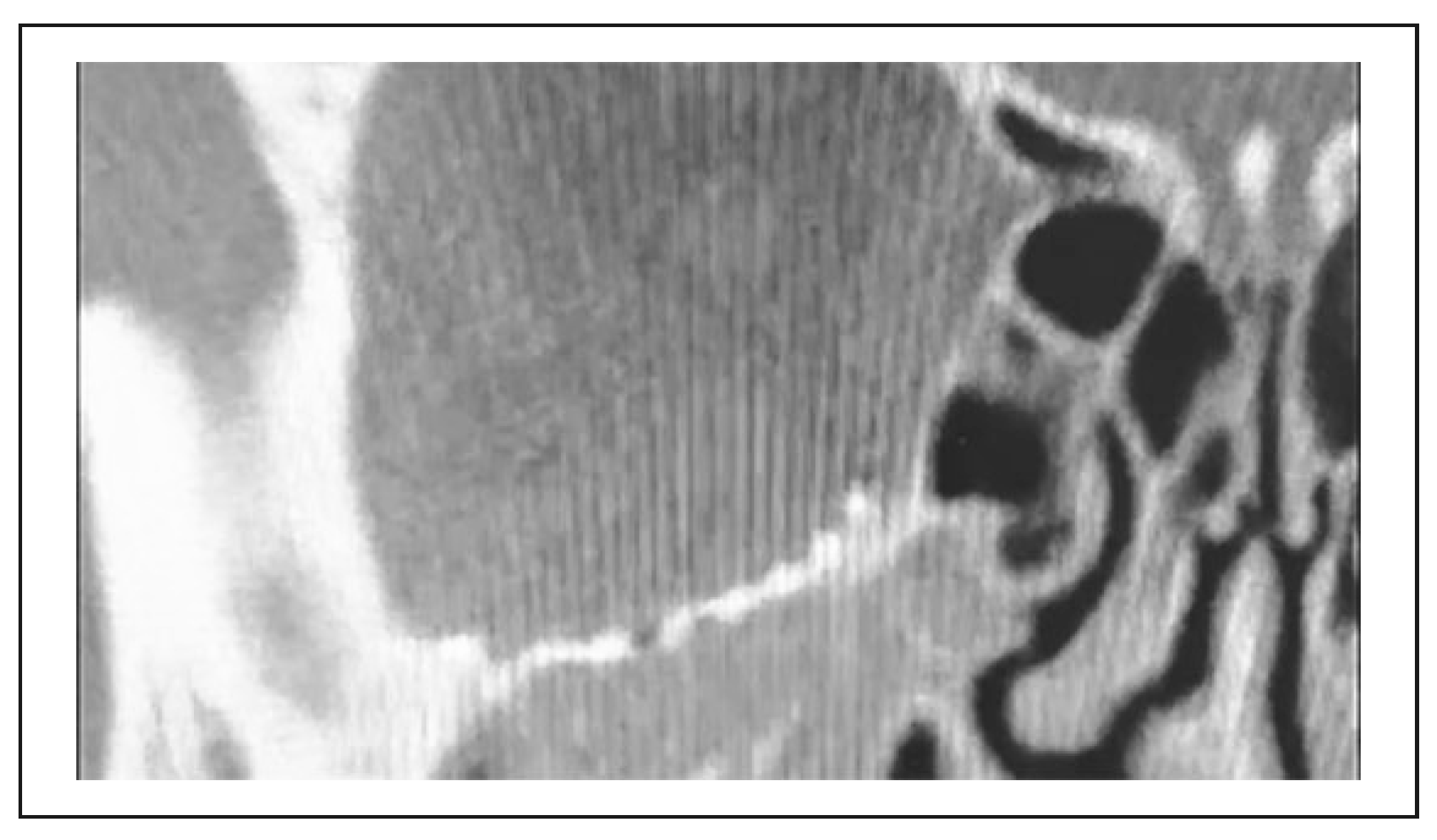
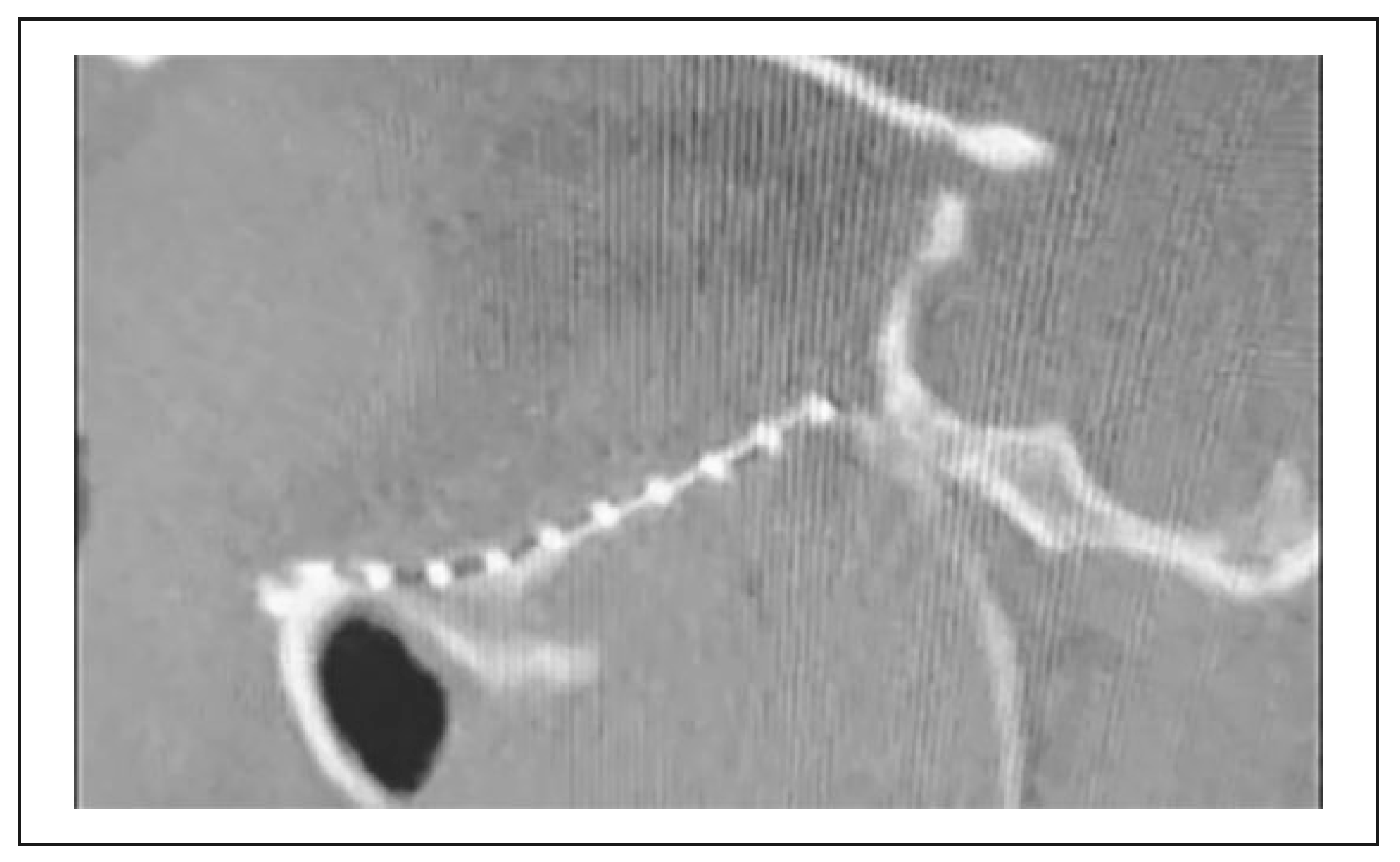
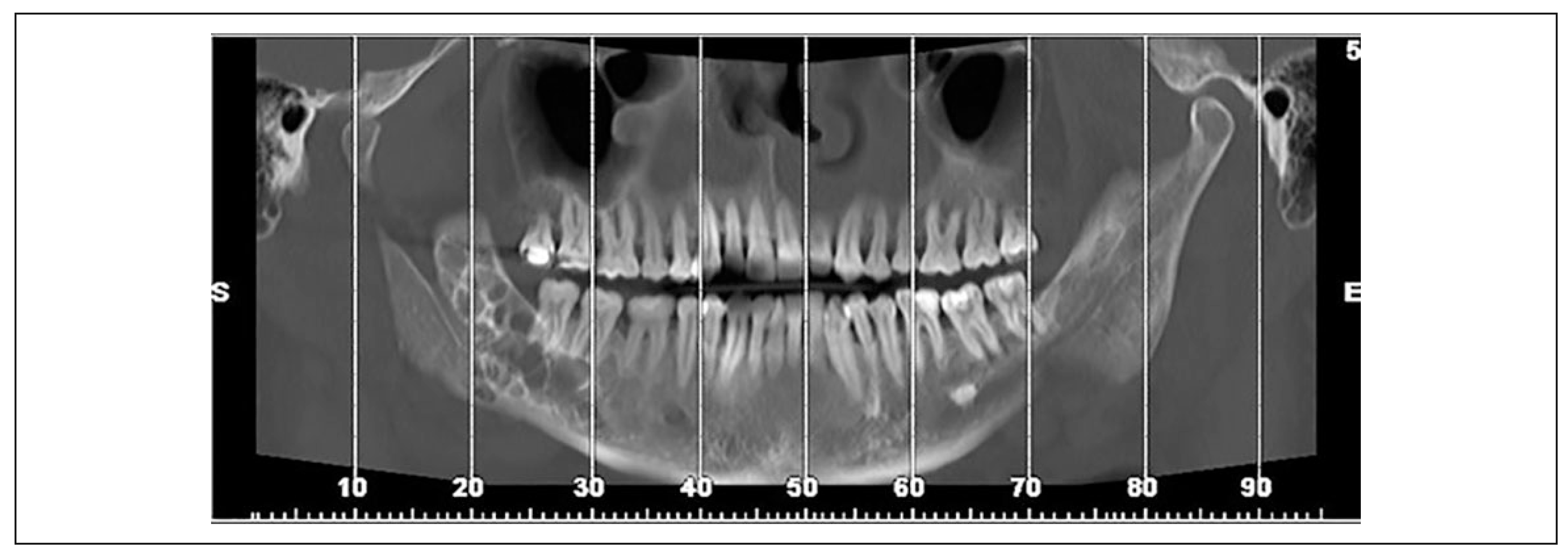
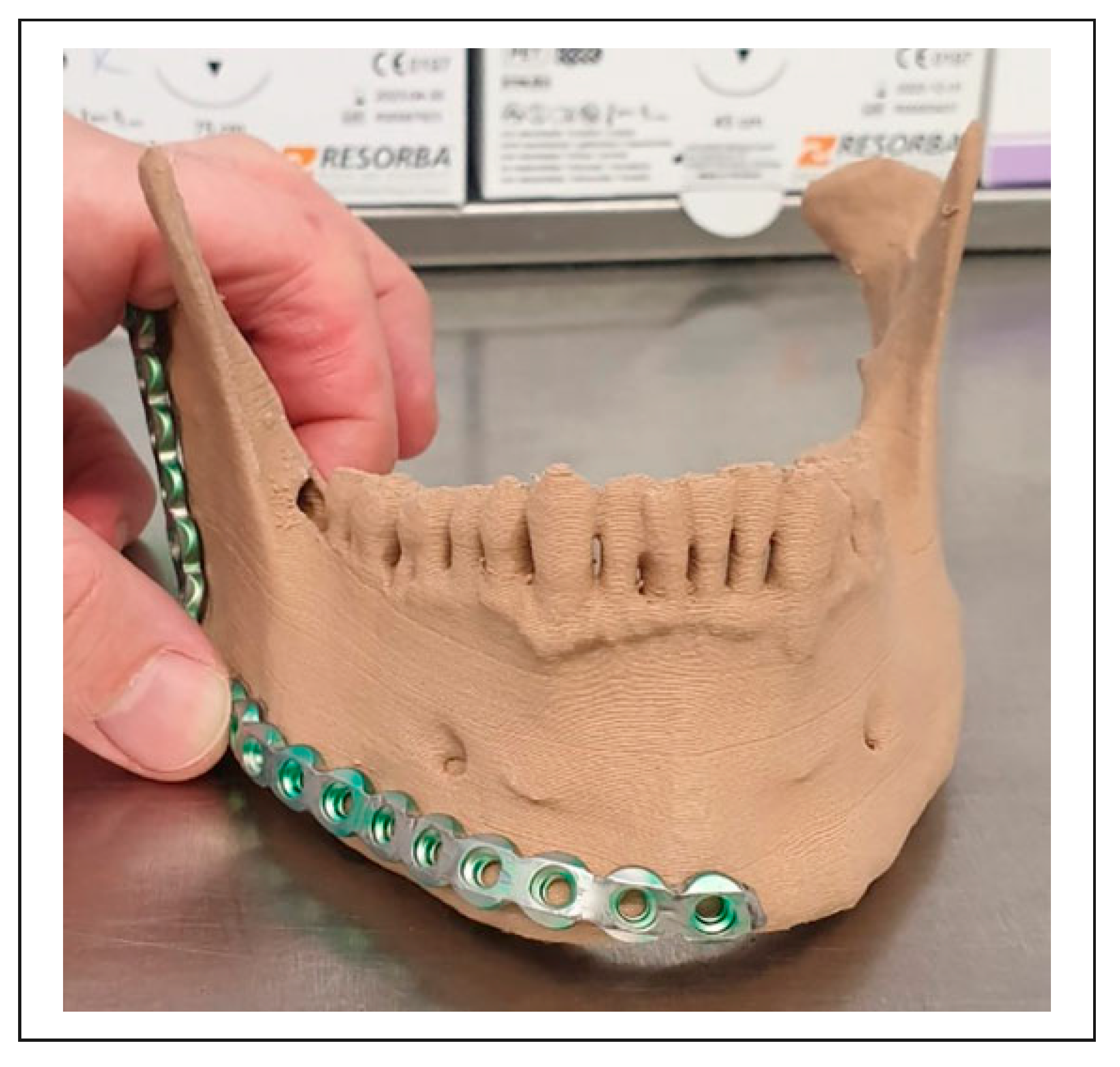
3. Results
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Ethics Approval
Informed Consent to Participate
Informed Consent for Publication
References
- Banks, J. Adding value in additive manufacturing: Researchers in the United Kingdom and Europe look to 3D printing for customization. IEEE Pulse. 2013, 4, 22–26. [Google Scholar]
- Mehra, P.; Miner, J.; D’Innocenzo, R.; Nadershah, M. Use of 3-d stereolithographic models in oral and maxillofacial surgery. J. Maxillofac. Oral. Surg. 2011, 10, 6–13. [Google Scholar]
- Winder, J.; Bibb, R. Medical rapid prototyping technologies: State of the art and current limitations for application in oral and maxillofacial surgery. J. Oral. Maxillofac. Surg. Off. J. Am. Assoc. Oral. Maxillofac. Surg. 2005, 63, 1006–1015. [Google Scholar]
- Valding, B.; Zrounba, H.; Martinerie, S.; May, L.; Broome, M. Should you buy a three-dimensional printer? A study of an orbital fracture. J. Craniofac. Surg. 2018, 29, 1925–1927. [Google Scholar] [CrossRef]
- Sinha, P.; Skolnick, G.; Patel, K.B.; Branham, G.H.; Chi, J.J. A 3-dimensional-printed short-segment template prototype for mandibular fracture repair. JAMA Facial Plast. Surg. 2018, 20, 373–380. [Google Scholar]
