Introduction
The zygomatic bone is closely associated with the skull and the facial skeleton through 4 sutures: the temporozygomatic, frontozygomatic, sphenozygomatic, and zygomaticomaxillary sutures. Therefore, isolated fractures of the zygomatic bone are rare, and fracture lines often run through the maxilla and orbit. Zygomaticomaxillary complex (ZMC) fractures comprise 5.7-44.3% of all facial bone fractures.[
1,
2,
3,
4,
5,
6,
7] Young men are predominantly affected, generally due to interpersonal violence or fall injuries. Fractures of the infraorbital rim are particularly frequent, with the fracture line most commonly involving the infraorbital canal or orbital floor.
Interestingly, post-traumatic infraorbital neurosensory disturbances occur in up to 94% of these cases.[
1,
8] Indeed, patients often describe a high degree of discomfort during follow-up. The infraorbital nerve (ION) may be damaged due to oedema, ischemia, bony compression, sharp edges of the fracture line, hematoma, or neurogenic inflammation. [
9,
10] More complex fractures are associated with more severe nerve injury, and more persistent hyper- or hypoaesthesia.[
1,
11]
The decisions of whether to operate and which surgical approach to follow are based on the degree of dislocation, surgeon’s experience, and treatment guidelines at the surgical center. Moreover, treatment planning must consider the patient’s opinion and medical condition, presence of facial asymmetry, presence of facial lacerations, functional problems, and ION damage.[
12]
ION injuries and the related medical costs constitute an important medical issue. Thus, the present study aimed to investigate the correlations between aetiologies, fracture types, and clinical and radiological features.
Methods
The clinical records and radiographs of 308 patients who presented with unilateral or bilateral fractures of the zygomaticomaxillary or orbitozygomatic complex between January 2014 and January 2019, were retrospectively analyzed. Patients with isolated blow-out fractures of the orbital floor were excluded. This research was conducted in compliance with the principles of the Declaration of Helsinki and in accordance with all applicable regulatory requirements. The study protocol was approved by the Ethics Committee UZ/KU Leuven (S62735).
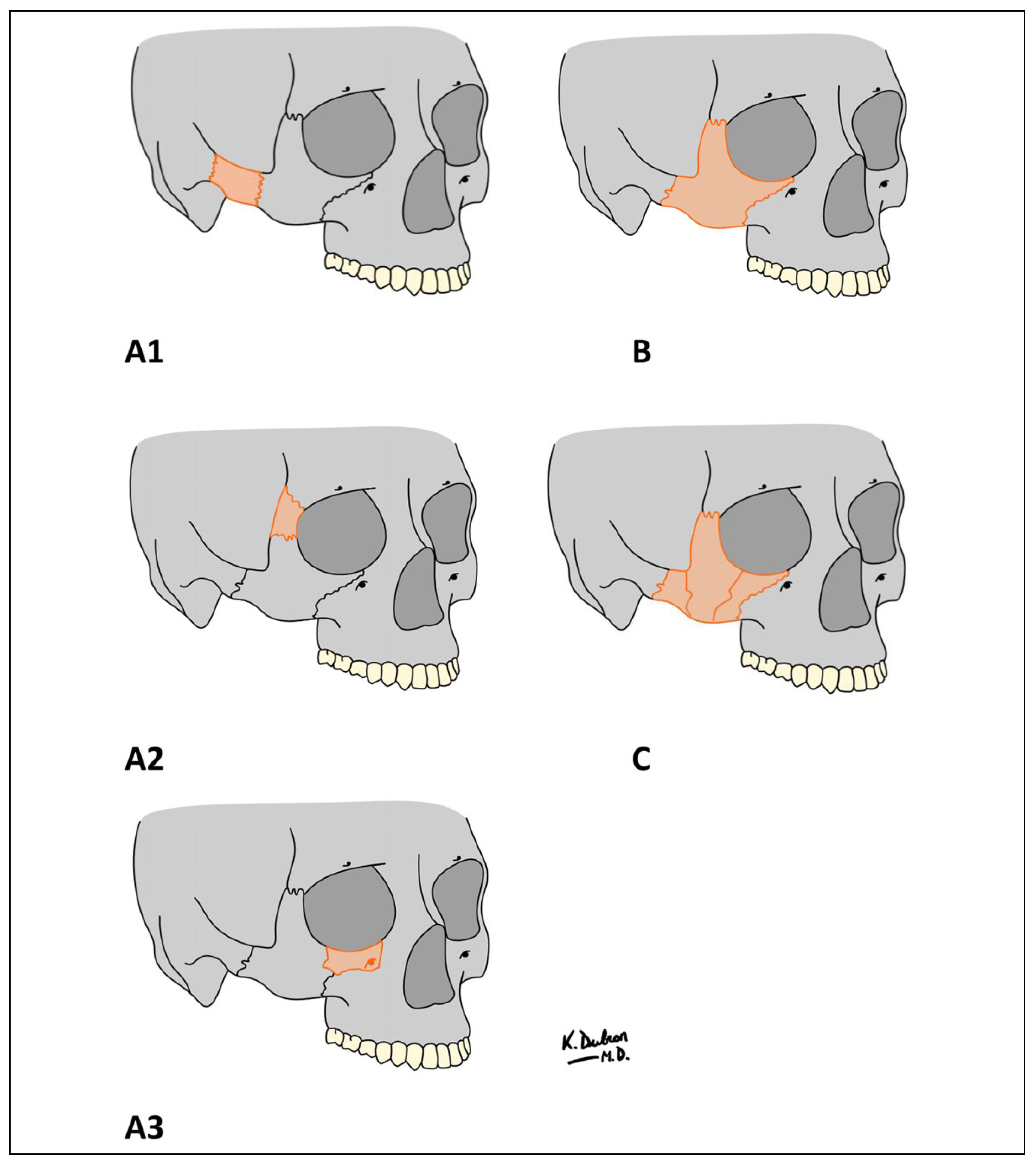
The patient files were reviewed for demographics, fracture aetiology, fracture type, treatment method, associated complications, and follow-up. Fracture patterns were classified based on anatomical landmarks, and fracture patterns according to the Zingg classification (
Figure 1).[
13,
14] Zingg type A fractures are isolated incomplete fractures of the zygoma, involving only the zygomatic arch (A1), lateral orbital wall (A2), or infraorbital wall (A3); Zingg type B fractures are classic tetrapod fractures; and Zingg type C fractures are comminuted fractures.
Fracture aetiology was classified as fall accident, interpersonal violence, road traffic accident (including bicycle, motor, and car accidents), sport accident (football, horse, hockey, or other sports), or work accident. A fall was defined as a sudden involuntary transfer of the body to the ground or to a lower level than its previous location.
