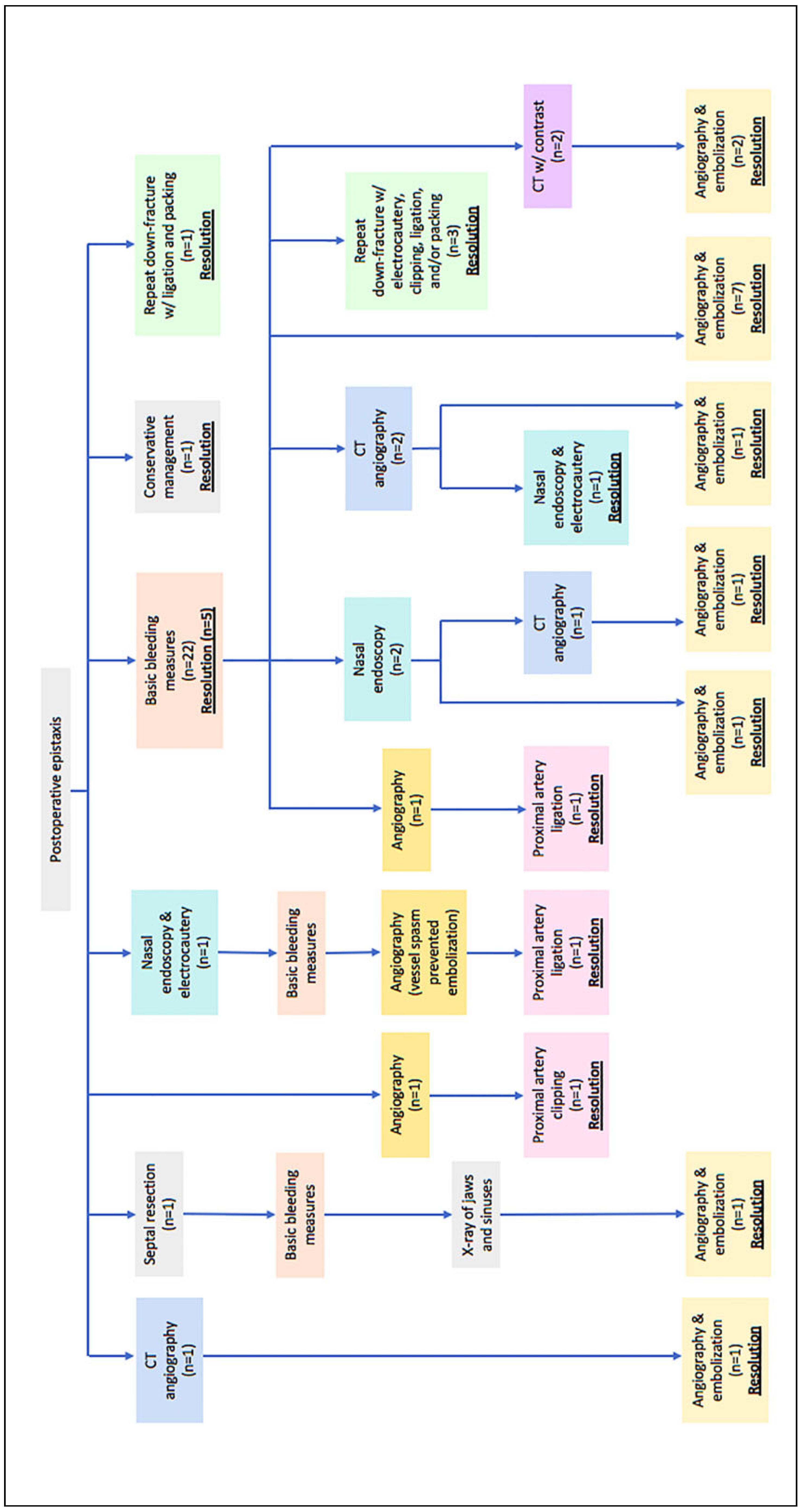
Introduction
Orthognathic surgical techniques improve occlusion, airway function, and facial aesthetics in patients with maxillofacial deformities, which affect approximately 5 percent of the US.[
1] In 2008, more than ten-thousand hospitalizations for orthognathic surgery were identified in the US.[
1] Among these procedures, one of the most commonly performed is the Le Fort I maxillary osteotomy.[
2] The Le Fort I procedure can correct malocclusion, vertical maxillary hypoplasia, and vertical maxillary excess.[
3,
4] This procedure is frequently utilized due to its short duration, straightforward anatomy, reliable long-term results, and versatile mechanism to reposition the maxilla in three-dimensional space.[
2,
5,
6] Over the past few decades, advances in Le Fort I surgical techniques have reduced complication rates associated with surgical mobilization, down-fracture, and repositioning of the maxilla.[
2,
3,
5] Despite these improvements, some perioperative risks persist. Complication rates of Le Fort I osteotomies range from 4-9%.[
3,
5,
6,
7] Perioperative bleeding is the most common complication of orthognathic surgery, and has the greatest potential to become life-threatening.[
7,
8]
Epistaxis is a well-known postoperative complication of orthognathic surgery. Indeed, some degree of blood-tinged nasal discharge is expected after Le Fort I osteotomy and may persist for 10-14 days.[
9] However, intraoperative vessel injury, traumatic nasal intubation, or separation of the nasal mucosa from underlying structures are common causes of early postoperative epistaxis.[
3] Most instances of postoperative epistaxis are addressed with nasal packing for 3 to 5 days,[
3,
10] and are so-called “isolated epistaxis.” Since there is no consensus as to the expected duration of isolated epistaxis,[
11] it can be difficult to discern between appropriate conservative management and delayed intervention of more severe bleeding, which has been observed as early as the first postoperative week.[
12]
Life-threatening hemorrhage is rare but has been documented.[
3,
10] The etiology of severe epistaxis includes pseudoaneurysms,[
11,
13,
14] arteriovenous fistulas,[
15] and hemorrhage secondary to intraoperative vascular injury.[
16] In the case of pseudoaneurysms, presentation ranges from repeated episodes of epistaxis to a single massive bleed.[
11,
13,
15] It is generalized that pseudoaneurysms usually present as unilateral posterior epistaxis, Pseudoaneurysminduced epistaxis is refractory to conservative management, presenting as either unilateral or bilateral nasal bleeding,[
17,
18] with onset anywhere from a few days to 11 weeks postoperatively. [
14,
15,
19] Pseudoaneurysms have been noted to require transfusions,15 multiple hospital readmissions,[
15] and even operative management with repeat down-fracture of the maxilla.[
20] Therefore, epistaxis that is recurrent, delayed, or resistant to conservative management warrants further investigation.[
3]
Clinically, it is challenging to distinguish between different causes of epistaxis. This is, in part, due to limited literature and poor characterization of less common causes. This increases the risk of a delay in diagnosis of serious causes of hemorrhage. Despite the rarity of massive postoperative hemorrhage in orthognathic surgery, the potential for mortality calls for a more consolidated and organized approach to managing epistaxis following orthognathic surgery. There are no evidence-based guidelines for evaluation or management of epistaxis following Le Fort I osteotomy.[
11] The purpose of this study is to 1) review the literature to characterize various presentations of epistaxis following orthognathic surgery, 2) identify management approaches and their associated consequences, and 3) to synthesize a treatment algorithm to guide future management of postoperative epistaxis.