- Ren, W.; Gao, L.; Li, S.; Chen, C.; Li, F.; Wang, Q.; Zhi, Y.; Song, J.; Dou, Z.; Xue, L.; et al. Virtual planning and 3D printing modeling for mandibular reconstruction with fibula free flap. Med. Oral. Patol. Oral. Cir. Bucal. 2018, 23, e359–e366. [Google Scholar]
- Fan, B.; Chen, H.; Sun, Y.-J.; Wang, B.-F.; Che, L.; Liu, S.-Y.; Li, G.-Y. Clinical effects of 3-D printingassisted personalized reconstructive surgery for blowout orbital fractures. Graefes Arch. Clin. Exp. Ophthalmol. [Albrecht Von Graefes Arch. Klin Exp. Ophthalmol.] 2017, 255, 2051–2057. [Google Scholar] [CrossRef]
- Chen, C.H.; Chen, C.T.; Wang, P.F.; Wang, Y.T.; Hsu, P.H.; Lin, C.L. A novel anatomical thin titanium mesh plate with patient-matched bending technique for orbital floor reconstruction. J. CranioMaxillofac. Surg. Off. Publ. Eur. Assoc. Cranio-Maxillofac. Surg. 2018, 46, 1526–1532. [Google Scholar] [CrossRef]
- Kozakiewicz, M.; Elgalal, M.; Loba, P.; Komuński, P.; Arkuszewski, P.; Broniarczyk-Loba, A.; Stefańczyk, L. Clinical application of 3D pre-bent titanium implants for orbital floor fractures. J. Cranio-Maxillofac. Surg. Off. Publ. Eur. Assoc. CranioMaxillofac. Surg. 2009, 37, 229–234. [Google Scholar] [CrossRef]