The main outcome variable was ION hypoaesthesia, described as a loss or abnormal sensation over the area of innervation of the maxillary branch of the trigeminal nerve, which could be detected using the 2-point discrimination test, with the nonaffected side serving as the control side. Secondary outcomes were ION hypoaesthesia recovery time, treatment options for ION injuries, and clinical and radiological patterns that were possibly correlated with ION injuries and facial nerve injuries. Mobility was clinically evaluated, and ZMC dislocation was assessed on radiological imaging.
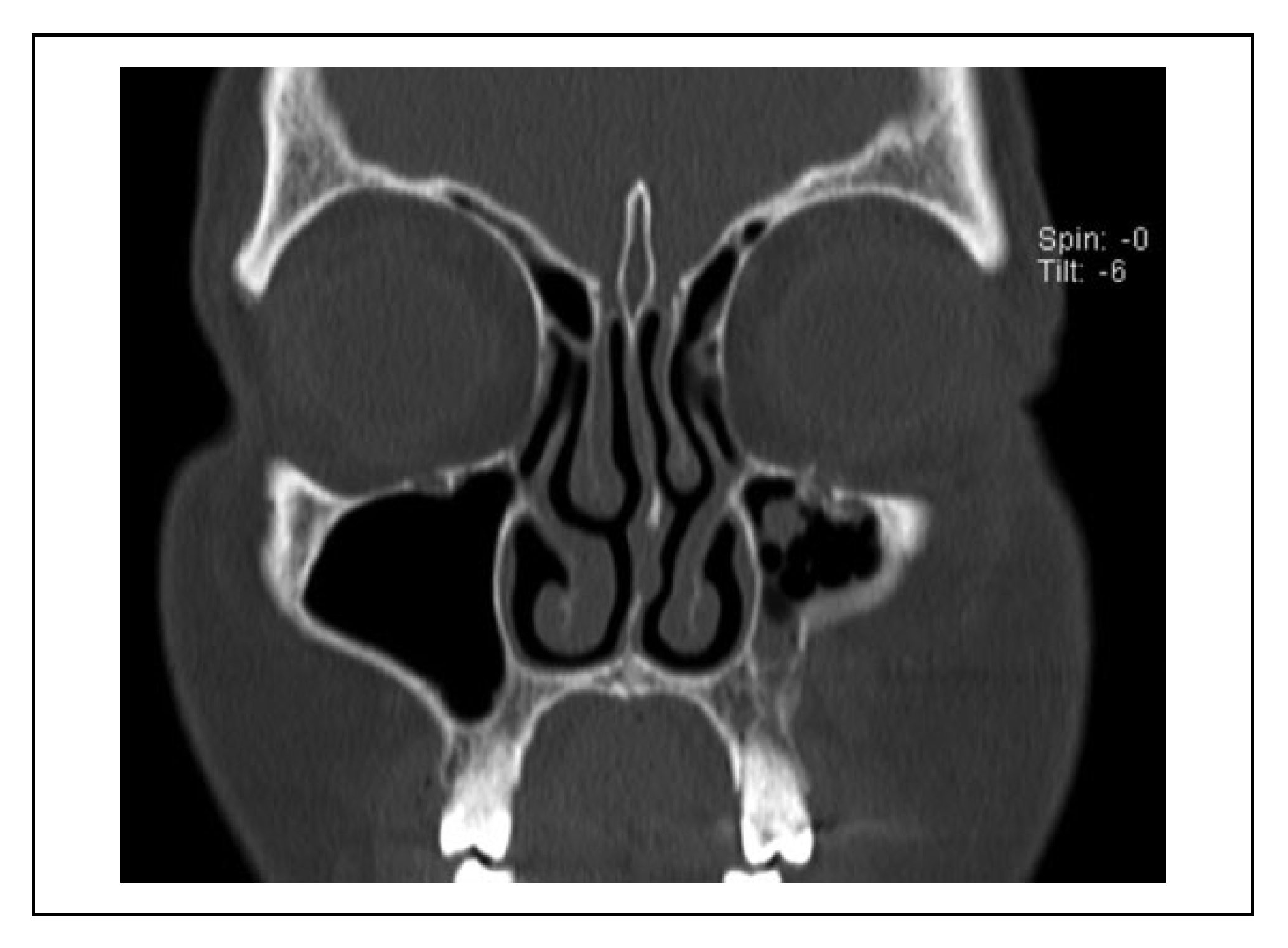
A case was considered to show involvement of the fracture course through the infraorbital canal (IOC) when the fracture line penetrated the cortex of the IOC at one site or more (
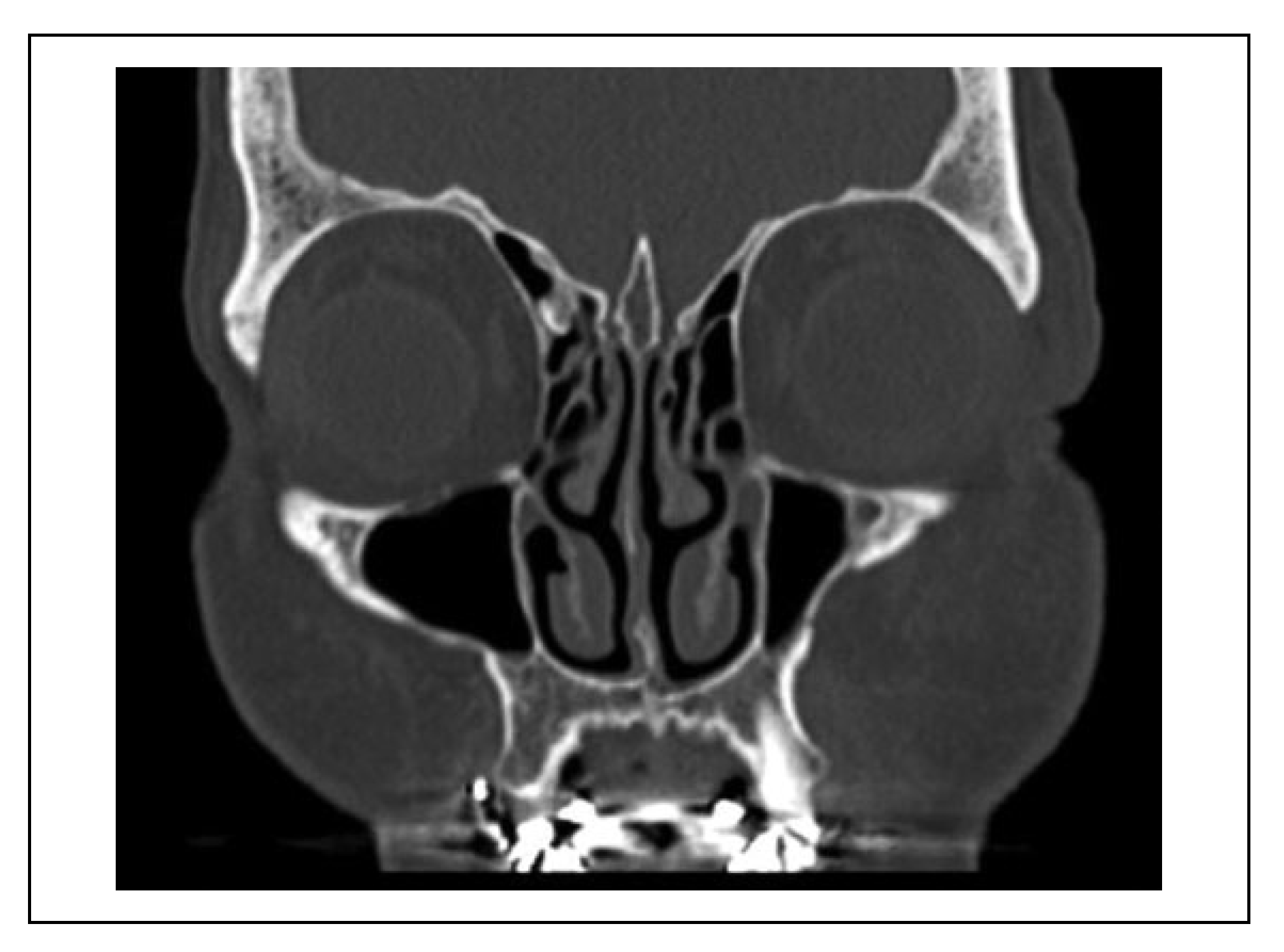
Figure 2). When the fracture line was near the IOC (within 1 mm), this indicated that the cortex of the IOC was still intact (
Figure 3).