Methods
A PubMed literature search was conducted for articles published between June 1990 and October 2020 investigating the management of epistaxis as a postoperative complication of orthognathic surgery. The search terms “epistaxis” AND “orthognathic surgery” were queried. The authors reviewed full texts and reference lists from relevant papers to identify further articles. Inclusion criteria consisted of articles in English, retrospective cohort studies, case reports, case series, and case reports with detailed literature review. Reports of patients with anatomic anomalies or prior orthognathic injuries or surgeries were included. Exclusion criteria consisted of non-English articles, review articles without case reports, technique papers, and perspective papers.
Data on postoperative epistaxis presentation, source of bleeding, diagnostic procedures, and treatment interventions were reported. Additional data collected included any mention of intraoperative complications, patient anatomical anomalies, and prior orthognathic surgeries or injuries that may have contributed to patient outcomes.
Results
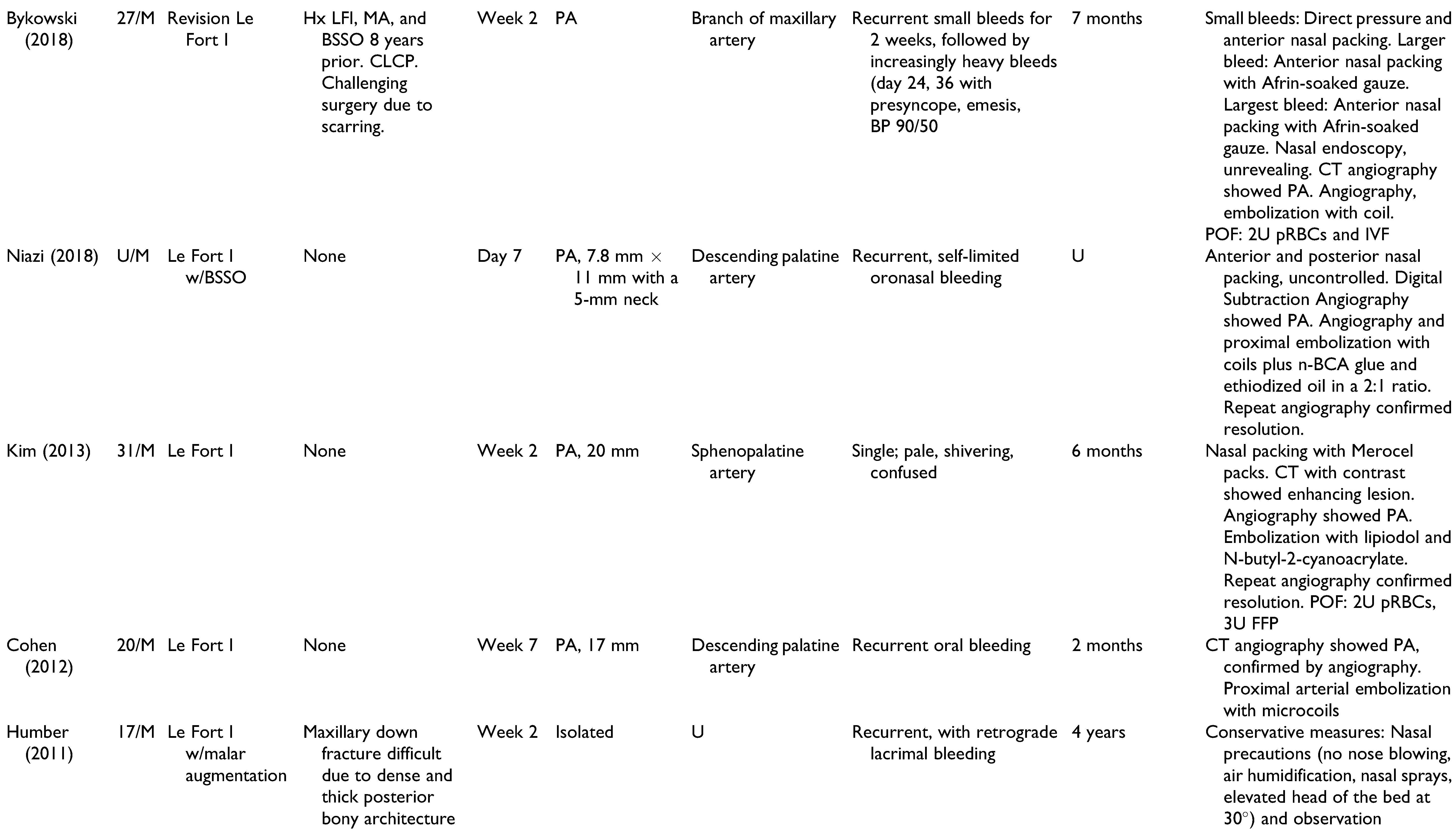
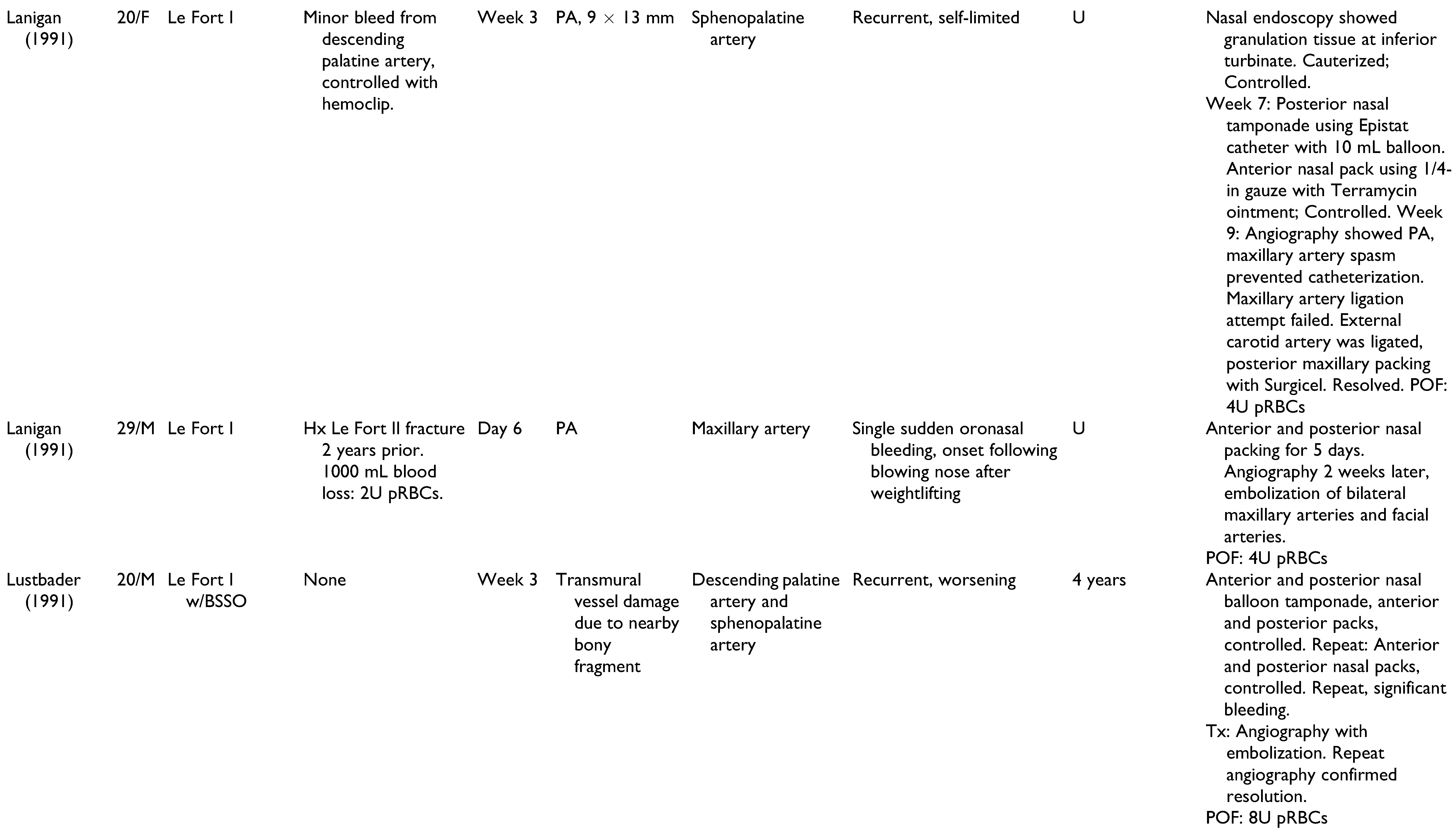
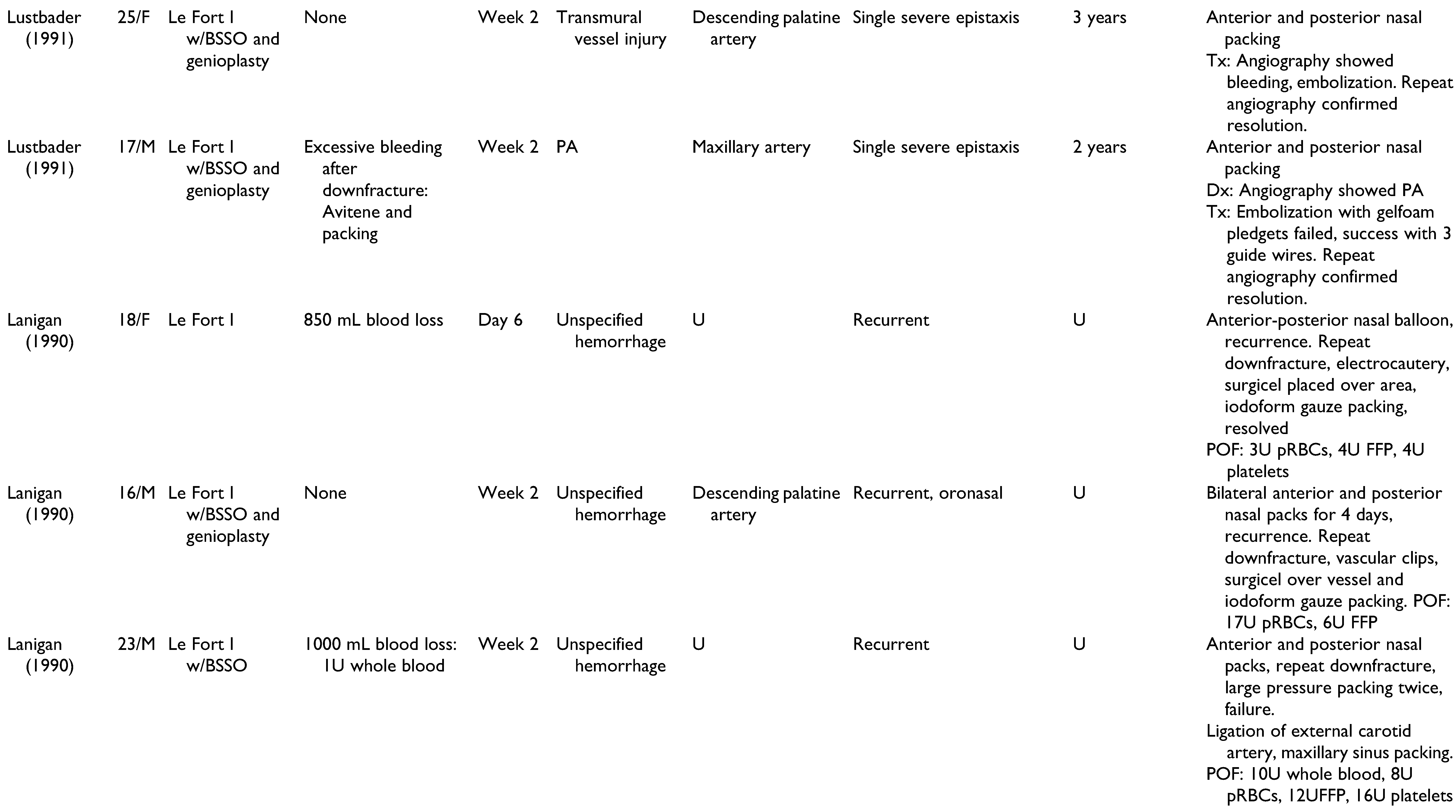
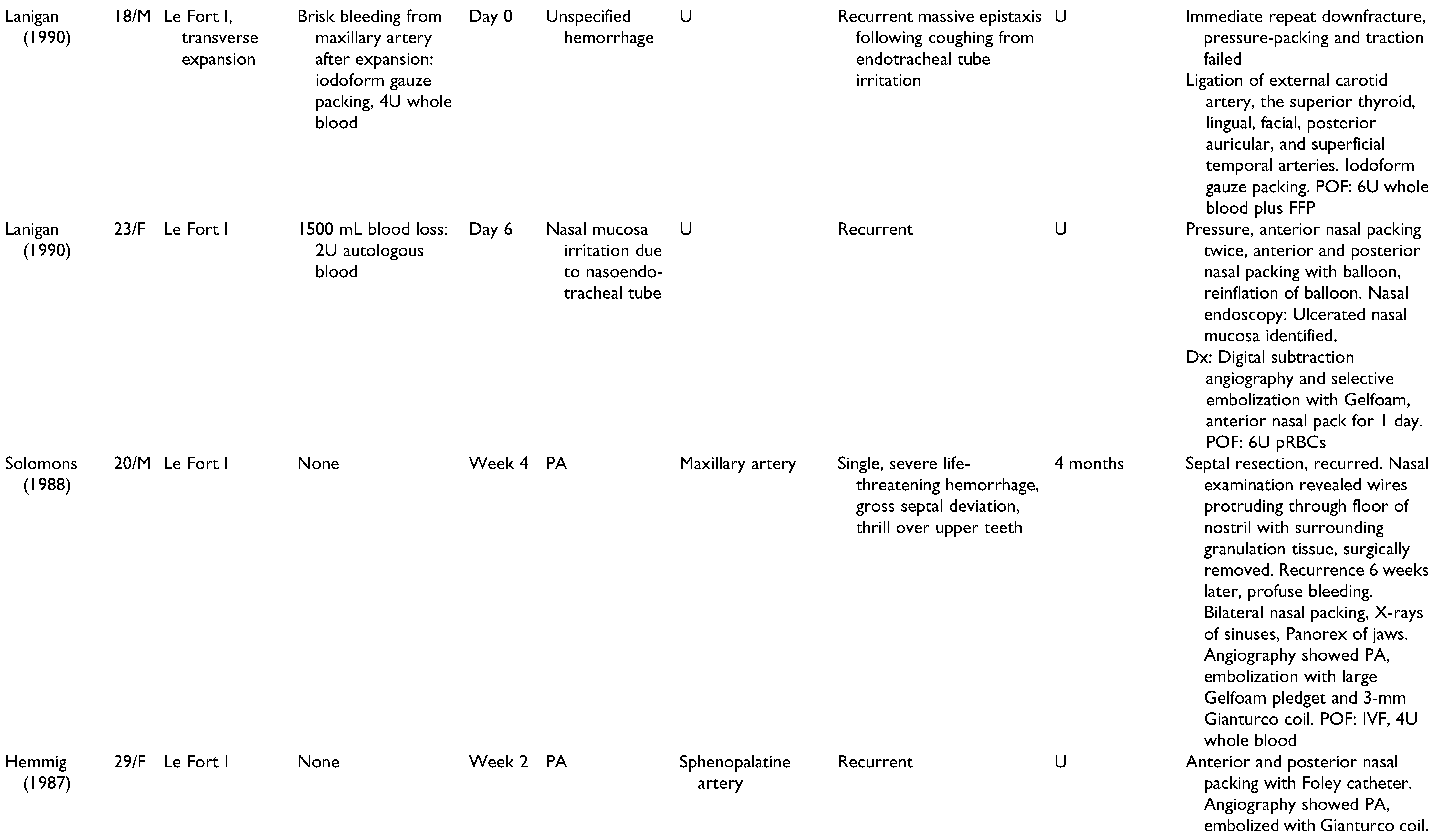
A total of 22 publications were full-text reviewed, and 17 studies qualified after exclusion criteria were applied (
Figure 1). These include 6 case reports,[
13,
16,
21,
22] 3 case series,[
15,
17,
18,
20,
23] 1 prospective study,[
7] 3 retrospective studies,[
24,
25,
26] and 4 case reports with a detailed literature review.[
11,
14,
27,