- Raisian, S.; Fallahi, H.R.; Khiabani, K.S.; Heidarizadeh, M.; Azdoo, S. Customized titanium mesh based on the 3D printed model vs. manual intraoperative bending of titanium mesh for reconstructing of orbital bone fracture: A randomized clinical trial. Rev. Recent. Clin. Trials. 2017, 12, 154–158. [Google Scholar] [CrossRef]
- Msallem, B.; Beiglboeck, F.; Honigmann, P.; Jaquie’ry, C.; Thieringer, F. Craniofacial reconstruction by a cost-efficient template-based process using 3D printing. Plast. Reconstr. Surg. Glob. Open 2017, 5, e1582. [Google Scholar]
- Yang, W.-F.; Choi, W.S.; Leung, Y.Y.; Curtin, J.P.; Du, R.; Zhang, C.-Y.; Chen, X.-S.; Su, Y.-X. Three-dimensional printing of patient-specific surgical plates in head and neck reconstruction: A prospective pilot study. Oral. Oncol. 2018, 78, 31–36. [Google Scholar] [PubMed]
- Batstone, M.D. Reconstruction of major defects of the jaws. Aust. Dent. J. 2018, 63 (Suppl 1), S108–S113. [Google Scholar] [PubMed]
- Orabona, G.D.; Abbate, V.; Maglitto, F.; Bonavolontà, P.; Salzano, G.; Romano, A.; Reccia, A.; Committeri, U.; Iaconetta, G.; Califano, L. Lowcost, self-made CAD/CAM-guiding system for mandibular reconstruction. Surg. Oncol. 2018, 27, 200–207. [Google Scholar]