Statistical analyses were performed using the statistical package SPSS version 20.0 for Windows (SPSS, Inc.). Data are reported as mean ± standard deviation (SD) or percentage, as appropriate. Patients were analyzed in separate groups, and divided according to associated nerve injuries. We assessed the level of association between categorical variables using Fisher’s exact tests. Cross-tabulations and univariate logistic regression were applied to evaluate significant associations between explanatory variables and outcome. Results are reported as odds ratio (OR) and 95% confidence interval (CI). Probabilities of <0.05 were considered statistically significant.
Results
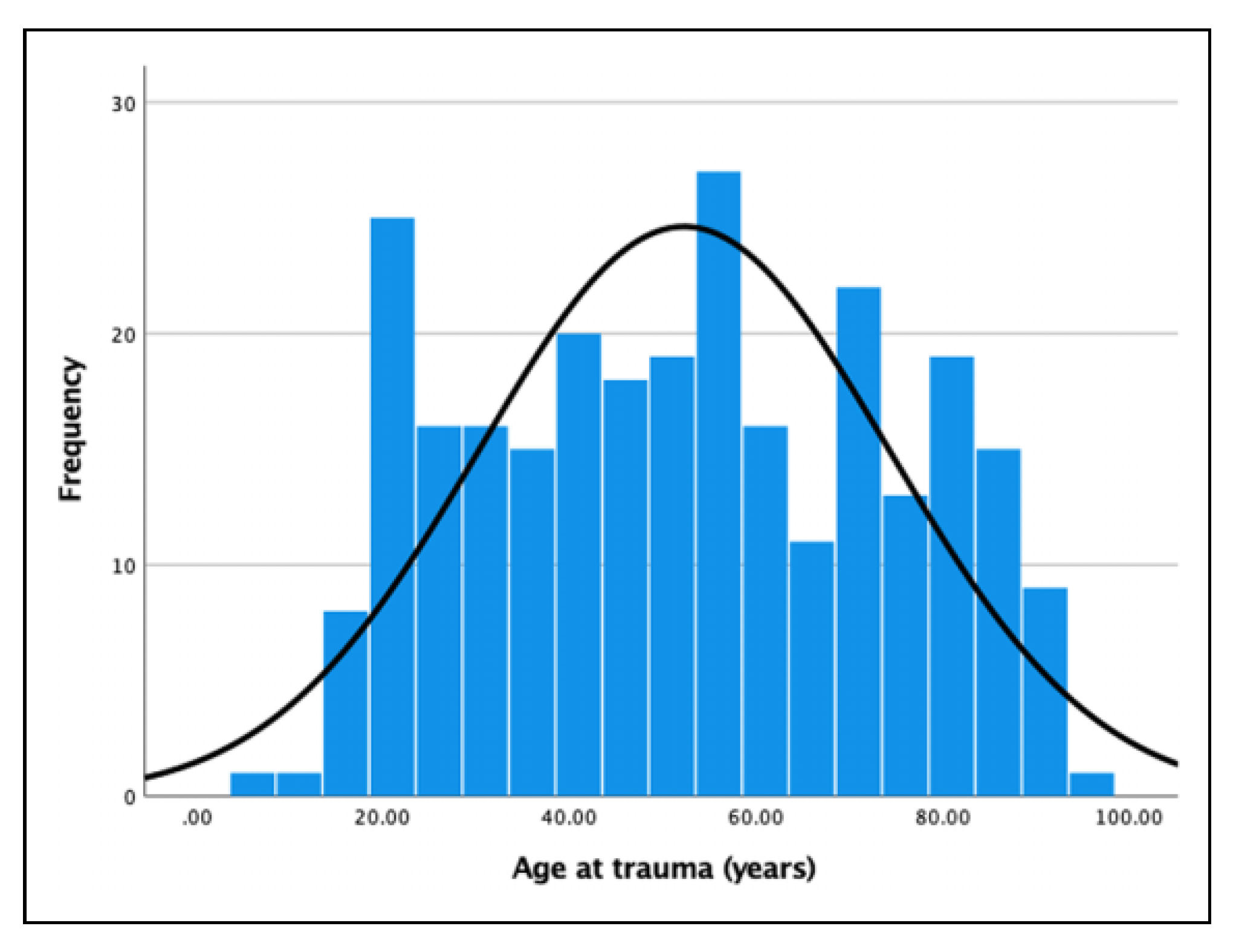
The medical files of 308 patients were analyzed. Of these patients, 36 were excluded because they had Le fort II or Le Fort III fractures. Thus, the total study group comprised 272 patients, including 169 males and 103 females. The mean age (SD) was 52.3 years (±22), with a range of 6- 97 years. Age was normally distributed (
Figure 4). The mean follow-up (SD) of all patients was 4.4 months (+9.2), with a range of 1 day to 5 years.