28] Lanigan et al included a survey of oral and maxillofacial surgeons regarding major postoperative hemorrhage following maxillary surgery in 21 patients.[
20] Cohen et al presented a retrospective study of 8 cases of postoperative epistaxis, although only 1 case occurred following orthognathic surgery.[
25] A total of 28 cases were assessed and summarized in
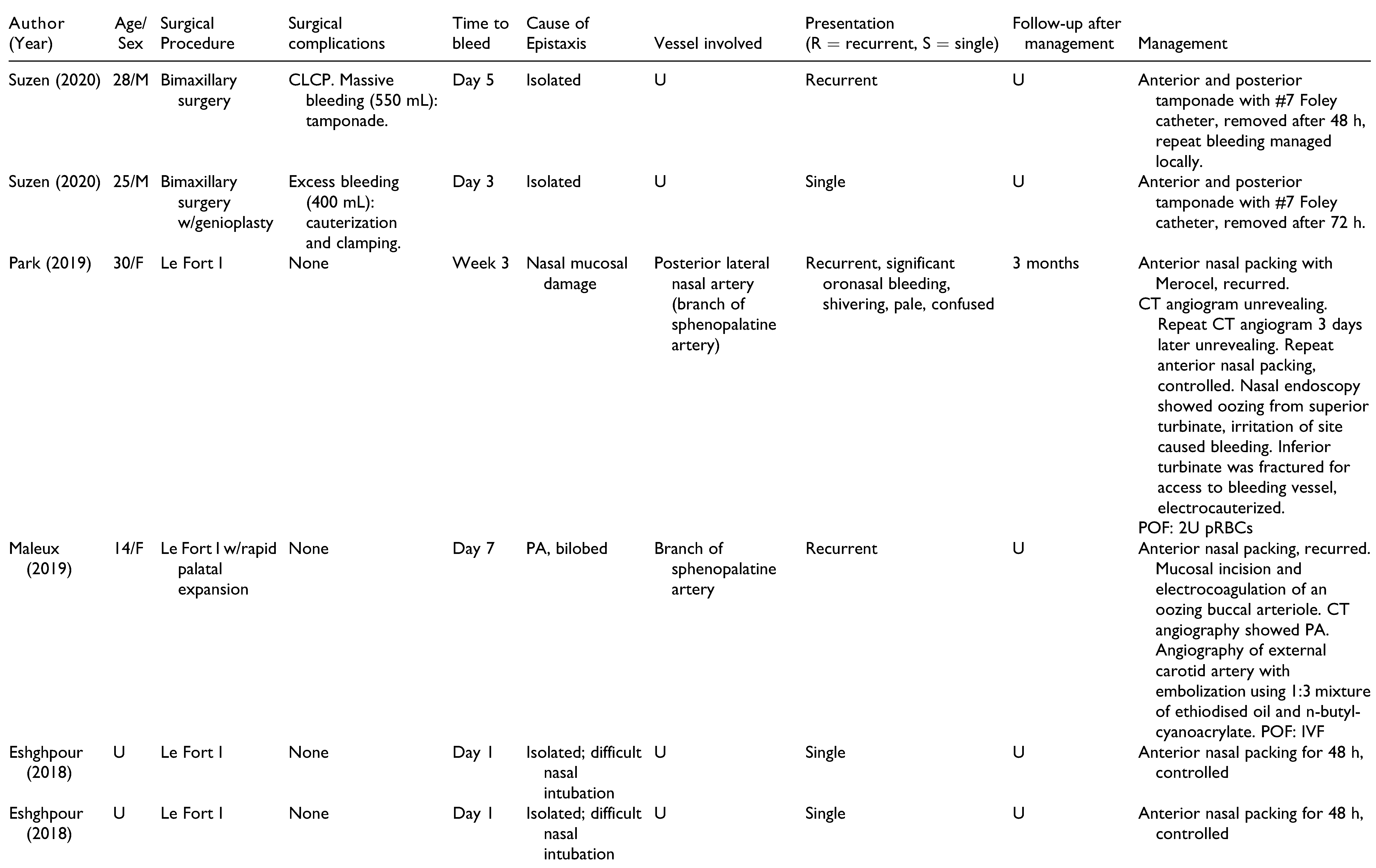
Table 1. Patients included 15 men, 11 women, and 2 unspecified. Patients had a mean age of 22.6 years (range 13-41 years), and onset of epistaxis occurred from 0 days to 9 weeks postoperatively. These studies offer a broad spectrum of epistaxis etiologies and presentations, highlighting the challenges of making an accurate diagnosis and appropriate treatment. All cases of epistaxis were controlled without long-term complications.