- Elegbede, A.; Diaconu, S.C.; McNichols, C.H.; Seu, M.; Rasko, Y.M.; Grant, M.P.; Nam, A.J. Officebased three-dimensional printing workflow for craniomaxillofacial fracture repair. J. Craniofac. Surg. 2018, 29, e440–e444. [Google Scholar] [CrossRef] [PubMed]
- Mendez, B.M.; Chiodo, M.V.; Patel, P.A. Customized in-office three-dimensional printing for virtual surgical planning in craniofacial surgery. J. Craniofac. Surg. 2015, 26, 1584–1586. [Google Scholar]
- Salgueiro, M.I.; Stevens, M.R. Experience with the use of prebent plates for the reconstruction of mandibular defects. Craniomaxillofacial Trauma. Reconstr. 2010, 3, 201–208. [Google Scholar] [CrossRef]
- Smithers, F.A.E.; Cheng, K.; Jayaram, R.; Mukherjee, P.; Clark, J.R. Maxillofacial reconstruction using in-house virtual surgical planning. ANZ, J. Surg. 2018, 88, 907–912. [Google Scholar] [CrossRef]
- Abo Sharkh, H.; Makhoul, N. In-house surgeon-led virtual surgical planning for maxillofacial reconstruction. J. Oral. Maxillofac. Surg. Off. J. Am. Assoc. Oral. Maxillofac. Surg. 2020, 78, 651–660. [Google Scholar] [CrossRef]
- Kamio, T.; Hayashi, K.; Onda, T.; Takaki, T.; Shibahara, T.; Yakushiji, T.; Shibui, T.; Kato, H. Utilizing a low-cost desktop 3D printer to develop a “one-stop 3D printing lab” for oral and maxillofacial surgery and dentistry fields. 3D Print. Med. 2018, 4, 6. [Google Scholar] [CrossRef]