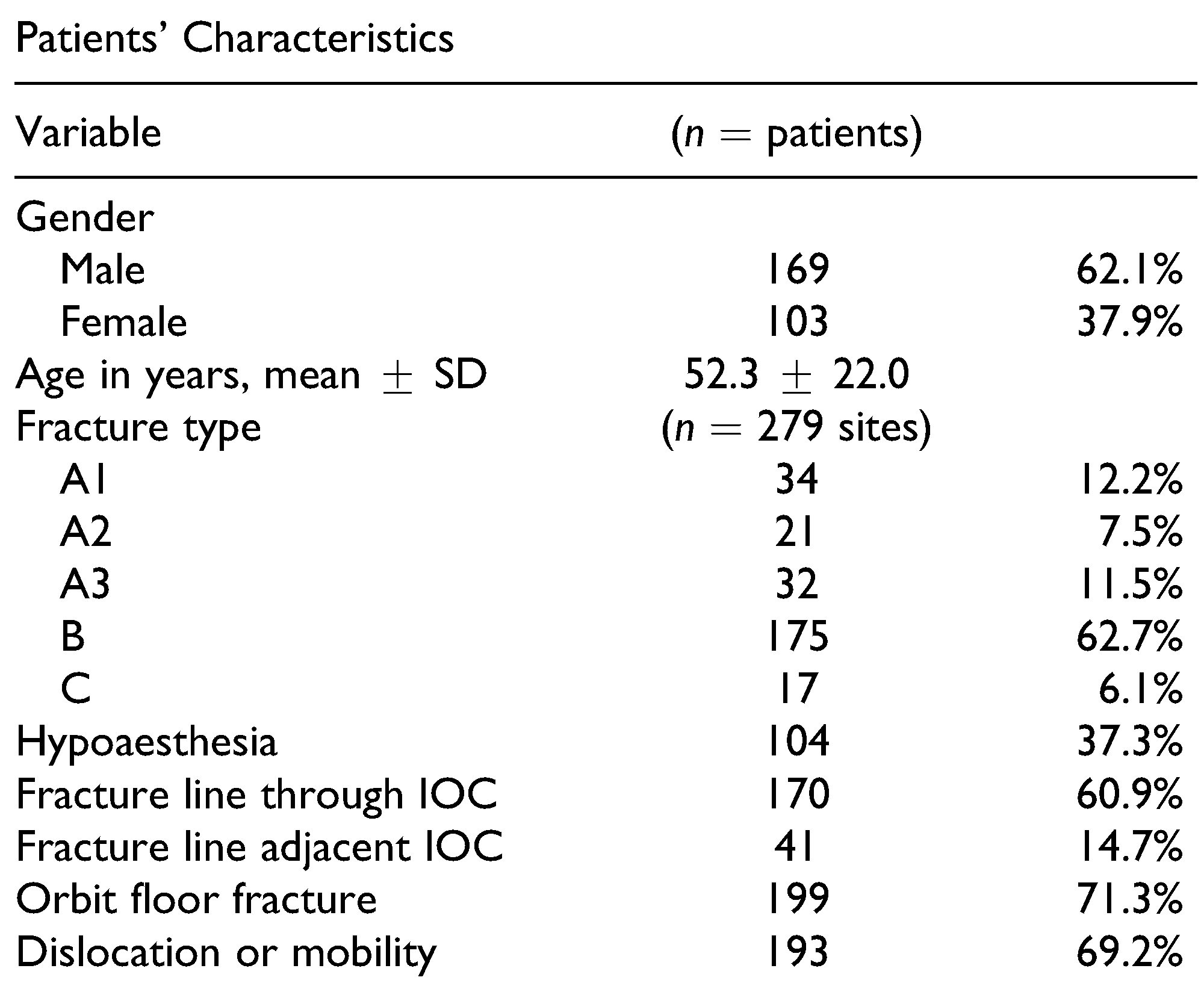
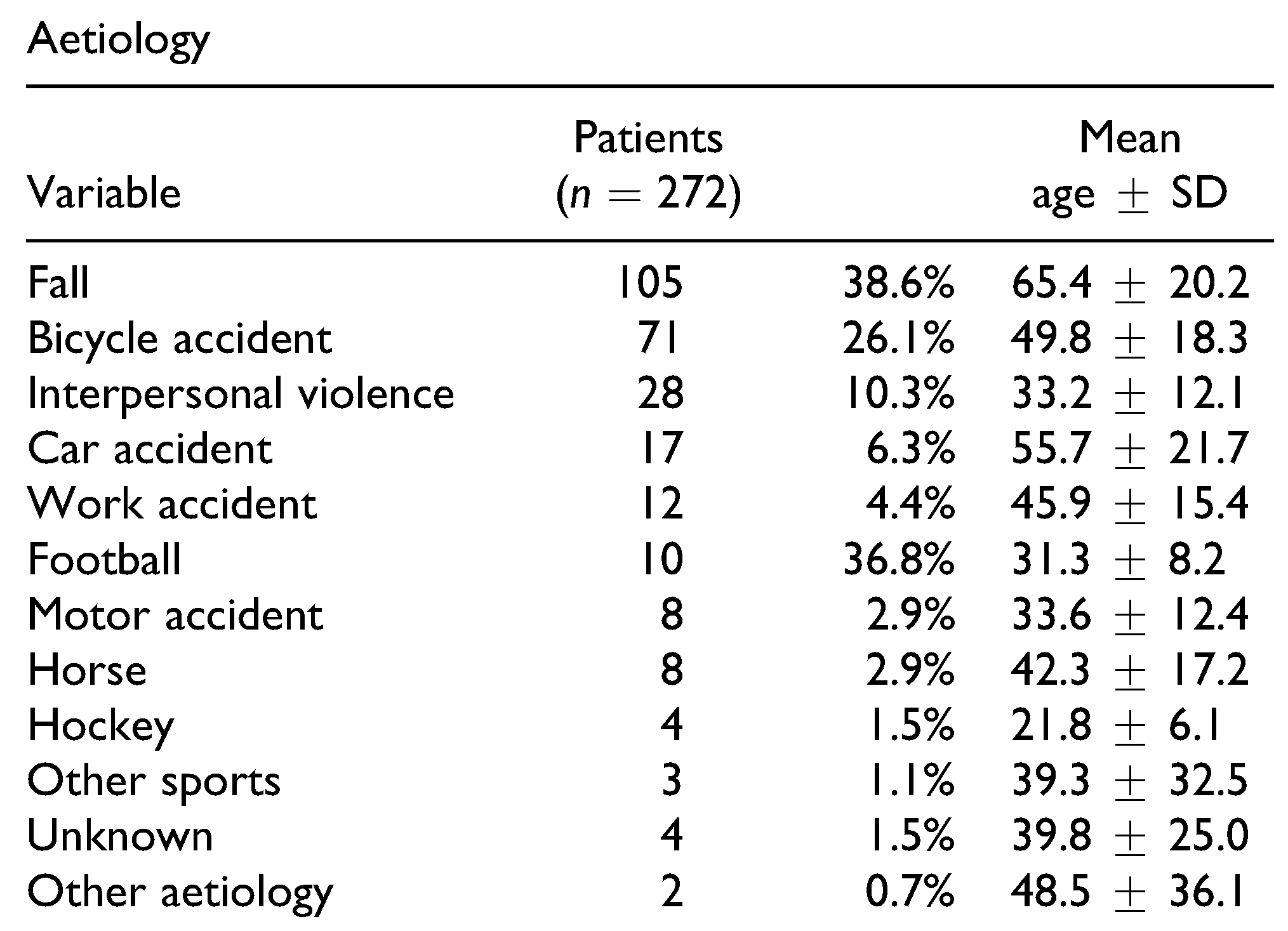
Table 1 shows the patients’ characteristics and distribution of ZMC fracture types. Fractures occurred with similar frequency on both sides of the face (138 right, 141 left), and 7 patients (2.6%) presented with bilateral ZMC fractures. The fractures resulted from falls (
n = 105; 38.6%), road traffic accidents (
n = 96; 35.3%), interpersonal violence (
n = 28; 10.3%), sport accidents (
n = 25; 9.2%), work accidents (
n = 12; 4.4%), train accident (
n = 1), gunshot (
n = 1), or unknown causes (
n = 4) (
Table 2). The fractures in 35 cases (12.9%) resulted from situations involving alcohol use. Among the total of 279 fracture sites, operations were performed at 107 sites (38.4%) in 103 patients. Among these patients, 96 underwent operation under general anesthesia, and 7 under intravenous sedation (fentanyl and midazolam). The mean time (SD) between the accident and surgery was 15.5 days (±2.1 months), with a range of 0-16.7 months.