Mechanism of Vascular Injury
In 10 cases, the source of bleeding was not identified (n = 10), due to either successful hemostasis with anterior and posterior nasal packing [
7,
24,
27] or poor visibility during massive hemorrhage warranting emergent ligation of the proximal arterial inflow.[
20] In cases without time for diagnostic imaging, the type of vessel damage (extensive mucosal injury vs. transmural vessel damage vs. pseudoaneurysm rupture) was also undetermined (n = 4).
Isolated epistaxis resolved with nasal packing in 5 cases. However, Park et al and Lanigan et al described cases of more extensive nasal mucosal injury, which persisted despite nasal packing.[
16,
20] Park et al identified the posterior lateral nasal artery, a branch of the sphenopalatine artery, to be the source of bleeding.[
16] Posterior vessels are larger in caliber than the distal arteries and capillaries of the anterior nasal cavity, and are more likely to cause extensive and persistent bleeding when damaged.[
27] Excessive intraoperative bleeding (n = 10), particularly following maxillary down-fracture or palatal expansion, indicates large vessel injury that may rebleed postoperatively.[
20] Similarly, thickened or scarred bone may require that excess force be used during surgery, predisposing to vessel injury.[
11] This was the cause of bleeding attributed to a case of prior facial fracture (n = 1) and previous orthognathic surgery (n = 1).
Half of cases were attributed to pseudoaneurysmal bleeding (n = 14), with epistaxis onset ranging from postoperative day 6 to week 9. Pseudoaneurysms are rare because the terminal branches of the maxillary artery are small in caliber and therefore are more likely to be completely transected.[
13] As a result, pseudoaneurysms are more common in the larger caliber vessels, which are located posteriorly.[
11,
13] Unfortunately, it is impossible to differentiate transmural vessel injury from a pseudoaneurysm without imaging. Although pseudoaneurysms may present with pulsatile swelling,[
10] this finding is often absent, particularly in deep vessels. The true incidence of asymptomatic pseudoaneurysm following orthognathic surgery is unknown because postoperative imaging is not routinely performed.[
11] Rupture may go unrecognized until massive epistaxis occurs, which may explain the range of time to first bleeding episode.[
11] Several authors recommended that pseudoaneurysms should be considered when: 1) bleeding recurs despite conservative management, 2) bleeding does not resolve following nasal packing/tamponade, or 3) bleeding onset is delayed.[
11,
16,
28] Of the patients presented here, all bleeding episodes presenting after postoperative week 3 were caused by pseudoaneurysms. However, pseudoaneurysms should still be considered in the differential diagnosis in early post-operative epistaxis. For example, Bykowski et al have reported a patient who presented 2 weeks after surgery with epistaxis, yet the of a pseudoaneurysm was delayed until week 5.[
11]
Management of Postoperative Epistaxis
Management of all 28 cases of postoperative epistaxis is outlined in
Figure 2. Humber et al presented one case in which recurrent non-brisk epistaxis and lacrimal bleeding during postoperative week 2 were resolved using conservative management alone: air humidification, nasal sprays, bed head elevation to 30 degrees, and sinus precautions.[
27] In most cases (n = 22) epistaxis is first treated with anterior and/or posterior nasal packing and direct external pressure for 24-72 hours. Eshghpour et al recommends nasal packing for 3-5 days.[
7] Nasal packing and external pressure effectively resolved bleeding in 5 of the cases, all of which presented within the first postoperative week. In the 17 cases not controlled with packing and external pressure, further work-up was performed.
Computed Tomography with Contrast and Computed Tomography Angiography
Although CT with contrast was used in 2 cases, it is not a preferred method for assessing postoperative epistaxis. CT with contrast has a limited ability to discriminate between a pseudoaneurysm and overt bleeding secondary to vascular injury, particularly when imaging small vessels such as the sphenopalatine artery.[
11] Further, this modality may miss small defects altogether; a 20 mm pseudoaneurysm was identified, but a 2 × 2 mm pseudoaneurysm was missed.[
28] CT with contrast may not allow direct visualization of a pseudoaneurysm if obscured by hematoma or oronasal bleeding.[
13] Additionally, mini-plates used for maxillary fixation create metal artifact, thereby obstructing view.[
11] For these reasons, CT with contrast is either followed by another imaging modality,[
13] or excluded altogether. CT angiography was the initial imaging modality in 4 cases, 3 of which identified pseudoaneurysms. However, Cohen et al reported that CT angiography does not detect pseudoaneurysms 4 mm or smaller, compared to a minimum dimension of 6 mm pseudoaneurysm that can be detected with CT with contrast.[
25] For both imaging modalities, the radiation burden should be considered, particularly in young patients.[
29] Despite the convenience of CT with contrast and the slightly greater sensitivity of CT angiography, neither imaging modality offers therapeutic benefit, necessitating subsequent imaging to embolize the injured vessel.