- Ghai, S.; Sharma, Y.; Jain, N.; Satpathy, M.; Pillai, A.K. Use of 3-D printing technologies in craniomaxillofacial surgery: A review. Oral. Maxillofac. Surg. 2018, 22, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Pawlaczyk-Luszczynska, M.; Dudarewicz, A.; Szymczak, W.; Sliwinska-Kowalska, M. Evaluation of annoyance from low frequency noise under laboratory conditions. Noise Health 2010, 12, 166–181. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; LeBouf, R.F.; Duling, M.G.; Nurkiewicz, T.; Chen, B.T.; Schwegler-Berry, D.; Virji, M.A.; Stefaniak, A.B. Emission of particulate matter from a desktop three-dimensional (3D) printer. J. Toxicol. Environ. Health A 2016, 79, 453–465. [Google Scholar] [CrossRef]
- Vance, M.E.; Pegues, V.; van Montfrans, S.; Leng, W.; Marr, L.C. Aerosol emissions from fuse-deposition modeling 3d printers in a chamber and in real indoor environments. Environ. Sci. Technol. 2017, 51, 9516–9523. [Google Scholar] [CrossRef]
- Steinle, P. Characterization of emissions from a desktop 3D printer and indoor air measurements in office settings. J. Occup. Environ. Hyg. 2016, 13, 121–132. [Google Scholar] [CrossRef]
- Stefaniak, A.B.; Bowers, L.N.; Knepp, A.K.; Luxton, T.P.; Peloquin, D.M.; Baumann, E.J.; Ham, J.E.; Wells, J.R.; Johnson, A.R.; LeBouf, R.F.; et al. Particle and vapor emissions from vat polymerization desktop-scale 3-dimensional printers. J. Occup. Environ. Hyg. 2019, 16, 519–531. [Google Scholar] [CrossRef]