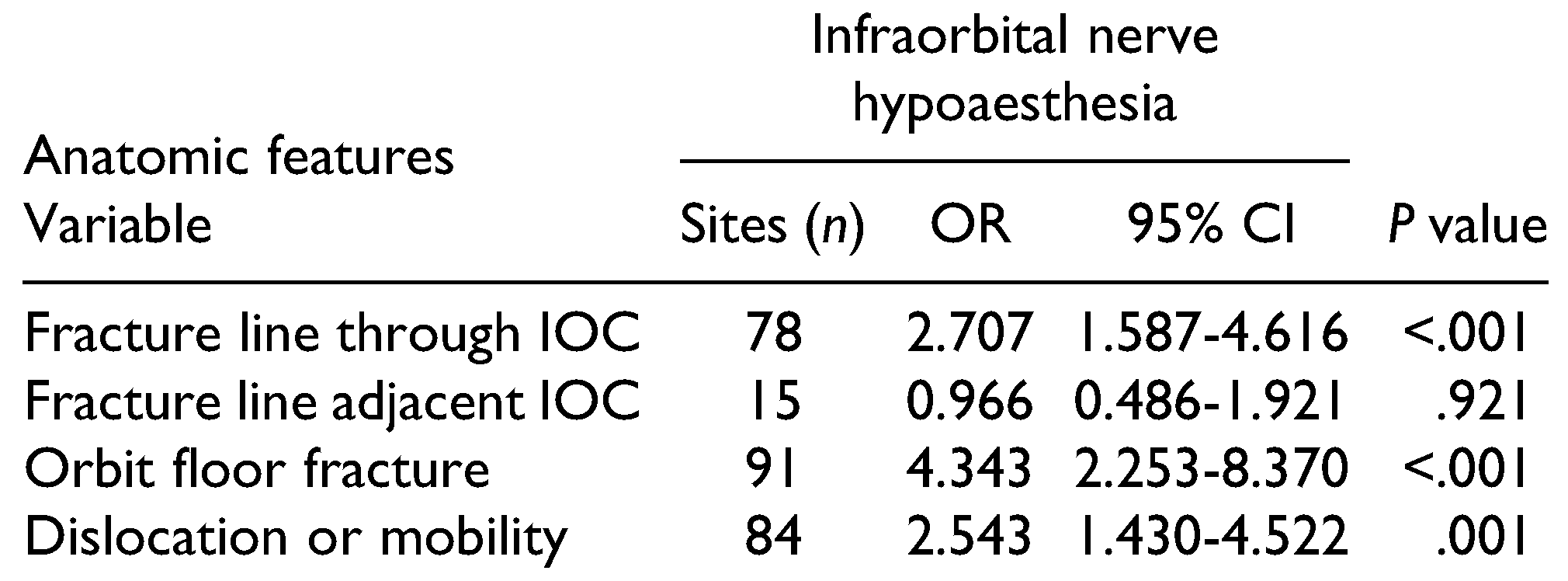
Infraorbital nerve sensory disturbances, such as hypoaesthesia, were reported at 104 sites (37.3%) in 100 patients (male/female ratio: 65/35). Hyperaesthesia was only observed unilaterally at 3 sites in 3 patients (1.1%). Among the 7 patients with bilateral fractures, 2 male patients and 2 female patients had bilateral hypoaesthesia. Of the 100 patients with hypoaesthesia, 59 underwent an operation.
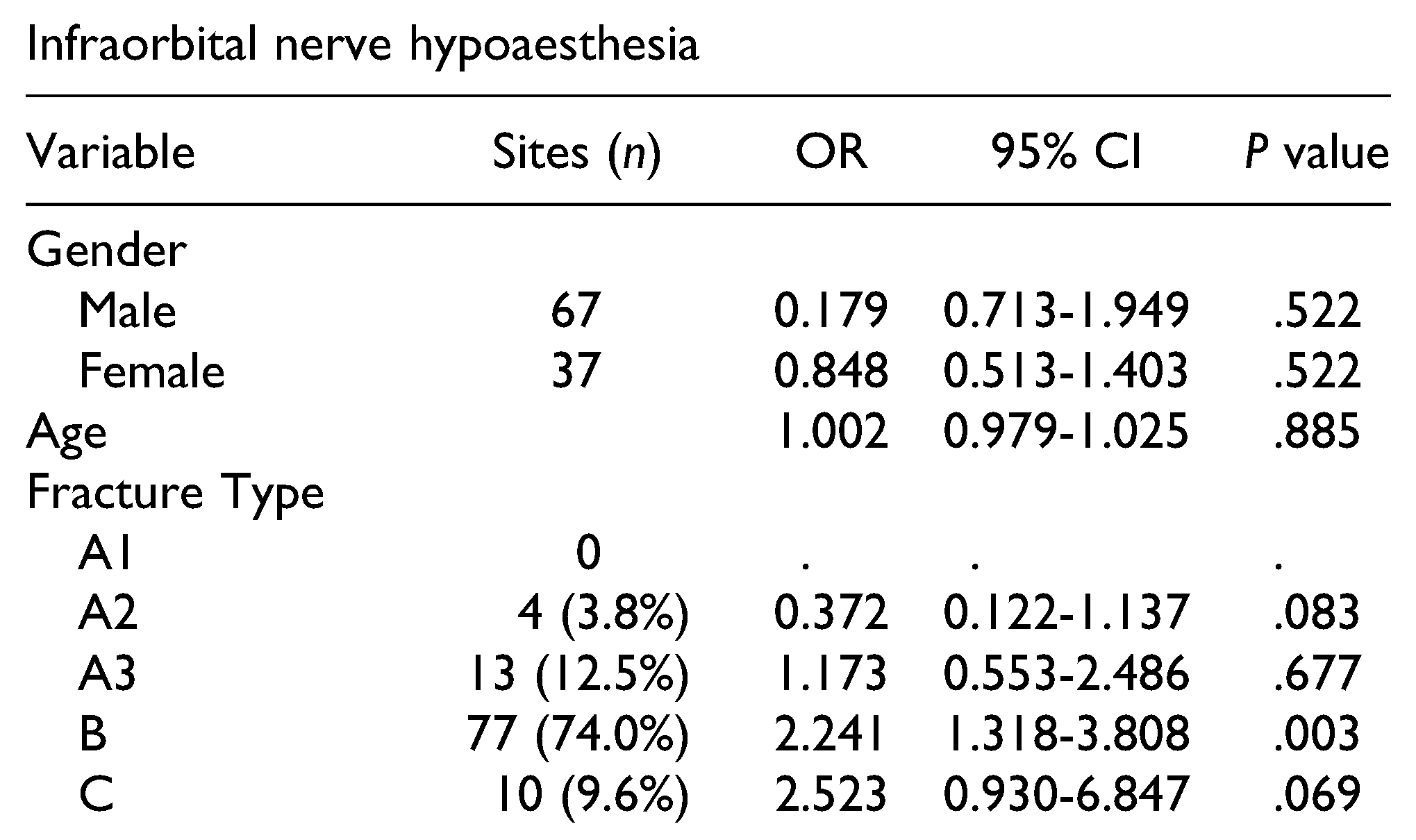
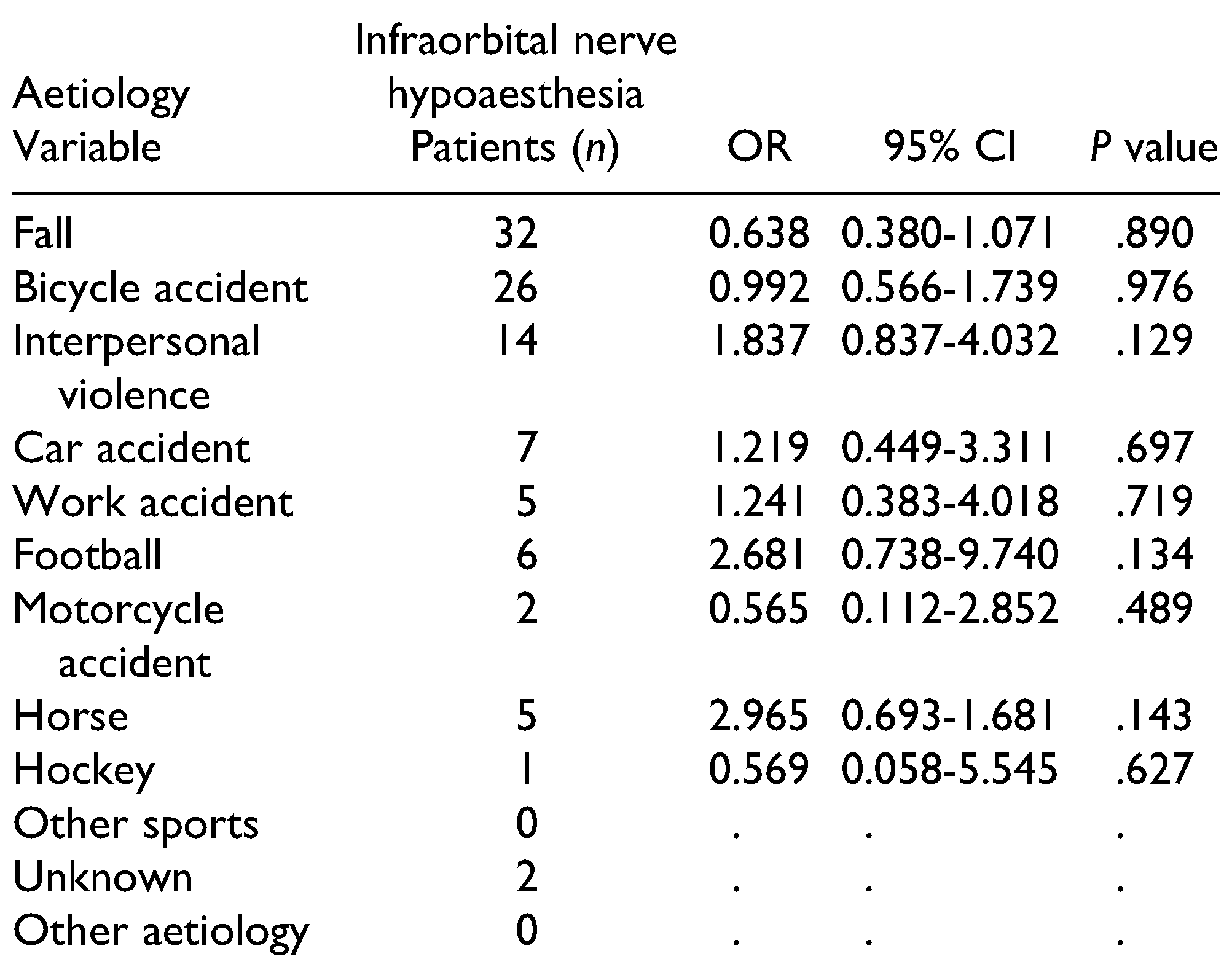
Table 3 and
Table 4 present the correlations of ION hypoaesthesia with patients’ characteristics, fracture type, and aetiology.
Hypoaesthesia of a ZMC fracture was significantly correlated with type B fractures. Non-significant correlations were also identified between hypoaesthesia absence and type A2 fractures, and between hypoaesthesia presence and type C fractures. Furthermore, ION hypoaesthesia was significantly correlated with a fracture line course through the IOC, with orbital floor fractures, and with clinical or radiographic dislocation or mobility (
Table 5). Patients without dislocation or mobility reported hypoaesthesia in 19.2%.