Angiography and Embolization
Several authors describe angiography to be the diagnostic method of choice in cases of severe bleeding.[
13,
16] Angiography (aka. conventional angiography) is a highly accurate method by which iodinated contrast is injected through a catheter into a particular target vessel and imaged with fluoroscopy.[
28] Digital subtraction angiography (DSA) is a subset in which a pre-contrast mask image is taken and “subtracted” from the post-contrast image, resulting in only contrast-enhanced vessel visibility.[
30] Angiography was used in most cases (n = 17), often as the primary imaging modality (n = 11). Avelar et al demonstrated that angiography could identify a 2 × 2 mm pseudoaneurysm previously missed by CT with contrast.[
28] Angiography also depicts collateral flow from other vascular trees that may contribute to bleeding.[
22] Potential complications include mucosal ischemia and ulceration, cerebral infarction, and iodinated contrast media-related problems (e.g. allergic reactions and nephrotoxicity).[
22] Additionally, vessel spasm would prohibit catheterization, as seen in one case described by Lanigan et al, requiring that an alternative therapeutic approach be used.[
15]
A unique benefit of angiography is the opportunity to perform embolization during the same session.[
21] Fourteen cases used this dual diagnostic-and-therapeutic approach. Angiography with embolization is the ideal treatment for pseudoaneurysms and cephalic vessel bleeding, because the embolized material occludes the outpouching vessel wall rather than completely occluding the injured vessel.[
17,
18,
19,
25] In the case that the vessel is subsequently narrowed, shearing stress of flow through a stenosed vessel will stimulate formation of ancillary collateral blood vessel networks to reconstitute flow to distal structures, as seen in cases of chronic coronary or cerebral artery stenosis.[
19,
31,
32] Vessel patency prevents necrosis of the maxilla,[
15,
19] minimizes the risk of persistent hemorrhage from collateral flow, and enables subsequent accessibility in case of treatment failure.[
22] A variety of embolization materials were utilized (
Table 1). Six cases repeated angiography to confirm successful embolization.
Nasal Endoscopy
Although angiography is a favorable modality for massive postoperative epistaxis, in settings when examination is unclear or a diagnosis cannot be determined in an emergent case, nasal endoscopy is a less invasive and effective alternative.[
16] Recent studies have reported that the endoscopic approach is superior to nasal packing and embolization.[
16] The sphenopalatine artery and the posterior nasal artery are easily accessible through the endoscopic nasal approach, and ligation or direct cauterization can be performed. Compared with other approaches, endoscopy is less painful, less invasive, more cost-effective, and more clinically effective.[
33] However, nasal endoscopy may not be feasible in cases where extensive bleeding limits visibility.[
16]
Surgical Approaches
Surgical approaches were typically reserved as last resort for the management of hemorrhage or pseudoaneurysm to either directly target the bleeding site with electrocautery and pressure packing or acquire proximal control of the bleeding vessel via clipping or ligation.[
15,
20] Repeat maxillary down-fracture with surgical exploration was described only in 4 cases presented by Lanigan et al.[
20] Having occurred 30 years ago, it is possible that interventional radiology services were not available, making surgical intervention the next best step following nasal packing. One of these cases presented with significant bleeding intraoperatively and in the early postoperative period, and immediate down-fracture was used to locate, pack and ligate the bleeding vessels. More recent studies involved ligating or clipping proximal arteries after angiography was used to identify pseudoaneurysms in specific vessels. One case by Lanigan et al used ligation solely because vessel spasm prohibited catheterization and embolization following angiography.[
15] Overall, proximal control was not a preferred method, because collaterals and anastomoses may reconstitute flow through the pseudoaneurysm and cause rebleeding. Ligation also eliminates any future opportunity for selective endovascular embolization of the vascular tree.[
25] Knowledge of potential anastomotic routes, identification of the cranial nerve supply from the external carotid artery, and proper choice of embolic material are crucial to avoid neurologic complications during ligation.[
25] In cases where the external carotid artery or maxillary artery was ligated, healing of the maxilla was not impaired, and no neurologic complications were reported.
Hospital Stay
A retrospective review by Suzen et al found that only 1% of patients with massive intraoperative bleeding were kept in the hospital for longer than the average length of stay, which is ranges from 1-3 days depending on whether the patient underwent singleor double-jaw procedure, operation time, and procedure complexity.[
24] Although there is no consensus as to the relationship between massive intra-operative hemorrhage and postoperative epistaxis,[
24] 8 of 9 cases complicated by large-volume intraoperative bleeding presented with epistaxis within the first postoperative week. Some studies therefore recommend that orthognathic patients be hospitalized for 8-10 days postoperatively, regardless of intraoperative blood loss.[
3,
12]
However, most of the cases reviewed in this study presented after this window, many requiring repeat hospitalizations due to epistaxis recurrence. Additionally, life-threatening postoperative epistaxis is rare, and all such cases in this study were well-managed without long-term complication. Further, such cases do not justify the incurred hospital costs and loss of patient autonomy that would result from routine extension of postoperative hospital stay.
An Algorithm for Management of Postoperative Epistaxis after Orthognathic Surgery
The cases of postoperative epistaxis reviewed in this paper demonstrated a variety of intraoperative complications, epistaxis presentations, and surgeon approaches to appropriate management. The goal of this review was to identify diagnostic and therapeutic modalities that are accurate and effective, while limiting interventions to avoid excessive financial, emotional, and time strain. With consideration of author discussions regarding decision-making and outcomes, an algorithm was constructed to summarize the steps of epistaxis management that are most supported by the literature (
Figure 3). Basic bleeding measures such as nasal packing, tamponade, and direct pressure are likely to resolve isolated cases of epistaxis. Nasal endoscopy with electrocautery is an effective local approach when mucosal or capillary damage is suspected. Angiography is superior to CT with contrast and CT angiography due to its ability to detect pseudoaneurysms as small as 2 mm. With a 0.03% mortality and a 1.73% morbidity rate,[
34] angiography is the imaging modality of choice in postoperative epistaxis, particularly in cases of delayed, recurrent, or massive hemorrhage when a pseudoaneurysm may be implicated. Surgical approaches are to be used as a last resort and include proximal vessel ligation or repeat maxillary down-fracture with surgical exploration.
Case 1
A 31-year-old transgender female patient with class 3 malocclusion underwent Le Fort I osteotomy with mandibular sagittal split osteotomy and genioplasty. Past surgical history included frontal sinus surgery 1 year prior, and facial feminization of the lower jaw and tracheal shave 2 years prior. Atraumatic right nasal endotracheal intubation was achieved upon first attempt, and good occlusion was achieved using intermaxillary fixation with the final splint. Liposuction of the abdomen and lateral thighs were performed and 10 cc of fat were then injected into the midface for projection and contouring. The patient was placed in elastics, and she was extubated without any complications. Intraoperative blood loss was estimated to be 300 mL, and she was discharged on postoperative day 2.