- Stefaniak, A.; Johnson, A.; du Preez, S.; Hammond, D.; Wells, J.; Ham, J.; LeBouf, R.; Menchaca, K.; Martin, S.; Duling, M.; et al. Evaluation of emissions and exposures at workplaces using desktop 3-dimensional printer. J. Chem. Health Saf. 2019, 26, 19–30. [Google Scholar] [CrossRef]
- Stefaniak, A.B.; LeBouf, R.F.; Yi, J.; Ham, J.; Nurkewicz, T.; Schwegler-Berry, D.E.; Chen, B.T.; Wells, J.R.; Duling, M.G.; Lawrence, R.B.; et al. Characterization of chemical contaminants generated by a desktop fused deposition modeling 3-dimensional printer. J. Occup. Environ. Hyg. 2017, 14, 540–550. [Google Scholar] [CrossRef] [PubMed]
- 3-D-Drucker Sicher Verwenden. Sichere Arbeitsplätze in Produktion und Industrie. Published 9 December 2019. Accessed 12 December 2019. Intranet. 9 December.
- Musterspule Splint Fill Filament, 2,85 mm—Shop 3D Agency. Available online: https://eshop-3d-agency.de/p/musterspule-splintfill-filament-2-85mm (accessed on 14 May 2020).
- Kunststoffverabeitung, B. Sicherheitsdatenblatt gemä ß 91/155/EWG. Available online: https://eshop-3dagency.de/WebRoot/Store16/Shops/e50a3f7b-8bd4-440da099-1dda18a98e9c/5CE2/94FF/438B/CCDF/18ED/0A48/3548/D079/SD-SplintFill-DE.pdf (accessed on 14 May 2020).