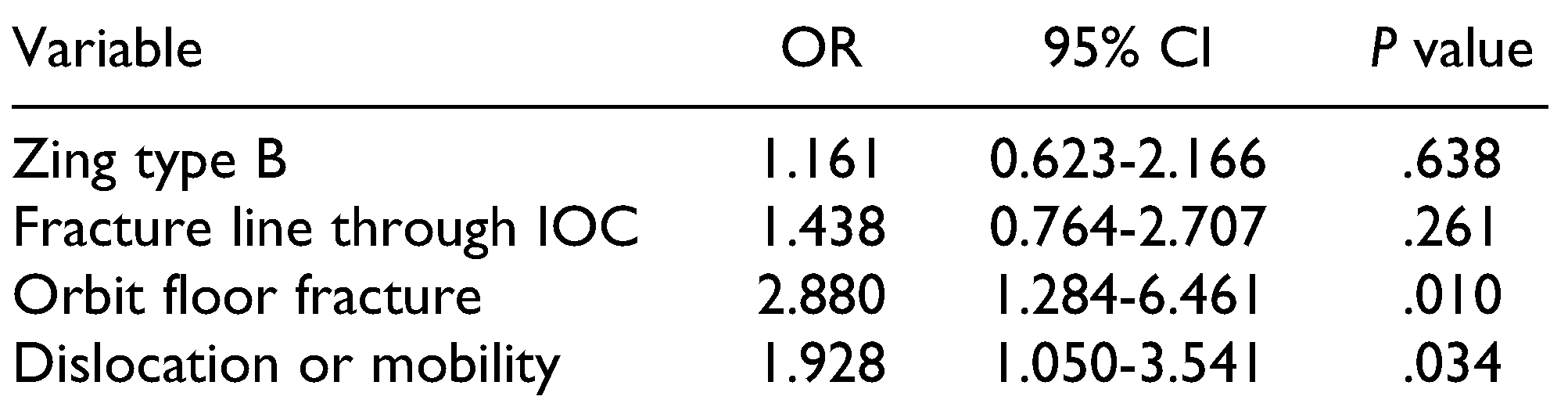
In 52 patients, ION hypoaesthesia recovered in a mean time period (SD) of 4.3 months (±5.7 months) (range: 7 days-23 months). Multivariate analysis revealed that ION hypoaesthesia was significantly associated with orbit floor fracture and with dislocation or mobility, with an R
2 of 0.103 (
Table 6). Among the 100 patients presenting with hypoaesthesia, 40 were treated with oral vitamin B1- B2-B6-B12, and 6 patients were treated with intramuscular vitamin B1-B6-B12. Six patients (2.2%; male/female ratio: 2/4) developed neurogenic pain symptoms, and were started on empirical treatment with either amitriptyline, lymphatic drainage, low-level laser therapy, cryotherapy, duloxetine, or pregabalin, or combinations of these treatments. Among the 6 patients who developed neuropathic pain, 5 had type B fractures and 1 had a type C fracture. Neuropathic pain symptoms were not reported with other types of fractures. Orbit floor fracture was associated with neuropathic pain symptoms in 5 patients. The development of neuropathic symptoms occurred within a mean (SD) of 1.5 months (±1.6 months) after trauma (range: 2.5 weeks to 4.0 months). Three patients were successfully treated and recovered after a mean (SD) of 9.0 months (±9.0 months). One patient was lost to follow-up.
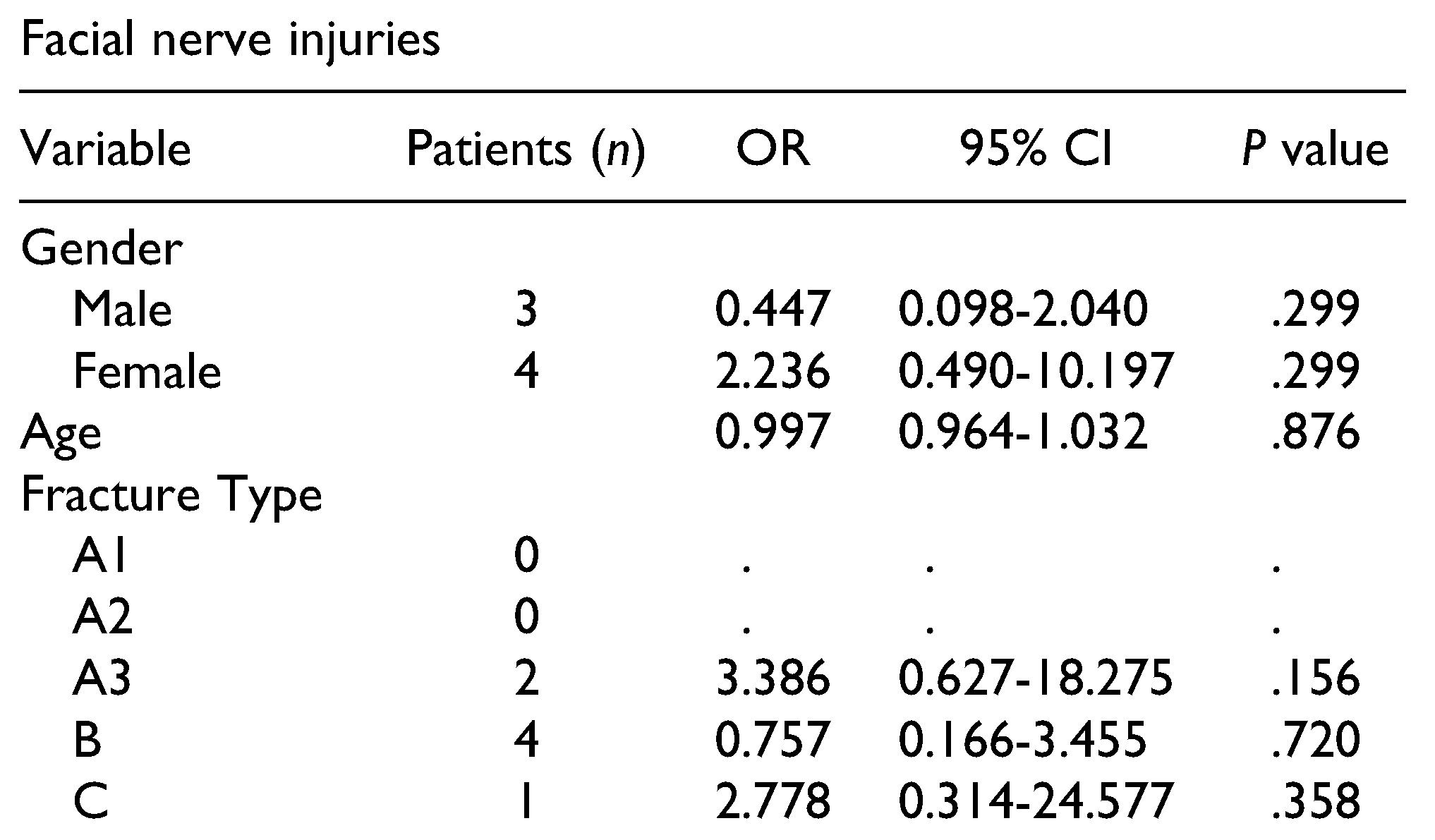
Post-traumatic facial nerve injuries were reported in 7 patients (2.6%), and were always unilateral.
Table 7 presents the results and associated fracture types. One patient exhibited complete recovery after 2.5 months, and 2 other patients showed functional improvement after a mean time of 5 months. One patient with a type B fracture showed no recovery, even after 4.8 years. The remaining 3 patients were lost to follow-up.
Discussion
Fractures of the ZMC are common injuries because the ZMC is a prominent part of the facial skeleton, and ION hypoaesthesia is the most reported posttraumatic complication of such injuries.[
1,
10,
15,
16] This present study aimed to investigate the presence of infraorbital nerve injury in relation to the aetiology and type of fracture. Additionally, correlations between ION hypoaesthesia and clinical/radiological findings were investigated.