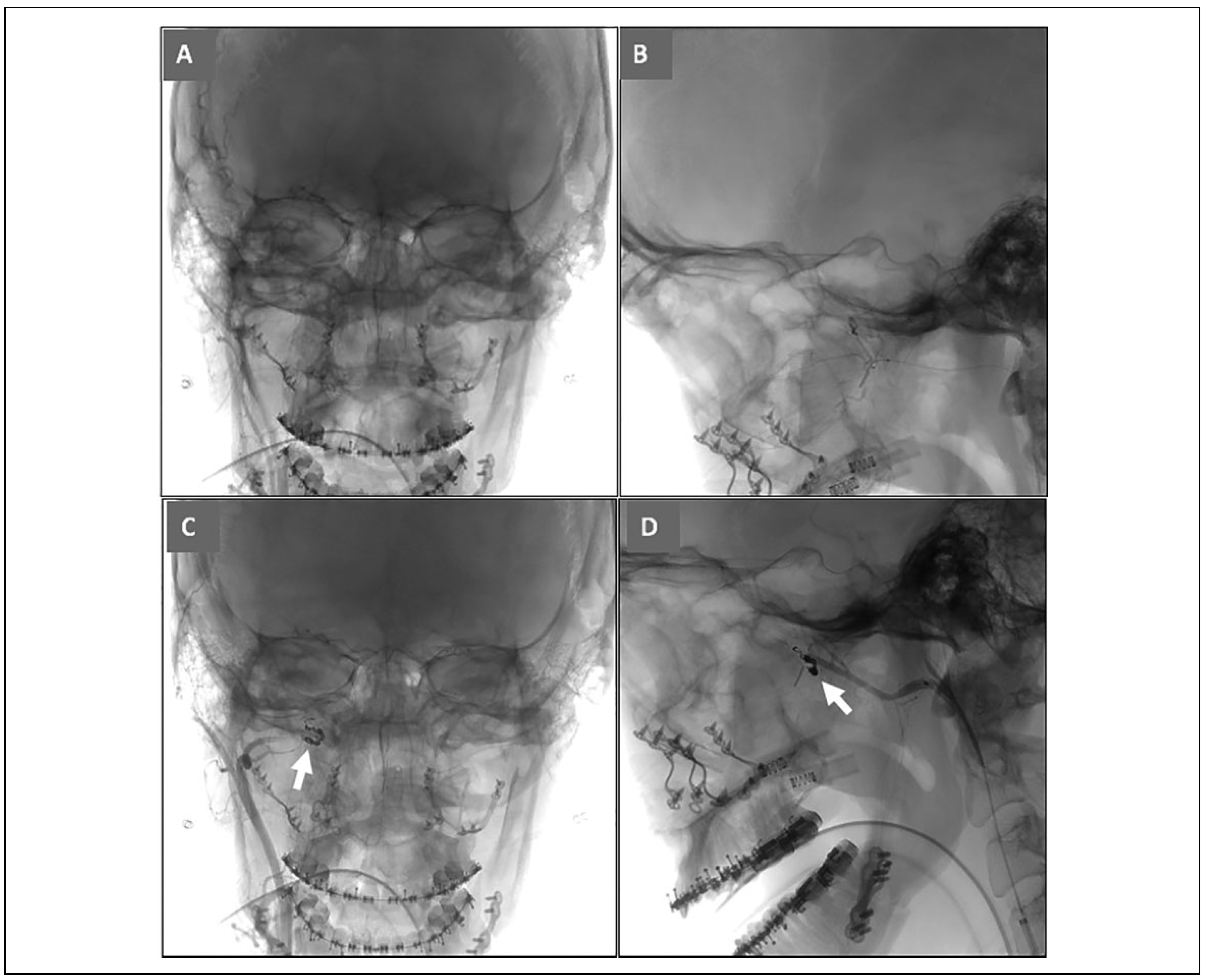
Nine days postoperatively, she experienced a short episode of bright red right nasal bleeding that progressed to bilateral when her head was bent down. Bleeding stopped after she pinched her nose and tipped her head back, but promptly recurred, causing her to come into a local emergency department. Anterior cotton pledgets soaked with 1:1 4% lidocaine and epinephrine were placed for 20 minutes, then soaked with transexamic acid, and repeated without hemostasis. Oral bands were removed, and bilateral posterior nasal tampons were inflated. Bleeding recurred a few minutes later, and a large clot was suctioned from her oropharynx. CBC, BMP, PT, INR, and aPTT were within normal limits. She had a near syncopal episode, and dizziness improved with repositioning and 2 L normal saline. She was discharged after 3 hours of observation. Later that day, she followed up with her surgeon due to recurrent persistent bilateral nasal and oral bleeding. Labs included a hemoglobin of 8.0 g/dL from 12.1 g/dL at the local emergency department earlier that day. Distal right internal maxillary artery embolization was performed using hydra coil 2 × 8 cm, 1.5 × 4 cm, 2 × 6 cm (
Figure 4). Distal left internal maxillary artery embolization was performed using polyvinyl alcohol (PVA) 500-700 um was performed until flow arrest, and she was kept for observation for 2 days. She has had no further bleeding events within the 2-month period following embolization.
Case 2
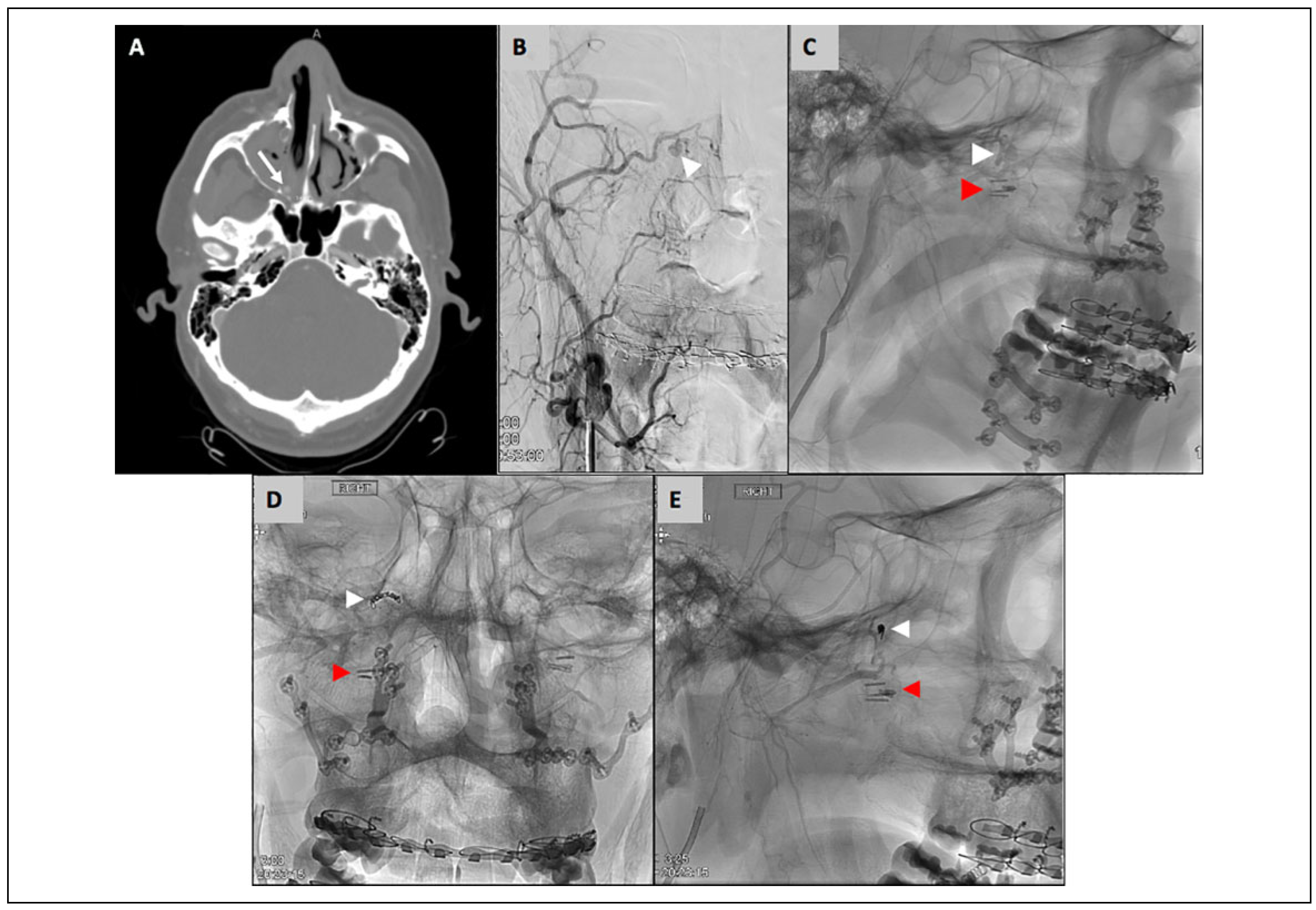
A 33-year-old male with obstructive sleep apnea underwent Le Fort 1 osteotomy with bilateral sagittal split osteotomies for maxillomandibular advancement. Notably, the descending palatine arteries were ligated intraoperatively, which is routinely done by the operating surgeon. The patient was discharged on postoperative day 1. He then presented to the emergency department 15 days postoperatively with moderate right-sided bright red nasal bleeding and mild right facial swelling. The right nasal cavity was packed with a 7.5 cm rhino-rocket. He was hemodynamically stable and had no other complaints at this time. Laboratory data were notable for a hemoglobin of 10.2 g/dL, which had decreased from a hemoglobin of 15.5 g/dL on the day of surgery. A CT angiogram was obtained, which showed a right sphenopalatine artery pseudoaneurysm (3 mm) with active blush (
Figure 5). Neurointerventional radiology was consulted, who ultimately embolized the pseudoaneurysm with 2 coils. The coils measured 1.5 mm × 3 cm and 1 mm × 3 cm. The nasal packing was left in place and he was observed overnight. He was discharged on postoperative day 1 and returned to clinic on postoperative day 3 for removal of the nasal packing. At 3 months postoperatively, there was no recurrent bleeding.
Case 3
A 24-year-old male with a history of unilateral cleft lip/ palate underwent single piece Le Fort 1 advancement/ impaction with bilateral sagittal split osteotomy and genioplasty. His surgical course was uneventful. Bilateral descending palatine arteries were not isolated or ligated during the Le Fort 1 disimpaction. He was discharged postoperative day 2. He returned to clinic 1 week later for adjustment of his postoperative rubber bands.
On postoperative day 11 he presented to the emergency room with an epistaxis episode that was initially controlled with placement of balloon rhinoplasty packing. He was discharged home for follow up and removal in clinic in 2 days, which resulted in recurrent bleeding that was controlled with more nasal packing. He was sent to the emergency room and neuro-interventional radiology was consulted. Emergent CT angiogram identified a ruptured pseudoaneurysm along the branch of the left internal maxillary artery (
Figure 6). Hemostasis was achieved with coiling and nasal packing, and he was admitted for observation. His nasal packing was removed the next day which did not have any subsequent bleeding episodes.
Discussion