- Arfona LLC. Arfona 3D Printing Materials. Arfona. Available online: https://www.arfona.com/materials (accessed on 14 May 2020).
- DAS FILAMENT Inh. Roman Stieben AS 6 91448 Emskirchen. PLA Filament—1,75 mm—Neonorange. Published 14 May 2020. Available online: https://www.dasfilament.de/filament-spulen/pla-1-75-mm/96/pla-filament-1-75-mm-neonorange?c¼11 (accessed on 14 May 2020).
- BDEW. BDEW-Strompreisanalyse Januar 2020. Available online: http://www.bdew.de/service/daten-und-grafiken/bdew-strompreisanalyse/ (accessed on 29 May 2020).
- 20200107_BDEW-Strompreisanalyse_Januar_2020.pdf. Available online: https://www.bdew.de/media/documents/20200107_BDEW-Strompreisanalyse_Januar_2020.pdf (accessed on 29 May 2020).
- Slicer Wiki contributors. Documentation/4.x/Acknowledgments. Published 9 April 2020. Available online: https://www.slicer.org/w/index.php?title¼Documentation/4.x/Acknowledgments&oldid¼61143 (accessed on 22 September 2021).
- Jolesz, F.A. Intraoperative Imaging and Image-Guided Therapy; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Kikinis, R.; Pieper, S.D.; Vosburgh, K.G. 3D slicer: A platform for subject-specific image analysis, visualization, and clinical support. In Intraoperative Imaging and Image-Guided Therapy; Jolesz, F.A., Ed.; Springer: New York, NY, USA, 2014; pp. 277–289. [Google Scholar]
- Kapur, T.; Pieper, S.; Fedorov, A.; Fillion-Robin, J.-C.; Halle, M.; O’Donnell, L.; Lasso, A.; Ungi, T.; Pinter, C.; Finet, J.; et al. Increasing the impact of medical image computing using community-based openaccess hackathons: The NA-MIC and 3D slicer experience. Med. Image Anal. 2016, 33, 176–180. [Google Scholar] [CrossRef]
- Fedorov, A.; Beichel, R.; Kalpathy-Cramer, J.; Finet, J.; Fillion-Robin, J.-C.; Pujol, S.; Bauer, C.; Jennings, D.; Fennessy, F.; Sonka, M.; et al. 3D Slicer as an image computing platform for the quantitative imaging network. Magn. Reson. Imaging. 2012, 30, 1323–1341. [Google Scholar] [PubMed]
- Velasco, I.; Vahdani, S.; Ramos, H. Low-cost method for obtaining medical rapid prototyping using desktop 3D printing: A novel technique for mandibular reconstruction planning. J. Clin. Exp. Dent. 2017, 9, e1103–e1108. [Google Scholar] [PubMed]
- Autodesk, Inc. Autodesk Meshmixer Free Software for Making Awesome Stuff. Available online: http://www.meshmixer.com/ (accessed on 14 May 2020).
- Foundation, B. blender.org—Home of the Blender Project—Free and Open 3D Creation Software. blender.org. Available online: https://www.blender.org/ (accessed on 14 May 2020).
- Ultimaker, B.V.; Ultimaker Cura: Leistungsstarke, Benutzerfreundliche 3D-Drucksoftware. ultimaker.com. Available online: https://ultimaker.com/de/software/ultimakercura (accessed on 14 May 2020).
- Hofmann, M. Formular Laufzettel/Implantat, M.K.G. Published online 27 May. 2019.
- DRG-Research Group Webgrouper. Available online: https://www.drg-research-group.de/index.php?option¼com_webgrouper&Itemid¼112&view¼webgrouperv (accessed on 31 July 2020).