ZMC fractures were predominantly caused by falls or road traffic accidents and interpersonal violence. Fractures caused by sports accidents and interpersonal violence exhibited the highest incidence of developing posttraumatic ION hypoaesthesia, which can be explained by the high energy of impact, corresponding with the literature. [
1,
11] Motor vehicle accidents are a well-known major cause of ZMC fractures.[
1] However, in this review, bicycle accidents were a more common cause of ZMC fractures than motor vehicle accidents (car and motorcycle).[
1,
11] Interpersonal violence has also been previously reported to play a significant role in ZMC fractures, with such fractures commonly located on the left side due to a righthanded stroke.[
17] However, in our present study, fractures due to interpersonal violence were equally located on the left and right side. The prior statement that sport accidents were related to ION hypoaesthesia[
1] could not be statistically confirmed in this study, possibly due to the relatively small and heterogeneous group of sports accidents.
While prior findings indicate that ION hypoaesthesia is mostly associated with B and C type fractures,[
1,
11] in this present study, only type B fractures (74.0%) were significantly more associated with ION hypoaesthesia. Likely due to the low number of subjects, only a trend of greater incidence of ION hypoaesthesia in type C fractures (9.6%) was observed. The infraorbital canal is a weak point in the midface, and is thus frequently involved in the fracture line. Bony compression, haematoma, or neurogenic inflammation in the IOC can lead to Wallerian degeneration which, in turn, can result in ION hypoaesthesia.[
15,
18] In the current study, fracture line involvement of the IOC was inherently associated with type B and C fractures, and was significantly associated with ION hypoaesthesia. In the literature, up to 95%of type B and type C fractures involve the IOC. On the other hand, the reported frequencies of ION hypoaesthesia are relatively low, meaning that not every fracture through the IOC causes ION hypoaesthesia. However, it is possible that hypoaesthesia is underreported in these study groups, or that there was a lack of assessments during follow-up. Notably, the hypoaesthesia incidence in the literature is highly dependent on the method of diagnosis.
The anatomic location of type B fracture types is also a reason why the multivariate analysis revealed significant findings for a fracture line course through the IOC, fracture of the orbit floor, and dislocation or mobility of the ZMC. However, multivariate analysis showed that displacement or presence of an orbital floor fracture had a higher impact on ION injury (IONI), with a loss of significance for a fracture through the IOC or a Zingg type B fracture. This indicates that the latter 2 factors alone do not increase the prevalence of IONI without the former 2 factors.
ION hypoaesthesia occurred in 37.3% of all ZMC fractures, which is in accordance with previous reported incidences of 30-80%.[
1,
15] The fracture line ran through the IOC in 60.9% of the fractures, and these cases were associated with significantly higher odds of having ION hypoaesthesia (OR
= 2.707;
p < .001). The same results were seen in cases with orbital floor involvement (OR
= 4.343) and in cases of dislocation or mobility (OR
= 2.543). In contrast, a fracture line adjacent to the IOC, and thus not perforating the cortex, was not significantly correlated with ION hypoaesthesia. These results indicated that ION hypoaesthesia was significantly correlated with anatomic features of the course of the fracture line itself, as well as the degree of displacement. These results are supported by evidence from the literature showing that ION hypoaesthesia is more common when the IOC is fractured, and that severe displacement is related to ION hypoaesthesia.[
10,
11,
15] Patients without dislocation or mobility reported significantly less hypoaesthesia (19.2%), which was in line with previous studies.[
15] However, these previous studies did not use the Zingg classification, and described the use of different methods to assess nerve function.
The surgical management of an ION injury remains controversial, although multiple authors recommend nerve decompression through ZMC reduction.[
16,
19,
20] Others suggest that the occurrence of ION hypoaesthesia alone is not an indication for operation.[
12,
21] In this present study, if hypoaesthesia did not recover after surgical reduction of the ZMC, the next step was administration of the oral vitamins B1-B2-B6-B12 and/or intramuscular vitamins B1-B6-B12, based on a proven treatment protocol for inferior alveolar nerve injuries.[
22] Significant improvement of neurological status after vitamin treatment could not be confirmed in this study due to the low number of patients in the respective groups. However, ION hypoaesthesia exhibited an overall mean recovery time of 4.3 months, and all reported recoveries occurred during a range of less than 2 years. Nevertheless, some patients with ION hypoaesthesia developed neuropathic pain symptoms, and received different treatment. Following ION injury, neuropathic pain can develop into a chronic pain condition with important psychosocial impacts and consequent influences on quality of life.[
23] Therefore, it is important to manage and treat these patients in a timely manner when possible. In this study, neuropathic pain symptoms developed in a mean time of 1.5 months after trauma, and 50% of patients recuperated after a mean of 9.0 months. Neuropathic pain symptoms were slightly more common among women, and were only associated with type B or C fractures.
Even though ZMC fractures disturb the anatomical location of the ZMC, there are preserved reliable fixed landmarks that maxillofacial surgeons can depend on to identify and preserve the ION. In the future it is likely that advanced 3D imaging and related analysis may aid in ION identification and preservation.[
12,
21]
A ZMC fracture can potentially lead to damage of the zygomatic branch of the facial nerve, which can result in disfigurement with cosmetic, functional, and psychologic repercussions.[
24] While injuries of the infraorbital nerve are commonly described, facial nerve complications related to ZMC fractures are rarely reported.[
25] Some authors have suggested that facial nerve disturbances might be a postsurgical complication,[
12,
25] but this could not be confirmed in our present study.
Over the last decade, several publications on maxillofacial traumatology have described the course of the fracture line in ZMC fractures and its relationship with ION hypoaesthesia. This present study provides additional perspectives regarding clinical and radiological predictors of ION hypoaesthesia. Although a number of studies have previously identified that ION hypoaesthesia is related to the energy of impact and fracture pattern, our present study differs in that we used the Zingg classification and distinguished between fracture course involvement through versus near the IOC. Identifying the relationship between the fracture course and ION sensory disturbances may provide valuable information to both practitioners and patients.
The major limitation of this study was its retrospective nature. There remains a need for longer, carefully designed, prospective, and multicentre studies with longer follow-up intervals to more thoroughly investigate the duration of ION hypoaesthesia. In the present retrospective research setting, we could examine the duration of ION hypoaesthesia after trauma in 52 patients. In nearly half (48%) of the cases, objective post-traumatic ION function was not retrievable, mostly because of loss to follow-up, and this bias increased with duration. Furthermore, the reported associations were found to be significant in the multivariate model, but the R
2 of the multivariate model was only modest, suggesting that other co-existing factors likely play roles in the mechanism of ION hypoaesthesia. Another limitation was the variety of fracture classifications and diagnostic methods in the literature, which makes it difficult to compare our findings with the literature. The use of objective methods, such as electrical testing or a standardized questionnaire as proposed by Homer et al,[
10] could improve the diagnostic outcome and facilitate comparisons between different studies.