Approximately 27.4 million orthodontic procedures are performed in the US annually.[
35] Up to 19% of individuals presenting for orthodontic treatment may require additional orthognathic surgery to fully address functional and aesthetic concerns caused by maxillofacial deformity.[
1] In recent decades, the number of patients seeking orthognathic surgery has increased, and it is projected that this rise will continue due to sociocultural prioritization of facial appearance.[
5,
36] Though commonly performed, orthognathic surgery is not without serious risk. A systematic review of 44 articles concluded that postoperative hemorrhage is the most common complication following maxillary surgery, occurring in 9% in patients undergoing Le Fort I osteotomy.[
37] Life-threatening postoperative hemorrhage is rare, with an incidence of 0.7% or less.[
37] It is challenging to differentiate between manageable and life-threatening causes of postoperative epistaxis. As a result, patients are at risk of a delayed diagnosis, unnecessary testing, and ongoing blood loss.
At present, there are no evidence-based guidelines for evaluation or management of epistaxis following Le Fort I osteotomy.[
11] To our knowledge, this study is the first to offer an evidence-based management algorithm. Gart et al offers a similar algorithm for management of intra- and postoperative hemorrhage in patients undergoing oral or maxillofacial surgery.[
38] The highest potential level of detail to be included in these algorithms is limited by the inconsistent presentation of different hemorrhage etiologies. For example, life-threatening pseudoaneurysm rupture may present anytime within the first 9 postoperative weeks with either sudden massive hemorrhage, recurrent small bleeds, or a combination. Therefore, the surgeon must assess the patient as an individual and use his or her best judgement when making diagnostic and therapeutic decisions.
Patients are traditionally monitored in the hospital for 1 to 3 days postoperatively. However, 82% of the cases reviewed in this study presented with epistaxis after this period. Although some studies have suggested extending the postoperative stay to 8-10 days,[
3,
12] half of the reviewed cases presented later than this still. Additionally, routinely longer inpatient stay would increase hospital costs and threaten patient autonomy, often without reasonable cause. Unfortunately, there are no well-supported predictors of postoperative epistaxis to assist in patient surveillance. Suzen et al recommends extended stay for patients who experienced massive intraoperative bleeding.[
24] Of the 9 reviewed cases that were complicated by excessive intraoperative bleeding, 6 presented with an initial epistaxis episode occurring more than 3 days after surgery and as late as the second postoperative week. Further, the low incidence of life-threatening epistaxis offers reason to limit postoperative hospital stay.
Most cases of delayed epistaxis were life-threatening (e.g. pseudoaneurysm rupture), so it is essential that patients are provided with sufficient information regarding potential hemorrhagic complications following discharge. Patients should be encouraged to go to the emergency room in situations where hemostasis cannot be achieved with conservative management. Additionally, the surgical team should be on-call and prepared for rehospitalization with any massive postoperative bleeding.[
24] Basic bleeding measures should be used initially, extending to include bilateral nares or both anterior and posterior tamponade should epistaxis persist. Nasal endoscopy is another approach that may identify mucosal damage or be used in conjunction with cautery to resolve bleeding. Should local approaches fail, angiography is the best imaging modality to follow, commonly used due to its high accuracy and opportunity for concurrent embolization. If embolization is unavailable, the patient should undergo proximal control and ligation of the involved branch of the external carotid artery.