- Hinzpeter, R.; Sprengel, K.; Wanner, G.A.; Mildenberger, P.; Alkadhi, H. Repeated CT scans in trauma transfers: An analysis of indications, radiation dose exposure, and costs. Eury, J. Radiol. 2017, 88, 135–140. [Google Scholar] [CrossRef]
- Bracco, D.; Deckelbaum, D.; Artho, G.; Khwaja, K.; Mulder, D.S.; Gruska, J.; Razek, T. Additional and repeated computed tomography in interfacility trauma transfers: Room for standardization. Surgery 2018, 164, 872–878. [Google Scholar] [CrossRef]
- Jones, A.C.; Woldemikael, D.; Fisher, T.; Hobbs, G.R.; Prud’homme, B.J.; Bal, G.K. Repeated computed tomographic scans in transferred trauma patients: Indications, costs, and radiation exposure. J. Trauma. Acute Care Surg. 2012, 73, 1564–1569. [Google Scholar] [CrossRef]
- Vereinigung der kommunalen Arbeitgeberverbände, M.B. Tarifvertrag für Ärztinnen und Ärzte an kommunalen Kranken-häusern im Bereich der kommunalen Arbeitgeberverbände (TV-Ärzte/VKA). Published 31 July 2020. Available online: https://bit.ly/316orRP (accessed on 31 July 2020).
- Werz, S.M.; Zeichner, S.J.; Berg, B.I.; Zeilhofer, H.F.; Thieringer, F. 3D Printed surgical simulation models as educational tool by maxillofacial surgeons. Eur. J. Dent. Educ. Off. J. Assoc. Dent. Educ. Eur. 2018, 22, e500–e505. [Google Scholar]
- Ploch, C.C.; Mansi, C.S.S.A.; Jayamohan, J.; Kuhl, E. Using 3D printing to create personalized brain models for neurosurgical training and preoperative planning. World Neurosurg. 2016, 90, 668–674. [Google Scholar]
- Nicot, R.; Druelle, C.; Schlund, M.; Roland-Billecart, T.; Gwénaël, R.; Ferri, J.; Gosset, D. Use of 3D printed models in student education of craniofacial traumas. Dent. Traumatol. Off. Publ. Int. Assoc. Dent. Traumatol. 2019, 35, 296–299. [Google Scholar]
- Hasan, O.; Atif, M.; Jessar, M.M.; Hashmi, P. Application of 3D printing in orthopaedic surgery. A new affordable horizon for cost-conscious care. JPMA, J. Pak. Med. Assoc. 2019, 69 (Suppl 1), S46–S50. [Google Scholar]
- Chen, K.; Yang, F.; Yao, S.; Xiong, Z.; Sun, T.; Zhu, F.; Telemacque, D.; Drepaul, D.; Ren, Z.; Guo, X. Application of computerassisted virtual surgical procedures and three-dimensional printing of patient-specific pre-contoured plates in bicolumnar acetabular fracture fixation. Orthop. Traumatol. Surg. Res. 2019, 105, 877–884. [Google Scholar] [PubMed]






 |
 |
© 2021 by the author. The Author(s) 2021.
Share and Cite
Gleissner, H.; Castrillon-Oberndorfer, G.; Gehrlich, S. Introduction of 3D Printing in a German Municipal Hospital—Practice Guide for CMF Surgery. Craniomaxillofac. Trauma Reconstr. 2022, 15, 369-378. https://doi.org/10.1177/19433875211050721
Gleissner H, Castrillon-Oberndorfer G, Gehrlich S. Introduction of 3D Printing in a German Municipal Hospital—Practice Guide for CMF Surgery. Craniomaxillofacial Trauma & Reconstruction. 2022; 15(4):369-378. https://doi.org/10.1177/19433875211050721
Chicago/Turabian StyleGleissner, H, G Castrillon-Oberndorfer, and St Gehrlich. 2022. "Introduction of 3D Printing in a German Municipal Hospital—Practice Guide for CMF Surgery" Craniomaxillofacial Trauma & Reconstruction 15, no. 4: 369-378. https://doi.org/10.1177/19433875211050721
APA StyleGleissner, H., Castrillon-Oberndorfer, G., & Gehrlich, S. (2022). Introduction of 3D Printing in a German Municipal Hospital—Practice Guide for CMF Surgery. Craniomaxillofacial Trauma & Reconstruction, 15(4), 369-378. https://doi.org/10.1177/19433875211050721




